Abstract
Background and objectives: Hypertension and proteinuria are common but poorly understood renal toxicities of vascular endothelial growth factor (VEGF) receptor signaling pathway inhibitors. In this phase II study of cediranib (AZD2171) for recurrent epithelial ovarian cancer, the time course and severity of BP changes and proteinuria were characterized.
Design, setting, participants, & measurements: 46 women ages 41 to 77 years were treated with cediranib. 26% had baseline hypertension. Twice-daily BP was recorded. Urinalyses were performed every 2 weeks, and in some patients proteinuria was further quantified.
Results: 31 women (67%) developed hypertension by day 3; 87% by the end of the study. 43% developed grade ≥3 hypertension. Mean systolic BP increase over 3 days was 18 mmHg. Women above the mean age (≥57 years) had a larger rise in systolic BP by day 3 (15.9 versus 7.0 mmHg). 14 women developed proteinuria. There was a dose response (45 versus 30 mg daily). Proteinuria also developed rapidly, with 7 of 14 women developing proteinuria within 2 weeks. Only 7 of 20 women who developed grade 3 hypertension developed proteinuria.
Conclusions: Cediranib induced a rapid but variable rise in BP within 3 days of initiation in most patients. Proteinuria was common and also developed rapidly. The rapid development of hypertension suggests that acute inhibition of VEGF-dependent vasodilation might explain the BP rise with VEGF inhibitors. Clinicians must be vigilant in early detection and management of toxicities of this expanding drug class, especially in older patients.
Inhibitors of the vascular endothelial growth factor (VEGF) receptor signaling pathway (VSP) are promising agents for treatment of malignancies. Bevacizumab, sorafenib, sunitinib, and pazopanib have all been approved to treat solid tumors. Cediranib (AZD2171) is an oral small-molecule inhibitor of VEGF receptor 1 (VEGFR-1), VEGFR-2, VEGFR-3, PDGFR, and c-kit that is currently in phase II testing for the treatment of tumors such as epithelial ovarian carcinoma, non-small cell lung cancer, glioblastoma, and colon cancer (1–4). Because the primary toxicities of this drug class are on-target effects related to VSP inhibition, toxicities may be more common or severe with the newer, more potent members of this drug class currently in development. Understanding the pathophysiology and management of these toxicities is required to optimize safety and tolerability of this promising class of chemotherapy.
Hypertension and proteinuria are known renal toxicities of VSP inhibitors (5). Hypertension is a mechanism-dependent class effect. Rates of hypertension are as high as 30% to 40% on many forms of these medications, including bevacizumab, sunitinib, and sorafenib (3–7). Phase I studies of cediranib demonstrated rates as high as 80%, with 35% of patients developing grade 3 or 4 hypertension (3,4). The incidence and degree of proteinuria on these agents are less well characterized but also appear to be a class effect mediated by inhibition of VEGF signaling between glomerular podocytes and endothelial cells, which together form the permeability barrier (8). Agents with higher potency, such as cediranib, might be expected to have a higher incidence of on-target side effects, such as hypertension and proteinuria, but no data exist beyond phase I trials to address this at present.
Because hypertension and proteinuria are mechanism-dependent toxicities, these biomarkers of VSP inhibition may be useful in optimizing individualized drug dosing (9,10). The development of hypertension has been associated with improved anticancer responses in breast, colon, and renal cell carcinomas (11–13). An association between proteinuria and outcomes has not been reported.
To assess the utility of hypertension and proteinuria as pharmacodynamic markers of VSP inhibition, the time course, risk factors, and pathophysiology for these toxicities must be characterized. In this study, we have investigated the time course for development of hypertension and proteinuria in patients receiving cediranib.
Patients and Methods
Study Population
Forty-six patients ages 41 to 77 years with recurrent epithelial ovarian carcinoma were enrolled in an open-labeled, phase II study of single-agent cediranib between September 2005 and November 2008, and they were followed for the entire duration of cediranib treatment (14). The primary objective of this study was assessment of overall response. Investigation of toxicities was a secondary objective. Inclusion and exclusion criteria have been previously described (14). Notably, exclusion criteria included >1+ albuminuria by dipstick and uncontrolled hypertension (systolic BP (SBP): >150 mmHg or diastolic BP (DBP): >90 mmHg). The initial daily dose of cediranib was 45 mg. After excessive toxicities were observed in the first 11 patients, this was decreased to 30 mg. The two most common reasons for dose reduction were fatigue and diarrhea (14).
