Abstract
OBJECTIVE
This study examines the usefulness of childhood glucose homeostasis variables (glucose, insulin, and insulin resistance index [homeostasis model assessment of insulin resistance {HOMA-IR}]) in predicting pre-diabetes and type 2 diabetes and related cardiometabolic risk factors in adulthood.
RESEARCH DESIGN AND METHODS
This retrospective cohort study consisted of normoglycemic (n = 1,058), pre-diabetic (n = 37), and type 2 diabetic (n = 25) adults aged 19–39 years who were followed on average for 17 years since childhood.
RESULTS
At least 50% of the individuals who ranked highest (top quintile) in childhood for glucose homeostasis variables maintained their high rank by being above the 60th percentile in adulthood. In a multivariate model, the best predictors of adulthood glucose homeostasis variables were the change in BMI Z score from childhood to adulthood and childhood BMI Z score, followed by the corresponding childhood levels of glucose, insulin, and HOMA-IR. Further, children in the top decile versus the rest for insulin and HOMA-IR were 2.85 and 2.55 times, respectively, more likely to develop pre-diabetes; children in the top decile versus the rest for glucose, insulin, and HOMA-IR were 3.28, 5.54, and 5.84 times, respectively, more likely to develop diabetes, independent of change in BMI Z score, baseline BMI Z score, and total-to-HDL cholesterol ratio. In addition, children with adverse levels (top quintile versus the rest) of glucose homeostasis variables displayed significantly higher prevalences of, among others, hyperglycemia, hypertriglyceridemia, and metabolic syndrome.
CONCLUSIONS
Adverse levels of glucose homeostasis variables in childhood not only persist into adulthood but also predict adult pre-diabetes and type 2 diabetes and relate to cardiometabolic risk factors.
Diabetes has become one of the most commonly prevalent chronic diseases with related mortality in the U.S. (1). There are ∼19 million people with type 2 diabetes and another 54 million people with impaired fasting glucose or pre-diabetes in the country (2). It is also widely recognized that diabetes is a major contributor to adult cardiovascular morbidity and mortality and often accompanies hypertensive renal disease (3).
Type 2 diabetes is preceded by a pre-diabetic state linked to relative insulin resistance associated with mild increases in blood glucose levels, despite hyperinsulinemia (4). A number of studies have indicated that hyperinsulinemia/insulin resistance is associated with cardiometabolic risk factors including obesity, dyslipidemia, and hypertension, a constellation of disorders characteristic of the metabolic syndrome (5,6). Previous findings, including our own, have shown that the elevations in insulin (7,8) and glucose (9,10) levels persist (track) over time in children and adults alike. We have reported that individuals with relatively high/low fasting plasma insulin levels tended to remain so 8 years later; and significant clustering of obesity, hypertension, and dyslipidemia occurred primarily among those with persistently elevated levels (8). However, information is scant regarding whether the adverse levels of glucose homeostasis variables (glucose, insulin, and insulin resistance index) in childhood persist over time and predict pre-diabetes and type 2 diabetes and other cardiometabolic risk factors in apparently healthy young adults. The present analysis examines this aspect as part of the Bogalusa Heart Study, a biracial (black and white), community-based investigation of the evolution of cardiovascular disease risk beginning in childhood (11).
RESEARCH DESIGN AND METHODS
The retrospective study cohort was derived from the two sets of the cross-sectional surveys conducted in the community (65% white and 35% black subjects) of Bogalusa, Louisiana, involving five cross-sectional surveys of children during 1981–1994 (n = 13,444; 40% black and 50% female) and three cross-sectional surveys of adults during 1995–2000 who remained in the community and participated in the study (n = 3,640). Subjects (n = 1,120; 36% black and 60% female) who participated in their childhood and adulthood and had fasting blood samples on both examinations were included in the study. At the baseline examination, the children with a history of the treatment of diabetes or who had a fasting glucose level ≥100 mg/dl (5.6 mmol/l) were excluded. These subjects were 4–18 years of age (means ± SD age 11.6 ± 3.6 years) at baseline and 19–39 years of age at follow-up (mean ± SD age in adulthood was 28.3 ± 5.1 years). With respect to age, race, sex, overall adiposity (BMI Z score) and lipid, glucose, and insulin profile, the baseline childhood characteristics of the study cohort, which represented 8% of the original ascertained childhood population, were similar to the characteristics of the subjects who did not participate in the follow-up survey as adults (data not shown).
