Abstract
Background and objectives: IgA nephropathy (IgAN) is the most common primary glomerular disease worldwide. Accurately identifying patients who are at risk for progressive disease is challenging. The extent to which histopathologic features improves prognostication is uncertain.
Design, setting, participants, & measurements: We studied a retrospective cohort with biopsy-proven IgAN in Calgary, Canada. Renal biopsies were reviewed by a nephropathologist with histopathologic data abstracted using a standardized form. The primary outcome was the composite of doubling of serum creatinine, ESRD, or death. Spline models defined significant levels of interstitial fibrosis, glomerulosclerosis, hypertension, proteinuria, and creatinine. The prognostic significances of clinical and histopathologic parameters were determined using Cox proportional hazards models.
Results: Data from 146 cases were available for analysis with a median follow-up of 5.8 years. Greater than 25% interstitial fibrosis, >40% glomerular sclerosis, and a systolic BP >150 mmHg were risk thresholds. In univariable analyses, baseline creatinine, proteinuria, systolic BP, glomerular sclerosis, interstitial fibrosis, and crescentic disease were predictors of the primary outcome. In multivariable models adjusted for clinical characteristics, interstitial fibrosis (hazard ratio [HR]2.7; 95% confidence interval [CI] 1.2 to 6.0), glomerular sclerosis (HR 2.6; 95% CI 1.2 to 4.5), and crescents (HR 2.4; 95% CI 1.2 to 5.1) remained independent predictors of the primary outcome and significantly improved model fit compared with clinical characteristics alone.
Conclusions: Baseline histopathologic parameters are independent predictors of adverse outcomes in IgAN even after taking into consideration clinical characteristics. Relatively small degrees of interstitial fibrosis confer an increased risk for progressive IgAN.
IgA nephropathy (IgAN) is the most common primary glomerulonephritis worldwide. Patients with IgAN have a variable clinical course with between 6 and 43% progressing to ESRD over 10 yr (1–5). Given this variability, identifying reliable prognostic factors is important to help stratify clinical monitoring and treatment regimens.
Previous studies have identified clinical features including high-grade proteinuria, reduced kidney function, and hypertension at the time of diagnosis as predictors of adverse outcomes (4–7). Studies have identified interstitial fibrosis and glomerular sclerosis as poor prognostic features (8–10). This is not surprising considering that these features are a common final result of damage from glomerulonephritis; however, histopathologic features frequently correlate with serum creatinine, and whether they add prognostic values beyond the measurement of serum creatinine is uncertain. Histopathologic features are commonly categorized on the basis of arbitrary thresholds to denote significant degrees of damage (3,5,11), a factor that may contribute to poor performance in multivariable models of risk prediction in IgAN and result in an underestimation of their ability independently to predict outcomes. We performed a retrospective study using detailed baseline clinical data and quantitative analysis of renal biopsies, including the degree of interstitial fibrosis and glomerulosclerosis, to assess the factors that determine adverse outcomes including chronic kidney disease (CKD) progression and death.
Materials and Methods
Patient Identification
Patients were identified from a prospective database of all renal biopsies performed in the Calgary Health Region in Calgary, Canada (catchment population 1.2 million). All patients who were older than 18 years and had a biopsy demonstrating IgAN between 1985 and 2000 were eligible for study. IgAN was defined by a renal biopsy demonstrating dominant or co-dominant IgA deposition in the mesangium on immunofluorescence microscopy in the absence of a clinical diagnosis suggesting a secondary cause for the biopsy finding (e.g., a clinical diagnosis of Henoch-Schönlein purpura, systemic lupus erythematosus, or liver cirrhosis).
Histopathology
All histopathologic samples were reviewed and scored independent of previous pathology reviews and by a single renal pathologist who was blinded to previous pathology reviews (in the context of usual clinical care) and to patients' clinical outcomes. Biopsy data were abstracted to a standardized form that included quantitative analysis of all major renal histopathologic compartments including but not limited to the number and percentage of glomeruli that were segmentally sclerosed, that were globally sclerosed, that had fibrous crescents, and that had cellular crescents; the maximum number of mesangial cells; the percentage of the interstitium that was fibrosed, the percentage of interstitium with cellular infiltrates, and the percentage of tubules that were atrophied. Continuous parameters (e.g., interstitial fibrosis) were estimated visually and without aids such as counting grids. Interstitial fibrosis was estimated as the area of the cortical interstitium affected by fibrosis excluding the area occupied by glomeruli. Twenty percent of biopsies were re-read by the study renal pathologist to test intrarater reliability. In addition, a second renal pathologist reviewed a random subset of 20% of biopsies to assess interrater reliability.
