Abstract
Background and objectives: Adequate early mycophenolic acid (MPA) exposure is an important determinant for effective rejection prophylaxis. This pharmacokinetic study investigated whether an intensified dosing regimen of enteric-coated mycophenolate sodium (EC-MPS) could achieve higher mycophenolic acid (MPA) exposure early after transplantation versus a standard dosing regimen.
Design, setting, participants, & measurements: De novo kidney transplant recipients (n = 75) who were treated with basiliximab induction and cyclosporine were randomly assigned to receive EC-MPS as either standard dosing (1440 mg/d; n = 37) or intensified dosing (days 0 through 14: 2880 mg/d; days 15 through 42: 2160 mg/d; followed by 1440 mg/d; n = 38). Full 12-hour pharmacokinetic and pharmacodynamic profiles were taken at six time points during the first 3 months. Exploratory analysis of inosine monophosphate dehydrogenase (IMPDH) activity was also performed for better understanding of the pharmacokinetic–pharmacodynamic relationship between MPA exposure and IMPDH activity in the early posttransplantation period. Preliminary efficacy parameters, safety, and tolerability were assessed.
Results: Exposure to MPA was significantly higher on days 3 and 10 after transplantation in the intensified versus standard EC-MPS group, with 52.9 versus 22.2% (P < 0.05) of patients reaching MPA exposure >40 mg/h per L in the first week. The intensified regimen resulted in significantly lower IMPDH activity on day 3 after transplantation, and the overall safety was comparable for both groups.
Conclusions: These pharmacokinetic and safety data support further research on the hypothesis that early adequate MPA exposure could improve clinical outcome.
The combination of mycophenolic acid (MPA), given as mycophenolate mofetil (MMF) or enteric-coated mycophenolate sodium (EC-MPS), with steroids and calcineurin inhibitors (either cyclosporine A [CsA] or tacrolimus) has become standard immunosuppressive therapy worldwide. MMF and EC-MPS have a similar efficacy and safety profile (1,2) but differ in their pharmacokinetic characteristics (3).
A large number of retrospective and prospective studies support the hypothesis that adequate early MPA exposure is an important determinant for effective rejection prophylaxis (4–13). Whereas the majority of tacrolimus-treated patients achieve adequate MPA exposure early after transplantation (13,14), studies have demonstrated that approximately 50% of patients who are treated with CsA and standard MPA dosages are underexposed (4,7,12,13). Larger initial MMF dosages (up to 4 g/d) have been suggested early after transplantation for achievement of sufficient MPA exposure in combination with CsA (13,15,16).
There are only limited data on the pharmacokinetics, safety, and efficacy of higher (>3 g/d) MMF dosages (4,5,17), and data on higher EC-MPS dosages are lacking. The aim of this pilot study was to investigate the feasibility and safety of achieving target MPA exposure levels (≥40 mg/h per L), measured as area under time-concentration curve (AUC), using an intensified EC-MPS dosing regimen, compared with a standard dosing regimen, in de novo CsA-treated renal transplant patients. In addition, an exploratory analysis of inosine-monophosphate dehydrogenase (IMPDH) activity was performed for better understanding of the pharmacokinetic–pharmacodynamic relationship between MPA exposure and IMPDH activity early after transplantation.
Materials and Methods
Patients and Study Design
This was an exploratory, multicenter, open-label, prospective, randomized, parallel-group 6-months study (EudraCT no. 2005-006138-14) designed to compare an intensified EC-MPS dosing regimen with a standard regimen in CsA-treated de novo renal transplant patients. This study was designed, implemented, and reported in accordance with ICH Guidelines for Good Clinical Practice and with the Declaration of Helsinki. The protocol was approved by the local ethics committees. All enrolled patients gave written informed consent. Study data were collected between June 2006 and November 2007 from three transplant centers in Germany.
All patients who were aged 18 to 70 yr and had received a first or second kidney transplant were eligible for inclusion. Important exclusion criteria were previous graft loss within 12 months after transplantation, multiorgan recipient, cardiac death donor, ABO-incompatible transplant, current panel-reactive antibody level >50%, and existing HLA antibodies against the transplant. Patients were randomly assigned using a validated, locked system to assign treatment groups to randomization numbers in a 1:1 ratio, stratified for donation from living and deceased donors, and received either an intensified (days 0 through 14: 1440 mg twice daily; days 15 through 42: 1080 mg twice daily; followed by 720 mg twice daily) or a standard (720 mg twice daily) EC-MPS dosing regimen (Myfortic; Novartis Pharma, Nuremberg, Germany). All patients were treated with basiliximab (Simulect; Novartis; 20 mg on days 0 and 4 after transplantation) and commenced on an immunosuppressive regimen of CsA microemulsion (Sandimmune Optoral; Novartis). The CsA dosage was adjusted to achieve a target trough level of 130 to 220 ng/ml for the first 3 months and 100 to 150 ng/ml thereafter. All patients received an intraoperative corticosteroid dose of 500 mg of methylprednisolone. Maintenance methylprednisolone dose was tapered to 40 mg on day 7, followed by a stepwise reduction to 16 mg at month 1 and 6 mg at month 4. Minimum daily dose of 4 mg of methylprednisolone or equivalent was administered until the end of the study.