According to the American Diabetes Association criteria (12), adult subjects were classified as normoglycemic (n = 1,058) if they had a fasting glucose level <100 mg/dl (5.6 mmol/l), pre-diabetic (n = 37) if they had a fasting glucose level between 100 and 125 mg/dl (5.6–6.9 mmol/l), and diabetic (n = 25) if they had a fasting glucose level ≥126 mg/dl (7 mmol/l) or were taking medication for diabetes. Informed consent was obtained from all participants, and the study was approved by the institutional review board of the Tulane University Health Sciences Center.
General examination
Standardized protocols were used by trained examiners across all surveys (13). Participants were instructed to fast for 12 h before the venipuncture, and compliance was ascertained by an interview on the day of examination. Information on personal health history (e.g., hypertension, dyslipidemia, or diabetes and medical treatment for these conditions) was obtained by questionnaires. Anthropometric and blood pressure measurements were made in replicate and mean values were used. BMI (in kg/m2 = weight in kilograms divided by the square of height in meters) was used as a measure of overall adiposity; waist circumference was used as an indicator of abdominal visceral fat. BMI Z scores for childhood were calculated from the 2000 Centers for Disease Control and Prevention (CDC) growth charts to account for the differences in BMIs by sex and age (14). These growth charts express the BMIs of children in the current study relative to their sex- and age-matched peers in the U.S. between 1963 and 1980; BMIs of 5 year olds in the CDC growth charts also include data from 1988 to 1994. BMIs for adulthood were standardized based on age- and sex-specific means and SDs. The calculated Z scores are termed “BMI Z score” in the current analyses. Right upper-arm length and circumference were used to select the cuff size for blood pressure measurements with mercury sphygmomanometers. Two randomly assigned nurses measured blood pressure (three replicates each) while subjects were in a relaxed, sitting position. Systolic and diastolic blood pressures were recorded at the first and fourth (children) or fifth (adults) Korotkoff, respectively. Mean arterial pressure (MAP), calculated as diastolic blood pressure plus one-third pulse pressure, was used in the analysis.
Laboratory analyses
Cholesterol and triglyceride levels were initially measured using chemical procedures on a Technicon Autoanalyzer II (Technicon Instruments) according to the laboratory manual of the lipid research clinics program. Later, these variables were determined by enzymatic procedures on the Abbott VP Instrument (Abbott Laboratories) between 1987 and 1996 and on the Hitachi 902 Automatic Analyzer (Roche Diagnostics) afterward. Both chemical and enzymatic procedures met the performance requirements of the lipid standardization program of the CDC, which has routinely monitored the precision and accuracy of cholesterol, triglycerides, and HDL cholesterol measurements since the beginning of this study. Serum lipoprotein cholesterol levels were analyzed by using a combination of heparin-calcium precipitation and agar–agarose gel electrophoresis procedures (15). The intraclass correlation coefficients between the blind duplicate (10% random sample) values ranged from 0.86 to 0.98 for HDL cholesterol, 0.86 to 0.98 for LDL cholesterol, and 0.88 to 0.99 for triglycerides.
From 1976 to 1991, plasma glucose was measured initially by a glucose oxidase method using a Beckman glucose analyzer (Beckman Instruments). Since then, it has been measured enzymatically as part of a multichemistry (SMA20) profile. Plasma immunoreactive insulin levels were measured by a commercial radioimmunoassay kit (Phadebas, Pharmacia Diagnostics). The intraclass correlation coefficients between blind duplicate values ranged from 0.94 to 0.98 for insulin and 0.86 to 0.98 for glucose. In addition, an index of insulin resistance was calculated according to the homeostasis model assessment (HOMA) formula: HOMA of insulin resistance (HOMA-IR) = (insulin [μU/ml] × glucose [mmol/l]/22.5).
Metabolic syndrome risk factors in adults were identified if subjects were centrally obese (waist circumference >102 cm for male subjects or >88 cm for female subjects), were dyslipidemic (LDL cholesterol ≥160 mg/dl [4.14 mmol/l], triglycerides ≥150 mg/dl [2.26 mmol/l], HDL cholesterol <40 mg/dl [1.03 mmol/l] for male or 50 mg/dl [1.29 mmol/l] for female subjects, or on medication for dyslipidemia), were hyperglycemic (fasting glucose ≥100 mg/dl [5.6 mmol/l] or on treatment for diabetes), or were hypertensive (systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or on antihypertensive medication) (16). Metabolic syndrome was defined as coexistence of three or more of the above risk factors (16).