The interstitial fibrosis results that were obtained by the renal pathologist were compared with results that were obtained from quantitative computerized image analysis in a random subset of 20% of biopsies. Image analysis techniques systematically assess entire biopsy slides and allow unbiased, accurate, and reproducible quantification of histopathologic parameters (12,13). These techniques require more time in reading the biopsies, extra processing of specimens, and specific computer software and may therefore be impractical for routine clinical care but serve as a reference point for clinical research. Quantification of the area of cortex with interstitial fibrosis was achieved by fully digitizing a representative section of each patient's renal biopsy stained with periodic acid-Schiff and methenamine silver. A Cavalieri estimator grid with nodes spaced 150 μm apart was superimposed on each image (14). The renal cortical area of the biopsy was estimated by multiplying the total number of nodes overlying the cortex (excluding glomeruli) by the area represented by each node (14). Nodes that overlay areas that were affected by interstitial fibrosis were summed, and an estimate of the fraction of cortical area affected was calculated on the basis of the renal cortical area excluding glomeruli. All image analysis was performed using Stereo Investigator 9 software (MicroBrightField).
Clinical Data
Baseline clinical characteristics were obtained from a review of the patient's outpatient nephrology clinic chart and hospital charts from the time of diagnosis. The primary outcome was time to the composite of the first of (1) doubling of baseline serum creatinine, (2) ESRD (permanent hemodialysis, peritoneal dialysis, or renal transplantation), or (3) death from any cause. Initial serum creatinine measurements were determined by reviewing patients' clinic and hospital charts; clinic charts and a database of all serum creatinine measurements for all patients in the Calgary Health Region was used after 2003. The serum creatinine measurement that was taken most recently before the renal biopsy was used as the baseline value. Proteinuria data were abstracted from patient charts. Twenty-four-hour urine protein values that most recently preceded the renal biopsy were used as baseline value. When 24-hour collections were not available, a protein-to-creatinine ratio was taken as an estimation of 24-hour proteinuria. The occurrence of ESRD was obtained from the prospective databases of the Southern Alberta Renal Program dialysis and transplant patients (15). All-cause mortality data were obtained from the previously mentioned sources, supplemented by the provincial Department of Vital Statistics. Patients who had no follow-up with a nephrologist and no follow-up creatinine values and did not have a renal transplant or dialysis initiated or a reported death were assumed not to have met the primary end point at the time the database was closed. Sensitivity analyses that excluded these patients were performed to ensure that the primary analysis results were robust.
Statistical Analysis
Baseline characteristics for patients who did and did not reach the primary outcome were compared using t tests or the Mann-Whitney U test for continuous variables as appropriate and χ2 tests for dichotomous variables. Inter- and intrarater reliabilities were measured using the intraclass correlation coefficient. Threshold values for the minimal levels of baseline creatinine, proteinuria, mean systolic BP (SBP), interstitial fibrosis, and glomerulosclerosis that predicted the primary outcome were determined by fitting b-splines to Cox proportional hazards models (16). A spline is a smooth function that is sensitive to changes in the relationship between a predictor variable and an outcome across the range of the predictor. This analysis permitted us to determine the functional form of the relationship between the level of the predictor variables (i.e., the threshold at which the risk increased) and the composite primary outcome. This analysis included a test for nonlinearity in the relationship as well as a graphic examination of the risk for the outcome (with 95% confidence intervals [CIs]) across the range of each of the predictor variables of interest. These continuous variables were then categorized on the basis of the levels of the continuous parameters that corresponded to an inflection in the hazard of the primary outcome. Variables with P < 0.10 in univariable Cox proportional hazards models were retained as potential predictors in multivariable models. Multivariable Cox proportional hazard models were first constructed using clinical parameters. Histopathologic parameters were then added to the clinical model. Clinical parameter models were compared with models that included histopathologic parameters using the Akaike information criteria with lower values representing better fitting models. Assumptions for the Cox models were tested and met. The study was approved by the institutional review board of the University of Calgary.