Measurement of BP
Baseline BP was measured in oncology clinic. Patients received a home BP cuff (provided by AstraZeneca) and instructions by study nurses on techniques for measuring BP twice daily according to The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure guidelines (15). BP management was dictated by study protocol using Common Terminology Criteria for Adverse Events v3.0 toxicity grades, and home BP measurements were used for grading of this toxicity. Grade 1 is a transient (<24 hours) BP elevation >150/100 or a rise in DBP of >20 mmHg. Grade 2 is defined by the same parameters as grade 1 but ≥24 hours. Grade 3 is defined as grade 2 hypertension not controlled after 48 hours of per-protocol antihypertensive treatment. Grade 4 is a hypertensive crisis. The study protocol had guidelines for medical management of BP, frequency of monitoring, and cediranib dose modifications on the basis of BP. The goal was to keep the BP <150/90, with medication choice at the discretion of the provider. If the BP remained elevated, the study medication was stopped for 24 to 48 hours and restarted at a lower dose as prespecified by the protocol.
Measurement of Proteinuria
A urinalysis was performed at baseline and every 2 weeks during the study period. If the dipstick urine albumin was >1+, a 24-hour urine protein or a spot urine protein-creatinine ratio (UPC) was performed to further quantify the proteinuria, assuming that a UPC of 1 was equivalent to 1 g of protein over 24 hours (16). Patients were diagnosed with Common Terminology Criteria for Adverse Events v3.0 grade 1 proteinuria for 1+ dipstick albumin or between 0.15 and 1.0 g per 24 hours, grade 2 for 2+ or 3+ on dipstick or between 1.0 and 3.5 g per 24 hours, grade 3 for 4+ on dipstick or >3.5 g per 24 hours, or grade 4 for development of the nephrotic syndrome. If the protein level was between 1 and 2 g per 24 hours, the medication dose was decreased. If it was >2 g per 24 hours, the medication was held until it fell back below 2 g per 24 hours and restarted at a lower dose. In some instances, patients with nephrotic syndrome were continued on the medication if there was clinical benefit.
Analysis of Covariates
Other covariates included age, body mass index, prior hypertension, family history of hypertension or cardiovascular disease, and estimated GFR (eGFR) by the Chronic Kidney Disease Epidemiology Collaboration equation, chosen instead of the Modification of Diet in Renal Disease equation because of increased accuracy with values of eGFR >60 ml/min per 1.73 m2 (17). Age was examined by dividing the patients into those older and younger than the median age of 57 years, and also by analyzing patients 65 years and older separately. Covariate information was obtained from medical records.
We also analyzed the relationship between hypertension and proteinuria and tumor responsiveness, but because of small numbers with data on tumor responsiveness (n = 35), this was limited.
Data and Statistical Analyses
Relative risks (RRs) and 95% confidence intervals (95% CIs) were calculated for comparison of two dichotomous variables. P values were obtained by χ2 analyses.
Because of the low threshold for addition of BP medications, presence or absence of hypertension was defined not only by actual BP values, but also by the number of additional BP medications or changes in medication doses. Development of hypertension was defined as the addition of at least one BP medication, a new SBP >140 mmHg or DBP >90 mmHg (the definition of hypertension by Joint National Committee 7 guidelines) (15), or if a patient had prior hypertension, a rise in SBP of >20 mmHg or DBP of >15 mmHg. Hypertension was analyzed dichotomously. Development of hypertension in 3 days was analyzed separately from overall development of hypertension.
Proteinuria was evaluated as a general category of all proteinuria, but also as proteinuria ≥grade 2.
The associations between proteinuria and hypertension and tumor responsiveness were analyzed with χ2 analyses. Tumor responsiveness was defined as a Response Evaluation Criteria in Solid Tumors (RECIST) response of either stable disease or partial remission, with progressive disease defined as “nonresponsiveness” (18).
BP values were normally distributed. Means at different time points were compared with a paired t test. BPs between age groups were analyzed by the standard t test.