Statistical analysis
All of the statistical analyses were performed with SAS version 9.1 (SAS Institute). Continuous variables were tested for normality using a Kolmogorov-Smirnov test. Values of triglycerides, glucose, insulin, and HOMA-IR variables used in the analyses were log-transformed to improve normality. To evaluate the persistence or tracking of elevated levels of glucose, insulin, and HOMA index from childhood to adulthood, the baseline (childhood) age-, race-, and sex-specific top quintile for each of these variables was used as a cutoff point to classify children as having abnormal glucose homeostasis variables and to examine the distribution of such children among the corresponding adulthood quintiles 17 years later.
Models assessing the independent relations between childhood cardiometabolic risk factor variables and follow-up (adulthood) levels of glucose, insulin, or HOMA-IR were constructed using a stepwise multiple linear regression. The childhood independent variables initially included in these models were age, race, sex, BMI Z score, BMI Z score change from childhood to adulthood, MAP, ratio of total cholesterol to HDL cholesterol, as well as glucose (for model 1), insulin (for model 2), and HOMA-IR (for model 3). Since waist circumference in childhood was not measured, BMI Z score values were used as a childhood measure of obesity. The ratio of total to HDL cholesterol was chosen as a measure of dyslipidemia because it is a marker of insulin resistance characteristic of the metabolic syndrome.
A stepwise logistic regression analysis including childhood age, race, sex, BMI Z score, BMI Z score change over time, MAP, and total-to-HDL cholesterol ratio was then used to determine the odds ratio and 95% CI of developing pre-diabetes and diabetes in adulthood on the basis of childhood levels (top decile versus the rest) of glucose and insulin (model 1) and HOMA-IR (model 2). Collinearity was checked in the fixed model. To assess the overall fit of the logistic regression model, a Hosmer-Lemeshow goodness-of-fit test was performed. Because there was no interaction effect between childhood race (or sex) and glucose (or insulin and HOMA-IR) levels, the race-sex groups were combined to increase statistical power and to simplify the presentation. Since the prevalence of both pre-diabetes and diabetes in the study cohort was low (<3.5%) and the alternate analysis using the Cox proportional hazard model gave essentially identical results, only the results of logistic regression analysis estimating the relative risk of diabetes status are presented.
Finally, the prevalence of cardiometabolic risk factors in adulthood was examined according to childhood levels (top quintile versus the rest, specific for age, race, and sex) of glucose and insulin. Significant differences in the prevalence of the metabolic syndrome and its cardiometabolic risk factors in adulthood by childhood glucose, insulin, and HOMA-IR status were tested by the Pearson's χ2 test.
RESULTS
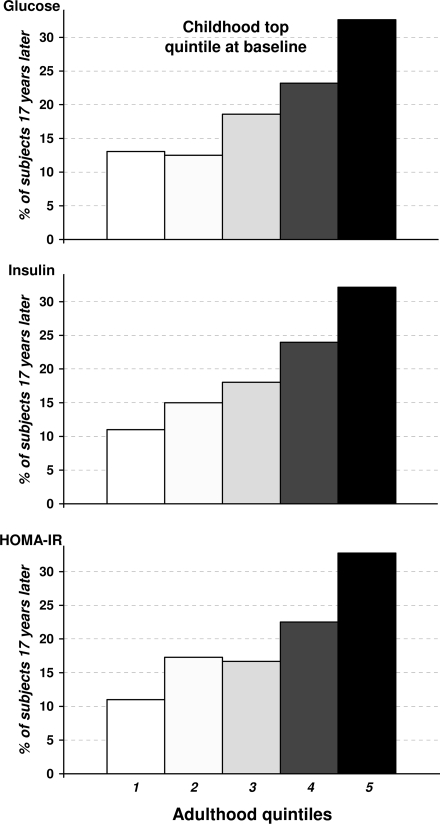
The persistence (tracking) of levels of glucose homeostasis variables (fasting glucose, insulin, and HOMA-IR) from childhood to adulthood was examined in terms of persistence of ranking in highest quintiles of the distribution over a 17-year period. If there is no persistence, 20% of those in a given quintile at baseline would persist in that ranking at the follow-up assessment by chance alone. As shown in Fig. 1, >32.1% of individuals who ranked highest (in the top quintile) with respect to glucose in childhood also did so in adulthood; another 22.5% remained in the next highest (fourth) quintile. In other words, 54.6% of individuals who ranked highest in childhood tended to maintain their high ranks by being above the 60th percentile in adulthood. Insulin and HOMA-IR levels showed similar trends for tracking over time. Further, these individuals (trackers) who maintained their high ranks by being above the 60th percentile in adulthood with respect to glucose, insulin, and HOMA-IR, compared with the nontracker group (individuals in top quintile at baseline and below the 60th percentile in adulthood), displayed consistently higher BMI Z score at baseline and BMI Z score change after 17-year follow-up (P < 0.05), after adjusting for age, race, and sex at baseline; no consistent trend was observed in other cardiometabolic variables (data not shown). With respect to tracking in the lowest quintiles from childhood to adulthood, the trends were essentially the same (data not shown).
