Abstract
Background and objectives: No large, randomized, double-blind trials in children with proteinuria treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers have previously been reported.
Design, setting, participants, & measurements: This 12-week, double-blind, multinational study investigated the effects of losartan 0.7 to 1.4 mg/kg per day compared with placebo (normotensive stratum) or amlodipine 0.1 to 0.2 mg/kg per day up to 5 mg/d (hypertensive stratum) on proteinuria (morning-void urinary protein-creatinine ratio, baseline ≥0.3 g/g) in 306 children up to 17 years of age.
Results: Twelve weeks of treatment with losartan significantly reduced proteinuria compared with amlodipine/placebo: losartan −35.8% (95% confidence interval: −27.6% to −43.1%) versus amlodipine/placebo 1.4% (95% confidence interval: −10.3% to 14.5%), P ≤ 0.001. Significance remained after adjustment for differences across treatment groups in change in BP (losartan produced incremental systolic and diastolic BP reductions versus amlodipine of 5.4 and 4.6 mmHg, respectively; and versus placebo of 3.8 and 4.0 mmHg, respectively). Proteinuria reduction was consistently observed in the normotensive (−34.4% losartan; 2.6% placebo) and hypertensive (−41.5% losartan; 2.4% amlodipine) strata, and in all prespecified subgroups, including age, gender, race, Tanner stage, weight, prior therapy with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, as well as among the most common etiologies of proteinuria. Adverse event incidence was low and comparable in all groups.
Conclusions: Losartan significantly lowered proteinuria and was well tolerated after 12 weeks in children aged 1 to 17 years with proteinuria with or without hypertension, a population that has not previously been rigorously studied.
In children with chronic kidney disease (CKD), the prevalence of significant proteinuria (>1 g/d) ranges from 5.8% in stage 1 CKD to 40% in stage 5 CKD (1), and lower-level proteinuria is even more prevalent. Persistent proteinuria is increasingly viewed not simply as a renal disease marker, but as being directly injurious to the kidneys (2,3), and may be a long-term risk factor for atherosclerosis (4,5).
Studies in adults with diabetic and nondiabetic renal disease have shown that angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin II type I receptor blockers (ARBs) delay progression of renal disease to end-stage renal failure and have antiproteinuric effects distinct from their effects on BP (6–11). Despite their different mechanisms of action, the two classes of drug appear to have comparable antiproteinuric and renoprotective properties, although a number of adverse effects, including hyperkalemia, occur less frequently with ARBs (12). Although these agents are now in routine use in adults, concerns persist about their efficacy and safety in children, where the causes of renal disease may be very different.
No prior large, placebo-controlled, randomized trials have investigated the efficacy and safety of ACE-Is or ARBs in the reduction of proteinuria in children with renal disease, although a number of small, uncontrolled or retrospective studies have been published (13–16). In the ongoing Effect of Strict Blood Pressure Control and ACE Inhibition on Progression of Chronic Renal Failure in Pediatric Patients (ESCAPE) study, treatment with the ACE-I ramipril was reported to lead to a 2.2-mmHg decrease in mean arterial BP and a 50% reduction in proteinuria in hypertensive children with CKD, with similar efficacy in patients with hypo/dysplastic kidneys and glomerulopathies (17).
This study evaluated losartan's effects on proteinuria in children and adolescents. Patients were divided into normotensive and hypertensive groups. Losartan was compared with placebo in the former, whereas in the latter, the calcium channel blocker (CCB) amlodipine was chosen as a comparator because of its known antihypertensive action in the absence of any significant effect on proteinuria.
Materials and Methods
Study Design and Participants
This double-blind, randomized, parallel-group, placebo- or amlodipine-controlled study was conducted in 50 clinical centers in 19 countries, and it included male or female children and adolescents with a documented history of proteinuria associated with CKD of any etiology (mean urinary protein-creatinine ratio (UPr/Cr) ≥0.3 g/g from three first-morning spot urine collections at baseline), with or without hypertension (hypertension defined as systolic BP (SBP) or diastolic BP (DBP) above the 95th percentile by National High Blood Pressure Education Program Working Group standards for the patient's gender, age, and height, or local standards, if required) (18). Patients had to have a GFR ≥30 ml/min per 1.73 m2 calculated by the Schwartz formula (19) and could not have taken ACE-Is, ARBs, or antihypertensive agent(s) other than study drug within 28 days of randomization. Antihypertensive therapies other than study medications were not allowed during the study. Children with renal transplants were excluded.