SAS v9.1 statistical software was used for statistical analyses (SAS Institute Inc., Cary, NC).
Results
Patient Characteristics
The cohort for analysis consisted of 46 women. The median age was 57 years, and 98% were white. At baseline, 26% of the women had hypertension, and all women had <1+ albuminuria on dipstick. Median study length was 84 days, with a minimum of 6 days and maximum of 544 days (Table 1).
Table 1.
Characteristics of the cohort (n = 46)
| Age, yr, median (IQR) | 56.5 (52.0, 61.8) |
| Race, n (%) | |
| Caucasian | 45 (98) |
| Asian | 1 (2) |
| Baseline eGFR, ml/min per 1.73 m2, median (IQR) | 82 (67, 88) |
| Diabetes, n (%) | 2 (4) |
| Baseline BMI, median (IQR) | 26.1 (22.6, 31.4) |
| Prior diagnosis of hypertension, n (%) | 12 (26) |
| Baseline SBP, mmHg, median (IQR) | 119 (110, 130) |
| Baseline DBP, mmHg, median (IQR) | 73 (69, 81) |
| Baseline dipstick proteinuria, n (%) | |
| negative | 43 (93) |
| trace | 3 (7) |
| Length of time on study, days, median (IQR) | 84 (55, 158) |
IQR, interquartile range; BMI, body mass index.
Kinetics of Hypertension and Proteinuria
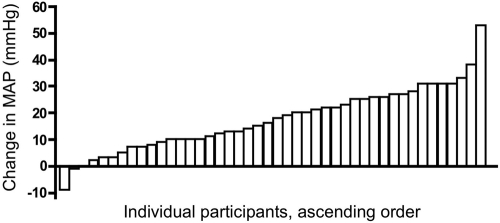
By day 3, 67% of women developed hypertension, 73% developed it by day 7, and 87% developed it by the end of the study. 43% of women developed grade 3 hypertension during the study. Additional BP medications were required by day 3 in 28% of women, in 43% by day 7, and in 70% by the end of the study (Figure 1). There was considerable variability in the degree of BP change throughout the study cohort, ranging from a change in mean arterial pressure between days 0 and 3 of −9 mmHg to +53 mmHg. (Figure 2).
Figure 1.
Kinetics of BP rise and addition of medications. Day 0 was before cedarinib treatment. The mean length of time in the study was 115 days, and the median was 84 days. Data are from all 46 patients, and each time point is for those patients still in the study at that time.
Figure 2.
Variability in change in mean arterial pressure (MAP) over 3 days. Change in MAP from baseline before initiation of cediranib to MAP on day 3 of the study medication. Data are from all patients from whom we have data from baseline and day 3 (n = 44).
The average SBP at baseline was 120 ± 16 mmHg, and by day 3 the average SBP had risen to 139 ± 18 mmHg (P < 0.0001 for the difference). Average DBP at baseline was 73 ± 10 mmHg and rose to 90 ± 11 mmHg by 3 days (P < 0.0001 for the difference). With the addition of BP medications, the average BP did not continue to rise over the rest of the study (Figure 3).
Figure 3.
SBP and DBP throughout the study. SBP and DBP mean values and SD at each time point. Data are from all patients remaining in the study at each time point.
Fourteen women (30%) developed proteinuria, with 7 (15%) developing grade 2 proteinuria. There were no instances of grade 3 or 4 proteinuria. Of those who developed proteinuria during the study, 50% developed this toxicity by 2 weeks, 92% by 4 weeks, and 100% by 6 weeks (Figure 4). Three women had follow-up 24-hour urines, with protein values of 1085, 222, and 35 mg, respectively. Two women had UPC ratios for 2+ proteinuria, with values of 0.2 and 0.211, respectively.
Figure 4.
Kinetics of development of proteinuria. Baseline was before initiation of cediranib. Urinalysis was performed every 2 weeks, and all patients who developed proteinuria during the study had developed this toxicity by 6 weeks. Data are from all patients for whom we have urinalysis data for at least one follow-up (n = 45).
Weights decreased in most participants over the course of the study. The median weight at baseline was 67.3 kg, at 2 weeks it was 66.0 kg, and at 4 weeks it was 64.6 kg.