Results
A total of 192 patients with IgAN were identified from the renal biopsy database. A total of 153 patients had their original biopsies available for review; seven of these patients had missing baseline clinical data and were excluded from analysis; therefore, 146 patients were retained for all primary analyses. The clinical characteristics of patients with missing biopsy specimens did not differ significantly from those with biopsy data. Nine patients had missing follow-up data and were assumed not to have reached the composite outcome; these patients were retained in primary analyses and excluded in sensitivity analyses.
The 146 included patients had a median follow-up time of 5.8 years. The clinical or biopsy characteristics of the patients who were included for analysis did not seem different from the patients with inadequate data for final analysis. Eighty percent of this cohort had significant proteinuria (>1 g/L), 38% had a serum creatinine >120 μmol/L, and 55% had a BP of >130/90 mmHg. Forty (26%) patients developed the composite outcome of a doubling of creatinine (28 patients), ESRD (six patients), and death (six patients). Of the patients who doubled their creatinine, 18 (64%) of 28 went on to develop ESRD, one of whom died. Patients who went on to the composite end point had higher baseline serum creatinine and were more likely to be treated with glucocorticoids and/or immunosuppressive medications at the time of diagnosis (Table 1).
Table 1.
Baseline clinical characteristics
| Characteristic | No Composite (n = 97) | Composite (n = 40) | P |
|---|---|---|---|
| Age (years; mean ± SD) | 38 ± 13 | 41 ± 15 | 0.30 |
| Female (%) | 35 | 40 | 0.58 |
| Creatinine (μmol/L; median [IQR]) | 92 (45 to 354) | 129 (35 to 900) | 0.01 |
| SBP (mmHg; mean ± SD) | 129 ± 20 | 133 ± 23 | 0.39 |
| DBP (mmHg; mean ± SD) | 80 ± 13 | 81 ± 12 | 0.59 |
| Proteinuria (g/d; median [IQR]) | 1.4 (0.0 to 21.0) | 2.0 (0.0 to 9.9) | 0.10 |
| Initial treatment (%) | |||
| glucocorticoids | 9 | 23 | 0.04 |
| immunosuppressives | 2 | 13 | 0.01 |
| angiotensin blockade | 43 | 53 | 0.33 |
| omega 3 fatty acids | 22 | 13 | 0.21 |
DBP, diastolic BP; IQR, interquartile range.
The baseline renal biopsies of patients who went on to reach the composite end point had significantly more segmental glomerular sclerosis, global glomerular sclerosis, interstitial fibrosis, tubular atrophy, and interstitial cellular infiltrates (Table 2). There were no significant differences in the mean degree of matrix expansion, the maximum number of mesangial cells per glomerulus, artery thickening, or arteriolar hypertrophy between groups. Interstitial fibrosis, tubular atrophy, and interstitial cellular infiltrates were highly correlated with one another (r2 = 0.82 to 0.92). Glomerular sclerosis (global or segmental) was only moderately correlated with interstitial fibrosis, tubular atrophy, and interstitial cellular infiltrates (r2 = 0.57 to 0.66). For this reason, only the presence of crescents, interstitial fibrosis, and global or segmental glomerular sclerosis (henceforth referred to collectively as glomerular sclerosis) were retained for regression analyses.
Table 2.
Baseline histopathologic features from renal biopsies of patients who did and did not reach the primary composite end point
| Histologic Feature | No Composite | Composite | Pa |
|---|---|---|---|
| Globally sclerosed glomeruli (% [IQR]) | 7 (0 to 24) | 27 (6 to 50) | 0.001 |
| Segmentally or globally sclerosis glomeruli (% [IQR]) | 13 (3 to 29) | 40 (8 to 60) | <0.001 |
| Any crescents present (%) | 26 | 43 | 0.05 |
| Area with interstitial fibrosis (% [IQR]) | 0 (0 to 15) | 30 (3 to 4) | <0.001 |
| Tubules atrophied (% [IQR]) | 5 (0 to 20) | 30 (5 to 50) | <0.001 |
| Area with interstitial infiltrate (% [IQR]) | 0 (0 to 5) | 20 (0 to 30) | <0.001 |
P values from Mann-Whitney U test (except for crescents, which are derived from a χ2 test).
