Abstract
Background and objectives: Long-term contact with mercury may induce membranous nephropathy (MN); however, the clinical pathologic features and pathogenesis of mercury-induced MN have not been investigated.
Design, setting, participants, & measurements: The present study retrospectively evaluated 11 cases of mercury-induced MN to analyze its causes and its clinical and pathologic features.
Results: A total of 10 women and 1 man ages 15 to 45 years were enrolled in the present study. Mercury exposure was caused by mercury-containing pills (five patients), skin lightening cream (four patients), hair-dyeing agents (one patient), and mercury vapor (one patient). The duration of contact with mercury ranged from 2 to 60 months, and the urinary mercury concentrations were 1.5 to 50 times higher than reference values. All patients presented with proteinuria and normal renal function; three had nephrotic syndrome. Light microscopy revealed thickened glomerular basement membrane and mildly proliferative mesangial cells. Acute tubulointerstitial injury occurred in three patients. The immunofluorescence findings showed granular deposits of IgG and C3 along the glomerular capillary wall, mostly accompanied by deposits of C4 and C1q. IgG1 and IgG4 (predominantly IgG1) deposits were observed along the glomerular capillary loops. Nine patients reached complete remission in follow-up after withdrawal from mercury exposure.
Conclusions: Deposits of IgG1 subclasses in renal tissues indicated that the pathogenesis of mercury-induced MN differs from that of idiopathic MN. It is important that clinicians are aware that mercury exposure should be considered a possible cause of membranous nephropathy.
Membranous nephropathy (MN), a disease characterized by an accumulation of immune deposits on the outer aspect of the glomerular basement membrane, is the common cause of idiopathic nephrotic syndrome in adults (1). MN may be classified as an idiopathic type and a secondary type associated with other clinical conditions or diseases, which include infections, autoimmune diseases, toxin or drugs, cancer, etc. (2).
Mercury-containing compounds have historically been used in dental amalgams, Chinese traditional medicines, and skin-lightening creams. Mercury can be absorbed into the human body by inhalation, ingestion, or intact skin. It has toxicities for kidneys, nerves, and gastrointestinal tracts. Some literature reported that exposure to mercury caused acute and chronic renal lesions (3). Long-term use of mercury-containing skin lightening cosmetics or occupational contact with mercury caused MN in sporadic cases (4–6). This study evaluated 11 cases of MN diagnosed by renal biopsy that were associated with chronic mercury exposure, to analyze its clinical and pathologic features, as well as the relationship between them to investigate the pathogenesis for better understanding of the mercury-induced MN.
Materials and Methods
Patient Selection
We retrospectively analyzed 11 cases of MN diagnosed by renal biopsy in our hospital from June 2004 to June 2008. The selected cases were required to meet the following criteria: (1) The histologic and immunopathologic changes of the patient were consistent with those of MN. (2) The patient had a clear history of contact with mercury-containing preparations or staying in a mercury-containing environment. (3) The patient had no history of renal diseases or abnormal urinary tests before mercury exposure. (4) Systemic lupus erythematosus, hepatitis B, hepatitis C, and tumor associated with MN were excluded; the patients who had a history of rheumatoid arthritis had no evidence of prior proteinuria before mercury exposure. No nonsteroidal anti-inflammatory agents, gold preparations, or penicillamine was ever used. (5) Quantification of urinary mercury concentration was >8 μg/L, and there was resolution of proteinuria after cessation of exposure to mercury.
Data Collection
General Information.
Gender, age, duration of contact with mercury-containing preparations, the duration of renal diseases, hypertension, ultrasound of bilateral kidneys, and extrarenal presentations were recorded.
Urinalysis.
Hematuria under light microscope was defined as a red blood cell count >10,000/ml in urinary sediment. Proteinuria was defined as urine protein >0.4 g/24 hours. The urinary N-acetyl-pd glucosaminidase enzyme (NAG) enzyme was normal when it was less than 16.5 U/g per creatinine. The urinary retinol-binding protein (RBP) was normal when it was less than 0.5 mg/dl. The normal urinary osmolality was higher than 800 mOsm/kg H2O after fasting.
Blood Tests.
Hemoglobin, serum creatinine, urea nitrogen, blood uric acid, albumin, and globulin were recorded. Antinuclear antibody (ANA), anti-double-stranded DNA antibody, rheumatoid factors (RFs), anti-ribonuclear protein antibody, anti-SSA antibody, anti-SSB antibody, anticardiolipin antibody, complements, and immunoglobulins were also recorded.
