Abstract
Background and objectives: Elevated serum calcium has been associated with increased mortality in dialysis patients, but it is unclear whether the same is true in non-dialysis-dependent (NDD) chronic kidney disease (CKD). Outcomes associated with low serum calcium are also not well-characterized.
Design, setting, participants, & measurements: We examined associations of baseline, time-varying, and time-averaged serum calcium with all-cause mortality in a historic prospective cohort of 1243 men with moderate and advanced NDD CKD by using Cox models.
Results: The association of serum calcium with mortality varied according to the applied statistical models. Higher baseline calcium and time-averaged calcium were associated with higher mortality (multivariable adjusted hazard ratio (95% confidence interval): 1.31 (1.13, 1.53); P < 0.001 for a baseline calcium 1 mg/dl higher). However, in time-varying analyses, lower calcium levels were associated with increased mortality.
Conclusions: Higher serum calcium is associated with increased long-term mortality (as reflected by the baseline and time-averaged models), and lower serum calcium is associated with increased short-term mortality (as reflected by the time-varying models) in patients with NDD CKD. Clinical trials are warranted to determine whether maintaining normal serum calcium can improve outcomes in these patients.
Mineral and bone disorders in chronic kidney disease (CKD) (1) have emerged as novel mortality risk factors in dialysis patients (2–8). Some of these abnormalities (such as serum phosphorus and parathyroid hormone (PTH) levels) have also been implicated in similar ways in patients with non-dialysis-dependent (NDD) CKD (9–12). Serum calcium's effect on outcomes has been the focus of attention mainly in dialysis patients, where calcium metabolism is significantly distorted (13–19). The use of calcium-containing phosphate binders further complicates the picture because these medications could be involved in the etiology of vascular calcification (20,21), and their roles as therapeutic agents have been intensely debated (22). Supporting the potential role for calcium in cardiovascular disease were epidemiologic studies showing an association between higher calcium and increased mortality (2–8). Some of the same studies have also suggested that extremely low calcium levels may themselves be deleterious (2,3), which has ultimately resulted in recommendations to attain a low-normal serum calcium level in dialysis patients (23). Studies examining the role of calcium in NDD CKD patients are fewer and failed to unequivocally show an association between abnormal calcium levels and vascular calcification (24–27). No study has yet examined the association of calcium levels with mortality in NDD CKD.
We examined the association of serum calcium levels with all-cause mortality in a large number of male US veterans with moderate and advanced NDD CKD at a single medical institution.
Materials and Methods
Study Population and Data Collection
We studied all 1259 patients evaluated for NDD CKD at Salem Veterans Affairs Medical Center (VAMC) between January 1, 1990, and June 30, 2007, and followed them until April 1, 2009. Ten women and six patients whose race was other than white or black were excluded, with the final study population consisting of 1243 patients.
Baseline characteristics recorded at the time of the initial evaluation in the nephrology clinic were extracted retrospectively, including demographic and anthropometric characteristics, comorbid conditions, including the Charlson Comorbidity Index (CCI), and laboratory results, as detailed elsewhere (28,29). Follow-up clinical and laboratory data recorded during outpatient encounters over the entire follow-up period were also extracted and used in time-varying analyses. All calcium levels were corrected for serum albumin levels. Medication use, including that of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, statins, calcium-containing phosphate binders, sevelamer hydrochloride, and calcitriol, was also assessed over the entire follow-up period. GFR was estimated using the abbreviated equation developed for the Modification of Diet in Renal Disease Study (30) and was categorized according to the staging system introduced by the Kidney/Dialysis Outcome Quality Initiative Clinical Practice Guidelines for CKD: Evaluation, Classification, and Stratification (31). All of the biochemical measurements were performed in a single laboratory at the Salem VAMC.
Statistical Analyses
Missing data points for CCI (1%), body mass index (14%), serum albumin (1%), phosphorus (2%), alkaline phosphatase (ALP; 9%), blood cholesterol (2% missing), hemoglobin (0.3%), white blood cell (WBC) count (0.9%), percent lymphocytes in WBC (1%), and 24-hour urine protein (3%) were imputed using multiple imputations. Sixty five percent of patients had one or more serum PTH measurements performed sometime during follow-up, but only 35% had these measured at baseline. Models were adjusted for PTH in sensitivity analyses by categorizing this variable into quartiles and adding missing values as a fifth dummy category. Smoking (5% missing) was also analyzed as a categorical variable with the creation of a dummy category corresponding to missing status. To assess both the short-term and the long-term effects of abnormal serum calcium levels, we used the baseline, time-varying, and time-averaged values in our analyses. Baseline models were assessed because of their practical relevance, because risk stratification is easiest to perform on the basis of single baseline values. However, because of potential misclassification by baseline values in a dynamically changing cohort, we also applied two different types of analyses using time-updated values of serum calcium. Time-varying analyses assess outcomes associated with the last observed value of serum calcium; hence, such models were used to assess the short-term impact of abnormal serum calcium levels. Conversely, because calcium may also have long-term deleterious effects where exposure to abnormal levels over prolonged periods of time may have additive effects, we also assessed associations with time-averaged serum calcium levels, which incorporate time-weighted average serum calcium levels derived from all serum calcium measurements done over the entire follow-up period for each patient (32). Multivariable models of time-varying and time-averaged calcium were adjusted for time-varying and time-averaged covariates (if appropriate).