Acute rejection episodes were verified with biopsies before or within 24 hours after commencement of antirejection therapy and assessed according to Banff 2003 classification (18). Prophylactic treatment of cytomegalovirus (CMV) with valganciclovir (Valcyte; Roche), adjusted for renal function, was recommended for all patients at the investigator's discretion, except for CMV-negative patients who had received a CMV-negative donor organ.
The primary end point of this exploratory study was to compare the MPA-AUC0 to 12 during a 12-hour dosing interval at steady state achieved by two different EC-MPS dosing regimens at different time points after transplantation, including time to achieve an MPA-AUC ≥40 mg/h per ml. The secondary aim of the study was to assess preliminary safety and tolerability of the intensified EC-MPS dosing regimen. Additional efficacy assessments included biopsy proven acute rejection (BPAR) (graded Banff I and higher) and graft loss. Adverse events (AEs) were reported, as determined by the investigator.
Pharmacokinetic Assessment
On the days of pharmacokinetic profiling, patients fasted overnight before dosing and until 1.5 hours after EC-MPS administration. Pharmacokinetic assessment was performed under steady-state conditions (i.e., there was no EC-MPS dose change for at least 72 hours before pharmacokinetic sampling). Blood was drawn on study days 3, 10, 21, 42, 56, and 84 for measurement of MPA and its metabolites (MPA-glucuronide [MPAG] and acyl-MPA-glucuronide [ac-MPAG]) and free fraction of MPA (fMPA). Blood (collected in EDTA-containing tubes) was taken before the morning dose and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, and 12 hours after dose. Analysis of MPA and metabolites was performed in a central laboratory (Department of Clinical Chemistry, Klinikum-Stuttgart, Germany) using validated HPLC (19). Noncompartment MPA pharmacokinetic parameters were derived from individual plasma concentration-time profiles using WinNonLin 5.2 (Pharsight, Mountain View, CA) software and included AUC0 to 12, (calculated by linear trapezoid method); maximum (Cmax), minimum (Cmin), and average (Cavg) plasma concentration during dosing interval; and the time (tmax) at which Cmax occurs.
Assessment of IMPDH Activity
Blood was drawn for measurement of IMPDH activity before transplantation and on study days 3, 10, 21, and 56. Blood (collected in heparin-containing tube) was taken before the morning dose and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, and 12 hours before dose. Peripheral mononuclear cells were isolated within 24 hours, and prepared cell extracts were frozen and analyzed in a central laboratory (20). The pharmacodynamic parameters derived from each enzyme activity-time profile were calculated using WinNonLin 5.2 software and included the area-under-enzyme-activity time curve (AEC; calculated by linear trapezoid method) and maximum (Amax), minimum (Amin), and average (Aavg) daytime enzyme activity during dosing interval. The relationship between plasma MPA concentrations and their effect on IMPDH activity was explored with an inhibitory effect model using WinNonLin 5.2. For this analysis, Cmin, Cavg, Cmax and the corresponding Amax, Aavg, Amin per patient and visit were included. The MPA concentration associated with half-maximal inhibitory effect on IMPDH activity (EC50) was calculated from the formula E = (Emax × C)/(EC50 + C), where E is effect and Emax is effect at 0 drug concentration.
Statistical Analysis
For assessment of the pharmacokinetic end points, complete pharmacokinetic data based on valid profiles must be available for 42 patients on days 3, 10, and 21 after transplantation. A valid profile (0 to 12 hours) for the calculation of pharmacokinetic parameters was defined as the availability of results for at least 11 of 12 sampling points. Depending on the dropout rate, more patients could be included. The sample size for this pharmacokinetic study was chosen with respect to the exploratory nature of this study and was not based on statistical power considerations. The pharmacokinetic population consisted of all patients for whom at least one valid pharmacokinetic profile had been collected. For patients who discontinued prematurely, only their available data were analyzed. The intention-to-treat population for the efficacy and safety analyses consisted of all randomly assigned patients who received at least one dose of study medication (EC-MPS) and received a transplant.
Frequency distributions were provided for categorical variables, and the two treatment groups were compared using the χ2 test. Continuous variables were described using mean, SD, minimum, median, and maximum, and comparisons between the two treatment groups were performed with suitable two-sample tests (Wilcoxon rank sum test for AUC and log-rank test for time to AUC ≥40 mg/h per L). Two-sided P < 0.05 was considered statistically significant. Data analyses were carried out using SPSS 16.0 software.
Results
A total of 75 patients underwent randomization and received a transplant. In 33 patients, at least one pharmacokinetic profile in the first month was not available for analysis. As specified in the protocol, study recruitment was halted after three complete pharmacokinetic profiles had been obtained from 42 patients during their first month after transplantation. A total of 65 patients completed at least one pharmacokinetic profile and were included in the pharmacokinetic population for the primary analysis. Patient demographics and baseline characteristics were comparable between treatment groups (Table 1) and were representative of a typical German transplant population.
Table 1.