Risk Factors for Development of Hypertension and Proteinuria
Age ≥57 years predicted development of hypertension in the first 3 days of treatment, and it also predicted a larger average increase in SBP at day 3 than in those <57 years (15.9 versus 7.0 mmHg; P = 0.02). Being age ≥65 years showed a trend toward rapid development of hypertension over the first 3 days (RR: 1.41; 95% CI: 0.98 to 2.02). Body mass index, eGFR, family history of hypertension or cardiovascular disease, and prior hypertension did not predict development of hypertension, nor did starting dose of cediranib (Table 2). The only significant predictor of proteinuria was the starting dose of cediranib. Those patients who received the 45-mg dose were 3.2 times more likely to develop proteinuria than those who received the 30-mg dose (P = 0.006).
Table 2.
Relative risk of development of hypertension and proteinuria on the basis of baseline risk factors (n = 46)
| Hypertension in 3 Days | Hypertension Overall | Proteinuria | |
|---|---|---|---|
| Age ≥57 yr | 1.57 (1.01 to 2.43) | 1.09 (0.87 to 1.36) | 1.09 (0.45 to 2.61) |
| Age ≥65 yr | 1.41 (0.98 to 2.02) | 1.01 (0.75 to 1.35) | 0.88 (0.50 to 1.56) |
| Overweight (BMI > 25) | 1.05 (0.60 to 1.62) | 1.07 (0.84 to 1.37) | 0.48 (0.20 to 1.16) |
| History of HTN | 1.10 (0.78 to 1.79) | 1.07 (0.86 to 1.34) | 1.13 (0.44 to 2.94) |
| Family history of HTN or cardiovascular disease | 1.09 (0.64 to 1.85) | 1.11 (0.80 to 1.52) | 0.86 (0.30 to 2.43) |
| 45-mg starting dosea | 1.12 (0.72 to 1.74) | 1.06 (0.84 to 1.34) | 3.18 (1.43 to 7.08) |
| HTN in 3 days | —— | —— | 0.90 (0.37 to 2.21) |
| HTN overall | —— | —— | 0.90 (0.26 to 3.07) |
| Grade 3 HTN | —— | —— | 1.30 (0.54 to 3.10) |
| Proteinuria | 0.95 (0.60 to 1.50) | 0.98 (0.76 to 1.26) | —— |
Values in table are relative risk (95% confidence interval). BMI, body mass index; HTN, hypertension.
Reference dose 30 mg.
We examined the association between development of hypertension and proteinuria. Overall, 12 of the women (86%) with proteinuria developed hypertension, whereas 28 women (88%) without proteinuria developed hypertension. All patients who developed grade 2 proteinuria developed hypertension. However, hypertension alone did not increase the risk of proteinuria (Table 2). Of the 20 patients who developed grade 3 hypertension, only 7 (35%) developed proteinuria. Given that urinalyses were not checked until 14 days on study medication, we cannot ascertain whether hypertension preceded proteinuria. However, in one patient, hypertension did not develop until day 44 and proteinuria was present at 2 weeks, suggesting that proteinuria preceded hypertension in this individual.
Neither hypertension nor proteinuria correlated with RECIST response, but the confidence intervals were wide for these analyses. For hypertension at 3 days, the RR of a RECIST response was 0.77 (95% CI: 0.32 to 1.80), and for grade ≥2 proteinuria the RR of a response was 1.32 (95% CI: 0.52 to 3.33). A combination of the toxicities also did not correlate with response (data not shown).
Discussion
Single-agent cediranib for recurrent epithelial ovarian cancer caused a rapid BP rise in a majority of patients. Older age was a risk factor for developing hypertension. Although not as common as hypertension, proteinuria was a toxicity of therapy, was dose-dependent, and rose rapidly.
In the only published study describing daily BP on VSP inhibitors, Maitland et al. (19) reported a rise in BP with sorafenib over the first 24 hours. Kinetics of proteinuria have not been previously published for any VSP inhibitor.
Our prevalence rates for hypertension and proteinuria are consistent with phase I trials of cediranib but are much higher than rates for older VSP inhibitors. In one study for treatment of non-small cell lung cancer, 80% of the patients initiated on cediranib developed hypertension, with grade ≥3 hypertension in 35% (3). Proteinuria was not reported. However, in another phase I study of 40 patients on cediranib for multiple solid tumors, proteinuria occurred in varying grades in 68% of patients and appeared to be dose-dependent, and grade 4 proteinuria was a dose-limiting toxicity in one patient. In this study, 80% of patients also developed hypertension, but only one patient developed grade 3 hypertension (4).