Outcome Analysis.
The starting time for outcome analyses was the date of the first encounter in the nephrology clinic. Patients were considered lost to follow-up if no contact was documented with them for more than 6 months, and they were censored at the date of the last documented contact. The primary outcome measure was overall (predialysis and postdialysis) all-cause mortality, which was ascertained from VA electronic records.
The associations of baseline, time-varying, and time-averaged serum calcium levels with mortality were evaluated in Cox models. Selection of variables to be included in the final multivariable models was done by determining probable confounders (33) on the basis of baseline characteristics and on theoretical considerations. To account for the different time periods when patients were enrolled in the study, multivariable models were also adjusted for a dummy variable corresponding to the enrollment period (1990 to 1995, 1996 to 2000, or after 2000). Final models were adjusted for age, race, CCI, diabetes mellitus, cardiovascular disease (CVD), BP, body mass index, smoking status, the use of calcium-containing phosphate binders, sevelamer hydrochloride and calcitriol, enrollment period, estimated GFR, bicarbonate, phosphorus, ALP, blood hemoglobin, WBC count, percentage of lymphocytes, and 24-hour urine protein. Nonlinearity of associations was tested by including polynomial terms, by categorizing serum calcium by quartiles, and by using restricted cubic splines; spline analyses were restricted to values above the first and below the 99th percentile of the predictor variable to increase the stability of the spline models. Nonlinear associations for independent covariates were also addressed by including polynomial terms in multivariable models, if appropriate.
Interactions in mortality analyses were assessed by including interaction terms with age, race, estimated GFR, presence or absence of diabetes mellitus and CVD, and the use or nonuse of calcium-containing phosphate binders and calcitriol, with subsequent subgroup analyses if these terms were statistically significant. Sensitivity analyses were performed by examining the composite outcomes of predialysis mortality or ESRD (defined as initiation of maintenance dialysis therapy and ascertained from local hospital records, including Medicare Forms 2728), by adjusting the multivariable models for PTH, by using only nonimputed values of independent variables, and by restricting analyses to a more contemporary cohort of patients enrolled after January 1, 2001. Proportionality of non-time-varying models was tested by using Schoenfeld residuals. P values less than 0.05 were considered significant. Statistical analyses were performed using STATA statistical software version 10 (STATA Corporation, College Station, TX). The study protocol was approved by the Research and Development Committee at the Salem VAMC.
Results
The mean (±SD) age of the cohort was 68 ± 11 years; 24% of patients were black and the mean estimated GFR was 37 ± 17 ml/min per 1.73 m2. Most patients had CKD stages 3 (57%) and 4 (30%), with few patients categorized as CKD stages 1 (2%), 2 (8%), and 5 (4%). The mean (±SD) baseline and time-averaged corrected serum calcium were 9.4 ± 0.5 mg/dl and 9.2 ± 1.3 mg/dl. Patients had a median of 18 calcium measurements performed during follow-up (interquartile range: 10 to 32). Seven hundred and fifty-two patients (61%) were enrolled in the study after January 1, 2001. A total of 698 patients died (mortality rate: 123 per 1000 patient-years, 95% confidence interval (CI): 114 to 132), and 778 patients reached the composite outcome (161 per 1000 patient-years, 95% CI: 150 to 172) during a median follow-up of 3.2 years. Forty-five patients (4%) were lost to follow-up; their characteristics were not significantly different (data not shown).
Baseline characteristics in patients divided by quartiles of calcium are shown in Table 1. Patients with higher calcium were younger, more likely to be black, more likely to use statins, less likely to use calcium-based phosphate binders and calcitriol, had higher estimated GFR, bicarbonate, hemoglobin and WBC count, and had lower systolic BP.
Table 1.
