Abstract
Background and objectives: During the past decade, nephrogenic systemic fibrosis (NSF) has been reported in patients who have severe renal impairment and have been exposed to a gadolinium (Gd)-based contrast agent during magnetic resonance imaging (MRI). As a result of positive reporting bias, many suitable patients with chronic kidney disease (CKD) are being denied a highly important form of investigation that can be safely undertaken. We analyzed the safety of Gd-MRI in patients with CKD and varying levels of estimated GFR (eGFR).
Design, setting, participants, & measurements: We performed a retrospective analysis of 2053 unselected patients who had CKD and had received Gd-MRI between 1999 and 2009, so as to determine the risk for NSF related to level of CKD, nature of Gd preparation, and Gd dosage.
Results: Overall, 2053 patients (63.5% men; mean age 60.6 ± 15.7 years) had 2278 Gd-MRI scans; their mean eGFR was 40.7 ± 23.7 ml/min. A total of 918 (44.7%) patients had stage 3, 491 (23.9%) had stage 4, and 117 (5.7%) had predialysis stage 5 CKD. No cases of NSF were identified during an average follow-up period of 28.6 ± 18.2 months.
Conclusions: In this study, no patients developed NSF during extended follow-up, even after multiple Gd doses in some. Gd-MRI can be safely undertaken in the majority of patients with CKD, but caution is merited for dialysis patients and those with acute kidney injury, with relative caution for predialysis patients with stage 5 CKD.
During the past decade, nephrogenic systemic fibrosis (NSF) has been reported in patients who have severe renal impairment and have been exposed to a gadolinium (Gd)-based contrast agent during magnetic resonance imaging (MRI). Although NSF is a rare condition, it has caused much controversy in the literature and sparked a dramatic change of MR investigational policy and protocols. We believe that, as a consequence, many suitable patients are being denied a highly important form of investigation that can be safely undertaken. For example, many radiology departments are precluding patients with estimated GFR (eGFR) of <30 ml/min and, remarkably, some patients with eGFR of <60 ml/min from undergoing Gd-enhanced MRI (Gd-MRI), despite critical review of the literature suggesting that in uncomplicated chronic kidney disease (CKD), NSF almost never occurs when eGFR is >15 ml/min (1). We wished to add to the literature by emphasizing the safety of Gd-MRI for patients who had CKD and varying levels of eGFR and had undergone the investigations at two large UK nephrology centers.
Materials and Methods
The two centers that participated in the audit were the renal departments of Salford Royal Hospital, Greater Manchester and the Hammersmith Hospital, London. Data were obtained from each center's radiology department records of all patients who had undergone either investigation for atheromatous renovascular disease with MR angiography (MRA) or MRA of the pelvic vessels (usually pretransplantation assessment) between 2000 and 2009. Data derived from a portion of the patients from Salford Royal Hospital, collected between 1999 and 2007, were reported in a previous article (1). This study includes an additional 24-month cohort of Gd-MRI–exposed patients at this center, as well as more prolonged follow-up and expanded information compared with the previous report from the cohort. The closest available blood test to the time of the Gd-MRI scan was used to calculate the eGFR, using the four-variable Modification of Diet in Renal Disease (MDRD) equation. Patients had their notes and electronic records scrutinized for any signs or symptoms resembling NSF after the MRA scan. This included any new dermatology or rheumatology referrals. Symptoms of NSF would include new dermatologic or rheumatologic complaints such as pruritus, dysesthesia, skin changes, or mobility restriction. Signs included skin tightening, thickening, tethering, or hyperpigmentation as well as joint contractures. Any patient who had received a skin biopsy for any indication after Gd exposure had this result scrutinized, even when this occurred years after Gd exposure. The data were collected and amalgamated in 2009. When the cause of death was not recorded on the hospital records, the records around the time of death were analyzed, and the patient's family physician was contacted for further information.
MRI Protocols
Salford Royal Hospital.
Patients received Magnevist (n = 572); Dotarem (n = 86); Omniscan (n = 40); and, more recently, Gadovist (n = 69), Vasovist (n = 5), and Multihance (n = 3), depending on the favored contrast modality at the time. All patients had been scanned according to the department's protocol whereby 0.2 mmol/kg Gd-containing contrast agent was administered; however, from July 2007, a single dose was administered (0.1 mmol/kg). No special prophylactic precautions were undertaken. All of the patients had undergone MRA directed at imaging the renal arteries.
Hammersmith Hospital.