Quantification of Urinary Mercury.
The normal concentration was less 8 μg/L by using the cold vapor atomic fluorescence spectrometry.
Pathology.
A percutaneous renal biopsy was performed under the guidance of the ultrasound. Each renal sample contained more than 10 glomeruli. The procedure of renal biopsy was specified as follows: the samples were embedded in paraffin and sectioned at 2 μm, followed by hematoxylin-eosin, Masson, periodic acid-Schiff, and periodic acid-silver methenamine (PASM) staining. For immunofluorescence staining, the samples were sectioned in frozen conditions, followed by staining for IgG, IgA, IgM, C3, C4, and C1q. The deposits, staining intensity, and distribution of various immunoglobulins and complements were observed. The immunofluorescence intensity of immunoglobulins and complements was graded as negative, 1+, or 2+. The electron microscopy observations were done with the Hitachi 7500 electron microscope after routine sections from renal tissues, followed by double staining with uranyl acetate and lead citrate.
Deposits of IgG Subclass.
Indirect immunofluorescence assay was adopted as previously reported. The slides were prepared in frozen conditions and dried, sealed with 10% fetal serum, and rinsed with PBS for 5 minutes. The primary antibodies, including mouse anti-human IgG1 monoclonal antibody (clone 8c/6-39; Sigma-Aldrich), mouse anti-human IgG3 monoclonal antibody (clone HP-6050; Sigma-Aldrich), and mouse anti-human IgG4 monoclonal antibody (clone HP-6025; Sigma-Aldrich), diluted at 1:400, were added respectively before culturing for 2 hours. After the slides were rinsed with PBS for 5 minutes, the FITC-labeled rabbit anti-mouse IgG secondary antibody (1:50; DAKO) was added before culturing for 30 minutes. The slides were dried and sealed with glycerin for observation under the fluorescence microscope. The immunofluorescence intensity of IgG subtypes was graded as negative, 1+, or 2+.
Results
General Information
The present research enrolled 10 women and one man ranging in age from 15 to 45 years. The duration of contact with mercury ranged from 2 to 60 months, and the urinary mercury concentration ranged from 12 to 400 μg/L after hospitalization. See Table 1.
Table 1.
General clinical characteristics and etiology in patients of mercury-induced MN
| Case | Gender | Age, yr | Primary Disease | Etiology of Mercury Poisoning | Duration of Mercury Exposure, mo | Urinary Mercury Concentration, μg/L | Duration of Renal Symptoms, mo |
|---|---|---|---|---|---|---|---|
| 1 | F | 24 | rheumatoid arthritis | antirheumatoid pill | 60 | >400 | 12 |
| 2 | F | 45 | rheumatoid arthritis | antirheumatoid pill | 6 | 200 | 7 |
| 3 | F | 42 | rheumatoid arthritis | antirheumatoid pill | 5 | 48 | 2 |
| 4 | M | 15 | still disease | antirheumatoid pill | 60 | 22 | 2 |
| 5 | F | 37 | no | skin-lightening cream | 12 | 100 | 6 |
| 6 | F | 39 | no | skin-lightening cream | 8 | 12 | 1 |
| 7 | F | 33 | no | skin-lightening cream | 4 | 120 | 4 |
| 8 | F | 43 | no | skin-lightening cream | 8 | 18 | 3 |
| 9 | F | 38 | no | mercury-containing hair dye | 36 | 27 | 12 |
| 10 | F | 30 | tuberculosis of breast | mercury-containing drugs | 2 | >400 | 2 |
| 11 | F | 18 | no | mercury vapor | 7 | 35 | 3 |
The causes of 11 cases of mercury-induced MN are listed in Table 1. Three patients with rheumatoid arthritis (who met American College of Rheumatology criteria for rheumatoid arthritis) and one patient with still disease were medicated with the “antirheumatoid pill” prepared by a hospital; the routine urine test results were normal before medication. One course of medication included 120 pills; laboratory analyses indicated that the pills contained mercury; six pills dissolved in 100 ml of water amounted to a concentration higher than 0.4 mg/L mercury. Four instances involved use of skin-lightening cream prepared by a beauty salon; the cream was confirmed to contain 0.8% mercury by weight. One instance involved administration of mercury-containing drugs (confirmed by the urinary mercury concentration) for tuberculosis of breast. One instance resulted from contact with mercury-containing hair-dyeing agents (confirmed by the urinary mercury concentration). One patient worked in a fluorescence light bulb factory and had contact with mercury vapor for 7 months.