Figure 1.
Tracking of glucose, insulin, and HOMA index over a 17-year period in young adults. The degree of tracking was evaluated in terms of distribution by adulthood quintiles at follow-up of subjects who were in the extreme top quintile specific for age, race, and sex at baseline in childhood. The percentage on the vertical axis denotes the proportion of subjects at baseline in childhood remaining in each quintile at follow-up in adulthood.
As shown in Table 1, based on a stepwise multivariate regression analysis, childhood levels of glucose (model 1), insulin (model 2), and HOMA-IR (model 3) were independent predictors of corresponding follow-up adulthood levels 17 years later. However, as shown by the standardized regression coefficients, the best predictors for adult glucose, insulin, and HOMA-IR levels were the change of BMI Z score from childhood to adulthood and baseline BMI Z score, in that order. The next best predictors for all these variables were the corresponding childhood level, followed by male sex (for glucose ad insulin), age (for glucose), and total-to-HDL cholesterol ratio (for glucose, insulin, and HOMA-IR), in that order. Overall, these variables accounted for 12.5, 45.4, and 43.9% of the variance in glucose, insulin, and HOMA-IR, respectively.
Table 1.
Childhood predictors of follow-up levels of glucose, insulin, and HOMA-IR in young adults after 17 years
| Glucose (model 1) | Insulin (model 2) | HOMA-IR (model 3) | |
|---|---|---|---|
| Childhood predictor variable* | β | β | β |
| Age | 0.11† | ||
| Sex (male > female) | 0.10† | 0.08† | |
| BMI Z score at baseline | 0.25‡ | 0.61‡ | 0.60‡ |
| BMI Z score change over time | 0.25‡ | 0.63‡ | 0.62‡ |
| Total-to-HDL cholesterol ratio | 0.06§ | 0.06§ | 0.07‖ |
| Glucose | 0.16‡ | ||
| Insulin | 0.10† | ||
| HOMA-IR | 0.11‡ | ||
| Model R2 (%) | 12.5 | 45.4 | 43.9 |
β is the standardized regression coefficient.
*Stepwise regression model includes age, race, sex, baseline BMI Z score, BMI Z score change over time, MAP, total-to-HDL cholesterol ratio, as well as baseline childhood glucose and insulin (models 1 and 2, respectively) and HOMA-IR (model 3).
†P < 0.001;
‡P < 0.0001;
§P < 0.05;
‖P < 0.01.
As shown in Table 2, among the childhood glucose homeostasis variables, insulin and HOMA-IR (top decile versus the rest) showed an odds ratio of 2.85 (P < 0.05) and 2.55 (P < 0.05), respectively, for developing pre-diabetes after 17 years. In addition, age, baseline BMI Z score, BMI Z score change over time, and total-to-HDL cholesterol ratio showed odds ratios of 1.14, ∼1.44, ∼1.85, and 1.05, respectively, for developing pre-diabetes (P < 0.05). With respect to developing diabetes, the odds ratios were 3.28 (P < 0.05) for glucose, 5.54 (P = 0.0001) for insulin, and 5.84 for HOMA-IR (P < 0.0001); neither BMI Z score nor BMI Z score change over time predicted the development of overt type 2 diabetes. Further, alternate logistic regression models including age, race, sex, BMI Z score, and BMI Z score change over time, along with either childhood glucose and insulin (model 1) or HOMA-IR (model 2), showed glucose homeostasis variables, BMI Z score, and BMI Z score change over time as significant predictors of adult pre-diabetes and diabetes (P < 0.05). P values of goodness-of-fit test for all models were >0.20 (data not shown).