All patients received contrast with Multihance Gd (gadobenate dimeglumine; 0.1 mmol/kg), and, again, no special prophylactic precautions were undertaken. A total of 94.1% of the 1328 patients who were studied had undergone MRA of the renal arteries, whereas 5.9% were investigated with pelvic Gd-MRA.
Statistical Analysis
Continuous data (e.g., age, follow-up) were expressed as mean ± SD and categorical data as n (%). All statistical analyses were performed using SPSS 15.0.
Results
A total of 690 patients (636 patients with complete data) were followed up at Salford Royal Hospital and an additional 89 patients in its peripheral referral center at Bolton Royal Hospital. Complete data were available for 1328 patients who had had MRA in the Department of Radiology at the Hammersmith Hospital. Thus, a total of 2053 patients were included in the study. Baseline demographics of age, gender, and renal function are provided in Table 1. A total of 63.5% were men, mean age was 60.6 ± 15.7 years, and among them they had 2278 Gd-MRI scans. Mean follow-up of the cohort was 28.6 ± 18.2 months (range 0.0 to 115.0 months), with a total follow-up period of 5126.3 patient-years. A total of 89 (4.3%) patients had stage 1 CKD 1 at the time of imaging, 305 (14.9%) had stage 2 CKD, 918 (44.7%) had stage 3 CKD, 491 (23.9%) had stage 4 CKD, and 250 (12.2%) had stage 5 CKD. Thus 741 (36.1%) patients had either stage 4 or 5 CKD, with 133 of the latter being dialysis patients (Table 1). A total of 453 (21.4%) patients had renal transplants. Patients were also subdivided by which agent they received, with further subdivision of stage 5 CKD into whether they were predialysis or receiving dialysis (Table 2 and Figure 1).
Table 1.
Patients who underwent Gd-containing scans subdivided by CKD stage
| Parameter | Age (Years; Mean ± SD [Range]) | Gender (M:F; %) | Creatinine (μmol/L; Mean ± SD [Range]) | eGFR (ml/min; Mean ± SD [Range]) | Follow-up (Months; Mean ± SD [Range]) | CKD (n [%]) |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | ||||||
| Hammersmith Hospital (n = 1328) | 57.3 ± 15.8 (18.0 to 93.0) | 848:480 (63.6:36.1) | 211.8 ± 169.7 (46.0 to 1416.0) | 42.1 ± 24.4 (3.0 to 135.0) | 28.0 ± 15.2 (0.0 to 71.0) | 60 (4.5) | 225 (16.9) | 600 (45.2) | 278 (20.9) | 165 (12.4) |
| Salford Royal Hospital (n = 725) | 66.8 ± 13.6 (19.0 to 92.0) | 455:270 (62.8:37.2) | 221.0 ± 173.9 (36.0 to 884.0) | 38.2 ± 22.1 (5.0 to 133.0) | 29.8 ± 22.6 (0.0 to 115.0) | 29 (4.0) | 80 (11.0) | 318 (43.9) | 213 (29.4) | 85 (11.7) |
| Total (n = 2053) | 60.6 ± 15.7 (18.0 to 93.0) | 1303:750 (63.5:36.5) | 212.9 ± 170.1 (36.0 to 1416.0) | 40.7 ± 23.7 (3.0 to 135.0) | 28.6 ± 18.2 (0.0 to 115.0) | 89 (4.3) | 305 (14.9) | 918 (44.7) | 491 (23.9) | 250 (12.2) |
Table 2.
