Abstract
Background and objectives: Anemia is iron responsive in 30 to 50% of nondialysis patients with chronic kidney disease (CKD), but the utility of bone marrow iron stores and peripheral iron indices to predict the erythropoietic response is not settled. We investigated the accuracy of peripheral and central iron indices to predict the response to intravenous iron in nondialysis patients with CKD and anemia.
Design, setting, participants, & measurements: A diagnostic study was conducted on 100 nondialysis patients who had CKD and anemia and were erythropoiesis-stimulating agent and iron naive. Bone marrow iron stores were evaluated by aspiration. Hemoglobin, transferrin saturation index (TSAT), and ferritin were measured at baseline and 1 month after 1000 mg of intravenous iron sucrose. Posttest predictive values for the erythropoietic response (≥1-g/dl increase in hemoglobin) of peripheral and central iron indices were calculated.
Results: The erythropoietic response was noted in a higher proportion in bone marrow iron-deplete than in iron-replete patients (63 versus 30%). Peripheral iron indices had a moderate accuracy in predicting response. The positive (PPV) and negative predictive values (NPV) were 76 and 72% for a TSAT of 15% and 74 and 70% for a ferritin of 75 ng/ml, respectively. In the final logistic regression model, including TSAT and ferritin, the chances of a positive response increased by 7% for each 1% decrease in TSAT.
Conclusions: Because an erythropoietic response is seen in half of patients and even one third of those with iron-replete stores responded whereas peripheral indices had only a moderate utility in predicting response, the therapeutic trial to intravenous iron seems to be a useful tool in the management of anemia in nondialysis patients with CKD.
Iron deficiency is common, and intravenous iron supplementation is a recognized therapy of anemia in long-term hemodialysis patients (HD), especially in those who are treated with epoetin (1–6). Several studies suggested that iron deficiency—evaluated by bone marrow iron examination—is common also in predialysis patients with chronic kidney diseases (CKD). In the reported series, the prevalence of bone marrow iron depletion varied widely from 23 to 90% (7,8). The role of iron supplementation in nondialysis patients with CKD is much less clear than in HD patients, although studies have reported a positive response to intravenous iron even without concomitant epoetin administration in 38 to 68% of patients (9–12).
The prediction of erythropoietic response is clinically important, because the conventional markers of iron status—serum ferritin level and transferrin saturation index (TSAT)—although simple and relatively inexpensive, are not highly accurate in predicting response to iron in this population (5,7,8,11), and the safety of intravenous iron is a matter of concern in patients with CKD (13,14). Moreover, there are no published data relating iron stores, peripheral iron indices, and the response to intravenous iron. We conducted a study to investigate the accuracy of peripheral and central iron status indices to predict the response to intravenous iron in nondialysis patients who had CKD and anemia and were erythropoiesis-stimulating agent and iron naive.
Materials and Methods
Study Design
This was a single-center diagnostic study with a duration of 3 months. In a 2-month run-in period, renal function, anemia, and the other admission criteria were assessed. At baseline, a bone marrow aspiration was performed, peripheral iron indices were evaluated, and 1000 mg of iron sucrose was administered intravenously in 5 days, irrespective of iron status. One month after the last intravenous iron dose, blood was drawn for hemoglobin (Hb) and peripheral iron index evaluation.
The reference test was the erythropoietic response, defined as a ≥1-g/dl increase in Hb level. Index tests were central (bone marrow iron stores, vide infra) and peripheral iron indices (TSAT and serum ferritin). The study was approved by the Helsinki committee of the hospital.
Selection Criteria
Anemia, defined as a Hb <11.0 g/dl (three measurements in the previous 8 weeks with ≤2.5-g/dl difference between highest and lowest values) and stages 3 through 5 CKD and mean estimated GFR (eGFR; abbreviated Modification of Diet in Renal Disease [MDRD] formula) (15) <60 ml/min per 1.73 m2 (three measurements in the previous 8 weeks each separated by at least 1 week) were the inclusion criteria. Patients with any previous iron or erythropoiesis-stimulating agent therapy, active infectious conditions, cancer, iron overload (ferritin >800 ng/ml), blood transfusions and/or active bleeding within the preceding 3 months, hemolytic anemia, folate or vitamin B12 deficiency, severe malnutrition (Subjective Global Assessment score of C on a scale of A through C) (16), or hypothyroidism were excluded. Similarly, patients with congestive heart failure (New York Heart Association class III or IV), severe uncontrolled high BP (systolic BP >190 mmHg and/or diastolic BP >115 mmHg), active liver disease (more than three times increase in alanine or aspartate amino transferase), uremic complications (pericarditis, polyneuropathy), severe hyperparathyroidism (intact parathyroid hormone >800 pg/ml), severe psychiatric disorders, or known hypersensitivity to any component of iron sucrose as well as those who were participating in other clinical trials or refused to sign an informed consent were not enrolled.
Patients
One hundred adults (age >18 yr) were selected from the patients who were admitted to a nephrology department during a 2-year period (Figure 1).
Figure 1.
Patient selection.
Laboratory Tests
Bone Marrow Parameters of Iron Status.