A 4-week, single-blind run-in period intended to wash patients off antihypertensive agents preceded a 12-week, double-blind period. At randomization, patients were stratified on the basis of the presence of hypertension and prior ACE-I/ARB use. Patients were assigned an allocation number according to a computer-generated, randomized allocation schedule. Exact matching placebo tablets for losartan were used to maintain the blind for patients able to swallow tablets. The amlodipine supplies did not include an exact matching placebo; therefore, all patients were given amlodipine suspension or amlodipine placebo to maintain the blind. An unblinded pharmacist or other qualified individual not directly involved with the patient's care prepared the suspensions to maintain the blind. After completing the double-blind period (or discontinuing early from increasing proteinuria, confirmed by repeat measurements), patients were eligible to participate in an open-label extension in which they were rerandomized to remain on losartan or start enalapril. The ongoing extension aims to ascertain the relative efficacies of these two RAS agents in sustaining proteinuria reduction and will continue until 100 patients complete 3 years of follow-up (approximately March 2011).
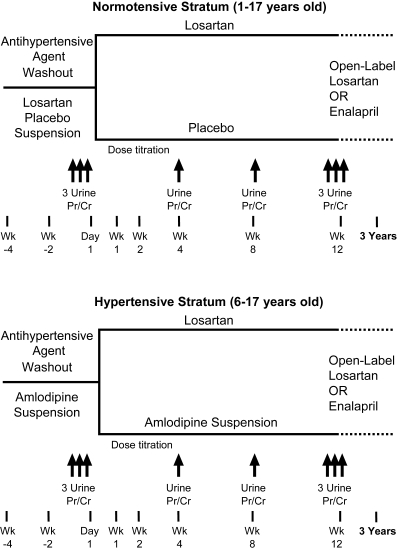
Normotensive Stratum (1 to 17 Years of Age).
Normotension was defined as (1) SBP and DBP <95th percentile in patients not receiving ACE-I/ARB for proteinuria at initial study visit, or (2) SBP and DBP <90th percentile and receiving ACE-I/ARB for proteinuria (a lower threshold was used for this group, whose BP off medication would likely have been <95th percentile) (Figure 1). During the 4-week run-in, all patients received placebo suspension. Subsequent randomization was 1:1 to placebo or losartan for 12 weeks.
Figure 1.
Study design.
Hypertensive Stratum (6 to 17 Years of Age).
Hypertension was defined as (1) SBP or DBP >90th percentile in patients receiving an ACE-I/ARB for proteinuria at the initial visit, (2) SBP or DBP >95th percentile in patients not receiving an ACE-I/ARB for proteinuria, or (3) patients with documented hypertension taking any antihypertensive medication at their initial visit (Figure 1). Patients with SBP or BP of >99th percentile plus 5 mmHg were excluded from the study, and if BP reached this level in randomized patients they were discontinued. During the 4-week run-in, all patients received amlodipine suspension 0.05 mg/kg per day (maximum 5 mg). Randomization was 1:1 to amlodipine or losartan for 12 weeks.
This study was conducted as Merck Losartan Protocol 326 and registered on www.clinicaltrials.gov as NCT00568178. It was approved by all relevant ethics review committees. Informed consent was obtained from parents or guardians. Patient assent (when feasible) was obtained as required by local regulations.
Procedures
UPr/Cr was measured on first-morning voided urine specimens in triplicate at randomization and week 12, and on a single specimen at weeks 4 and 8. At every visit, sitting BP was measured, adverse events (AEs) were collected, and compliance was assessed by pill count or visual inspection of the suspension bottle. A central laboratory performed all laboratory measurements. Serum creatinine was measured at week 2 (at investigator discretion) and week 12, and urine protein and creatinine were measured at weeks 4, 8, and 12. Because of the inclusion of young children, in whom 24-hour urine collections are difficult to obtain accurately, morning spot urines were used instead, as suggested in the PARADE publication, (2) and as used in the ESCAPE study (17).
Study Medications
Normotensive Stratum.
Losartan dosing was calculated on the basis of data from pediatric pharmacokinetic and dose-response studies (20). The daily starting dose was approximately 0.7 mg/kg. Losartan was up-titrated at 2 weeks to a maximum of approximately 1.4 mg/kg per day. Although the goal was to reach the maximal dose, individual investigators established this for each patient, because it was recognized that tolerance levels (determined by symptomatology) could differ among individuals. Patients randomized to placebo or amlodipine had sham up-titration at week 2.
Hypertensive Stratum.
The dose of amlodipine was chosen on the basis of product labeling. Because dosing information for amlodipine is not available for children <6 years old, only children ≥6 years old were enrolled in this stratum. During the 4-week run-in, patients were given single-blind amlodipine suspension to take once daily at the following starting dose: patients on one antihypertensive medication with SBP or DBP <95th percentile started on 0.05 mg/kg per day; patients on two antihypertensive medications or on one antihypertensive medication with SBP or DBP ≥95th percentile started on 0.1 mg/kg per day. This was up-titrated to 0.2 mg/kg (maximum 5 mg/day) after 2 weeks, if necessary, to control BP to <90th percentile. If the dose was up-titrated during the run-in, the higher dose was the starting dose of amlodipine (or its placebo) for the double-blind phase.