Individual patients' renal function at the time of MRI according to the Gd agents received
| Parameter | Agent |
|||||
|---|---|---|---|---|---|---|
| Gadobenate Dimeglumine (Bracco Diagnostics) | Gadopentetate Dimeglumine (Schering) | Gadodiamide (GE Healthcare) | Gadoterate Meglumine (Guerbay) | Gado-butrol (Bayer Schering Pharma) | Gadofosveset Trisodium (Bayer Schering Pharma) | |
| Trade name | Multihance | Magnevist | Omniscan | Dotarem | Gadovist | Vasovist |
| Type of agent | Ionic linear chelate | Ionic linear chelate | Nonionic linear chelate | Ionic cyclic chelate | Nonionic cyclic chelate | Ionic linear chelate |
| Patients (n [%]) | 1331 (64.8) | 522 (25.4) | 40 (1.9) | 86 (4.2) | 69 (3.4) | 5 (0.2) |
| eGFR of patients who received the agent (ml/min; mean ± SD [range]) | 42.1 ± 24.4 (3.0 to 135.0) | 36.2 ± 23.0 (5.0 to 133.0) | 38.1 ± 18.0 (8.0 to 87.0) | 40.9 ± 15.4 (14.0 to 82.0) | 50.0 ± 21.5 (14.0 to 90.0) | 31.2 ± 11.0 (16.0 to 40.0) |
| Receiving double dose of the Gd agent (n [%]) | 0 (0.0) | 495 (94.8) | 40 (100.0) | 0 (0.0) | 0 (0.0) | 5 (100.0) |
| No. of patients with stage 4 CKD | 272 | 165 | 9 | 23 | 12 | 2 |
| No. of patients with stage 5 CKD | 74 | 51 | 1 | 2 | 1 | 0 |
| No. of patients on dialysis | 99 | 30 | 4 | 0 | 0 | 0 |
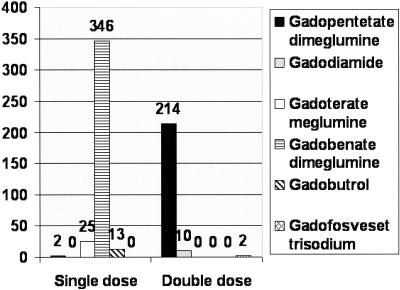
Figure 1.
Numbers of patients with CKD stage 4 and 5 pre-dialysis who received single or double dose gadolinium agents.
No patients developed any symptoms or signs suggestive of NSF during follow-up; 400 (19.5%) patients had died, but there was no record of any of the deaths being related to NSF. A total of 190 patients had received two MRA scans.
From the Salford Royal database, 21 patients were found to have had a follow-up appointment for a known rheumatologic or dermatologic condition, and 14 had routine dermatologic follow-up as part of their posttransplantation skin surveillance protocol. Twenty patients had a new referral to dermatology or rheumatology after imaging. The new diagnoses after referral were basal cell carcinoma (n = 6), osteoporosis (n = 3), actinic keratosis (n = 1), shingles (n = 1), lymphomatoid papulosis (n = 1), bullous pemphigoid (n = 1), paraesthesia-associated carpal tunnel syndrome (n = 1), musculoskeletal pain resolved spontaneously (n = 1), gout (n = 1), skin squamous cell carcinoma (n = 1), chondrodermatitis of the ear (n = 1), diabetic foot ulcer (n = 1), and tuberculosis-associated skin change (n = 1). There were no indeterminate diagnoses or diagnoses confirming cutaneous sclerosis or systemic fibrosis. Eighteen new skin biopsies were performed on these patients after referral. The histologic diagnoses identified on biopsy were basal cell carcinoma (n = 5), actinic keratosis (n = 2), squamous cell carcinoma (n = 1), bullous pemphigoid (n = 1), psoriasis (n = 1), and chondrodermatitis (n = 1).
Discussion
NSF is undeniably a rare but devastating disease, presenting with a spectrum of clinical and histopathologic manifestations (2,3). It is associated with increased tissue deposition of collagen in the skin, subcutaneous fascia, striated muscles, joints, diaphragm, lungs, liver, and myocardium (4). Five percent of cases run a fulminant course (5), and the severity of the cutaneous lesions correlates with poor prognosis and death (6). Our study, which incorporates >2053 patients encompassing 5126 patient-years of follow-up, provides more evidence that Gd-MRI can be used safely for patients with stages 4 and 5 (nondialysis) CKD.
To date, 315 cases of NSF have been reported in the literature (5); however, in the past 20 or so years, >200 million patients have been exposed to Gd-based contrast agents worldwide; >30 million have received Omniscan, and at least 80 million have received Magnevist. The majority of cases of NSF have been in patients with severe renal impairment, with >90% having dialysis-dependent CKD and the remaining 10% having acute kidney injury (AKI) or hepatorenal failure (7–9). In addition, the least stable Gd-based MR contrast agents, those that are “linear,” or nonionic (e.g., Omniscan, Magnevist), have been more commonly linked to the development of NSF, which often occurs on the background of an inflammatory process (10).