The evaluation of interstitial fibrosis had high interrater reliability (intraclass correlation coefficient 0.88) and intrarater reliability (intraclass correlation coefficient 0.92). The evaluation of interstitial fibrosis by the study pathologist was also highly correlated to image analysis (intraclass correlation coefficient 0.90). Pathologist estimates of interstitial fibrosis tended to be lower than image analysis estimates, particularly at lesser degrees of overall fibrosis.
Threshold Levels for Predictor Variables
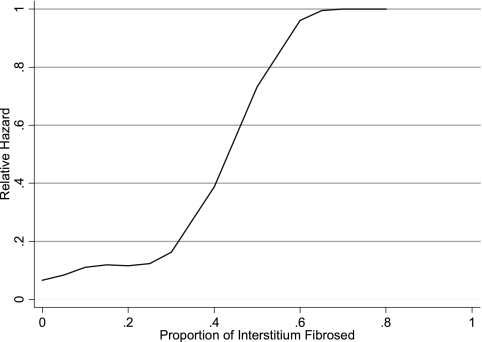
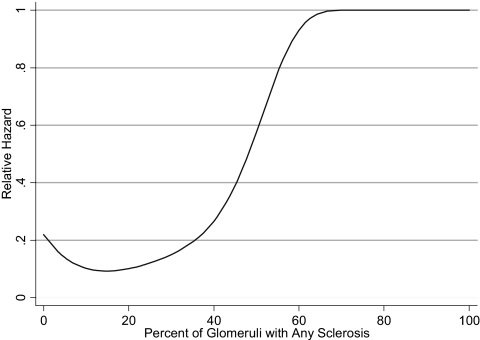
Spline models were fit to the continuous parameters of percentage of glomerular sclerosis, percentage of interstitial fibrosis, baseline serum creatinine, proteinuria, and mean SBP. With the exception of baseline proteinuria and serum creatinine, each variable seemed to have a significant inflection point above which the risk for the composite end point increased. For interstitial fibrosis, the inflection was at 25% (Figure 1); for glomerular sclerosis, the inflection was at 40% (Figure 2); and for mean SBP, the inflection was at 150 mmHg. There was a linear relationship between proteinuria and the risk for the composite outcome; therefore, proteinuria was included as a continuous variable in the final model. We were unable to detect a threshold creatinine value at which the risk for the composite outcome increased as a result of nonconvergence of the model. Serum creatinine was therefore dichotomized on the basis of previously published data suggesting that values of >135 μmol/L are associated with increased risk (1). The proportion of glomeruli with crescents of any kind occurred over a very compressed range. Seventy-five percent of the study population had no crescents, whereas the remaining patients had between 5 and 60% of glomeruli affected. Patients were therefore classified dichotomously as having either no crescents or any crescents.
Figure 1.
Spline model of the proportion of interstitial fibrosis used to predict the hazard of the composite end point of time to doubling of creatinine, ESRD, or death. An inflection at approximately 25% fibrosis demonstrates increased risk above this level.
Figure 2.
Spline model of the proportion of glomeruli with sclerosis used to predict the hazard of the primary composite end point. An inflection at approximately 40% fibrosis demonstrates a large increased level of risk.
Univariable and Multivariable Models
In univariable models, all variables except proteinuria were significantly associated with an increased risk for the composite end point (Table 3). In multivariable models that used only clinical parameters (age, gender, proteinuria, BP, and baseline creatinine), a potential interaction (P = 0.05) was found between serum creatinine and mean SBP in which patients with both high BP and an elevated creatinine were at much higher risk than those with either risk factor alone (hazard ratio [HR] 9.6 for both factors compared with 2.14 for elevated creatinine alone and 1.09 for elevated BP alone).
Table 3.