Baseline characteristics in patients grouped by quartiles of their baseline albumin-corrected serum calcium levels
| Serum calcium, mg/dl |
P for Trend | ||||
|---|---|---|---|---|---|
| <9.1 (n = 285) | 9.1 to 9.4 (n = 287) | 9.41 to 9.7 (n = 330) | >9.7 (n = 341) | ||
| Age, yr | 69.8 ± 10.3 | 68.5 ± 10.9 | 67.9 ± 10.3 | 67.4 ± 11.6 | 0.005 |
| Race (black), n (%) | 55 (19) | 66 (23) | 81 (25) | 98 (29) | 0.006 |
| DM, n (%) | 145 (51) | 156 (54) | 186 (56) | 195 (57) | 0.1 |
| ASCVD, n (%) | 169 (59) | 161 (56) | 179 (54) | 198 (58) | 0.7 |
| Smoking, n (%) | 59 (22) | 66 (24) | 92 (29) | 86 (27) | 0.1 |
| CCI | 2.6 ± 1.8 | 2.4 ± 1.8 | 2.5 ± 1.6 | 2.4 ± 1.7 | 0.4 |
| Future Ca-based binder use, n (%) | 103 (36) | 74 (26) | 92 (28) | 67 (26) | 0.01 |
| Future sevelamer HCl use, n (%) | 37 (13) | 25 (9) | 35 (11) | 37 (11) | 0.6 |
| Future calcitriol use, n (%) | 101 (35) | 96 (33) | 97 (29) | 95 (28) | 0.023 |
| Future aspirin use, n (%) | 167 (59) | 176 (61) | 202 (61) | 192 (56) | 0.5 |
| Future ACEI/ARB use, n (%) | 210 (74) | 219 (76) | 260 (79) | 254 (74) | 0.7 |
| Future statin use, n (%) | 161 (56) | 205 (71) | 217 (66) | 228 (67) | 0.045 |
| BMI, kg/m2 | 28.5 ± 5.3 | 29.6 ± 6.0 | 29.9 ± 6.3 | 29.2 ± 5.9 | 0.19 |
| SBP, mmHg | 153 ± 26 | 149 ± 24 | 152 ± 28 | 146 ± 29 | 0.01 |
| DBP, mmHg | 75 ± 16 | 74 ± 15 | 76 ± 15 | 73 ± 16 | 0.4 |
| Estimated GFR, ml/min per 1.73 m2 | 33.1 ± 15.9 | 36.8 ± 15.7 | 39.4 ± 15.7 | 39.8 ± 17.9 | <0.001 |
| Serum albumin, g/dl | 3.8 ± 0.4 | 3.7 ± 0.4 | 3.6 ± 0.5 | 3.5 ± 0.6 | <0.001 |
| Serum ALP, U/L | 98 ± 43 | 85 ± 34 | 95 ± 51 | 89 ± 41 | 0.24 |
| Total cholesterol, mg/dl | 190 ± 59 | 189 ± 57 | 188 ± 49 | 192 ± 59 | 0.7 |
| Serum phosphorus, mg/dl | 3.9 ± 0.9 | 3.8 ± 0.8 | 3.8 ± 0.7 | 3.8 ± 0.8 | 0.8 |
| Serum bicarbonate, mEq/L | 24.9 ± 3.7 | 25.5 ± 3.4 | 26.0 ± 3.1 | 26.1 ± 3.4 | <0.001 |
| Blood hemoglobin, g/dl | 12.4 ± 2.0 | 12.6 ± 1.9 | 12.7 ± 1.7 | 12.9 ± 2.0 | 0.001 |
| Blood WBC count, 1000/mm3 | 7.2 ± 2.1 | 7.4 ± 2.3 | 7.7 ± 2.1 | 8.0 ± 2.5 | <0.001 |
| Blood lymphocytes, % WBC | 22.6 ± 8.3 | 23.0 ± 9.3 | 23.7 ± 8.0 | 23.6 ± 8.5 | 0.12 |
| Proteinuria, mg/24 h, geometric mean (95% CI) | 871 (729 to 1041) | 615 (518 to 730) | 782 (657 to 931) | 721 (612 to 849) | 0.4 |
Data without parentheses are presented as mean ± SD. Comparisons were made by χ2 test for linear trend. DM, diabetes mellitus; ASCVD, atherosclerotic cardiovascular disease; CCI, Charlson Comorbidity Index; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; GFR, glomerular filtration rate; ALP, alkaline phosphatase.
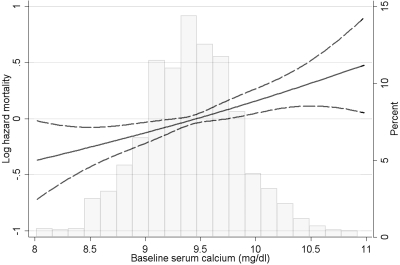
The mortality associated with calcium levels varied according to the statistical models applied. Baseline calcium levels showed a significant association with higher mortality (Figure 1 and Table 2). This association was linear and without a visible threshold level for the increased risk, with calcium 1 mg/dl higher being associated with a multivariable adjusted hazard ratio (95% CI) of 1.31 (1.13, 1.53); P < 0.001. There was a significant interaction according to prevalent CVD status (P = 0.004 for the interaction term with mortality); baseline calcium levels showed no association with mortality in patients without CVD (hazard ratio (95% CI): 1.00 (0.77, 1.29); P = 0.9) and a significant association in those with CVD (hazard ratio (95% CI): 1.58 (1.29, 1.94); P < 0.001).
Figure 1.
Multivariable-adjusted log hazards (solid lines) and 95% CI (dashed lines) of all-cause mortality associated with fixed baseline levels of serum calcium concentration in Cox models adjusted for age, race, CCI, diabetes mellitus, CVD, BP, body mass index, smoking status, the use of phosphate binders and calcitriol, enrollment period, estimated GFR, bicarbonate, phosphorus, ALP, blood hemoglobin, WBC count, percentage of lymphocytes and 24-hour urine protein. Superimposed is a histogram showing the distribution of baseline serum calcium levels.