Bone marrow was collected by aspiration from the sternum. The smears were prepared from marrow fragments, colored with Perls Prussian blue stain, and interpreted by a senior hematologist who had no knowledge of the patients' iron status. Positive and negative controls were used for calibration. Additional slides from aspirate were prepared when necessary, and at least nine bone marrow particles were reviewed for a final diagnosis (17).
The occurrence of siderotic granules in macrophages was graded on a scale from 0 to 6 (18). Patients with a score of 0 or 1 were considered iron deficient, those graded from 2 to 4 as having normal macrophage iron, and those graded from 5 to 6 as having macrophage iron overload.
Erythroblasts with green-blue particles on Perls stain were defined as sideroblasts. According to the number of siderotic granules, sideroblasts were classified as type 0 (no granules), type 1 (one to three granules), or type 2 (three or more granules), and their percentages were computed (19).
The pattern of bone marrow iron distribution was classified as normal (macrophage iron 2 to 4, sideroblasts in normal percentage), iron deficiency (macrophage iron 0 or 1, sideroblasts absent or in a very low percentage), iron overload (macrophage iron ≥5, sideroblasts in normal or increased percentage), and anemia of chronic inflammation (macrophage iron ≥5, sideroblasts absent or in very low percentage). The patients were classified according to bone marrow iron as deplete (bone marrow iron deficiency) or replete (normal iron stores, iron overload, or anemia of chronic inflammation).
Peripheral Parameters of Iron Status.
Serum ferritin and serum transferrin were measured by immunoturbidometric methods (Good Biotech, Taichung, Taiwan; Giesse Diagnostics, Rome, Italy) on an autoanalyzer Olympus AU400. Total serum iron-binding capacity was calculated as serum transferrin × 1.25. Serum iron was assessed by a colorimetric method (Giesse Diagnostics). TSAT was calculated as the percentage of serum iron from total serum iron-binding capacity.
Other Parameters.
C-reactive protein (CRP) was assessed by a high-sensitivity latex immunoturbidometric method (Giesse Diagnostics).
Statistical Analysis
Data are presented as mean ± SD, 95% confidence interval (95% CI), or median and interquartile range, according to their distribution. A t test, paired or not, Mann-Whitney, χ2, or Wilcoxon test was used to compare groups, as required.
Receiver operating characteristic (ROC) curves (20–22) were created using the erythropoietic response as reference test (dichotomous variable) and macrophage iron score, ferritin, and TSAT and their combination as continuous variables. The effect of combining tests (TSAT and ferritin) was tested according to Trieppi et al. (23). The diagnostic accuracy was evaluated by sensitivity and specificity. Posttest positive probability of response in case of a positive test and of a nonresponse in case of a negative test was calculated at various cutoff values (24,25). Because the reported prevalence of nondialysis patients who had CKD and responded to intravenous iron therapy varied between 38 and 68% (9–12), a prevalence of 40% was used to calculate the posttest probability of response. The relationship between the erythropoietic response (present versus absent) and relevant parameters was investigated by multivariable binominal logistic regression.
Considering a probability of 50% for a ≥1-g/dl increase in Hb and a presumed clinically significant difference among groups of 10%, at least 27 patients per group would be required for a 95% probability and a power of 95%. Statistical analyses were performed with Analyze-it (Analyze-it Software, Ltd., Leeds, UK) and SPSS (SPSS Inc., Chicago, IL) packages.
Results
One hundred patients were enrolled and accomplished the study. Median age was 62 years (range 24 to 84); 55% were older than 60 years. There was a slight male preponderance (55%). Vascular nephropathies and primary glomerulonephritis were main causes of CKD, whereas diabetic nephropathy was rare (43, 36, and 3%, respectively). Median eGFR was 14 ml/min per 1.73 m2 (interquartile range 10–24); most patients were in stage 4 or 5 CKD (28 and 56%). Inflammation was highly prevalent, but nutritional status was good, because >60% of patients had a serum CRP >10 mg/L, whereas 65% had a Subjective Global Assessment score of A and 75% a serum albumin >4 g/dl (Table 1).
Table 1.