Clinical Presentations
Five patients had red needlepoint-like skin rash with mild pruritus after administration or contact with mercury-containing compounds, of whom two patients used skin-lightening cream, two patients were administered the antirheumatoid pill, and one patient had contact with hair-dyeing agents. A patient using skin-lightening cream apparently suffered from a sleep disorder. One patient with tuberculosis of the breast had an apparent alanine aminotransferase/aspartate aminotransferase increase (no history of receiving antituberculosis antibiotics, such as rifampin). The other patients had no nervous system symptoms, such as apparent abnormal feeling, convulsion, tremor, etc. No patients had dental ulcer, alopecia, or digestive or respiratory irritation. Apparent anemia occurred in two female patients with rheumatoid arthritis; normal peripheral blood white cell count and platelet count were observed in all patients. Four patients were ANA-positive. All patients were negative for anti-double-stranded DNA, anti-SSA, anti-SSB, and anti-ribonuclear protein antibody. Two patients with rheumatoid arthritis were rheumatoid factor-positive. Normal C3 and C4 were noted in all patients.
All patients initially reported edema and proteinuria. No microscopic or macroscopic hematuria was observed. No patients had hypertension. The serum creatinine was normal in all patients. Nine patients had hypoalbuminemia; three patients had nephrotic syndrome. The urinary protein spectrum indicated that low-molecular weight proteins existed in nine patients, in two of whom low-molecular weight proteins accounted for more than 10%. All patients had elevated a urinary NAG enzyme content. Increased urinary RBP was observed in two patients. The urinary osmolality in nine patients was lower than the normal range. No urinary glucose or amino acid was found in any patients (Table 2).
Table 2.
Clinical findings at biopsy and follow-up in patients of mercury-induced MN
| Case | Hb, g/dl | BUN, mg/dl | SCr, mg/dl | UA, μmol/L | Alb, g/L | Glo, g/L | TC, mmol/L | TG, mmol/L | Urinary Protein, g/24 h | Urinary NAG, U/g per creatinine | Urinary RBP, mg/L | Osmolality, mOsm/kg H2O | ANA | Treatment | Follow-Up, mo | Last Follow-Up |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urinary Protein, g/24 h | Alb, g/L | SCr, mg/dl | ||||||||||||||||
| 1 | 9.7 | 6.35 | 0.41 | 244 | 27.5 | 24.8 | 4.03 | 1.29 | 2.08 | 40.4 | 0.38 | 594 | 1:128 | DMPS | 48 | 0.18 | 45.1 | 0.50 |
| 2 | 9.0 | 28.2 | 0.70 | 180 | 31.6 | 17.2 | 5.54 | 0.79 | 1.86 | 42.2 | 0.23 | 541 | N | DMPS | 6 | 0.78 | 38.5 | 0.61 |
| 3 | 13.6 | 13.5 | 0.47 | 257 | 35.5 | 24.3 | 9.78 | 1.83 | 1.2 | 22.2 | 0.6 | 1002 | 1:16 | 30 | 0.11 | 49.0 | 0.52 | |
| 4 | 14.8 | 10.2 | 0.51 | 286 | 42 | 27.5 | 4.79 | 2.24 | 1.49 | 35.3 | 0.21 | 1158 | N | 18 | 0.12 | 43.9 | 0.61 | |
| 5 | 12.8 | 10.5 | 0.56 | 347 | 21.3 | 16.2 | 7.25 | 1.86 | 3.82 | 29.3 | 0.17 | 782 | N | ACEI | 24 | 0.15 | 47.1 | 0.43 |
| 6 | 12.5 | 8.52 | 0.63 | 369 | 29.9 | 23.2 | 3.86 | 2.77 | 3.17 | 46.3 | 0.05 | 772 | 1:512 | ARB | 12 | 0.13 | 41.2 | 0.58 |
| 7 | 12.2 | 7.34 | 0.49 | 213 | 25 | 17.6 | 5.25 | 0.91 | 2.39 | 37.1 | 0.08 | 739 | N | DMPS | 14 | 0.36 | 41.2 | 0.58 |