Patient demographics and donor characteristics (intention-to-treat population)
| Parameter | Intensified Regimen (n = 38) | Standard Regimen (n = 37) | Total (N = 75) |
|---|---|---|---|
| Age (years) | |||
| mean ± SD | 51.7 ± 12.7 | 48.0 ± 16.1 | 49.9 ± 14.5 |
| range | 22.0 to 71.0 | 19.0 to 69.0 | 19.0 to 71.0 |
| Male/female (n [%]) | 19 (50)/19 (50) | 21 (57)/16 (43) | 40 (53)/35 (47) |
| Body mass index (mean ± SD) | 26.3 ± 5.0 | 24.9 ± 4.9 | 25.6 ± 5.0 |
| Ethnicity (n [%]) | |||
| white | 38 (100) | 36 (97) | 74 (99) |
| Asian | 0 (0) | 1 (3) | 1 (1) |
| Previous renal transplants (n [%]) | |||
| 0 | 35 (92) | 33 (89) | 68 (91) |
| 1 | 3 (8) | 4 (11) | 7 (9) |
| Donor age (years) | |||
| mean ± SD | 53.4 ± 16.1 | 53.3 ± 15.3 | 53.3 ± 15.6 |
| range | 16.0 to 79.0 | 19.0 to 79.0 | 16.0 to 79.0 |
| Donor age >65 yr (n [%]) | 4 (10.5) | 5 (13.5) | 9 (12.0) |
| Cold ischemia time (h; mean ± SD) | 10.0 ± 7.3 | 9.3 ± 6.1 | 9.6 ± 6.7 |
| Donor source (n [%]) | |||
| deceased | 27 (71) | 28 (76) | 55 (73) |
| living | 11 (29) | 9 (24) | 20 (27) |
| Delayed graft function (n [%]) | 6 (15.8) | 6 (16.2) | 12 (16) |
| HLA mismatches (n [%]) | |||
| 0 | 7 (18.4) | 2 (5.4) | 9 (12.0) |
| 1 and 2 | 13 (34.3) | 11 (29.7) | 24 (32.0) |
| 3 and 4 | 13 (34.2) | 16 (43.2) | 29 (38.7) |
| 5 and 6 | 5 (13.2) | 8 (21.6) | 13 (17.3) |
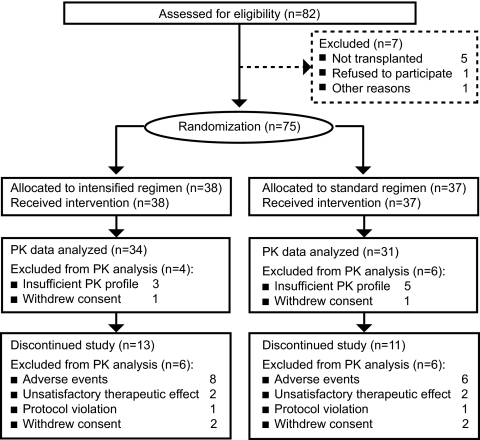
Patient disposition and withdrawals throughout the study are shown in Figure 1. Premature withdrawal from the study as a result of AEs occurred in eight (21.1%) of 38 patients from the intensified dosing group and in six (16.2%) of 37 patients from the standard dosing group. The most frequent types of AE leading to premature discontinuation were gastrointestinal disorders (intensified three of 38 versus standard one of 37), rejection (intensified one of 38 versus standard three of 37), and infections (intensified three of 38 versus standard one of 37). During the first 2 weeks, six patients in the intensified group required dosage reduction as a result of AEs, versus two patients in the standard dosing group. The leading cause was a gastrointestinal event (n = 4 intensified versus n = 1 standard). During the subsequent 4 weeks, dosage reductions were necessary for four patients from the intensified dosing group and for five patients from the standard dosing group.
Figure 1.
Patient flow diagram and analysis populations. PK, pharmacokinetic.
CsA starting dosages (intensified 7.5 ± 2.7 mg/kg versus standard 7.1 ± 2.8 mg/kg) and trough levels were similar throughout the study (day 3: 231 ± 107 versus 224 ± 119 ng/ml; day 14: 214 ± 70 versus 226 ± 73 ng/ml; day 56: 177 ± 60 versus 203 ± 178 ng/ml; month 6: 132 ± 38 versus 129 ± 38 ng/ml for intensified versus standard dosing groups, respectively). All patients received basiliximab and corticosteroids according to the protocol.
Pharmacokinetic and Pharmacodynamic Parameters
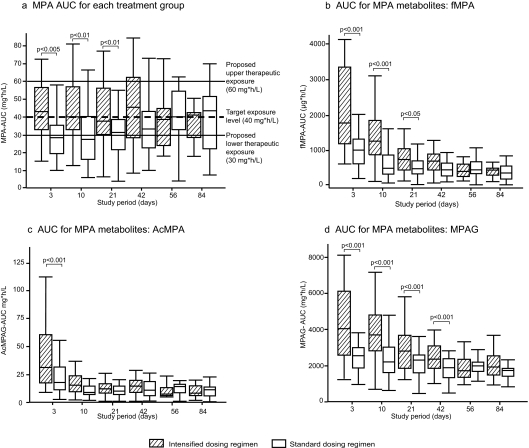
Steady-state pharmacokinetic parameters of MPA are shown in Table 2. Mean dosages of EC-MPS during the intensified treatment periods closely resembled the targeted regimen. Cmax showed no statistical differences, whereas Cmin and Cavg were significantly higher in the intensified dosing group. Exposure to MPA was significantly higher in the intensified group on days 3, 10, and 21 after transplantation in comparison with the standard group (Figure 2A). Similar results were observed for exposure to fMPA and to MPA metabolites (MPAG and ac-MPAG), with significantly higher exposure in the intensified dosing group on days 3 and 10 after transplantation versus the standard dosing group (Figure 2, B through D). In contrast to total MPA, exposure to fMPA and MPA metabolites was highest in both groups in the first week after transplantation and decreased over time. During the first 3 months after transplantation, MPA exposure in the intensified dosing group remained stable with decreasing MPA dosages, whereas MPA exposure in the standard dosing group increased over time. A greater proportion of patients in the intensified dosing group reached target MPA exposure (≥40 mg/h per L) early after transplantation, with a median time of 6 days compared with 23 days. More than half (52.9%) of patients in the intensified dosing group reached target MPA exposure on day 3 after transplantation, compared with 22.2% who received standard dosing (P < 0.05). More than 80% of patients in the intensified dosing group had reached MPA-AUC >30 mg/h per L by day 3 after transplantation (intensified versus standard day 3: 81.8 versus 40.7%; P < 0.001). All six patients in the intensified group with MPA-AUC <30 mg/h per L had poor renal function (<15 ml/min; n = 5) and/or low albumin (<36 mg/dl; n = 6). Most patients were within the proposed therapeutic window, with only a small proportion having MPA-AUC >60 mg/h per L (Table 3).