Table 2.
Hazard ratios (95% CI) of all-cause mortality associated with baseline serum calcium levels and various confounders in unadjusted and multivariable-adjusted Cox models
| Variable | Unadjusted Model | Multivariable-Adjusted Model |
|---|---|---|
| Baseline serum calcium | ||
| <9.1 mg/dl (referent) | 1.0 | 1.0 |
| 9.1 to 9.4 mg/dl | 0.88 (0.71, 1.10) | 1.16 (0.92, 1.46) |
| 9.41 to 9.7 mg/dl | 0.99 (0.80, 1.21) | 1.34 (1.08, 1.68) |
| >9.7 mg/dl | 1.02 (0.83, 1.26) | 1.38 (1.11, 1.72) |
| Age, 1 yr older | 1.03 (1.02, 1.04) | |
| Race, black versus white | 0.90 (0.74, 1.11) | |
| CCI, 1 unit higher | 1.11 (1.05, 1.16) | |
| DM versus no DM | 1.25 (1.05, 1.50) | |
| ASCVD versus no ASCVD | 1.33 (1.12, 1.58) | |
| SBP | ||
| Linear term, 1 mmHg higher | 0.981 (0.960, 1.003) | |
| Quadratic term, 1 mmHg higher | 1.0001 (0.9999, 1.0002) | |
| DBP, 1 mmHg higher | 0.999 (0.992, 1.006) | |
| Active smoking versus nonsmoking | 1.57 (1.30, 1.89) | |
| Calcitriol use versus no use | 0.46 (0.38, 0.56) | |
| Calcium-based binder use versus no use | 0.93 (0.77, 1.13) | |
| Sevelamer HCl use versus no use | 0.54 (0.40, 0.76) | |
| Estimated GFR, 1 ml/min/1.73m2 higher | 0.986 (0.980, 0.991) | |
| ALP, 1 U/L higher | 1.001 (0.999, 1.003) | |
| Serum phosphorus, 1 mg/dl higher | 1.01 (0.91, 1.13) | |
| Serum bicarbonate | ||
| Linear term, 1 mEq/L higher | 0.76 (0.64, 0.94) | |
| Quadratic term, 1 mEq/L higher | 1.005 (1.001, 1.008) | |
| Blood hemoglobin, 1 g/dl higher | 0.91 (0.86, 0.95) | |
| WBC count, 1000/mm3 higher | 1.02 (0.98, 1.06) | |
| Percent lymphocytes in WBC, 1% higher | 0.987 (0.976, 0.997) | |
| 24-h urine protein, 1 mg higher | 1.11 (1.04, 1.19) | |
| Enrollment period | ||
| 1990 to 1995 | 0.64 (0.49, 0.84) | |
| 1996 to 2000 | 0.91 (0.75, 1.11) | |
| 2001 to 2007 (referent) | 1.0 |
DM, diabetes mellitus; ASCVD, atherosclerotic cardiovascular disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; CCI, Charlson comorbidity index; ALP, alkaline phosphatase.
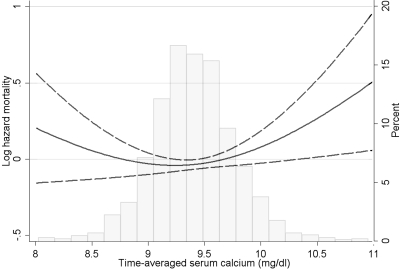
The associations of time-averaged calcium with mortality were significant but nonlinear (P = 0.004 for the joint significance of the polynomial terms), with higher adjusted hazards for mortality seen especially with increased serum calcium levels; the risk of mortality appeared to increase at serum calcium levels above approximately 9.5 mg/dl (Figure 2 and Table 3). Compared with the group with time-averaged serum calcium levels of 9.1 to 9.4 mg/dl, those with levels of <9.1, 9.41 to 9.7, and >9.7 mg/dl had adjusted hazard ratios (95% CI) of mortality of 1.32 (1.06, 1.64), 1.18 (0.96, 1.45), and 1.42 (1.13, 1.78), respectively (Table 3). There were no significant interactions.
Figure 2.
Multivariable-adjusted log hazards (solid lines) and 95% CI (dashed lines) of all-cause mortality associated with time-averaged levels of serum calcium concentration in Cox models adjusted for age, race, CCI, diabetes mellitus, CVD, BP, body mass index, smoking status, the use of phosphate binders and calcitriol, enrollment period, estimated GFR, bicarbonate, phosphorus, ALP, blood hemoglobin, WBC count, percentage of lymphocytes, and 24-hour urine protein. Superimposed is a histogram showing the distribution of time-averaged serum calcium levels.
Table 3.