Table 2.
Odds ratios (95% CIs) for developing pre-diabetes and diabetes in adulthood on the basis of childhood levels of glucose, insulin, and HOMA-IR
| Childhood deciles (top versus the rest)* | Pre-diabetes I | P | Diabetes | P |
|---|---|---|---|---|
| Model 1 | ||||
| Age | 1.14 (1.03–1.26) | <0.01 | ||
| BMI Z score at baseline | 1.43 (1.04–1.96) | <0.05 | ||
| BMI Z score change over time | 1.84 (1.21–2.78) | <0.01 | ||
| Total-to-HDL cholesterol ratio | 1.05 (1.00–1.09) | <0.05 | ||
| Glucose | 3.28 (1.29–8.33) | <0.05 | ||
| Insulin | 2.85 (1.22–6.66) | <0.05 | 5.54 (2.33–13.19) | 0.0001 |
| Model 2 | ||||
| Age | 1.14 (1.03–1.26) | <0.05 | ||
| BMI Z score at baseline | 1.44 (1.05–1.98) | <0.05 | ||
| BMI Z score change over time | 1.85 (1.23–2.79) | <0.01 | ||
| Total-to-HDL cholesterol ratio | 1.05 (1.01–1.09) | <0.05 | ||
| HOMA-IR | 2.55 (1.10–5.95) | <0.05 | 5.84 (2.51–13.60) | <0.0001 |
*Stepwise logistic regression model includes age, race, sex, baseline BMI Z score, BMI Z score change over time, MAP, total-to-HDL cholesterol ratio, as well as baseline childhood glucose and insulin (top decile versus the rest) for model 1 and HOMA-IR (top decile versus the rest) for model 2.
On the basis of glucose homeostasis variable levels (top quintile versus the rest), children were classified into low-risk versus high-risk group and the prevalence rates of obesity, hypertension, dyslipidemia, hyperglycemia, hyperinsulinemia, and metabolic syndrome in adulthood after 17 years of follow-up were compared between the two groups (supplementary Table 1 of the online appendix [available at http://care.diabetesjournals.org/cgi/content/full/dc09-1635/DC1]). The prevalence of adulthood metabolic syndrome and its variables in the high-risk versus low-risk group was significantly greater with respect to hyperglycemia, hypertriglyceridemia (marginal significant in childhood insulin group), hypertension (except childhood glucose group), obesity (except childhood glucose group), low HDL cholesterol (except childhood glucose group), high LDL cholesterol (childhood glucose group only), and metabolic syndrome.
CONCLUSIONS
This community-based study demonstrates that elevated levels of glucose, insulin, and insulin resistance index (HOMA-IR) in childhood track and persist in ranking over a 17-year period. Childhood levels relate independently to corresponding adulthood levels and predict pre-diabetes (except childhood glucose) and diabetes conditions in adulthood, independent of age, race, sex, change in BMI Z score over time, childhood BMI Z score, MAP, and total-to-HDL cholesterol ratio. In addition, childhood high- versus low-risk status (top quintile versus the rest) with respect to glucose homeostasis variables was associated with increased prevalences of the metabolic syndrome and its component cardiometabolic risk factors. Of particular interest, childhood glucose levels clinically considered within the normal range persist into adulthood and can predict diabetes.
The concept of tracking of cardiometabolic risk factors over time is well recognized. The current findings showing the persistence of adverse levels of glucose homeostasis variables since childhood (7–10), and related predictability of adult pre-diabetes and diabetes conditions, are in agreement with previous reports (6,17,18). Bao et al. (8) have demonstrated that the individuals with relatively high/low insulin levels trended to retain such levels over an 8-year follow-up. Of those who had insulin levels ranked in the top quartile at baseline, 40% remained so after 8 years (8). Elevations in fasting plasma glucose within the normoglycemic range indeed may track from childhood to adulthood and reflect the progression from normal glucose tolerance before the onset of impaired glucose regulation as a continuous process in the development of diabetes (10,18,19).