Univariable survival analysis to predict the primary composite endpoint
| Parameter | HR (95% CI) | P |
|---|---|---|
| Creatinine >135 μmol/L | 3.0 (1.6 to 5.6) | 0.001 |
| Proteinuria (per 1 g/d) | 1.08 (1.00 to 1.20) | 0.21 |
| SBP >150 mmHg | 3.3 (1.6 to 7.0) | 0.001 |
| Interstitial fibrosis >25% | 4.5 (2.4 to 8.4) | <0.001 |
| Glomerulosclerosis >40% | 4.7 (2.4 to 9.1) | <0.001 |
| Any crescents | 2.5 (1.3 to 4.8) | 0.006 |
In the full model including both clinical and histopathologic parameters, glomerular sclerosis (HR 2.6; 95% CI 1.2 to 5.7; P = 0.01), interstitial fibrosis (HR 2.7; 95% CI 1.2 to 6.0; P = 0.01), and the presence of crescents (HR 2.4; 95% CI 1.2 to 5.1; P = 0.02) all were independently associated with the composite end point (Table 4). The combination of high BP and creatinine (HR 12.6; 95% CI 3.9 to 41; P < 0.001) was also associated with the primary outcome, whereas proteinuria was not (P = 0.60). The addition of histopathologic parameters was a significant improvement over the model that used only clinical parameters (Akaike Information Criteria 285 compared with 305; likelihood ratio test for difference between models P < 0.001).
Table 4.
Risk for the primary composite end point associated with histopathologic findings in a multivariate model adjusted for age, gender, proteinuria, SBP, and baseline creatinine
| Parameter | HR (95% CI) | P |
|---|---|---|
| Interstitial fibrosis >25% | 2.7 (1.2 to 6.0) | 0.01 |
| Glomerulosclerosis >40% | 2.6 (1.2 to 5.7) | 0.01 |
| Any crescents | 2.4 (1.2 to 5.1) | 0.02 |
Discussion
IgAN is the most common form of glomerulonephritis worldwide. Predicting the clinical course of this heterogeneous disease has been difficult, and the contribution of histopathologic parameters have not been well characterized. We demonstrated, in a single-center cohort, that quantified measures of three simple histopathologic parameters—fibrosis of >25% of the interstitial area, sclerosis of >40% of glomeruli, and presence of crescents—are independently associated with a greater than two-fold increased risk for the composite end point of doubling of creatinine, ESRD, and death. These simple parameters also seem to improve significantly the fit of Cox proportional hazard models that are used to predict the time to doubling of creatinine, ESRD, or death when compared with clinical parameters alone.
IgAN can lead to ESRD in up to 40% of cases, and few treatments have demonstrated a significant reduction in progressive disease. Accurately predicting which individuals will go on to develop progressive disease would allow physicians to target high-risk patients for aggressive treatment or monitoring. Our study is consistent with other studies that showed interstitial fibrosis or glomerular sclerosis as independent risk factors for ESRD or death (1,2,17–19). Our study extends these findings by identifying empiric thresholds that define a high-risk population. This threshold for interstitial fibrosis seems lower than previously described in scoring systems for IgAN (3,5,19–23). Our findings also complement those of other groups that used an arbitrary 25% threshold for interstitial fibrosis (22,24).
It is notable that an international panel of experts recently produced guidelines on the reporting of biopsies that demonstrate IgAN (22,25). The Oxford classification system suggests that six features are relevant: (1) Mesangial cellularity, (2) segmental sclerosis, (3) endocapillary hypercellularity, (4) cellular/fibrocellular crescents, (5) percentage of interstitial fibrosis/tubular atrophy, and (6) score. Our study supports the importance of interstitial fibrosis, presence of crescents, and glomerular sclerosis, although we did not find mesangial cellularity, endocapillary hypercellularity, or arteriosclerosis significant predictors of outcome. Our study also supports the reproducibility of quantitative reporting and the significance of as little as 25% interstitial fibrosis. Differences in study results may be due to the use of different outcomes (doubling of creatinine/ESRD/death versus slope renal function and 50% decline of GFR/ESRD), case mix (our patients were older and included more women but had similar BP, renal function, proteinuria, and duration of follow-up), or sample size (146 versus 265 patients). The differences between these results highlight the need for further large, independent samples to confirm the relative importance of these histopathologic features in addition to clinical features.
We found that even a small amount of interstitial fibrosis increases the subsequent risk for ESRD and that this can be detected many years before the development of ESRD. This may therefore identify patients who are at high risk for progressive disease at a time when interventions may still be effective (i.e., before there is sufficient renal damage to lead to inevitable progression). The possibility of early intervention is of considerable interest because there is speculation that there may be a “point of no return” in CKD beyond which interventions are unlikely to affect prognosis significantly (19). Furthermore, because interstitial fibrosis and glomerular sclerosis are common final pathways in all kidney diseases, the b-spline methods that we used to determine what constitutes a significant degree of fibrosis or sclerosis may be useful in other CKDs.