Statistical Analyses
The primary endpoint was the change in UPr/Cr on the natural logarithm scale from baseline (end of run-in) to the end of 12 weeks of therapy, which was analyzed using a mixed model with fixed effect terms for treatment, stratum factor 1 (hypertensive/normotensive), and stratum factor 2 (prior ACE-I/ARB use or not), time, treatment-by-time interaction, and baseline UPr/Cr, a random effect for patient, and an unstructured variance-covariance. The primary hypothesis assessing the reduction in proteinuria after 12 weeks of treatment of losartan versus control was addressed on the basis of the ratio of geometric means (obtained after antilog transformation of the difference in least-square means) at week 12, with its 95% confidence interval (95% CI) and associated P value. Geometric means (obtained after antilog transformation of the least-square means), along with the percent reduction and its 95% CI in each treatment group, are also presented. The natural logarithm transformation was used to normalize the distribution of the data.
With 300 patients randomized, the study had 90% power to detect a 30% reduction in UPr/Cr at the 5% level (assuming an SD of 0.9 for the change in UPr/Cr on the log scale and a 10% discontinuation rate). Safety and tolerability were assessed by reviewing all safety parameters, including AEs, laboratory safety parameters, and vital signs. For safety events of clinical interest and predefined limits of change (PDLCs), statistical significance levels for between-group comparisons were provided by inferential testing.
Results
Patient Population
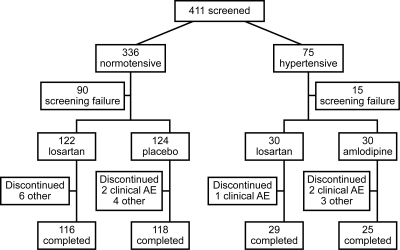
Of 411 patients screened, 306 were randomized (Figure 2): 246 in the normotensive stratum received losartan or placebo, and 60 in the hypertensive stratum received losartan or amlodipine. Of the 306 randomized, 302 took at least one dose of study drug, had at least one postrandomization efficacy measurement (UPr/Cr), and were included in the full analysis set population. Two hundred seventy patients completed the 12-week, double-blind treatment and had an efficacy measurement in the predefined time window for the week 12 visit.
Figure 2.
Disposition of patients.
Table 1 displays baseline characteristics. Male patients outnumbered female patients, 80% of the patients were normotensive, 54% had received ACE-Is/ARBs within 3 months before visit 1, and 48% were Tanner stage I. Mean age was 10 years (9.5 years in the normotensive stratum and 12.5 years in the hypertensive stratum). Proteinuria etiologies and baseline mean SBP and DBP in the treatment groups within each stratum were similar (although a slightly higher proportion of hypertensive children randomized to losartan had BP <90th percentile). Mean baseline GFR was similar in the treatment groups (losartan 119.6 ml/min per 1.73m2 and amlodipine/placebo 117.9 ml/min per 1.73 m2) and varied widely in both groups (16.3 to 295.6 ml/min per 1.73 m2).
Table 1.
Baseline characteristics
| Characteristic | Normotensive |
Hypertensive |
||
|---|---|---|---|---|
| Losartan (n = 122) | Placebo (n = 124) | Losartan (n = 30) | Amlodipine (n = 30) | |
| Age, yr, mean (SD) | 10.0 (4.9) | 8.9 (4.6); | 12.3 (3.4) | 12.8 (2.9) |
| range | 1.0 to 17.0 | 1.0 to 17.0 | 6.0 to 17.0 | 7.0 to 17.0 |
| ≤6 | 32 (26.2) | 42 (33.9) | 1 (3.3) | 0 (0.0) |