| 8 | 13.1 | 11.3 | 0.85 | 406 | 26.1 | 15.1 | 5.15 | 0.96 | 3.52 | 27.5 | 0.09 | 666 | N | ARB | 12 | 0.10 | 43.5 | 0.68 |
| 9 | 13.2 | 14.1 | 0.87 | 396 | 34.1 | 22.8 | 5.47 | 1.01 | 1.43 | 23.9 | 0.12 | 667 | 1:4 | ARB | 48 | 0.10 | 45.8 | 0.58 |
| 10 | 12.1 | 8.12 | 0.64 | 348 | 27.2 | 22.8 | 4.4 | 2.17 | 3.1 | 85.5 | 2.77 | 604 | N | DMPS | 36 | 0.10 | 48.6 | 0.64 |
| 11 | 14.1 | 5.23 | 0.51 | 271 | 21.1 | 21.7 | 10.56 | 2.96 | 4.76 | 52.3 | 0.13 | 682 | N | ARB | 6 | 1.78 | 36.9 | 0.46 |
Hb, hemoglobin; BUN, blood urea nitrogen; SCr, serum creatinine; UA, uric acid; Alb, albumin; Glo, globulin; TC, total cholesterol; TG, total triglyceride; ANA, anti-nuclear antibody; NAG, N-acetyl-pd-glucosaminidase enzyme; N, negative; DMPS, sodium dimercaptopropane sulfonate; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Histologic Findings
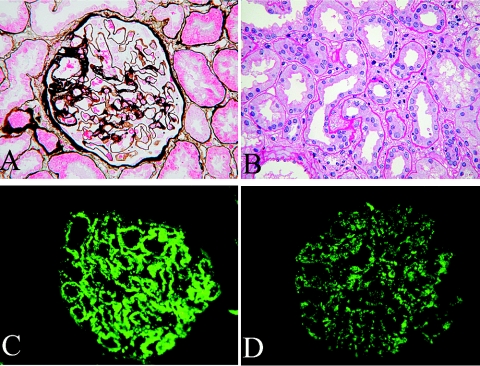
Stiff glomerular peripheral capillary loops, slightly thickened glomerular basement membrane and, occasionally, subepithelial fuchsinophilic deposits on PASM-Masson staining were observed under light microscopy (Figure 1A). Mild proliferation of mesangial cells and matrix was observed. No evidence of fibrinoid necrosis, crescents, or endocapillary proliferation was observed. Patient nos. 1, 2, 3, and 11 had few glomerular infiltrating cells, among which neutrophil granulocytes and monocytes counted for the majority. Observed for patient nos 1, 2, and 10 were acute focal tubulointerstitial injuries, partial loss of the tubular brush border, granular degeneration of epithelial cells, and focal concentration of monocyte and neutrophil granulocyte infiltration (Figure 1B). The other patients had no apparent tubulointerstitial injury. Some patients had hyaline degeneration of afferent arterioles but without infiltration of inflammatory cells.
Figure 1.
Pathologic findings in mercury-induced MN. (A) Thickened glomerular basement membrane, as well as subepithelial fuchsinophilic deposits along the epithelium. Patient no. 2 (PASM staining; magnification, ×400). (B) Loss of the tubular brush border and focal concentration of interstitial infiltrating cells. Patient no. 10 (periodic acid-Schiff staining; magnification, ×400). (C) Deposits of IgG1 in the glomerular basement membrane (2+). Patient no. 2 (immunofluorescence staining; magnification, ×400). (D) Deposits of IgG4 in the glomerular basement membrane (1+). Patient no. 2 (immunofluorescence staining; magnification, ×400).
All patients had granular deposits of IgG and C3 along the capillary loop of glomeruli on immunofluorescence analysis. Additional codeposits of C4 were observed in two patients, whereas additional codeposits of C4 and C1q were observed in another six patients. The finding of positivity for all of IgG, IgA, and IgM, together with C3, C4, and C1q, was present in two patients (See Table 3). Two patients only had deposits of IgG1, whereas there were deposits of both IgG1 and IgG4 (predominantly IgG1) in the others. Two patients showed deposits of IgG1, IgG3, and IgG4 along the capillary loop of glomeruli on immunofluorescence analysis (Figure 1, C and D; Table 3).
Table 3.