We have demonstrated that quantitative measures of these histopathologic parameters can be reliably performed with minimal inter- and intraobserver variability. Pathologist estimates of interstitial fibrosis demonstrated a high degree of concordance with an objective image analysis–based method of quantification. The reliability of pathologist-based estimates of interstitial fibrosis and glomerular sclerosis improve the likelihood that these measures can be accurately reproduced in other centers and for other studies. Furthermore, simplicity of measuring these two histopathology parameters would make their integration into routine clinical biopsy reporting easy to implement and to interpret. This is in contrast to more complicated biopsy grading schemes that may offer little extra in terms of prognostic information.
Although our study provides important information on the value of histopathologic parameters in IgAN, the results should be interpreted within the context of the study's limitations. As in all observational studies, the possibility of residual confounding or misclassification of outcomes cannot be excluded. Our end points, however, were objective and supported by robust administrative and research databases. In addition, patients with missing data had very similar demographic and baseline data to our included patients, suggesting the included patients are representative of the overall cohort. Importantly, we were able to obtain information on the major predictors of progression of kidney dysfunction, namely baseline creatinine, proteinuria, and BP. Our study used only data that were available at the time when the diagnosis of IgAN was ascertained, whereas other observational studies have reported improved prognostic abilities using data from follow-up visits rather than initial presentation (6). Although follow-up data may improve the ability to prognosticate, physicians and patients often prefer estimates of prognosis as early as possible, and reliance on follow-up parameters may allow the accrual of additional chronic renal damage that reduces the window of opportunity for successful interventions. Last, our study results were derived from a single cohort and will require validation in an external cohort. An external cohort will also provide a better measure of improvement in prognostic discrimination with the addition of histopathologic parameters.
Conclusions
Simple histopathologic measures of interstitial fibrosis and glomerular sclerosis improve the identification of patients who have IgAN and are at high risk for progressive loss of kidney function. Lesser degrees of interstitial fibrosis than previously recognized are independently associated with adverse outcomes in patients with IgAN. These results require confirmation and validation in an independent cohort of patients with biopsy-proven IgAN.
Disclosures
None.
Acknowledgments
M.W. is supported by the Kidney Foundation of Canada, Canadian Institutes of Health Research, and Canadian Society of Nephrology through the KRESCENT program and by the Alberta Heritage Foundation for Medical Research through the Clinical Fellowship program. B.M. and B.H. are supported by the Canadian Institutes of Health Research through New Investigator Awards. B.H. is also supported by the Alberta Heritage Foundation for Medical Research through a Population Health Investigator award.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Alamartine E, Sabatier JC, Guerin C, Berliet JM, Berthoux F: Prognostic factors in mesangial IgA glomerulonephritis: An extensive study with univariate and multivariate analyses. Am J Kidney Dis 18: 12–19, 1991 [DOI] [PubMed] [Google Scholar]
- 2.D'Amico G: Influence of clinical and histological features on actuarial renal survival in adult patients with idiopathic IgA nephropathy, membranous nephropathy, and membranoproliferative glomerulonephritis: Survey of the recent literature. Am J Kidney Dis 20: 315–323, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Haas M: Histologic subclassification of IgA nephropathy: A clinicopathologic study of 244 cases. Am J Kidney Dis 29: 829–842, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Johnston PA, Brown JS, Braumholtz DA, Davison AM: Clinico-pathological correlations and long-term follow-up of 253 United Kingdom patients with IgA nephropathy: A report from the MRC Glomerulonephritis Registry. Q J Med 84: 619–627, 1992 [PubMed] [Google Scholar]