| 7 to 12 | 43 (35.2) | 45 (36.3) | 14 (46.7) | 12 (40.0) |
| 13 to 17 | 47 (38.5) | 37 (29.8) | 15 (50.0) | 18 (60.0) |
| Gender, n (%) | ||||
| male | 69 (56.6) | 72 (58.1) | 17 (56.7) | 13 (43.3) |
| female | 53 (43.4) | 52 (41.9) | 13 (43.3) | 17 (56.7) |
| Race, n (%) | ||||
| Asian | 24 (19.7) | 23 (18.5) | 2 (6.7) | 3 (10.0) |
| Black | 4 (3.3) | 3 (2.4) | 1 (3.3) | 2 (6.7) |
| multiracial | 28 (23.0) | 25 (20.2) | 8 (26.7) | 9 (30.0) |
| White | 61 (50.0) | 69 (55.6) | 16 (53.3) | 16 (53.3) |
| other | 5 (4.1) | 4 (3.2) | 3 (10.0) | 0 (0.0) |
| Tanner stage, n (%) | ||||
| I to III | 98 (80.3) | 99 (79.8) | 19 (63.3) | 18 (60.0) |
| IV to V | 24 (19.7) | 25 (20.2) | 11 (36.7) | 12 (40.0) |
| Prior ACE-I/ARB use, n (%) | ||||
| yes | 61 (50.0) | 62 (50.0) | 22 (73.3) | 19 (63.3) |
| no | 61 (50.0) | 62 (50.0) | 8 (26.7) | 11 (36.7) |
| Body mass index, kg/m2 | ||||
| mean (SD) | 19.3 (5.5) | 18.8 (4.0) | 21.7 (5.0) | 20.8 (4.4) |
| range | 12.0 to 55.2 | 12.2 to 35.6 | 14.6 to 35.2 | 12.6 to 33.8 |
| Duration of hypertension, yr | ||||
| mean (SD) | NA | NA | 4.8 (4.1); | 6.1 (4.8); |
| range | NA | NA | 1.0 to 17.0 | 1.0 to 16.0 |
| Protein/creatinine ratio, g/g | ||||
| mean (SD) | 2.0 (2.6) | 2.8 (4.0) | 2.9 (2.7) | 2.9 (3.1) |
| range | 0.1 to 11.8 | 0.1 to 26.6 | 0.3 to 9.4 | 0.4 to 16.6 |
| GFR, ml/min per 1.73 m2 | ||||
| mean (SD) | 120.5 (50.2) | 119.5 (51.4) | 116.0 (45.9) | 110.9 (60.6) |
| range | 16.3 to 295.6 | 29.4 to 283.3 | 30.7 to 201.4 | 32.1 to 289.4 |
| Cystatin-C, mg/L | ||||
| mean (SD) | 11.4 (5.9) | 12.5 (7.1) | 11.8 (4.8) | 13.4 (7.0) |
| range | 6.0 to 45.0 | 5.0 to 43.0 | 6.0 to 30.0 | 6.0 to 33.0 |
| Sitting systolic blood pressure, mmHg, mean (SD) | 103.2 (12.5) | 103.7 (12.2) | 117.4 (10.9) | 121.6 (10.5) |
| Age/gender/height-adjusted percentile, mean (SD) | 54.6 (29.1) | 60.6 (27.8) | 78.8 (19.5) | 84.4 (19.4) |
| Sitting diastolic blood pressure, mmHg, mean (SD) | 64.4 (10.0) | 65.1 (9.6) | 76.4 (8.1) | 79.1 (12.4) |
| Age/gender/height-adjusted percentile, mean (SD) | 64.7 (25.3) | 69.8 (22.3) | 84.7 (13.5) | 84.7 (17.3) |
| Etiologies of proteinuria, n | 246 | 60 | ||
| glomerular | 113 | 35 | ||
| reflux nephropathy | 27 | 2 | ||
| hemolytic uremic syndrome | 25 | 6 | ||
| Alport syndrome | 24 | 6 | ||
| hypoplasia/dysplasia/aplasia | 14 | 2 | ||
| obstruction | 5 | 0 | ||
| other | 14 | 1 | ||
| unknown | 24 | 8 | ||
Proteinuria
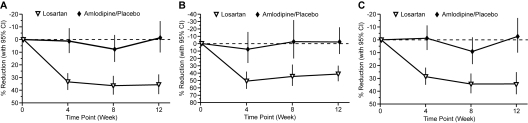
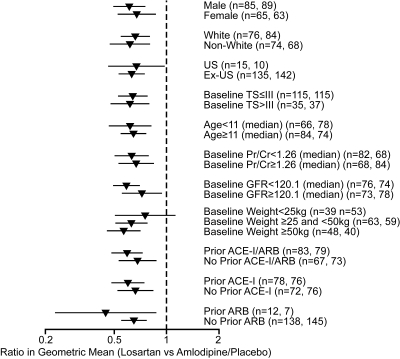
Twelve weeks of treatment with losartan significantly reduced proteinuria compared with amlodipine/placebo. There was a 35.8% (95% CI: 27.6% to 43.1%) reduction in mean (SD) UPr/Cr from 1.27 (2.30) to 0.83 (2.48) in losartan-treated patients compared with a 1.4% (−10.3% to 14.5%) increase with amlodipine/placebo from 1.55 (4.81) to 1.60 (6.73), P ≤ 0.001. Proteinuria reduction was consistently observed in the normotensive (−34.4% losartan; 2.6% placebo) and hypertensive (−41.5% losartan; 2.4% amlodipine) patients (Figure 3), and in all prespecified subgroups, including age, gender, race, Tanner stage, weight, and prior therapy with ACEIs/ARBs (Figure 4). Among the most commonly occurring proteinuria etiologies in this study (focal segmental glomerulosclerosis, hemolytic uremic syndrome, Alport syndrome, and reflux nephropathy), the reduction in proteinuria was also consistently observed (data not shown).