Table 2.
Steady-state pharmacokinetic parameters of MPA in patients who were treated with intensified versus standard dosing regimens of EC-MPS [arithmetic mean ±SD] (pharmacokinetic population; n = 65)
| MPA | Regimen | Study Visit |
|||||
|---|---|---|---|---|---|---|---|
| Day 3 (33/27) | Day 10 (30/24) | Day 21 (25/25) | Day 42 (25/21) | Day 56 (22/18) | Day 84 (19/14) | ||
| Dose (mg/d) | Intensified | 2869a | 2724a | 2174a | 1915a | 1440 | 1383 |
| Standard | 1440a | 1440a | 1397a | 1234a | 1380 | 1276 | |
| AUC (mg/h per L) | Intensified | 45.00 ± 15.70b | 42.80 ± 17.40c | 41.60 ± 17.20c | 45.40 ± 22.00 | 36.80 ± 14.30 | 36.70 ± 12.20 |
| Standard | 32.60 ± 18.71b | 31.30 ± 18.40c | 31.60 ± 15.80c | 37.50 ± 18.40 | 42.60 ± 20.60 | 40.30 ± 24.10 | |
| Cmin (mg/L) | Intensified | 1.22 ± 0.55b | 0.94 ± 0.47d | 1.17 ± 0.65c | 1.10 ± 0.66 | 0.89 ± 0.38 | 1.04 ± 0.49 |
| Standard | 0.92 ± 0.67b | 0.62 ± 0.45d | 0.76 ± 0.37c | 0.77 ± 0.35 | 1.04 ± 0.60 | 0.97 ± 0.77 | |
| Cmax (mg/L) | Intensified | 14.50 ± 12.10 | 15.40 ± 10.50 | 13.50 ± 9.70 | 16.50 ± 12.50 | 13.20 ± 9.10 | 11.30 ± 6.00 |
| Standard | 10.60 ± 6.40 | 13.00 ± 9.20 | 13.20 ± 9.70 | 16.50 ± 11.10 | 14.80 ± 10.00 | 11.50 ± 9.40 | |
| tmax (hours) | Intensified | 3.80 ± 2.70 | 3.50 ± 2.88 | 3.30 ± 2.96 | 3.29 ± 3.66 | 3.75 ± 2.56 | 5.05 ± 3.30 |
| Standard | 3.30 ± 2.59 | 3.02 ± 2.62 | 2.86 ± 2.37 | 3.57 ± 3.12 | 3.19 ± 2.38 | 3.14 ± 2.37 | |
| Cavg (mg/L) | Intensified | 3.75 ± 1.31b | 3.57 ± 1.45c | 3.47 ± 1.44c | 3.78 ± 1.83 | 3.07 ± 1.19 | 3.06 ± 1.02 |
| Standard | 2.72 ± 1.56b | 2.61 ± 1.54c | 2.63 ± 1.31c | 3.12 ± 1.53 | 3.55 ± 1.72 | 3.36 ± 2.00 | |
P < 0.001;
P < 0.005;
P < 0.05;
P < 0.01.
Figure 2.
(A through D) AUC for total MPA (A), fMPA (B), and metabolites (C and D) over time in patients who were treated with intensified versus standard dosing regimens of EC-MPS. Exposure to MPA was significantly higher in the intensified dosing group in the early posttransplantation period. Furthermore, significantly higher levels of fMPA and MPAG and ac-MPAG were observed in the intensified dosing group in the early posttransplantation period. Pharmacokinetic population (n = 65).
Table 3.
AUC for MPA according to the proposed therapeutic window (pharmacokinetic population; n = 65)
| Study Visit | Intensified Dosing Group MPA-AUC (mg/h per L) |
Standard Dosing Group MPA-AUC (mg/h per L) |
||||
|---|---|---|---|---|---|---|
| <30 | 30 to 60 | >60 | <30 | 30 to 60 | >60 | |
| Day 3 | 6 (18.2%) | 20 (60.6%) | 7 (21.2%) | 16 (59.3%) | 9 (33.3%) | 2 (7.4%)a |
| Day 10 | 7 (23.3%) | 18 (60.0%) | 5 (16.7%) | 13 (54.2%) | 9 (37.5%) | 2 (8.3%)b |
| Day 21 | 6 (24.0%) | 16 (64.0%) | 3 (12.0%) | 11 (44.0%) | 13 (52.0%) | 1 (4.0%) |
| Day 42 | 9 (34.6%) | 9 (34.6%) | 8 (30.8%) | 9 (42.9%) | 9 (42.9%) | 3 (14.3%) |
| Day 56 | 8 (34.8%) | 14 (60.9%) | 1 (4.3%) | 3 (16.7%) | 13 (72.2%) | 2 (11.1%) |
| Day 84 | 5 (26.3%) | 13 (68.4%) | 1 (5.3%) | 6 (40.0%) | 6 (40.0%) | 3 (20.0%) |
P < 0.01;
P < 0.05 (χ2 test).