Hazard ratios (95% CI) of all-cause mortality associated with time-averaged serum calcium levels and various confounders in unadjusted and multivariable-adjusted Cox models
| Variable | Unadjusted Model | Multivariable-Adjusted Model |
|---|---|---|
| Time-averaged serum calcium | ||
| <9.1 mg/dl | 1.45 (1.17, 1.80) | 1.33 (1.07, 1.66) |
| 9.1 to 9.4 mg/dl (referent) | 1.0 | 1.0 |
| 9.41 to 9.7 mg/dl | 1.12 (0.92, 1.36) | 1.18 (0.96, 1.44) |
| >9.7 mg/dl | 1.31 (1.06, 1.63) | 1.41 (1.13, 1.77) |
| Age, 1 yr older | 1.02 (1.01, 1.03) | |
| Race, black versus white | 0.86 (0.70, 1.05) | |
| CCI, 1 unit higher | 1.09 (1.04, 1.15) | |
| DM, versus no DM | 1.16 (0.98, 1.39) | |
| ASCVD, versus no ASCVD | 1.38 (1.16, 1.64) | |
| SBP | ||
| Linear term, 1 mmHg higher | 0.93 (0.90, 0.96) | |
| Quadratic term, 1 mmHg higher | 1.0002 (1.0001, 1.0003) | |
| DBP, 1 mmHg higher | 0.98 (0.97, 0.99) | |
| Active smoking, versus nonsmoking | 1.41 (1.16, 1.70) | |
| Calcitriol use, versus no use | 0.45 (0.37, 0.54) | |
| Calcium-based binder use, versus no use | 0.79 (0.65, 0.97) | |
| Sevelamer HCl use, versus no use | 0.46 (0.33, 0.63) | |
| Estimated GFR, 1 ml/min per 1.73 m2 higher | 0.989 (0.982, 0.995) | |
| ALP, 1 U/L higher | 1.004 (1.002, 1.005) | |
| Serum phosphorus, 1 mg/dl higher | 1.08 (0.94, 1.24) | |
| Serum bicarbonate | ||
| Linear term, 1 mEq/L higher | 0.53 (0.40, 0.71) | |
| Quadratic term, 1 mEq/L higher | 1.005 (1.001, 1.008) | |
| Blood hemoglobin, 1 g/dl higher | 0.81 (0.76, 0.86) | |
| WBC count, 1000/mm3 higher | 1.06 (1.02, 1.11) | |
| Percent lymphocytes in WBC, 1% higher | 0.984 (0.972, 0.996) | |
| 24 h urine protein, 1 mg higher | 1.05 (0.97, 1.13) | |
| Enrollment period | ||
| 1990 to 1995 | 0.43 (0.32, 0.57) | |
| 1996 to 2000 | 0.78 (0.64, 0.95) | |
| 2001 to 2007 (referent) | 1.0 |
DM, diabetes mellitus; ASCVD, atherosclerotic cardiovascular disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; CCI, Charlson comorbidity index; ALP, alkaline phosphatase.
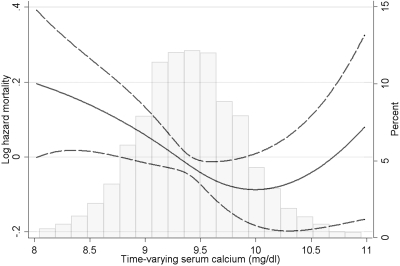
Time-varying calcium also showed a nonlinear association with mortality (Figure 3; P = 0.03 for the joint significance of the polynomial terms), with only the lower levels of calcium showing a trend toward higher mortality: the risk of mortality appeared to increase at serum calcium levels below approximately 10 mg/dl. Compared with the group with time-varying calcium of 9.41 to 9.7 mg/dl, those with levels of <9.1, 9.1 to 9.4, and >9.7 mg/dl had adjusted hazard ratios (95% CI) of mortality of 1.21 (0.97, 1.51), 1.07 (0.85, 1.36), and 1.06 (0.85, 1.33), respectively (Table 4). The mortality risk associated with lower calcium was more pronounced in patients with estimated GFR >30 ml/min per 1.73 m2 (P = 0.006 for the interaction term) and in patients who did not receive calcium-containing phosphate binders (P = 0.048 for the interaction term).
Figure 3.
Multivariable-adjusted log hazards (solid lines) and 95% CI (dashed lines) of all-cause mortality and the composite outcome of predialysis mortality or ESRD associated with time-varying levels of serum calcium concentration in Cox models adjusted for time-varying age, race, CCI, diabetes mellitus, CVD, BP, body mass index, smoking status, the use of phosphate binders and calcitriol, enrollment period, estimated GFR, bicarbonate, phosphorus, ALP, blood hemoglobin, WBC count, percentage of lymphocytes, and 24-hour urine protein. Superimposed is a histogram showing the distribution of time-varying serum calcium levels.
Table 4.