Figure 3.
Change from baseline in protein/creatinine ratio (g/g) at week 12 in (A) hypertensive and normotensive children (n = 302), (B) hypertensive children (n = 59), and (C) normotensive children (n = 243).
Figure 4.
Ratio in geometric mean protein/creatinine ratio (g/g at week 12 by subgroup, point estimate and 95% CI. n = number in losartan group, number in amlodipine/placebo group. TS, Tanner stage; US, United States.
Impact of Reduction in BP on Proteinuria
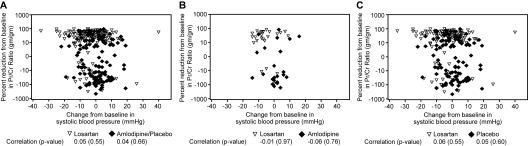
In hypertensive children, 12 weeks of treatment with losartan led to a 5.5-mmHg decrease in SBP compared with a 0.1-mmHg decrease with amlodipine. Treatment with losartan led to a 3.8-mmHg decrease in DBP compared with a 0.8-mmHg increase with amlodipine. In the normotensive stratum, losartan lowered SBP and DBP by 3.7 and 3.4 mmHg, respectively, compared with increases of 0.1 and 0.6 mmHg, respectively, in the placebo arm. Because of the between-group differences in BP changes, an exploratory sensitivity analysis was performed to see how much the treatment effect on proteinuria changed after adjusting the model for the primary endpoint for time-varying changes in BP. Results of that sensitivity analysis were consistent with the primary analysis (geometric mean ratio: 0.66 (95% CI: 0.56 to 0.77); P ≤ 0.001). The very similar results between the primary and sensitivity analyses were consistent with the lack of relationship between proteinuria reduction and BP lowering (Figure 5).
Figure 5.
Relationship between reduction in proteinuria and systolic BP in (A) hypertensive and normotensive children (n = 302), (B) hypertensive children (n = 59), and (C) normotensive children (n = 243).
Safety
Discontinuation because of AEs occurred in 0.7% of patients on losartan versus 6.7% on amlodipine and 3.2% on placebo (Table 2). Two drug-related serious clinical AEs were reported, both on placebo. AEs of clinical interest (predefined as angioedema, hyperkalemia, renal dysfunction, and hypotension) occurred at similarly low rates across the treatment groups. Patients on losartan and amlodipine/placebo had mean serum creatinine levels of 0.826 and 0.852 mg/dl at baseline, respectively, and 0.862 and 0.925 at week 12, respectively; serum potassium levels were 4.30 and 4.36 at baseline, respectively, and 4.38 and 4.32 at week 12, respectively. The most common PDLC was increased serum potassium (defined as ≥0.5 mEq/L), observed in 12 patients on losartan and 17 on placebo in the normotensive stratum; five on losartan and two on amlodipine among the hypertensive patients.
Table 2.
Patients with adverse events (AEs)
| Losartan (N = 152), n (%) | Amlodipine/Placebo (N = 154), n (%) | Difference Losartan versus Amlodipine/Placebo, % (95% CI)a | |
|---|---|---|---|
| Clinical AEs | |||
| one or more AEs | 93 (61.2) | 85 (55.2) | 6.0 (−5.1 to 16.9) |
| no AE | 59 (38.8) | 69 (44.8) | |
| drug-relatedb AEs | 8 (5.3) | 8 (5.2) | 0.1 (−5.3 to 5.5) |
| serious AE | 7 (4.6) | 5 (3.2) | 1.4 (−3.4, 6.4) |
| serious drug-related AEs | 0 | 2 (1.3) | −1.3 (−4.6 to 1.2) |
| died | 0 | 0 | – |
| discontinuedd due to AE | 1 (0.7) | 6 (3.9) | −3.2 (−7.7 to 0.2) |
| discontinued due to drug-related AE | 0 | 3 (1.9) | – |
| discontinued due to a serious AE | 1 (0.7) | 2 (1.3) | – |
| discontinued due to a serious drug-related AE |
0 | 2 (1.3) | – |
| Laboratory AEs | |||
| one or more AEs | 8 (5.3) | 12 (7.8) | −2.5 (−8.5 to 3.3) |
| no AE | 144 (94.7) | 142 (92.2) | – |
| drug-relatedb AEs | 0 | 3 (1.9) | −1.9 (−5.6 to 0.6) |
| serious AE | 0 | 0 | – |
| serious drug-related AEs | 0 | 0 | – |
| discontinuedc due to AE | 0 | 0 | – |
| discontinued due to drug-related AE | 0 | 0 | – |
| discontinued due to a serious AE | 0 | 0 | – |
| discontinued due to a serious drug-related AE |
0 | 0 | – |
| AEs of special interestd | |||
| renal dysfunction | 2 (1.3) | 3 (1.9) | −0.63 (−4.42 to 2.95) |
| hyperkalemia | 0 | 0 | – |
| hypotension | 1 (0.7) | 0 | 0.7 (−1.8 to 3.6) |
| angioedema | 0 | 0 | – |
Based on Miettinen-Nurminen method.