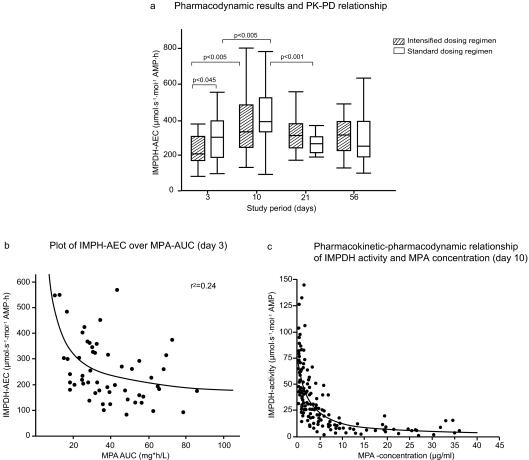
Analysis of pharmacodynamic parameters after transplantation is shown in Table 4. The intensified dosing regimen resulted in lower IMPDH activity on days 3 and 10 compared with standard dosing; however, this difference was significant only on day 3. The IMPDH-AEC over time is shown in Figure 3A. IMPDH-AEC significantly increased on day 10 compared with day 3 in both dosing groups, whereas the between-group difference remained similar (Table 4). On day 21, IMPDH-AEC decreased to values that were seen on day 3. The relationship between IMPDH-AEC and MPA-AUC was best described with an inverse function (r2 = 0.276; P < 0.0001; Figure 3B). IMPDH-AEC also correlated poorly with free MPA-AUC (r2 = 0.127). Estimated Emax values corresponding to the enzyme activity in absence of the inhibitor were 78.7 μmol/s per mol AMP (95% confidence interval [CI] 58.2 to 98.9) on day 3, 82.9 μmol/s per mol AMP (95% CI 70.3 to 95.5) on day 10, and 75.4 μmol/s per mol AMP (95% CI 62.5 to 88.2) on day 21 and similar to pretransplantation baseline IMPDH activity in the intensified and standard dosing groups (83.5 ± 44.1 [n = 32] versus 81.4 ± 34.6 μmol/s per mol AMP [n = 27], respectively). No difference was found in calculated EC50 values during the first 3 weeks (day 3: 1.24 mg/L [95% CI 0.59 to 1.89]; day 10: 1.76 mg/L [95% CI 1.06 to 2.97]; and day 21: 1.44 mg/L [95% CI 0.89 to 2.0]). The plot of IMPDH activity over MPA concentration on day 10 is shown in Figure 3C.
Table 4.
Pharmacodynamic parameters for IMPDH in patients who were treated with intensified versus standard dosing regimens of EC-MPS (pharmacokinetic population; n = 65)
| IMPDH | Regimen | Study Visit (Arithmetic Mean ± SD) |
|||
|---|---|---|---|---|---|
| Day 3 (30/27) | Day 10 (29/27) | Day 21 (25/20) | Day 56 (19/16) | ||
| AEC (μmol/s per mol AMP*h) | Intensified | 233 ± 103a | 371 ± 161 | 326 ± 111 | 314 ± 99 |
| Standard | 297 ± 132 | 421 ± 158 | 270 ± 89 | 291 ± 142 | |
| Amin (μmol/s per mol AMP) | Intensified | 8.1 ± 5.3 | 10.1 ± 7.1 | 9.8 ± 6.5 | 8.2 ± 5.3 |
| Standard | 7.0 ± 3.8 | 10.4 ± 7.2 | 6.6 ± 4.0 | 7.1 ± 5.2 | |
| Amax (μmol/s per mol AMP) | Intensified | 44.0 ± 22.4 | 62.6 ± 30.7 | 53.4 ± 20.7 | 54.0 ± 21.3 |
| Standard | 56.6 ± 30.0 | 66.8 ± 28.6 | 49.2 ± 16.2 | 46.9 ± 20.0 | |
| Aavg (μmol/s per mol AMP) | Intensified | 19.4 ± 8.6 | 35.0 ± 13.2 | 27.1 ± 9.2 | 26.1 ± 8.2 |
| Standard | 24.8 ± 11.0 | 30.9 ± 13.4 | 22.5 ± 7.4 | 24.2 ± 11.9 | |
P < 0.05.
Figure 3.
(A) Pharmacodynamic results and pharmacodynamic–pharmacokinetic relationship. Reduced IMPDH activity was observed in intensified dosing regimen in the early posttransplantation period compared with that from the standard dosing regimen. (B) Plot of the IMPDH-AEC over the MPA-AUC on day 3. The association of IMPDH-AEC over MPA-AUC was best described by an inverse function (r2 = 0.24); however, the relationship was weak, and it might be concluded that the IMPDH-AEC was not the ideal derived pharmacodynamic parameter. (C) Pharmacokinetic–pharmacodynamic relationship of IMPDH activity and MPA concentration on day 10. The relationship between MPA concentration and IMPDH activity at day 10 was explored by modeling pharmacodynamic parameters Amax, Amin, and Aavg with the corresponding pharmacokinetic parameters Cmin, Cmax, and Cavg per patient.