The association between hypertension and proteinuria on VSP inhibitors has only been explored in one study. In a phase III trial of capecitabine compared with bevacizumab plus capecitabine for metastatic breast cancer including 462 patients, Miller et al. (20) showed that patients who developed proteinuria were more likely to develop hypertension than those who did not develop proteinuria (47% versus 16%; P < 0.001). In our study, 86% of patients who developed proteinuria also developed hypertension, whereas 88% of those who did not develop proteinuria developed hypertension. The prevalence of hypertension in our study was higher, but cediranib has a higher potency for VSP inhibition than bevacizumab.
In our study, up to 65% of patients who developed hypertension ≥grade 3 did not develop proteinuria, and in at least one patient, the proteinuria preceded the hypertension. This suggests that mechanisms of VSP inhibitor-induced hypertension and proteinuria differ, despite the fact that each is likely a mechanism-dependent toxicity. It also indicates that proteinuria is not simply a secondary consequence of hypertension, which is consistent with the important role of podocyte-derived VEGF in maintaining the glomerular filtration barrier (21).
The mechanism by which VSP inhibitors induce hypertension in humans is not yet clear. Inhibition of VEGF-dependent vasodilatory pathways such as nitric oxide (NO) has been proposed, but capillary rarefaction causing increased systemic vascular resistance has also been proposed. Preclinical evidence supports both hypotheses (10,22). Our results, in which a majority of patients developed hypertension within 3 days of treatment, suggest that acute inhibition of NO signaling is playing an important role. The ability of infused VEGF to directly induce hypotension in an NO-dependent fashion supports this model (23–25). Although early stages of capillary regression can be measured as early as 24 h after drug initiation in mouse models, capillary density is not decreased by >50% until after at least 7 to 14 days (26,27). This has not been tested in humans earlier than 5 weeks into drug therapy, but there was shown to be evidence of decreased capillary density at 5 weeks with telatinib treatment (28). It is unlikely, given this evidence, that such a significant degree of BP elevation within 3 days would be due to capillary rarefaction alone. However, this needs further investigation.
Another potential cause of hypertension is activation of the renin-angiotensin-aldosterone axis by decreased renal blood flow in the setting of thrombotic microangiopathy. Our finding that weights decreased while BP increased argues against this hypothesis, because hyperreninemia should result in decreased natriuresis, and therefore increased weight. In a study by Facemire et al. (25) using DC101, a VEGF receptor inhibitor in mice, renin mRNA and aldosterone urinary excretion were actually decreased compared with control mice. In the only published study examining this question in humans, in 20 patients taking sorafenib, renin and aldosterone levels were not increased (29). This was a small study, and further investigation is needed, but these findings are consistent with systemic vasoconstriction as the mechanism of VSP-induced hypertension.
Hypertension from any of these causes could be further perpetuated by a blunting of pressure natriuresis due to VSP inhibition (30). Cyclic GMP, a downstream effector of NO, is required for the natriuretic response of the kidney proximal tubule in both rats and humans in the setting of elevated renal perfusion pressure (31,32). VSP inhibitors may therefore blunt pressure natriuresis through endothelial NO synthase inhibition, further exacerbating hypertension. Although the weight loss observed in this cohort could be due to pressure natriuresis, we think it more likely reflects medication side effects, such as diarrhea, which was seen in 31% of the cohort (14).
Evidence to date indicates that VSP inhibitor-mediated proteinuria reflects disrupted glomerular podocyte to endothelial VEGF signaling, although the precise mechanism for this effect has not yet been elucidated (5). Podocytes express high levels of VEGF. In animal models, podocyte-specific VEGF deletion causes endotheliosis, proteinuria, and thrombotic microangiopathy—the same pathologic lesion seen in human kidney biopsy specimens from patients with proteinuria on VSP inhibitors (21). Our observation that proteinuria may develop within weeks of starting therapy is considerably earlier than previously reported, and proteinuria below dipstick levels may appear even earlier. This indicates either that subclinical thrombotic microangiopathy may develop rapidly in some patients or, alternatively, that VSP inhibition alters the permeability of the glomerulus in unanticipated ways. In support of the latter possibility, NO inhibition directly increases proteinuria in some animal models (33). Podocytes express soluble guanylate cyclase, the NO receptor. The resultant cyclic GMP molecule may regulate the podocyte cytoskeleton, determining glomerular permeability. Thus, NO inhibition may underlie podocyte damage and albuminuria from VSP inhibition (34). More systematic measurements of the time course and magnitude of proteinuria on VSP inhibitors are required to approach these questions. Our findings lend credence to the necessity of these future studies.