Determined by the investigator to be related to the drug.
Study medication withdrawn.
Renal dysfunction, hyperkalemia, hypotension, and angioedema: Five instances of renal dysfunction were reported: two on losartan (one hypertensive patient with GFR decrease from 80 to 58 ml/min per 1.73 m2, deemed study drug-related, and one normotensive patient with a GFR drop from 161 to 76 ml/min per 1.73 m2, felt to be related to worsening of nephrotic syndrome but not to study drug); one on placebo (GFR change 54 to 30 ml/min per 1.73 m2), considered drug-related; and two in the amlodipine group, which were not drug-related (GFR decreases of 47 to 32 ml/min per 1.73 m2 and 40 to 36 ml/min per 1.73 m2). One case of non-drug-related hyperkalemia occurred at the end of the 4-week run-in on the day of randomization before study drug (losartan) was started. One report of hypotension in a normotensive patient on losartan, considered drug-related: a 7-year-old was “quieter than usual,” and upon reducing the losartan dose, the patient resumed his previous level of activity.
Discussion
This is the first large, randomized, controlled, double-blind study to evaluate the antiproteinuric efficacy of a renin-angiotensin system (RAS) agent, losartan, in children. More than 300 children aged 1 to 17 years with proteinuria of a broad spectrum of etiologies with or without hypertension were treated for 12 weeks. Treatment with losartan at approximately 0.7 to 1.4 mg/kg per day led to a reduction in proteinuria of 35.8% compared with a 1.4% increase on amlodipine/placebo. Whereas treatment with losartan resulted in a significant BP decline in the hypertensive stratum, amlodipine had an unexpected neutral effect on BP. However, the difference in proteinuria change from baseline between losartan and amlodipine/placebo remained highly significant after adjustment for change in SBP and DBP.
The neutral effect of amlodipine on proteinuria observed in this study was consistent with published data (9,21), although the lack of further BP lowering with amlodipine after randomization in the hypertensive stratum was surprising. The most likely explanation for not achieving BP goal in patients on amlodipine was titration inertia. By the end of the 4-week run-in, only 50% of the 60 hypertensive patients had been titrated up to amlodipine 5 mg. In practice, higher absolute and weight-based doses of amlodipine are commonly used by pediatricians (up to 20 mg/d), exceeding the maximum approved dose (5 mg) for children (22). Because a majority of the hypertensive patients had been on antihypertensive agents before the study, the starting amlodipine doses in the run-in were likely sufficient only to maintain prestudy BP levels but were probably not sufficient to lower BP further after patients began the study. Apparently, rather than up-titrating amlodipine during the study, investigators chose to continue the run-in dose as long as BP did not worsen from prestudy levels.
Losartan generally achieves maximal BP lowering by 3 weeks in adults with essential hypertension. In this study, losartan produced its maximal BP-lowering effect by 1 week. One possible explanation for this rapid BP response is that children with CKD/proteinuria may have a more “activated” RAS at baseline compared with adults with essential hypertension because of renal tissue injury/scarring or a direct effect of proteinuria, possibly rendering them more sensitive to RAS inhibition (22–24). A study of hypertensive adult CKD patients showed that maximal/near-maximal BP lowering appeared to be achieved after roughly 1 week on losartan (although these patients had not been switched from amlodipine) (25). Another possible explanation is that amlodipine in the run-in may have “primed” the RAS for an enhanced hemodynamic response to losartan. Shorter-acting dihydropyridine CCBs are known to stimulate the sympathetic nervous system and lead to increases in renin activity. Conflicting data exist on whether amlodipine, a longer-acting CCB, produces similar sympathetic and renin responses, but increased local RAS activity has been reported in patients with essential hypertension on amlodipine (no data are available in children with CKD) (26,27).