Hazard ratios (95% CI) of all-cause mortality associated with time-varying serum calcium levels and various confounders in unadjusted and multivariable-adjusted Cox models
| Variable | Unadjusted Model | Multivariable-Adjusted Model |
|---|---|---|
| Time-averaged serum calcium | ||
| <9.1 mg/dl | 1.42 (1.15, 1.76) | 1.22 (0.98, 1.53) |
| 9.1 to 9.4 mg/dl (referent) | 1.11 (0.88, 1.40) | 1.08 (0.85, 1.37) |
| 9.41 to 9.7 mg/dl | 1.0 | 1.0 |
| >9.7 mg/dl | 1.25 (0.99, 1.56) | 1.08 (0.86, 1.36) |
| Age, 1 yr older | 1.04 (1.03, 1.05) | |
| Race, black versus white | 0.92 (0.76, 1.13) | |
| CCI, 1 unit higher | 1.06 (1.01, 1.12) | |
| DM, versus no DM | 1.18 (0.99, 1.40) | |
| ASCVD, versus no ASCVD | 1.33 (1.11, 1.58) | |
| SBP | ||
| Linear term, 1 mmHg higher | 0.94 (0.92, 0.97) | |
| Quadratic term, 1 mmHg higher | 1.001 (1.000, 1.002) | |
| DBP, 1 mmHg higher | 1.009 (1.002, 1.016) | |
| Active smoking, versus nonsmoking | 1.39 (1.15, 1.68) | |
| Calcitriol use, versus no use | 0.73 (0.59, 0.91) | |
| Calcium-based binder use, versus no use | 0.79 (0.63, 0.99) | |
| Sevelamer HCl use, versus no use | 0.84 (0.60, 1.18) | |
| Estimated GFR, 1 ml/min per 1.73 m2 higher | 0.989 (0.982, 0.996) | |
| ALP, 1 U/L higher | 1.003 (1.002, 1.004) | |
| Serum phosphorus, 1 mg/dl higher | 1.11 (1.03, 1.20) | |
| Serum bicarbonate | ||
| Linear term, 1 mEq/L higher | 0.78 (0.68, 0.90) | |
| Quadratic term, 1 mEq/L higher | 1.005 (1.002, 1.008) | |
| Blood hemoglobin, 1 g/dl higher | 0.83 (0.79, 0.87) | |
| WBC count, 1000/mm3 higher | 1.08 (1.05, 1.11) | |
| Percent lymphocytes in WBC, 1% higher | 0.97 (0.96, 0.98) | |
| 24 h urine protein, 1 mg higher | 1.04 (0.97, 1.11) | |
| Enrollment period | ||
| 1990 to 1995 | 0.18 (0.09, 0.34) | |
| 1996 to 2000 | 0.84 (0.67, 1.04) | |
| 2001 to 2007 (referent) | 1.0 |
DM, diabetes mellitus; ASCVD, atherosclerotic cardiovascular disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; CCI, Charlson comorbidity index; ALP, alkaline phosphatase.
The associations with the composite outcome were similar to those with mortality (data not shown). The results did not change substantially in sensitivity analyses when the models were adjusted for PTH or for unimputed variables, or when the associations were examined in a more contemporary cohort enrolled after January 1, 2001 (data not shown).
Discussion
We examined associations between calcium levels and all-cause mortality in a large group of men with moderate and advanced NDD CKD. We found that in models that examined long-term average exposure to calcium (baseline fixed-covariate and time-averaged models), higher calcium was associated with increased mortality. Conversely, in time-varying models that associated outcomes with the last observed values of the dependent variable, lower calcium levels were associated with higher mortality. These findings underscore calcium's complex pathophysiologic role in humans, which varies from that of a rapidly fluctuating intracellular messenger to that of a stable component of skeletal structure (13). Calcium stabilizes the membranes of excitable cells; thus, lower serum calcium can increase neuromuscular excitability (34), which may explain why lower calcium levels in our time-varying models could have been associated with higher short-term death rates, possibly through a higher incidence of cardiac arrhythmias. Calcium homeostasis is significantly altered in CKD: lower intestinal absorption due to decreased 1,25(OH)2 vitamin D or increased intestinal absorption in those treated with active vitamin D (14), decreased incorporation in the bones or increased release from the bones in those with abnormally low or high PTH levels, (35,36), and the decreased renal excretion of calcium, combined with unregulated calcium exchange through dialysis membranes all contribute to a distorted homeostasis in CKD, and especially in ESRD (15–19). On the basis of these considerations, it has been postulated that elevated calcium may play an integral role in engendering cardiovascular calcification in uremic patients (37,38). Such a mechanism of action could be a possible explanation for the increased mortality associated with higher time-averaged calcium levels in our study, because it appears more likely that prolonged exposure to higher serum calcium levels would be more likely to promote vascular calcification. Epidemiologic studies in dialysis patients indeed found significant associations between higher calcium, cardiovascular calcification, and mortality (2,3,5–8). Similar studies in NDD CKD only examined cardiovascular calcification, finding equivocal associations with calcium levels (24–27). This may have been because of a true lack of association because of the lesser degree by which serum calcium levels are affected in NDD CKD (39) and/or a shorter exposure to abnormal calcium levels or, alternatively, because of an inability to detect significant calcification because of the small size and select nature of the studied populations. Ours is, to our knowledge, the first study to examine mortality associated with abnormal calcium levels in NDD CKD patients, and our findings suggest that lower calcium levels can have short-term deleterious effects, but abnormally high calcium levels can also be harmful over a longer period of time. The complex nature of these associations was also emphasized by our finding of significant interactions with several covariates. Higher baseline calcium levels' association with mortality was restricted to patients with prevalent CVD, suggesting that the effect of long-term exposure to higher calcium may be modified by a propensity for cardiovascular calcification. Conversely, the association of lower calcium with mortality in time-varying models showed separate interactions with the intake of calcium-containing phosphate binders and with estimated GFR, but the latter was affected by the fact that virtually no patients with CKD stage 3 were receiving binders (data not shown). The fact that lower calcium levels' association with increased mortality was not present in the subgroup receiving calcium-containing phosphate binders could have been because of less hypocalcemia and, consequently, fewer arrhythmias in this group, but such a hypothesis would have to be tested in prospective trials. We detected no interactions with calcitriol use in any of the studied models. Calcitriol use was also associated with significantly better survival (40), and the application of calcitriol could have confounded our results on the basis of its known effects on calcium homeostasis. The observed associations between calcium and outcomes were, however, independent of calcitriol, which may have been because of the low doses of this medication that were universally applied in this cohort (40).