Immunofluorescence findings in patients with mercury-induced MN
| Case | IgG | IgA | IgM | C3 | C4 | C1q | IgG1 | IgG3 | IgG4 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1+ | N | N | 2+ | 1+ | 1+ | 2+ | N | 1+ |
| 2 | 2+ | 1+ | 1+ | 1+ | 1+ | 1+ | 2+ | N | 1+ |
| 3 | 2+ | N | N | 2+ | N | N | 2+ | 1+ | 1+ |
| 4 | 2+ | N | N | 2+ | 1+ | N | 2+ | N | 1+ |
| 5 | 2+ | 1+ | 1+ | 2+ | 1+ | 1+ | 2+ | N | N |
| 6 | 2+ | N | N | 2+ | N | N | 1+ | 1+ | 1+ |
| 7 | 2+ | N | N | 1+ | 1+ | 1+ | 2+ | N | 1+ |
| 8 | 2+ | N | N | 2+ | 1+ | N | 2+ | N | 1+ |
| 9 | 2+ | N | N | 1+ | N | N | 2+ | N | N |
| 10 | 2+ | N | N | 2+ | 1+ | 1+ | 2+ | N | 1+ |
| 11 | 2+ | N | N | 1+ | 1+ | 1+ | 2+ | N | 1+ |
N, negative.
Among 11 patients, two were classified as stage I MN, seven were stage II MN, one was stage III MN, and one was stage IV MN according to the electron microscopic finding. Electron microscopy showed numerous scattered subepithelial deposits in all patients; some of the deposits were separated from each other by basement membrane spikes, whereas five patients had electron-dense deposits in the mesangium. Intramembranous and subendothelial deposits were not present. There was extensive effacement of the foot processes of the visceral epithelial cells.
Treatment and Prognosis
All patients abstained from mercury-containing preparations after definitive diagnosis. Four patients with a urinary mercury concentration higher than 100 μg/L were treated with sodium dimercaptopropane sulfonate for mercury detoxification. Five patients were treated with angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker. In follow-up, two patients were lost in 6 months, for whom the urine protein decreased, the serum albumin recovered to a normal range, and the renal functions remain normal. Nine patients reached complete remission in follow-up 1 to 4 years (see Table 2).
Discussion
Mercury-containing compounds have been used commercially and medically for centuries; it was a common constituent of Chinese traditional medicines (7). Long-term mercury poisoning usually damages several systems, and common symptoms include nervous system symptoms, digestive symptoms, respiratory symptoms, and kidney symptoms (3). In this study, a few patients had skin rash, elevated liver enzymes, or insomnia, and the others did not have apparent nervous system symptoms, such as abnormal feeling, convulsion, and tremor, indicating that the majority of the patients' extrarenal organs or systems were not obviously implicated. Epidemiologic data showed that mercury poisoning mainly resulted from occupational contact with mercury, abuse of mercury-containing compounds, or skin-lightening cosmetics (8,9). In recent years, renal diseases from mercury poisoning have apparently decreased in developed countries, but mercury exposure is still not rare in developing countries, which deserves concern. In this study, the mercury poisoning instances mainly were related to mercury-containing skin-lightening cream or the mercury-containing Chinese traditional medicine antirheumatoid pill. The urinary concentrations of mercury in our patients were 1.5 to 50 times higher than reference values for nonexposed populations. The diagnosis of mercury poisoning rests mainly on a history of mercury-containing compound exposure; supporting evidence may be provided by a raised urinary concentration of mercury.
Kidney is the main mercury accumulation and excretion organ. Acute mercury poisoning mainly results in acute tubular necrosis, especially with involvement of the proximal tubules (10), whereas chronic mercury exposure mainly causes immune complex nephritis, especially MN (11). In this study, all patients showed membranous nephropathy in pathologic findings; most of the patients in this study were young and female, which is not consistent with the epidemiologic finding for idiopathic MN (2). It is thus hypothesized that they had secondary MN. In our patients, all patients had long-standing mercury exposure before proteinuria appeared; none of them had any other secondary cause of nephropathy, such as systemic lupus erythematosus, malignancies, or viral hepatitis, and no nonsteroidal anti-inflammatory agent, gold, or penicillamine was ever used. Therefore, membranous nephropathy was probably related to mercury exposure. It must be indicated that three patients had a history of rheumatoid arthritis. Because both rheumatoid arthritis and mercury poisoning may cause MN, whether their MN results from rheumatoid arthritis or mercury poisoning is not clear in light of clinical presentations or pathologic changes. However, considering the fact that no abnormal urine tests existed before administration of the mercury-containing traditional medicine antirheumatoid pill, and that edema, proteinuria, and increased urinary mercury appear within 6 to 60 months of exposure, one is able to relate the MN to chronic mercury poisoning rather than rheumatoid arthritis. In this study, the urinary mercury concentration has predictive values in diagnosis and treatment for chronic mercury poisoning. The patients were in complete remission after withdrawal from mercury exposure or the mere therapy of mercury detoxification without immune suppression, indicating that chronic mercury poisoning is the ultimate cause of MN.