Losartan was well tolerated. No significant differences were seen in the incidence of AEs between losartan and amlodipine/placebo. Drug-related incidences of hyperkalemia and renal dysfunction were very low and were comparable between losartan and placebo/amlodipine. The safety profiles of the treatment groups in the two BP strata were similar to that of the overall study population. No significant differences in the occurrence of events of clinical interest or of PDLCs were seen between losartan and amlodipine/placebo. GFR changes during the 12-week double-blind treatment period were not significantly different between the treatment groups. Changes in weight and height were minimal and were similar in the losartan and amlodipine/placebo groups.
In addition to the lack of equivalent BP control discussed above, another potential limitation of this study is the relatively small number of hypertensive patients (n = 59; 20%). The study was not powered to detect treatment differences within each BP stratum or any other subgroup. Nonetheless, the observed proteinuria reductions with losartan in both strata and in the subgroups point to a remarkably consistent magnitude of antiproteinuric effect of losartan across all of these pediatric subsets.
Table 3.
Study investigators
| Investigators |
|---|
| Chile: Angela Delucchi, Santiago. |
| Colombia: Pilar M Amado, Bucaramanga; Oscar A. Hernandez, Bogotá; Ricardo Gastelbondo, Bogotá. |
| Germany: Katalin Dittrich, Erlangen; Juergen Strehlau, Leipzig; Martin Pohl, Freiburg. |
| Guatemala: Luis F. Arroyo-Garcia, Guatemala City; Randall Lou, Guatemala City. |
| Hungary: György Reusz, Budapest; László Szabó, Miskolc. |
| India: Rajiv Aggarwal, Karnataka; Urmila Anandh, Karnataka; Shiela Bhave, Maharashtra; Padmanabha P. Maiya; Karnataka; Kishore Phadke, Karnataka. |
| Lithuania: Birute Pundziene, Kaunas; Augustina Jankauskiene, Vilnius. |
| Mexico: Silvestre Garcia, México; Froylán E. Hernández-Lara, Puebla; Ricardo López, Morelos. |
| Norway: Anna Bjerre, Oslo; Damien Brackman, Bergen. |
| Panama: Florencio A. McCarthy, Panama City. |
| Peru: Reyner F. Loza-Munarriz, Lima; Graciela Sakihara-Asato, Lima. |
| Philippines: Maria-Angeles Marbella, Quezon City; Rosario Cruz, Quezon City. |
| Puerto Rico: Melvin Bonilla-Felix, San Juan. |
| Romania: Aurel Bizo, Cluj; Gheorghe Chiriac-Babei, Cluj; Ioan Sabau, Cluj; Victor M. Nanulescu, Cluj. |
| Russian Federation: Yuri B. Belousov, Moscow; Vladimir V. Dlin, Moscow; Alexey N. Tsygin, Moscow. |
| Spain: Laura Espinosa-Román, Madrid. |
| Taiwan: Jeng-Daw Tsai, Taipei; Chi-Hui Cheng, Kweishan Taoyuan. |
| United Kingdom: William van't Hoff, London; Nicholas Webb, Manchester. |
| United States: Virnal Chadha, Richmond, VA; Vikas R. Dharnidharka, Gainesville, FL; Robert M. Haws, Marshfield, WI; Ronald J. Hogg, Temple, TX; Craig B. Langman, Chicago, IL; Kenneth V. Lieberman, Hackensack, NJ; Victoria Norwood, Charlottesville, VA; Cynthia G. Pan, Milwaukee, WI; Howard Trachtman, New Hyde Park, NY; Constancia Uy, Newark, NJ. |
Conclusion
This is the first large, randomized, double-blind, controlled trial to be published on the antiproteinuric effect of any RAS agent in children and demonstrates that losartan was well tolerated and significantly lowered proteinuria after 12 weeks in children aged 1 to 17 years with proteinuria of a wide range of etiologies.
Disclosures
N.J.A.W. has received consulting, advisory board, lecture fees, and any combination of the three from Astellas, Merck, Novartis, Roche, and Wyeth, and has received grant support from Astellas and Novartis. S.S. has received consulting fees from Amgen, Merck, and Johnson and Johnson. J.S. has received consulting fees from Merck. T.G.W. has received consulting fees from Merck, Novartis, Boehringer Ingelheim, Takeda, and Daiiachi Sankyo. C.L., E.S., D.M., and G.W.G. are employees of Merck and Co. Inc. and own stock in Merck. T.L. is an employee of Merck and Co. Inc.
Role of the funding source: This study was sponsored by Merck & Co., Inc., who provided all funding and study drugs.
Acknowledgments
We are grateful to Dr. Alan Meehan for editorial and administrative support of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This study is registered at www.clinicaltrials.gov (identifier NCT00568178).