Evaluating the precise risk for developing NSF is hampered by a lack of prospective studies, which, given the strong causal relationship between Gd and NSF, would now be considered unethical; however, retrospective studies have provided some insight into the risk for dialysis patients. For example, in a cohort of 102 patients with varying degrees of renal function, NSF was found in 18% after exposure to gadodiamide (Omniscan). All patients with NSF had stage 5 CKD, and most had received high dosages of gadodiamide (typically 0.3 mmol/kg) (11). In another study, Prince et al. (12) assessed the incidence of NSF in two large US medical centers. Of 83,121 patients who received Gd-based contrast agents, 15 (0.02%) developed NSF. These patients all had received a higher-than-standard dosage of Gd and had either AKI or acute deterioration of CKD at the time of their scan. The prevalence of NSF within dialysis groups has been variably reported from 1.6% (13) to 4% (9) of patients, but these figures need to be examined with consideration for the frequency of MRI use in individual dialysis centers and the risk of positive reporting bias. Repeated doses of Gd seem to increase the risk for NSF, but no association has been found between residual urine volume or creatinine clearance in dialysis patients and the risk for NSF (13).
There is no definitive cure for NSF. Anecdotal reports suggested partial responses to various therapies such as plasmapheresis, steroids, extracorporeal photopheresis, or treatment with thalidomide (5). Some have reported slowing or even reversal of the disease after renal transplantation or accompanying improvements in renal function (14,15). Dialysis after Gd-MRA/MRI does not always prevent occurrence of NSF (9) but is nonetheless recommended promptly after imaging for patients who are already receiving dialysis. Selective removal of both free and chelated Gd (gadopentetate dimeglumine and gadodiamide) by nanostructure silica materials in dialysis and hemoperfusion systems (16) has shown some experimental promise, potentially allowing for Gd removal from high-risk patients after Gd-MR exposure.
As a result of the adverse literature linking CKD and Gd exposure to risk for NSF, various radiologic guidelines that initially recommended avoidance of Gd-MRI for patients with eGFR <30 ml/min and the exercising of caution for patients who are being investigated with eGFR between 30 and 60 ml/min were developed (17). This has provided a major investigative problem for clinicians, because Gd-MRI examinations have become indispensable to clinicians for certain indications. In addition, the functional information that is provided by MRI extends and complements the usefulness of MR studies (18).
Although caution should be retained when undertaking Gd-MRI in the high-risk dialysis group, there is minimal evidence in the literature to suggest similar risk for patients with stages 1 through 4 CKD. Within the multicenter Angioplasty and STent for Renal Artery Lesions (ASTRAL) trial (19), in which 30% of the patients had stage 4 or 5 CKD, there was only one case of NSF in 1735 of the screened patients (incidence 0.06%), and that patient had acutely deteriorating stage 5 CKD (1). The only case series that described patients who had stages 2 and 3 CKD and had NSF has been criticized because of inaccuracies in the calculation of the eGFR (20). Further confirmation of the safety of Gd-MRI in earlier stages of CKD comes from a recent prospective study (21) that examined the incidence of NSF in 168 patients with stage 3 CKD after intravenous exposure to gadobenate dimeglumine (Multihance; linear, ionic structure). No cases of NSF were reported in this cohort.
As a result of the heightened awareness of NSF in patients with CKD, less stable Gd agents are now no longer being used in advanced CKD; Omniscan in particular is not recommended in any stage of renal impairment (22), because it is reported to carry a 3 to 7% risk for causing NSF in at-risk (stages 4 and 5 CKD) patients. Magnevist carries a 0.1 to 1.0% risk (23). In addition, radiology reports now indicate whether Gd has been administered during a scan as well as the dosage used. The American College of Radiology Blue Ribbon Panel on MR Safety has updated its MR Safe Practice Guidelines, which exist to ensure safe and responsible practices in clinical and research MR environments (22). They advise that patients who have moderate CKD to ESRD (stages 3 through 5) and are to undergo Gd-MRI examinations of any kind have a written approval order from the radiologist involved. The guidelines also recommend refraining from administration of any Gd-containing contrast agents in patients with CKD unless a risk–benefit assessment for a particular patient indicates that the benefits clearly outweigh the potential risk (22); however, no unambiguous distinction is made between patients with more advanced (stages 4 and 5) and milder forms (stage 3) of CKD.
Knowledge of a patient's renal function and exclusion of AKI would be considered good practice before undertaking Gd-MRI and considered mandatory before administration of a Gd agent that has been associated with development of NSF by the European Society of Urogenital Radiology (23). All requests for MR should state whether a history of kidney disease or dialysis exists; further pragmatic guidance includes use of the lowest dosage of Gd that would provide the diagnostic benefit being sought and allowing a sufficient period of time for elimination of Gd from the body before re-administration in cases for which repeat scans are required.