Efficacy Evaluations
During the 6-month study period, a total of six (8%) of 75 patients experienced BPAR (standard dosing group five [13.5%] of 37 versus intensified dosing group one [2.6%] of 38). BPARs were grade 1A (n = 5) or 1B (n = 1) according to the Banff classification (18). Pharmacokinetic and pharmacodynamic data were available only for four patients with BPAR. For two patients from the standard dosing group (BPAR on days 14 and 26), MPA-AUC was ≤30 mg/h per L in the first 3 weeks after transplantation. The MPA-AUC of a third patient from this group was 51 mg/h per L on day 3, and BPAR was suspected on day 4. All three patients who developed BPAR in the first month had high IMPDH activity (above median) before rejection. Two patients had IMPDH-AECs in the upper quartile on day 10. In the intensified dosing group, the patient with BPAR had three valid profiles during the first weeks, and MPA-AUC was always >30 mg/h per L. BPAR was first suspected in this patient on day 101, and on day 84 the MPA-AUC was 42 mg/h per L. In contrast to a strong inhibited IMPDH activity on day 3 (lowest quartile), this patient had a high IMPDH-AEC (in the highest quartile) at the last determination before experiencing rejection (day 56).
No graft losses occurred in either treatment group. One patient from the intensified group died from hemorrhagic shock during the study. This was caused by blood leak at the anastomosis (unrelated to study medication). Estimated mean creatinine clearances (last observation carried forward; Cockcroft-Gault formula [21]) at month 6 were similar in both groups (intensified 58.5 ± 23.2 ml/min versus standard 49.4 ± 23.4 ml/min).
Safety Evaluations
All patients in both groups reported comparable AEs (Table 5). Hematologic AEs and infections occurred in a similar percentage of patients from both dosing groups. CMV infections were infrequent as a result of prophylaxis but were numerically greater in the standard dosing group. Only one patient (from the intensified dosing group) had documented BK infection. Gastrointestinal symptoms occurred in a higher proportion of patients who received the intensified dosing regimen (74 versus 65%). The number of serious AEs (SAEs) was similar for both groups. Gastrointestinal SAEs occurred in five (13.2%) of 38 patients in the intensified dosing group and four (10.8%) of 37 of those in the standard group. Infection SAEs occurred in two (5.3%) of 38 patients in the intensified dosing group and in one (2.7%) of 37 of those from the standard group. The safety laboratory results (including hematology and liver function tests) were similar between groups.
Table 5.
AEs that occurred in ≥5% patients, regardless of relationship to study medication (safety population)
| System Organ Class, Preferred Term | Number of AEs (% Patients) |
||
|---|---|---|---|
| Intensified Regimen (n = 38) | Standard Regimen (n = 37) | Total (N = 75) | |
| Patients with ≥1 AE | 38 (100) | 37 (100) | 75 (100) |
| Blood and lymphatic system disorders | |||
| total no. of AEs (n [% all AEs]) | 26 (6) | 21 (5) | 47 (5.5) |
| total no. of patients with AEs | 17 (46) | 16 (42) | 33 (44) |
| anemia | 8 (21) | 10 (27) | 18 (24) |
| leukocytosis | 2 (5) | 1 (3) | 3 (4) |
| leukopenia | 8 (21) | 8 (22) | 16 (21) |
| thrombocytopenia | 3 (8) | 0 (0) | 3 (4) |
| Gastrointestinal disorders | |||
| total no. of AEs (n [% all AEs]) | 76 (18) | 58 (14) | 134 (16) |
| total no. of patients with AEs | 28 (74) | 24 (65) | 52 (69) |
| abdominal pain | 8 (21) | 4 (11) | 12 (16) |
| constipation | 10 (26) | 11 (30) | 21 (28) |
| diarrhea | 16 (42) | 13 (35) | 29 (39) |
| flatulence | 8 (21) | 2 (5) | 10 (13) |
| gingival hyperplasia | 2 (5) | 0 (0) | 2 (3) |
| nausea | 12 (32) | 11 (30) | 23 (31) |
| vomiting | 7 (18) | 9 (24) | 16 (21) |
| Infections and infestations | |||
| total no. of AEs (n [% all AEs]) | 62 (14) | 65 (15) | 127 (15) |
| total no. of patients with AEs | 25 (66) | 31 (84) | 56 (75) |
| BK virus | 1 (3) | 0 | 1 (1) |
| CMV | 1 (3) | 4 (11) | 5 (7) |
Discussion
The majority of patients in the intensified EC-MPS dosing regimen reached target MPA exposure (≥40 mg/h per L) on day 3 after transplantation. Although intensified dosing increased the percentage of adequately exposed patients, a significant number (20 to 30%) remained under- or overexposed. An intensified dosing regimen may also have potential drawbacks: It increases the risk for overexposure and toxicity if the starting dosage is either too high or given for too long. However, the protocol-driven dosage tapering used here was sufficient to avoid overexposure in the vast majority of patients. EC-MPS was generally well tolerated during the higher dosage phases, and the majority of patients stayed on the intensified dosing scheme.