References
- 1.Wong H, Mylrea K, Feber J, Drukker A, Filler G: Prevalence of complications in children with chronic kidney disease according to KDOQI. Kidney Int 70: 585–590, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Hogg RJ, Portman RJ, Milliner D, Lemley KV, Eddy A, Ingelfinger J: Evaluation and management of proteinuria and nephrotic syndrome in children: Recommendations from a pediatric nephrology panel established at the National Kidney Foundation conference on proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE). Pediatrics 105: 1242–1249, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Remuzzi G, Ruggenenti P, Benigni A: Understanding the nature of renal disease progression. Kidney Int 51: 2–15, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Grimm RH, Jr., Svendsen KH, Kasiske B, Keane WF, Wahi MM: Proteinuria is a risk factor for mortality over 10 years of follow-up. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Kidney Int ( Suppl) 63: S10–S14, 1997 [PubMed] [Google Scholar]
- 5.Portman R, Hawkins E, Verani R: Premature atherosclerosis (PA) in pediatric renal patients (PRP): Report of the southwest pediatric nephrology study group [Abstract]. Pediatr Res 29: 349A, 1991 [Google Scholar]
- 6.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Zucchelli P: Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med 334: 939–945, 1996 [DOI] [PubMed] [Google Scholar]
- 8.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349: 1857–1863, 1997 [PubMed] [Google Scholar]
- 9.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I; Collaborative Study Group: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Andrade C, Russo D, Iversen B, Zuchelli P, Aranda P, Guerra L, Casado S: Comparison of losartan and amlodipine in renally impaired hypertensive patients. Kidney Int Suppl 68: S120–124, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Zaffanello M, Franchini M, Fanos V: New therapeutic strategies with combined renin-angiotensin system inhibitors for pediatric nephropathy. Pharmacotherapy 28: 125–130, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Franscini LM, Von Vigier RO, Pfister R, Casaulta-Aebischer C, Fossali E, Bianchetti MG: Effectiveness and safety of the angiotensin II antagonist irbesartan in children with chronic kidney disease. Am J Hypertens 15: 1057–1063, 2002 [DOI] [PubMed] [Google Scholar]
- 14.White CT, Macpherson CF, Hurley RM, Matsell DG: Antiproteinuric effects of enalapril and losartan: A pilot study. Pediatr Nephrol 18: 1038–1043, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Ellis D, Vats A, Moritz ML, Reitz S, Grosso MJ, Janosky JE: Long term antiproteinuric and renoprotective efficacy and safety of losartan in children with proteinuria. J Pediatr 143: 89–97, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Seeman T, Dusek J, Vondrák K, Flogelova H, Geier P, Janda J: Ramipril in the treatment of hypertension and proteinuria in children with chronic kidney disease. Am J Hypertens 17: 415–420, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Wühl E, Mehls O, Schaefer Fthe ESCAPE Trial Group: Antihypertensive and antiproteinuric efficacy of ramipril in children with chronic renal failure. Kidney Int 66: 768–76, 2004 [DOI] [PubMed] [Google Scholar]
- 18.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report in the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114: 555–576, 2004 [PubMed] [Google Scholar]
- 19.Schwartz GJ, Haycock GB, Edelmann CM, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 20.Shahinfar S, Cano F, Soffer BA, Ahmed T, Santoro EP, Zhang Z, Gleim G, Miller K, Vogt B, Blumer J, Briazgounov I: A double-blind, dose-response study of losartan in hypertensive children. Am J Hypertens 18: 183–190, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Flynn JT, Pasko DA: Calcium channel blockers: pharmacology and place in therapy of pediatric hypertension. Pediatr Nephrol 15: 302–316, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BA; Chronic Kidney Disease in Children Study Group: Blood pressure in children with chronic kidney disease: A report from the chronic kidney disease in children study. Hypertension 52: 631–637, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf G, Ritz E: Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: Pathophysiology and indications. Kidney Int 67: 799–812, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Hadtstein C, Schaefer F: Hypertension in children with chronic kidney disease: pathophysiology and management. Pediatr Nephrol 23: 363–371, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toto R, Shultz P, Raij L, Mitchell H, Shaw W, Ramjit D, Toh J, Shahinfar S: Efficacy and tolerability of losartan in hypertensive patients with renal impairment. Hypertension 31: 684–691, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Lamarre-Cliché M, de Champlain J, Lacourcière Y, Poirier L, Karas M, Larochelle P: Effects of circadian rhythms, posture, and medication on renin-aldosterone interrelations in essential hypertensives. Am J Hypertension 18: 56–64, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Sakata K, Shirotani M, Yoshida H, Nawada R, Obayashi K, Togi K: Effects of amlodipine and cilnidipine on cardiac sympathetic nervous system and neurohormonal status in essential hypertension. Hypertension 33: 1447–1452, 1999 [DOI] [PubMed] [Google Scholar]