Of note, gain in adiposity (BMI), a modifiable risk factor, from childhood to adulthood along with childhood adiposity were the best predictors of the adult glucose homeostasis variables in this study. Because obesity is pathologically linked to insulin resistance/hyperinsulinemia, it plays a crucial role as an initiating factor in the development of dysglycemia. This is consistent with earlier observations showing temporal associations between the degree of baseline adiposity and the incidence of hyperinsulinemia (20) or metabolic syndrome (21), independently of baseline insulin levels. Studies (6,9,18) have also shown baseline obesity to be an independent risk factor for type 2 diabetes.
The observational nature of the current study can not address the issue of causality but only suggests putative mechanisms for the observed relationships. Intra-abdominal and intramyocellular lipid accumulation along with adipocyte-derived cytokines have been involved in the development of insulin resistance and the attendant type 2 diabetes (22). It is also apparent from the present study that children with top quintile (high risk) of glucose, insulin, and HOMA-IR levels displayed increased prevalence of metabolic syndrome and are associated with type 2 diabetes. As mentioned earlier, excess adiposity, especially visceral fat, may be the initiating factor in the observed adverse relationships (21). Excess fat and related insulin resistance/hyperinsulinemia increase triglyceride (VLDLs) levels as a result of abnormal fatty acid metabolism and excess hepatic triglyceride synthesis and/or low clearance of triglycerides from the circulation (23). In turn, increases in LDL cholesterol and decreases in HDL cholesterol levels ensue (24).
With respect to blood pressure, hyperinsulinemia could relate to raises in levels by 1) increasing renal sodium retention, 2) stimulating the sympathetic nervous system, 3) disturbing cell membrane calcium transport, and 4) increasing the smooth muscle cell proliferation (5,25). Alternatively, excess adiposity, per se, increases blood pressure by adversely altering, among others, intravascular volume, cardiac output, renal pressure natriuresis, and the adipose renin-angiotensin-aldosterone system (25). Taken together, it appears that excess levels of glucose homeostasis variables within the normoglycemic range even in childhood is a biomarker of risk for developing adverse cardiometabolic conditions including diabetes and subtle abnormalities of the cardiovascular system.
The present study has certain limitations in that it lacks direct assessments of postchallenge glucose, in vivo insulin action and secretion, glycosylated hemoglobin, and body fat mass and distribution. Instead, we used well-established simple surrogate measures of glucose homeostasis that are applicable to population studies. The fasting status for metabolic variables including glucose was based on self-report. However, it should be mentioned that nonsystematic misclassification of self-reports would actually tend to underestimate the outcome. Further, the current findings should be viewed with caution in view of the modest number of events, especially diabetes.
In summary, the present findings indicate the importance of even moderately elevated levels of childhood glucose homeostasis variables (glucose, insulin, and HOMA-IR) considered within the normoglycemic range in terms of predicting pre-diabetes, diabetes, and metabolic syndrome and its cardiometabolic risk factors in apparently healthy young adults, with obesity and the change of obesity levels over time being the major contributors. Additional longitudinal population-based studies are obviously needed to validate the current findings and to develop the common glucose homeostasis variable cutoff values for type 2 diabetes and other cardiometabolic risk assessment and intervention in pediatric population.
Supplementary Material
Acknowledgments
Supported by grants AG16592 from the National Institute on Aging and 0855082E from American Heart Association.
No potential conflicts of interest relevant to this article were reported.