Previous studies that used higher MMF dosages in combination with CsA (4,22–25) or standard dosages in combination with tacrolimus (15,26) reported similar MPA exposure (30 to 60 mg/h per L). Median MPA-AUC was only 33.7 mg/h per L on day 14 in the Apomygre study, and levels >40 mg/h per L were not achieved until month 1 using a concentration-controlled approach (12). Similarly, mean MPA-AUC of CsA-treated patients on day 10 was 34.4 mg/h per L in the Fixed-Dose Concentration-Controlled (FDCC) Study (13). The authors concluded that higher MPA dosages seemed to be necessary to reach early target AUC in CsA-treated populations (13).
Another potential problem for successful MPA therapeutic drug monitoring (TDM) is limited dosage proportionality for MPA early after engraftment (11). In this study, EC-MPS dosage was doubled but MPA exposure increased only by 50%, which may explain some of the problems encountered with TDM. Although it is obvious that an initially intensified dosing regimen is needed to achieve adequate MPA exposure in the first week after transplantation in CsA-treated patients, intensified MPA dosing schemes and TDM are not necessarily mutually exclusive but may well complement each other.
We found large interpatient variability of IMPDH activity before and after transplantation. Intensified dosing resulted in stronger inhibition of IMPDH activity in the first week, which could be an important determinant of outcome (27,28). Although there was a statistically significant difference in IMPDH-AEC between days 3 and 10, the EC50 values remained stable and Amax was comparable to baseline pretransplantation IMPDH activity. We assume that the changes in IMPDH-AEC despite comparable MPA-AUC might be related to decreasing fMPA and ac-MPAG from days 3 to 10. IMPDH activity at Cmin (= Amax) below EC50 reflects more the interpatient variability of IMPDH without MPA; therefore, it is not clear whether AEC is the ideal parameter to describe the pharmacodynamics of MPA or whether other parameters (e.g., Amin, Amax, percentage inhibition, area under a certain threshold) might be more suitable. An important finding of the pharmacodynamic modeling with the inhibitory Emax model is that the maximal inhibition of IMPDH occurred at low drug concentrations, which were significantly lower than those targeted in clinical practice. EC50 values ranged between 1.24 and 1.76 μg/ml, which has important implications for our understanding of MPA therapy. As a consequence, Cmax values, which were >10 μg/ml, may not be important at all, because the IMPDH enzyme is already completely inhibited at much lower MPA concentrations. Thus, Cmin and Cavg, which displayed significant differences between treatment groups, are potential pharmacokinetic parameters of greater importance.
On the basis of the data presented here, an intensified regimen of EC-MPS given in combination with CsA leads to a significantly higher MPA exposure and significantly stronger inhibition of IMPDH early after transplantation. These results support the overall hypothesis that early adequate MPA exposure in renal transplant recipients can be achieved with a higher starting dosage and are consistent with findings from previous studies (4,7,12); however, data from larger studies are needed to confirm whether the pharmacokinetic and pharmacodynamic differences reported here can be translated into improvements in efficacy and safety.
Disclosures
This study was sponsored by Novartis Pharma (Nuremberg, Germany). P.G. receives speaker fees from Novartis; W.A. receives speaker fees from Novartis and is a member of a Novartis advisory board; C.S., M.S., and M.Z. receive research funding from Novartis; K.B. receives research funding and honoraria from Novartis. S.K., E.-M.V., and W.F. are full-time employees of Novartis; K.B., W.A., and C.S. also receive funding from other companies that are unrelated to this study; T.A. has no financial relationship with Novartis or with any other company.
Acknowledgments
Dr. Debra Brocksmith (Brocksmith Scientific Ltd, UK) provided assistance in preparing this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Salvadori M, Holzer H, de Mattos A, Sollinger H, Arns W, Oppenheimer F, Maca J, Hall MERL B301 Study Group: Enteric-coated mycophenolate sodium is therapeutically equivalent to mycophenolate mofetil in de novo renal transplant patients. Am J Transplant 4: 231–236, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Budde K, Curtis J, Knoll G, Chan L, Neumayer HH, Seifu Y, Hall MERL B302 Study Group: Enteric-coated mycophenolate sodium can be safely administered in maintenance renal transplant patients: results of a 1-year study. Am J Transplant 4: 237–243 2004 [DOI] [PubMed] [Google Scholar]
- 3.Arns W, Breuer S, Choudhury S, Taccard G, Lee J, Binder V, Roettele J, Schmouder R: Enteric coated mycophenolate sodium delivers bioequivalent MPA exposure compared with mycophenolate mofetil. Clin Transplant 19: 199–206, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Van Gelder T, Hilbrands LB, Vanrenterghem Y, Weimar W, de Fijter JW, Squifflet JP, Hené RJ, Verpooten GA, Navarro MT, Hale MD, Nicholls AJ: A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation 68: 261–266, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Hale MD, Nicholls AJ, Bullingham RE, Hene R, Hoitsma A, Squifflet JP, Weimar W, Vanrenterghem Y, Van de Woude FJ, Verpooten GA: The pharmacokinetic-pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin Pharmacol Ther 64: 672–683, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Weber LT, Shipkova M, Armstrong VW, Wagner N, Schütz E, Mehls O, Zimmerhackl