Our study has several limitations. First, the eGFR was based on a single serum sample before imaging. As such, the eGFR provides only an “estimate” of renal function, even in patients with steady-state CKD, and this should never supervene clinical judgment. In addition, this value may be unrepresentative in patients with AKI and oliguria, with the result that the renal function may be much worse than the serum creatinine indicates for that point in time. In such cases, the advisability of using Gd should be very carefully considered. Second, this was a retrospective analysis because NSF was described as a complication related to imaging with MRI-Gd only in 2006. A flaw of the retrospective design is that the diagnosis of NSF would have depended on the keenness of insight and accurate documentation of the clinicians who saw the patients, as well as the availability of adequate long-term follow-up of patients, and it is possible that there may have been cases of NSF that were undetected or incorrectly diagnosed. To improve confidence in the accuracy of our data relating to this, in one of the centers, we analyzed all clinical entries, biopsy results, and new referral letters that were available on our computerized records so as to detect any signs or symptoms that may have been overlooked as NSF as a result of lack of awareness of its existence. Third, it is likely that there was underestimation of the amount of Gd to which some of our patients were exposed, because only renal and abdominal MRA scans were included.
Nevertheless, we believe that our study serves to fill a gap in the literature concerning the safety of Gd in large cohorts of patients with stages 4 and 5 CKD before dialysis. The fear of NSF should not result in denial of Gd-MRI when clinically indicated. Our view is shared by the UK Royal College of Radiologists (24), whose guidelines allow for selective use of Gd-MRI in patients with low GFR when the clinical need for information is considered greater than that of NSF risk (24), albeit patients with stages 4 and 5 CKD are regarded as high risk for NSF in their guidelines. This last clause may result in clinicians being reluctant to image patients with stage 4 CKD and stable stage 5 CKD because of an unjustified concern of high NSF risk in nondialysis patients with CKD.
Following this guidance and further to our two centers' experience of outcomes, we would make the following recommendations:
Knowledge of not just a patient's eGFR but also the trend in eGFR should be sought before imaging patients with Gd-MRI.
Caution is merited for patients who have dialysis-requiring CKD or AKI especially with concomitant vascular comorbidities and are being considered for Gd-MRI. The latter should be undertaken only when the advantages of imaging far outweigh the risk for NSF.
Relative caution is required for patients who have stable stage 5 CKD and are not on dialysis, and, when possible, other imaging modalities should be considered (e.g., non–Gd-MRI, computed tomographic angiography), or Gd-MRI with safer, cyclic agents (see point 6) should be used.
We believe that the risk for NSF is minimal for patients with stable stages 3 and 4 CKD (eGFR >15 ml/min) and that these patients can safely be subjected to Gd-MRI when clinically indicated, notwithstanding the need for an informed discussion between clinician and patient and the application of other broad safety principles.
The minimum dosage of Gd required to obtain the required information should be used. We recommend one standard dosage of Gd or lower.
Safer structural Gd compounds (cyclic, ionic structure) should be used when possible, especially when Gd-MRI needs to be performed in the highest risk patients (dialysis-requiring CKD, AKI) and ideally in patients with non–dialysis-treated stage 5 CKD.
A candid discussion between the physician and the patient with CKD is therefore necessary. Our study is supportive of the low risk for NSF in patients with stages 1 through 4 CKD and those with predialysis stage 5 CKD, some of whom even received multiple and repeated doses of Gd. We believe that the risk for NSF is minimal for patients with stage 3 and 4 CKD (eGFR >15 ml/min), provided that their renal function is stable, notwithstanding the need for application of other, broad safety principles.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Chrysochou C, Buckley DL, Dark P, Cowie A, Kalra PA: Gadolinium-enhanced magnetic resonance imaging for renovascular disease and nephrogenic systemic fibrosis: Critical review of the literature and UK experience. J Magn Reson Imaging 29: 887–894, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Deng A, Bilu MD, Spillane A, Chwalek J, Surin-Lord S, Brooks S, Petrali J, Sina B, Gaspari A, Kao G: Nephrogenic systemic fibrosis with a spectrum of clinical and histopathological presentation: A disorder of aberrant dermal remodeling. J Cutan Pathol March31, 2009. [ epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Marckmann P, Skov L, Rossen K, Thomsen HS: Clinical manifestation of gadodiamide-related nephrogenic systemic fibrosis. Clin Nephrol 69: 161–168, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Gibson SE, Farver CF, Prayson RA: Multiorgan involvement in nephrogenic fibrosing dermopathy: An autopsy case and review of the literature. Arch Pathol Lab Med 130: 209–212, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Cowper SE. Nephrogenic Fibrosing Dermopathy [ICNSFR Website]. 2001–2009. Available at: http://www.icnfdr.org Accessed August 13, 2009