We were unable to effectively study the association between hypertension and proteinuria and tumor response. Larger studies are needed to investigate the utility of quantitative proteinuria as a biomarker for VSP inhibition, and potentially clinical efficacy, in patients receiving cediranib. Proteinuria is simple to measure quantitatively, unlike BP, which can vary considerably in outpatients unless measured using ambulatory blood pressure monitoring, which is not amenable to common clinical practice.
Our study is limited by size. As a consequence, some of our findings showed trends but did not reach statistical significance. Power was further decreased with tumor response analyses. Given the observed trends, future research with larger studies is warranted. Although BP was measured twice daily, we did not use ambulatory blood pressure monitoring to quantify average BP over a 24-hour period, increasing the variability of the BP measurements. We did not further quantify the proteinuria data by use of UPC ratios in all women, so we may have missed proteinuria at its early stages. Urine dipstick albumin levels can be influenced by urine concentration, thus there may have been some misclassification of proteinuria.
Conclusions
Understanding the time course, risk factors, and mechanisms of hypertension and proteinuria induced by VSP inhibition will be critical for optimizing the safety, tolerability, and perhaps efficacy of this promising drug class. Clinicians, especially nephrologists, need to be aware that hypertension and proteinuria can occur early in the course of therapy and should monitor and manage this toxicity appropriately with antihypertensive therapies. Greater awareness of proteinuria as a mechanism-dependent toxicity of VSP inhibition is needed, because proteinuria occurs early after treatment initiation and is less cumbersome to accurately quantify than BP. More studies are necessary to explore mechanisms of these toxicities, and larger studies are required to identify additional risk factors, define optimal dosing and toxicity management strategies, and correlate hypertension and proteinuria with outcomes.
Disclosures
None.
Acknowledgments
Support for this research was provided by a National Kidney Foundation Fellowship Grant (to E.S.R.), The Madeline Franchi Ovarian Cancer Fund, Ovations for the Cure, National Institutes of Health grant DK073628 (to B.D.H.), and a pilot grant from Harvard Catalyst: National Institutes of Health grant UL1 RR 025758-02 with financial contributions from participating institutions (to B.D.H.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Dietrich J, Wang D, Batchelor TT: Cediranib: Profile of a novel anti-angiogenic agent in patients with glioblastoma. Expert Opin Investig Drugs 18: 1549–1557, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M, Curwen JO, Chester R, Jackson JA, Boffey SJ, Kilburn LL, Barnett S, Richmond GH, Wadsworth PF, Walker M, Bigley AL, Taylor ST, Cooper L, Beck S, Jurgensmeier JM, Ogilvie DJ: AZD2171: A highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res 65: 4389–4400, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Laurie SA, Gauthier I, Arnold A, Shepherd FA, Ellis PM, Chen E, Goss G, Powers J, Walsh W, Tu D, Robertson J, Puchalski TA, Seymour L: Phase I and pharmacokinetic study of daily oral AZD2171, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with carboplatin and paclitaxel in patients with advanced non-small-cell lung cancer: the National Cancer Institute of Canada clinical trials group. J Clin Oncol 26: 1871–1878, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto N, Tamura T, Yamada K, Yamada Y, Nokihara H, Fujiwara Y, Takahashi T, Murakami H, Boku N, Yamazaki K, Puchalski TA, Shin E: Phase I, dose escalation and pharmacokinetic study of cediranib (RECENTIN), a highly potent and selective VEGFR signaling inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 64: 1165–1172, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Izzedine H, Rixe O, Billemont B, Baumelou A, Deray G: Angiogenesis inhibitor therapies: Focus on kidney toxicity and hypertension. Am J Kidney Dis 50: 203–218, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA: A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349: 427–434, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel TV, Morgan JA, Demetri GD, George S, Maki RG, Quigley M, Humphreys BD: A preeclampsia-like syndrome characterized by reversible hypertension and proteinuria induced by the multitargeted kinase inhibitors sunitinib and sorafenib. J Natl Cancer Inst 100: 282–284, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE: VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphreys BD, Atkins MB: Rapid development of hypertension by sorafenib: Toxicity or target? Clin Cancer Res 15: 5947–5949, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snider KL, Maitland ML: Cardiovascular toxicities: Clues to optimal administration of vascular endothelial growth factor signaling pathway inhibitors. Target Oncol 4: 67–76, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scartozzi M, Galizia E, Chiorrini S, Giampieri R, Berardi R, Pierantoni C, Cascinu S: Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol 20: 227–230, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Rini BI, SJ, Fruehauf JP, Cohen EE, Tarazi JC, Rosbrook B, et al. : Association of diastolic blood pressure (DBP)>90mmHg with overall survival (OS) in patients treated with axitinib (AG-013736) [Abstract]. J Clin Oncol 26[ Suppl], 2008 [Google Scholar]
- 13.Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, Flockhart DA, Hancock B, Davidson N, Gralow J, Dickler M, Perez EA, Cobleigh M, Shenkier T, Edgerton S, Miller KD: Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol 26: 4672–4678, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matulonis UA, Berlin S, Ivy P, Tyburski K, Krasner C, Zarwan C, Berkenblit A, Campos S, Horowitz N, Cannistra SA, Lee H, Lee J, Roche M, Hill M, Whalen C, Sullivan L, Tran C, Humphreys BD, Penson RT: Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol 27: 5601–5606, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ: Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Ginsberg JM, Chang BS, Matarese RA, Garella S: Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med 309: 1543–1546, 1983 [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG: New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Maitland ML, Kasza KE, Karrison T, Moshier K, Sit L, Black HR, Undevia SD, Stadler WM, Elliott WJ, Ratain MJ: Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res 15: 6250–6257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, Langmuir V, Rugo HS: Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23: 792–799, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mourad JJ, des Guetz G, Debbabi H, Levy BI: Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol 19: 927–934, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Sane DC, Anton L, Brosnihan KB: Angiogenic growth factors and hypertension. Angiogenesis 7: 193–201, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK: Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A 98: 2604–2609, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM: Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension 54: 652–658, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, McDonald DM: Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol 290: H547–H559, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G, Yancopoulos GD, McDonald DM: Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol 165: 35–52, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steeghs N, Gelderblom H, Roodt JO, Christensen O, Rajagopalan P, Hovens M, Putter H, Rabelink TJ, de Koning E: Hypertension and rarefaction during treatment with telatinib, a small molecule angiogenesis inhibitor. Clin Cancer Res 14: 3470–3476, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Veronese ML, Mosenkis A, Flaherty KT, Gallagher M, Stevenson JP, Townsend RR, O'Dwyer PJ: Mechanisms of hypertension associated with BAY 43–9006. J Clin Oncol 24: 1363–1369, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Guyton AC, Coleman TG, Cowley AV, Jr., Scheel KW, Manning RD, Jr., Norman RA, Jr.: Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 52: 584–594, 1972 [DOI] [PubMed] [Google Scholar]
- 31.Lieb DC, Kemp BA, Howell NL, Gildea JJ, Carey RM: Reinforcing feedback loop of renal cyclic guanosine 3′ 5′-monophosphate and interstitial hydrostatic pressure in pressure-natriuresis. Hypertension 54: 1278–1283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki S, Siragy HM, Gildea JJ, Felder RA, Carey RM: Production and role of extracellular guanosine cyclic 3′, 5′ monophosphate in sodium uptake in human proximal tubule cells. Hypertension 43: 286–291, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Datta PK, Sharma M, Duann P, Lianos EA: Effect of nitric oxide synthase inhibition on proteinuria in glomerular immune injury. Exp Biol Med (Maywood) 231: 576–584, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Lewko B, Stepinski J: Cyclic GMP signaling in podocytes. Microsc Res Tech 57: 232–235, 2002 [DOI] [PubMed] [Google Scholar]