LB, Oellerich M, Tönshoff B: The pharmacokinetic-pharmacodynamic relationship for total and free mycophenolic acid in pediatric renal transplant recipients: A report of the German Study Group of Mycophenolate Mofetil Therapy. J Am Soc Nephrol 13: 759–768, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Kiberd BA, Lawen J, Fraser AD, Keough-Ryan T, Belitsky P: Early adequate mycophenolic acid exposure is associated with less rejection in kidney transplantation. Am J Transplant 4: 1079–1083, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Van Gelder T, Le Meur Y, Shaw LM, Oellerich M, DeNofrio D, Holt C, Holt DW, Kaplan B, Kuypers D, Meiser B, Toenshoff B, Mamelok RD: Therapeutic drug monitoring of mycophenolate mofetil in transplantation. Ther Drug Monit 28: 145–154, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Knight SR, Morris PJ: Does the evidence support the use of mycophenolate mofetil therapeutic drug monitoring in clinical practice? A systematic review. Transplantation 85: 1675–1685, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Jeong H, Kaplan B: Therapeutic monitoring of mycophenolate mofetil. Clin J Am Soc Nephrol 2: 184–191, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Staatz CE, Tett SE: Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin Pharmacokinet 46: 13–58, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Le Meur Y, Büchler M, Thierry A, Caillard S, Villemain E, Lavaud S, Etienne I, Westeel PF, Hurault de Ligny B, Rostaing L, Thervet E, Szelag JC, Rérolle JP, Rousseau A, Touchard G, Marquet P: Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant 7: 1–8, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Van Gelder T, Silva HT, de Fijter JW, Budde K, Kuypers D, Tyden G, Lohmus A, Sommerer C, Hartmann A, Le Meur Y, Oellerich M, Holt DW, Tönshoff B, Keown P, Campbell S, Mamelok RD: Comparing mycophenolate mofetil regimens for de novo renal transplant recipients: The Fixed-Dose Concentration-Controlled Trial. Transplantation 86: 1043–1051, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Kuypers DR, Vanrenterghem Y, Sqifflet JP, Mourad M, Abramowicz D, Oellerich M, Armstrong V, Shipkova M, Daems J: Twelve-month evaluation of the clinical pharmacokinetics of total and free mycophenolic acid and its gluronide metabolites in renal allograft recipients on low dose tacrolimus in combination with mycophenolate mofetil. Ther Drug Monit 25: 609–622, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Van Hest R, Mathot R, Vulto A, Weimar W, Van Gelder T: Predicting the usefulness of therapeutic drug monitoring of mycophenolic acid: A computer simulation. Ther Drug Monit 27: 163–167, 2005 [DOI] [PubMed] [Google Scholar]
- 16.West-Thielke P, Kaplan B: Therapeutic monitoring of mycophenolic acid: Is there clinical utility? Am J Transplant 7: 2441–2442, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Bullingham RE, Nicholls AJ, Kamm BR: Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet 34: 429–455, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Racusen LC, Halloran PF, Solez K: Banff 2003 meeting report: New diagnostic insights and standards. Am J Transplant 4: 1562–1566, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Shipkova M, Schütz E, Armstrong VW, Niedmann PD, Oellerich M, Wieland E: Determination of the acyl glucuronide metabolite of mycophenolic acid in human plasma by HPLC and Emit. Clin Chem 46: 365–372, 2000 [PubMed] [Google Scholar]
- 20.Glander P, Sombogaard F, Budde K, van Gelder T, Hambach P, Liefeldt L, Lorkowski C, Mai M, Neumayer HH, Vulto AG, Mathot RA: Improved assay for the nonradioactive determination of inosine 5′-monophosphate dehydrogenase activity in peripheral blood mononuclear cells. Ther Drug Monit 31: 351–359, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–34, 1976 [DOI] [PubMed] [Google Scholar]
- 22.Sollinger HW: Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. Transplantation 60: 225–232, 1995 [DOI] [PubMed] [Google Scholar]
- 23.European Mycophenolate Mofetil Study Group: Placebo-controlled study of mycophenolate mofetil combined with cyclosporin and corticosteroids for prevention of acute rejection. Lancet 345: 1321–1325, 1995 [PubMed] [Google Scholar]
- 24.The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group: A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation 61: 1029–1037, 1996 [PubMed] [Google Scholar]
- 25.Pallet N, Anglicheau D, Martinez F, Mamzer M-F, Legendre C, Thervet E: Comparison of sequential protocol using basiliximab versus antithymocyte globulin with high-dose mycophenolate mofetil in recipients of a kidney graft from an expanded-criteria donor. Transplantation 81: 949–952, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kuypers DR, de Jonge H, Naesens M, de Loor H, Halewijck E, Dekens M, Vanrenterghem Y: Current target ranges of mycophenolic acid exposure and drug-related adverse events: A 5-year, open-label, prospective, clinical follow-up study in renal allograft recipients. Clin Ther 30: 673–683, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Glander P, Hambach P, Braun K-P, Fritsche L, Glessing M, Mai I, Einecke G, Waiser J, Neumayer HH, Budde K: Pre-transplant inosine monophosphate dehydrogenase activity is associated with clinical outcome after renal transplantation. Am J Transplant 4: 2045–2051, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Sombogaard F, Mathot R, Le HY, Glander P, Weimer W, Van Gelder T: IMPDH activity on day 6 after kidney transplantation is significantly related to the risk of acute rejection in MMF treated patients [Abstract]. Am J Transplant 8[ Suppl 2]: 280, 2008 [Google Scholar]