The Bogalusa Heart Study is a joint effort of many investigators and staff members, whose contributions are gratefully acknowledged. We especially thank the study participants.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Hoyert DL, Heron MP, Murphy SL, Kung H: Deaths: Final Data for 2003: National Vital Statistics Reports. Vol. 54, No. 13. Hyattsville, MD, National Center for Health Statistics, 2006. [PubMed] [Google Scholar]
- 2. Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Geiss LS, Gregg EW: Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care 2006;29:1263–1268 [DOI] [PubMed] [Google Scholar]
- 3. Fox CS, Sullivan L, D'Agostino RB, Sr, Wilson PW: the Framingham Heart Study. The significant effect of diabetes duration on coronary heart disease mortality: the Framingham Heart Study. Diabetes Care 2004;27:704–708 [DOI] [PubMed] [Google Scholar]
- 4. Abdul-Ghani MA, Tripathy D, DeFronzo RA: Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006;29:1130–1139 [DOI] [PubMed] [Google Scholar]
- 5. Reaven GM: Banting Lecture 1988: role of insulin resistance in human disease Diabetes 1988;37:1595–607 [DOI] [PubMed] [Google Scholar]
- 6. Li C, Ford ES, Zhao G, Mokdad AH: Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005–2006. Diabetes Care 2009;32:342–347. Epub 2008 Oct 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rönnemaa T, Knip M, Lautala P, Viikari J, Uhari M, Leino A, Kaprio EA, Salo MK, Dahl M, Nuutinen EM, Pesonen E, Pietikäinen M, Åkerblom HK: Serum insulin and other cardiovascular risk indicators in children, adolescents and young adults. Ann Med 1991;23:67–72 [DOI] [PubMed] [Google Scholar]
- 8. Bao W, Srinivasan SR, Berenson GS: Persistent elevation of plasma insulin levels is associated with increased cardiovascular risk in children and young adults: the Bogalusa Heart Study. Circulation 1996;93:54–59 [DOI] [PubMed] [Google Scholar]
- 9. Goran MI, Lane C, Toledo-Corral C, Weigensberg MJ: Persistence of pre-diabetes in overweight and obese Hispanic children: association with progressive insulin resistance, poor β-cell function, and increasing visceral fat. Diabetes. 2008;57:3007–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R: Baltimore Longitudinal Study of Aging: the natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes 2003;52:1475–1484 [DOI] [PubMed] [Google Scholar]
- 11. The Bogalusa Heart Study 20th Anniversary Symposium. Am J Med Sci 1995;310 (Suppl. 1):S1–S138 [DOI] [PubMed] [Google Scholar]
- 12. American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 2008;31 Suppl. 1:S55–S60 [DOI] [PubMed] [Google Scholar]
- 13. Berenson GS, McMahan CA, Voors AW, Webber LS, Srinivasan SR, Frank GC, Foster TA, Blonde CV: Cardiovascular Risk Factors in Children: The Early Natural History of Atherosclerosis and Essential Hypertension. New York, Oxford University Press, 1980. [Google Scholar]
- 14. Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL: 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11 2002;246:1–190 [PubMed] [Google Scholar]
- 15. Srinivasan SR, Berenson GS: Serum lipoproteins in children and methods for study. In Handbook of Electrophoresis. Lewis LA. Ed. Boca Raton, FL, CRC Press, 1983, p. 185–204 [Google Scholar]
- 16. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 17. Franks PW, Hanson RL, Knowler WC, Moffett C, Enos G, Infante AM, Krakoff J, Looker HC: Childhood predictors of young-onset type 2 diabetes. Diabetes 2007;56:2964–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS: Changes in risk variables of metabolic syndrome since childhood in pre-diabetic and type 2 diabetic subjects: the Bogalusa Heart Study. Diabetes Care 2008;31:2044–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, Kochba I, Rudich A: the Israeli Diabetes Research Group. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med 2005;353:1454–1462 [DOI] [PubMed] [Google Scholar]
- 20. Srinivasan SR, Myers L, Berenson GS: Temporal association between obesity and hyperinsulinemia in children, adolescents, and young adults: the Bogalusa Heart Study. Metabolism 1999;48:928–934 [DOI] [PubMed] [Google Scholar]
- 21. Srinivasan SR, Myers L, Berenson GS: Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes 2002;51:204–209 [DOI] [PubMed] [Google Scholar]
- 22. Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, Boselli L, Barbetta G, Allen K, Rife F, Savoye M, Dziura J, Sherwin R, Shulman GI, Caprio S: Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 2003;362:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nikkilä EA: Regulation of hepatic production of plasma triglycerides by glucose and insulin. In Regulation of Hepatic Metabolism. Lundquist F, Tygstrup N. Eds. Copenhagen, Denmark, Munksgaard, 1974, p. 360–387 [Google Scholar]
- 24. Tall AR: Plasma high density lipoproteins: metabolism and relationship to atherogenesis. J Clin Invest 1990;86:379–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Srinivasan SR, Myers L, Berenson GS: Changes in metabolic syndrome variables since childhood in prehypertensive and hypertensive subjects: the Bogalusa Heart Study. Hypertension 2006;48:33–39 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.