All patients presented normal kidney function and an increased level of urinary NAG enzyme. The NAG enzyme is a lysosomal enzyme that is commonly regarded to be predictive of renal tubular epithelial cell injuries when its level escalates. In the research, the consistent increase of urinary NAG enzyme in all patients may be associated with the direct poisoning of mercury compounds for renal tubular epithelial cells. The urinary NAG enzyme thus tends to be more sensitive than urinary RBP, and it is thus a preferred factor reflecting the intensity of injuries of renal tubular epithelial cells in cases of mercury poisoning (12). Three patients who were recently managed with mercury-containing traditional medicines demonstrated acute focal injuries of renal tubules. The other patients had no apparent tubular interstitial lesions, which is consistent with the finding in a previous study that the interstitial lesions were mild in patients using skin-lightening cosmetics (4).
The immunofluorescence findings showed that eight patients had codeposits of C4 and/or C1q associated with Ig in glomerular deposits. In two patients, a “full house” pattern, which is often observed in lupus nephritis (none of our patients showed any feature of systemic lupus erythematosus at the time of biopsy or follow-up), indicating activation of the classical complement pathway, as opposed to only deposits of IgG and C3 in idiopathic MN. It is considered that the pathogenesis of mercury-induced MN differs from that of idiopathic MN. To further investigate the pathogenesis of mercury-induced MN, the glomerular IgG subclass was observed. The deposits of both IgG1 and IgG4 (predominantly IgG1) were observed for mercury-induced MN, which differed from the feature of deposits of IgG4 in idiopathic MN (13) but was similar to the secondary MN due to malignancy (14). The distribution of IgG subclass varies because of the Th1 and Th2 predominant immune responses: IgG1 and IgG3 are involved in the Th1-type predominant immune response, whereas IgG4 is more active in the Th2-type predominant immune response (15). In idiopathic MN, the Th2-type response predominates and moderates the humoral immune response, whereas the deposits of glomerular both IgG1 and IgG4 in mercury-induced MN indicate that both Th1 and Th2 participate in the immune response. The research on the animal model also demonstrated that Th1 and Th2 were both involved in mercury-induced autoimmunity (16). The distribution of glomerular IgG subclass further confirms the diagnosis of mercury-induced MN rather than idiopathic MN for patients in the research.
The mechanism of mercury-induced MN is still uncertain. In highly susceptible mice, the mercury-induced autoimmune disease is characterized by a T cell-dependent polyclonal B cell activation, by mainly increased serum levels of IgG and IgE antibodies, by the production of ANA, and by the formation of renal immune complex deposits, and thus induced MN in susceptible mice and rats (17–20). In this study, four female patients were ANA-positive, of whom two were rheumatoid arthritis patients, whereas the other two suffered no autoimmune diseases before mercury exposure, implying that mercury exposure might induce autoimmune antibodies. Theoretically, a small fraction of humans may be susceptible to the development of autoimmunity; T cell-dependent polyclonal B cell activation might result in the production of antibodies against membrane proteins of podocyte similar to those in idiopathic MN. Such antibodies render specificity to M-type phospholipase A2 receptor, an antigen normally expressed on the podocyte cell membrane in humans (21). Whether the mercury-induced renal immune complex deposits are formed by accumulation of circulating immune complexes in the kidney or by direct binding of autoantibodies to the self-components of podocytes, then in situ immune complex formation remains to be elucidated.