Our study should be qualified for several potential limitations. Its historical and observational nature only allows us to establish associations but not causality. Our study was limited to male patients from a single institution; hence, our results may not apply to the larger population with NDD CKD. The retrospective nature of our study could have resulted in unequal numbers of calcium levels included in the time-varying and time-averaged models, which could have biased the findings of these models. The results of the various statistical models are, however, physiologically plausible, which makes it less likely that differences between them are purely a result of bias. Time-varying models could be affected by the impact of acute illnesses and associated malnutrition, which could independently affect both serum calcium levels and outcomes. We addressed this by excluding data collected during hospitalizations and by adjusting for time-varying markers of protein-energy wasting (serum albumin, WBC count, percentage of lymphocytes), but the effect of residual confounding cannot be ruled out. The enrollment of patients over an extended period of time makes it possible that secular trends in medical practices could have affected outcomes on the basis of the time of enrollment. To address this, we adjusted for time of enrollment and examined more contemporary patients separately, and we found no differences in outcomes. We hypothesized that associations between lower and higher calcium levels and mortality are related to cardiovascular events, but we did not have cause-specific mortality available for analyses to test this hypothesis. We only had data on PTH levels in a subgroup of patients; hence, we could not account for the confounding effects of it in the entire population, but we were able to use instead serum ALP as a marker of increased bone formation and an independent risk factor of mortality in maintenance hemodialysis patients (41,42). Furthermore, adjustment for PTH in sensitivity analyses did not change the results.
Conclusions
Chronic hypercalcemia and acute hypocalcemia are both associated with increased mortality in male patients with moderate and advanced NDD CKD. Thus, maintaining normal serum calcium levels may be beneficial in this patient population, but prospective studies will be needed to determine what the target range for serum calcium should be and how such a target should be achieved to derive the best therapeutic potential. Therapeutic regimens inducing either hypercalcemia or hypocalcemia should be assessed for any potentially deleterious effects in properly designed clinical trials.
Disclosures
Drs. Kovesdy and Kalantar-Zadeh have received grant support and/or honoraria from Genzyme, Shire, and Fresenius.
Acknowledgments
Parts of this material were presented at the American Society of Nephrology Renal Week 2009, October 27 to November 1, 2009, San Diego, California. This study was supported by an investigator-initiated grant from Genzyme to Dr. Kovesdy (without salary support) and by NIDDK grant 1R01DK078106–01 to Drs. Kovesdy and Kalantar-Zadeh.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Moe SM, Drueke T, Lameire N, Eknoyan G: Chronic kidney disease-mineral-bone disorder: A new paradigm. Adv Chronic Kidney Dis 14: 3–12, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Slinin Y, Foley RN, Collins AJ: Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: The USRDS waves 1, 3, and 4 study. J Am Soc Nephrol 16: 1788–1793, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK: Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52: 519–530, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Kimata N, Albert JM, Akiba T, Yamazaki S, Kawaguchi T, Fukuhara S, Akizawa T, Saito A, Asano Y, Kurokawa K, Pisoni RL, Port FK: Association of mineral metabolism factors with all-cause and cardiovascular mortality in hemodialysis patients: The Japan dialysis outcomes and practice patterns study. Hemodial Int 11: 340–348, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Noordzij M, Korevaar JC, Boeschoten EW, Dekker FW, Bos WJ, Krediet RT: The Kidney Disease Outcomes Quality Initiative (K/DOQI) Guideline for Bone Metabolism and Disease in CKD: Association with mortality in dialysis patients. Am J Kidney Dis 46: 925–932, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK: Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 67: 1179–1187, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Kovesdy CP, Kalantar-Zadeh K: Bone and mineral disorders in pre-dialysis CKD. Int Urol Nephrol 40: 427–440, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Voormolen N, Noordzij M, Grootendorst DC, Beetz I, Sijpkens YW, Manen JG, Boeschoten EW, Huisman RM, Krediet RT, Dekker FW: High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant 22: 2909–2916, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K: Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int 73: 1296–1302, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Bourdeau JE, Attie MF: Calcium metabolism. In: Maxwell and Kleeman's Clinical Disorders of Fluid and Electrolyte Metabolism, 5th ed., edited by Narins RG.New York, McGraw-Hill Inc. pp 243–306, 1994 [Google Scholar]