- 5.Katafuchi R, Oh Y, Hori K, Komota T, Yanase T, Ikeda K, Omura T, Fujimi S: An important role of glomerular segmental lesions on progression of IgA nephropathy: A multivariate analysis. Clin Nephrol 41: 191–198, 1994 [PubMed] [Google Scholar]
- 6.Bartosik LP, Lajoie G, Sugar L, Cattran DC: Predicting progression in IgA nephropathy. Am J Kidney Dis 38: 728–735, 2001 [DOI] [PubMed] [Google Scholar]
- 7.D'Amico G, Ragni A, Gandini E, Fellin G: Typical and atypical natural history of IgA nephropathy in adult patients. Contrib Nephrol 104: 6–13, 1993 [DOI] [PubMed] [Google Scholar]
- 8.D'Amico G: Natural history of idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am J Kidney Dis 36: 227–237, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Donadio JV, Bergstralh EJ, Offord KP, Holley KE, Spencer DC: Clinical and histopathologic associations with impaired renal function in IgA nephropathy. Mayo Nephrology Collaborative Group. Clin Nephrol 41: 65–71, 1994 [PubMed] [Google Scholar]
- 10.Ibels LS, Gyory AZ: IgA nephropathy: Analysis of the natural history, important factors in the progression of renal disease, and a review of the literature. Medicine (Baltimore) 73: 79–102, 1994 [PubMed] [Google Scholar]
- 11.Lee SM, Rao VM, Franklin WA, Schiffer MS, Aronson AJ, Spargo BH, Katz AI: IgA nephropathy: Morphologic predictors of progressive renal disease. Hum Pathol 13: 314–322, 1982 [DOI] [PubMed] [Google Scholar]
- 12.Furness PN: The use of digital images in pathology. J Pathol 183: 253–263, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Furness PN, Rogers-Wheatley L, Harris KP: Semiautomatic quantitation of macrophages in human renal biopsy specimens in proteinuric states. J Clin Pathol 50: 118–122, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubinova L, Janacek J, Guilak F, Opatrny Z: Comparison of several digital and stereological methods for estimating surface area and volume of cells studied by confocal microscopy. Cytometry 36: 85–95, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Manns BJ, Mortis GP, Taub KJ, McLaughlin K, Donaldson C, Ghali WA: The Southern Alberta Renal Program database: A prototype for patient management and research initiatives. Clin Invest Med 24: 164–170, 2001 [PubMed] [Google Scholar]
- 16.Ziegler Z: One-sided L1-approximation by splines of an arbitrary degree. In: Approximation with Special Emphasis on Spline Functions, edited by Schoenberg IJ.New York, Academic Press, 1969, pp 405–413 [Google Scholar]
- 17.Bogenschutz O, Bohle A, Batz C, Wehrmann M, Pressler H, Kendziorra H, Gartner HV: IgA nephritis: On the importance of morphological and clinical parameters in the long-term prognosis of 239 patients. Am J Nephrol 10: 137–147, 1990 [DOI] [PubMed] [Google Scholar]
- 18.Bohle A, Wehrmann M, Bogenschutz O, Batz C, Vogl W, Schmitt H, Muller CA, Muller GA: The long-term prognosis of the primary glomerulonephritides: A morphological and clinical analysis of 1747 cases. Pathol Res Pract 188: 908–924, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Lai FM, Szeto CC, Choi PC, Li PK, Tang NL, Chow KM, Lui SF, Wong TY, Ho KK, To KF: Primary IgA nephropathy with low histologic grade and disease progression: Is there a “point of no return”? Am J Kidney Dis 39: 401–406, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Alamartine E, Sabatier JC, Berthoux FC: Comparison of pathological lesions on repeated renal biopsies in 73 patients with primary IgA glomerulonephritis: Value of quantitative scoring and approach to final prognosis. Clin Nephrol 34: 45–51, 1990 [PubMed] [Google Scholar]
- 21.Okada H, Suzuki H, Konishi K, Sakaguchi H, Saruta T: Histological alterations in renal specimens as indicators of prognosis of IgA nephropathy. Clin Nephrol 37: 235–238, 1992 [PubMed] [Google Scholar]
- 22.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D'Agati V, D'Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H: The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 23.D'Amico G, Minetti L, Ponticelli C, Fellin G, Ferrario F, Barbiano di Belgioioso G, Imbasciati E, Ragni A, Bertoli S, Fogazzi G: Prognostic indicators in idiopathic IgA mesangial nephropathy. Q J Med 59: 363–378, 1986 [PubMed] [Google Scholar]
- 24.Howie AJ, Ferreira MA, Adu D: Prognostic value of simple measurement of chronic damage in renal biopsy specimens. Nephrol Dial Transplant 16: 1163–1169, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D'Agati V, D'Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H: The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]