Conclusions
The clinical and pathologic features of mercury-induced MN are not significantly different from those of idiopathic MN. Because mercury-containing compounds are still widely available in developing countries, it is important that clinicians are aware that mercury exposure should be considered a possible cause of membranous nephropathy, because it is likely to be missed unless specifically enquired for. In clinical practice, it is thus necessary to observe deposits of complements and IgG subclasses, inquire specifically about the history of use of mercury-containing skin lightening cosmetics or traditional medicines, and conduct urine tests in appropriate patients to exclude renal injuries due to mercury exposure. Once a definitive diagnosis is reached, patients should abstain from the mercury exposure, and immune suppression should be carefully avoided.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Li LS, Liu ZH: Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney Int 66: 920–923, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Zeng CH, Chen HM, Wang RS, Chen Y, Zhang SH, Liu L, Li LS, Liu ZH: Etiology and clinical characteristics of membranous nephropathy in Chinese patients. Am J Kidney Dis 52: 691–698, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Clarkson TW, Magos L, Myers GJ: The toxicology of mercury–current exposures and clinical manifestations. N Engl J Med 49: 1731–1737, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Soo YO, Chow KM, Lam CW, Lai FM, Szeto CC, Chan MH, Li PK: A whitened face woman with nephrotic syndrome. Am J Kidney Dis 41: 250–253, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Aymaz S, Gross O, Krakamp B, Ortmann M, Dienes HP, Weber M: Membranous nephropathy from exposure to mercury in the fluorescent-tube-recycling industry. Nephrol Dial Transplant 16: 2253–2255, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Oliveira DBG, Foster G, Savill J, Syme PD, Taylor A: Membranous nephropathy caused by mercury-containing skin lightening cream. Postgrad Med J 63: 303–304, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Shi JZ, Yu LM, Goyer RA, Waalkes MP: Mercury in traditional medicines: Is cinnabar toxicologically similar to common mercurials? Exp Biol Med (Maywood) 233: 810–817, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weldon MM, Smolinski MS, Maroufi A, Hasty BW, Gilliss DL, Boulanger LL, Balluz LS, Dutton RJ: Mercury poisoning associated with a Mexican beauty cream. West J Med 173: 15–18, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sin KW, Tsang HF: Large-scale mercury exposure due to a cream cosmetic: Community-wide case series. Hong Kong Med J 9: 329–334, 2003 [PubMed] [Google Scholar]
- 10.Kanluen S, Gottlieb CA: A clinical pathologic study of four adult cases of acute mercury inhalation toxicity. Arch Pathol Lab Med 115: 56–60, 1991 [PubMed] [Google Scholar]
- 11.Becker CG, Becker EL, Maher JF, Schreiner GE: Nephrotic syndrome after contact with mercury. A report of five cases, three after the use of ammoniated mercury ointment. Arch Intern Med 110: 178–186, 1962 [DOI] [PubMed] [Google Scholar]
- 12.Jarosińska D, Horvat M, Sällsten G, Mazzolai B, Dabkowska B, Prokopowicz A, Biesiada M, Barregård L: Urinary mercury and biomarkers of early renal dysfunction in environmentally and occupationally exposed adults: A three-country study. Environ Res 108: 224–232, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Imai H, Hamai K, Komatsuda A, Ohtani H, Miura AB: IgG subclasses in patients with membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis. Kidney Int 51: 270–276, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Ohtani H, Wakui H, Komatsuda A, Okuyama S, Masai R, Maki N, Kigawa A, Sawada K, Imai H: Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol Dial Transplant 19: 574–579, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Holdsworth SR, Kitching R, Tipping PG: Th1 and Th2 helper cell subsets affect patterns of injury and outcomes in glomerulonephritis1 Kidney Int 55: 1198–1216, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Hu H, Möller G, Abedi-Valugerdi M: Mechanism of mercury-induced autoimmunity: Both T helper 1- and T helper 2-type responses are involved. Immunology 96: 348–357, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua J, Pelletier L, Berlin M, Druet P: Autoimmune glomerulonephritis induced by mercury vapour exposure in the Brown Norway rat. Toxicology 79: 119–129, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Abedi VM, Hu H, Möller G: Mercury-induced renal immune complex deposits in young (NZB x NZW)F1 mice: Characterization of antibodies/autoantibodies. Clin Exp Immunol 110: 86–91, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Häggqvist B, Hultman P: Effects of deviating the Th2-response in murine mercury-induced autoimmunity towards a Th1-response. Clin Exp Immunol 134: 202–209, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Icard P, Pelletier L, Vial MC, Mandet C, Pasquier R, Michel A, Druet P: Evidence for a role of antilaminin-producing B cell clones that escape tolerance in the pathogenesis of HgCl2-induced membranous glomerulopathy. Nephrol Dial Transplant 8: 122–127, 1993 [PubMed] [Google Scholar]
- 21.Beck LH, Jr., Bonegio RGB, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]