- 6.Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA: Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum 35: 238–249, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marckmann P: An epidemic outbreak of nephrogenic systemic fibrosis in a Danish hospital. Eur J Radiol 66: 187–190, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Shabana WM, Cohan RH, Ellis JH, Hussain HK, Francis IR, Su LD, Mukherji SK, Swartz RD: Nephrogenic systemic fibrosis: A report of 29 cases. AJR Am J Roentgenol 190: 736–741, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I, Kirk GA: Gadodiamide-associated nephrogenic systemic fibrosis: Why radiologists should be concerned. AJR Am J Roentgenol 188: 586–592, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Swaminathan S, Shah SV: New insights into nephrogenic systemic fibrosis. J Am Soc Nephrol 18: 2636–2643, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Rydahl C, Thomsen HS, Marckmann P: High prevalence of nephrogenic systemic fibrosis in chronic renal failure patients exposed to gadodiamide, a gadolinium-containing magnetic resonance contrast agent. Invest Radiol 43: 141–144, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Prince MR, Zhang H, Morris M, MacGregor JL, Grossman ME, Silberzweig J, DeLapaz RL, Lee HJ, Magro CM, Valeri AM: Incidence of nephrogenic systemic fibrosis at two large medical centers. Radiology 248: 807–816, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Heinz-Peer G, Neruda A, Watschinger B, Vychytil A, Geusau A, Haumer M, Weber M: Prevalence of NSF following intravenous gadolinium-contrast media administration in dialysis patients with endstage renal disease. Eur J Radiol July18, 2009. [ epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Maloo M, Abt P, Kashyap R, Younan D, Zand M, Orloff M, Jain A, Pentland A, Scott G, Bozorgzadeh A: Nephrogenic systemic fibrosis among liver transplant recipients: A single institution experience and topic update. Am J Transplant 6: 2212–2217, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Cowper SE: Nephrogenic fibrosing dermopathy: The first 6 years. Curr Opin Rheumatol 15: 785–790, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Yantasee W, Fryxell GE, Porter GA, Pattamakomsan K, Sukwarotwat V, Chouyyok W, Koonsiripaiboon V, Xu J, Raymond KN: Novel sorbents for removal of gadolinium-based contrast agents in sorbent dialysis and hemoperfusion: Preventive approaches to nephrogenic systemic fibrosis. Nanomedicine May15, 2009. [ epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Public Assessment Report: Increased risk of nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis and gadolinium-containing MRI contrast agents. Available at: http://www.ismrm.org/special/MHRA_Report.pdf Accessed August 13, 2009
- 18.Michaely HJ, Sourbron S, Dietrich O, Attenberger U, Reiser MF, Schoenberg SO: Functional renal MR imaging: An overview. Abdom Imaging December7, 2006. [ epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J: Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 361: 1953–1962, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Saab G, bu-Alfa A: Are patients with moderate renal failure at risk for developing nephrogenic systemic fibrosis? Radiology 244: 930–931, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Bryant BJ, Im K, Broome DR: Evaluation of the incidence of nephrogenic systemic fibrosis in patients with moderate renal insufficiency administered gadobenate dimeglumine for MRI. Clin Radiol 64: 706–713, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Kanal E, Barkovich AJ, Bell C, Borgstede JP, Bradley WG, Jr, Froelich JW, Gilk T, Gimbel JR, Gosbee J, Kuhni-Kaminski E, Lester JW, Jr, Nyenhuis J, Parag Y, Schaefer DJ, Sebek-Scoumis EA, Weinreb J, Zaremba LA, Wilcox P, Lucey L, Sass N: ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol 188: 1447–1474, 2007 [DOI] [PubMed] [Google Scholar]
- 23.ESUR Guideline: Gadolinium based contrast media and nephrogenic systemic fibrosis, July 17, 2007. Available at: http://www.esur.org/fileadmin/NSF/NSF-ESUR_Guideline_Final.pdf Accessed September 3, 2009 [DOI] [PubMed]
- 24.The Royal College of Radiologists, Board of the Faculty of Clinical Radiology: Gadolinium-based Contrast Media and Nephrogenic Systemic Fibrosis, London, The Royal College of Radiologists, November 2007. Available at: http://www.rcr.ac.uk/docs/radiology/pdf/BFCR0714_Gadolinium_NSF_guidanceNov07.pdf Accessed August 6, 2009