- 14.Dusso AS, Brown AJ, Slatopolsky E: Vitamin D. Am J Physiol Renal Physiol 289: F8–F28, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Gotch F, Kotanko P, Handelman G, Levin N: A kinetic model of calcium mass balance during dialysis therapy. Blood Purif 25: 139–149, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Hou SH, Zhao J, Ellman CF, Hu J, Griffin Z, Spiegel DM, Bourdeau JE: Calcium and phosphorus fluxes during hemodialysis with low calcium dialysate. Am J Kidney Dis 18: 217–224, 1991 [DOI] [PubMed] [Google Scholar]
- 17.Kancir CB, Wanscher M, Petersen PH: Ionized and total calcium variations induced by haemodialysis. Nephron 48: 319–323, 1988 [DOI] [PubMed] [Google Scholar]
- 18.McIntyre CW: Calcium balance during hemodialysis. Semin Dial 21: 38–42, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Parker TF, Vergne-Marini P, Hull AR, Pak CY, Fordtran JS: Jejunal absorption and secretion of calcium in patients with chronic renal disease on hemodialysis. J Clin Invest 54: 358–365, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P: Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 68: 1815–1824, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Chertow GM, Burke SK, Raggi P: Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Kovesdy CP, Mehrotra R, Kalantar-Zadeh K: Battleground: Chronic kidney disorders mineral and bone disease–calcium obsession, vitamin d, and binder confusion. Clin J Am Soc Nephrol 3: 168–173, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Eknoyan G, Levin A, Levin N: Bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42: 1–201, 2003 [PubMed] [Google Scholar]
- 24.Mehrotra R, Budoff M, Christenson P, Ipp E, Takasu J, Gupta A, Norris K, Adler S: Determinants of coronary artery calcification in diabetics with and without nephropathy. Kidney Int 66: 2022–2031, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Qunibi WY, Abouzahr F, Mizani MR, Nolan CR, Arya R, Hunt KJ: Cardiovascular calcification in Hispanic Americans (HA) with chronic kidney disease (CKD) due to type 2 diabetes. Kidney Int 68: 271–277, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Russo D, Palmiero G, De Blasio AP, Balletta MM, Andreucci VE: Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis 44: 1024–1030, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Tomiyama C, Higa A, Dalboni MA, Cendoroglo M, Draibe SA, Cuppari L, Carvalho AB, Neto EM, Canziani ME: The impact of traditional and non-traditional risk factors on coronary calcification in pre-dialysis patients. Nephrol Dial Transplant 21: 2464–2471, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kovesdy CP, Trivedi BK, Anderson JE: Association of kidney function with mortality in patients with chronic kidney disease not yet on dialysis: A historical prospective cohort study. Adv Chronic Kidney Dis 13: 183–188, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, Anderson JE: Association of low blood pressure with increased mortality in patients with moderate to severe chronic kidney disease. Nephrol Dial Transplant 21: 1257–1262, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 31.K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 32.Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, Anderson JE: Association of anemia with outcomes in men with moderate and severe chronic kidney disease. Kidney Int 69: 560–564, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Thadhani R, Tonelli M: Cohort studies: Marching forward. Clin J Am Soc Nephrol 1: 1117–1123, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Carafoli E, Penniston JT: The calcium signal. Sci Am 253: 70–78, 1985 [DOI] [PubMed] [Google Scholar]
- 35.Parfitt AM: Bone and plasma calcium homeostasis. Bone 8[ Suppl 1]: S1–S8, 1987 [PubMed] [Google Scholar]
- 36.Ersoy FF, Passadakis SP, Tam P, Memmos ED, Katopodis PK, Ozener C, Akcicek F, Camsari T, Ates K, Ataman R, Vlachojannis JG, Dombros AN, Utas C, Akpolat T, Bozfakioglu S, Wu G, Karayaylali I, Arinsoy T, Stathakis PC, Yavuz M, Tsakiris JD, Dimitriades CA, Yilmaz ME, Gultekin M, Karayalcin B, Yardimsever M, Oreopoulos DG: Bone mineral density and its correlation with clinical and laboratory factors in chronic peritoneal dialysis patients. J Bone Miner Metab 24: 79–86, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Yang H, Curinga G, Giachelli CM: Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Kidney Int 66: 2293–2299, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM: Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87: E10–E17, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K: Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med 168: 397–403, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Blayney MJ, Pisoni RL, Bragg-Gresham JL, Bommer J, Piera L, Saito A, Akiba T, Keen ML, Young EW, Port FK: High alkaline phosphatase levels in hemodialysis patients are associated with higher risk of hospitalization and death. Kidney Int 74: 655–663, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Regidor DL, Kovesdy CP, Mehrotra R, Rambod M, Jing J, McAllister CJ, Van WD, Kopple JD, Kalantar-Zadeh K: Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol 19: 2193–2203, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]