Characteristics of patients, according to the response to intravenous iron IV (n = 100)
| Characteristic | All (n = 100) | Responders (n = 48) | Nonresponders (n = 52) | Pa |
|---|---|---|---|---|
| Age (years) | ||||
| mean ± SD | 62.0 ± 13.0 | 59.6 ± 13.7 | 63.5 ± 11.9 | 0.1 |
| >60 yr (%) | 55 | 46 | 63 | 0.02 |
| Male gender (%) | 55 | 52 | 58 | 0.6 |
| Primary renal disease (%) | 0.9 | |||
| glomerulonephritis | 36 | 33 | 40 | |
| interstitial nephropathies | 10 | 10 | 17 | |
| ADPKD | 8 | 8 | 8 | |
| vascular disease | 43 | 45 | 31 | |
| diabetic nephropathy | 3 | 4 | 4 | |
| eGFR (ml/min; median [IQR]) | 14 (10–24) | 17 (11–24) | 13 (8–25) | 0.8 |
| CKD stage (%) | 0.06 | |||
| 3 | 18 | 10 | 21 | |
| 4 | 28 | 44 | 23 | |
| 5 | 56 | 46 | 56 | |
| Body mass index (kg/m2) | ||||
| mean ± SD | 24.0 ± 4.0 | 23.6 ± 3.7 | 25.2 ± 4.4 | 0.06 |
| 18.5 to 25.0 (%) | 46 | 46 | 44 | 0.6 |
| Subjective global assessment score A (% patients) | 65 | 62 | 67 | 0.7 |
| Serum albumin (g/dl) | ||||
| mean ± SD | 3.7 ± 0.5 | 3.6 ± 0.5 | 3.7 ± 0.5 | 0.4 |
| serum albumin >4 g/dl (%) | 75 | 77 | 73 | 0.6 |
| CRP (mg/L) | ||||
| median (IQR) | 15 (6–30) | 15 (7–41) | 15 (6–25) | 0.8 |
| >10 mg/L (%) | 60 | 62 | 60 | 0.9 |
| Hb (g/dl) | ||||
| mean ± SD | 8.99 ± 1.07 | 8.97 ± 1.12 | 9.01 ± 1.03 | 0.87 |
| <10 g/dl (%) | 70 | 75 | 68 | 0.2 |
| TSAT (%) | ||||
| median (IQR) | 23 (13–30) | 18 (10–25) | 26 (22–32) | <0.001 |
| <20% | 33 | 50 | 17 | <0.001 |
| Serum ferritin (ng/ml) | ||||
| median (IQR) | 176 (79–300) | 114 (51–197) | 230 (132–359) | <0.001 |
| <100 ng/ml (%) | 31 | 48 | 15 | <0.001 |
| TSAT <20% and ferritin <100 ng/ml (% patients) | 17 | 33 | 2 | <0.001 |
| Macrophage iron score (% patients) | ||||
| 1 | 48 | 63 | 33 | <0.001 |
| 2 | 34 | 31 | 37 | 0.4 |
| ≥3 | 18 | 6 | 29 | <0.001 |
| Bone marrow iron deplete (%) | 48 | 63 | 30 | 0.005 |
| Erythropoietic response (g/dl) | ||||
| Hb (at assessment) | 10.0 ± 1.1 | 10.2 ± 1.0 | 9.7 ± 1.0 | 0.02 |
| ΔHb | 1.0 ± 0.4 | 1.3 ± 0.3 | 0.7 ± 0.2 | <0.001 |
Response defined as ≥1-g/dl increase in Hb. ADPKD, autosomal dominant polycystic kidney disease; IQR, interquartile range.
Iron deplete versus iron replete.
Anemia was severe: the mean Hb was 8.99 g/dl, and in 70% of cases, the initial Hb was <10 g/dl. Bone marrow iron stores were depleted in 48% of patients. Using conventional cutoffs for peripheral iron indices (TSAT <20% and ferritin <100 ng/ml), the proportions of patients below cutoff were 33, 31, and 17% for the combination of tests (TSAT <20% and ferritin <100 ng/ml), respectively.
One month after 1000 mg of intravenous iron, the mean Hb attained was 10.0 g/dl, with a 1.0 ± 0.4-g/dl mean increase. Hb increased by >1 g/dl in 48% of patients. In univariable analysis, the responders were younger and a higher proportion were in stage 4 CKD, but otherwise their general characteristics were similar to those of the nonresponders (Table 1).
A macrophage iron score of ≤1, reflecting deplete iron stores, was more frequent in responders than in nonresponders (63 versus 33%), whereas the opposite was true in the case of patients with bone marrow iron overload (a macrophage iron score ≥3): 6 versus 29%. The proportion of responders was twice as high in patients with iron-deplete versus iron-replete stores (63 versus 30%). Similarly, the peripheral iron indices were different. Median TSAT and ferritin were lower in responders. Using cutoff values of 20% for TSAT and 100 ng/ml for ferritin, the percentages of patients with lower than cutoff values were significantly higher in responders than in nonresponders: 50 versus 17%, 48 versus 15%, and 33 versus 2% for the combination of tests, respectively (Figure 2).
Figure 2.
Percentages of positive peripheral or bone marrow iron tests, according to the response to 1000 mg of intravenous iron (≥1-g/dl increase in Hb) in 100 nondialysis patients with CKD. sFerr, serum ferritin. *P < 0.05.
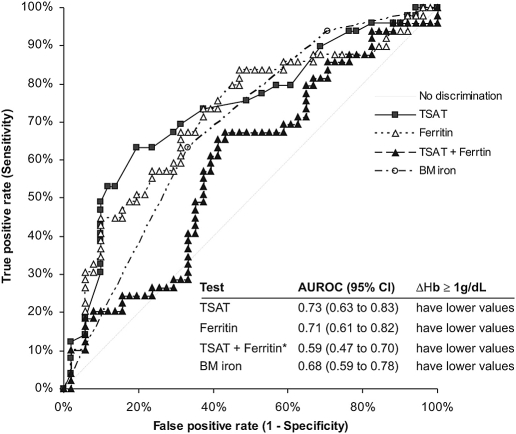
The sensitivity and the specificity of central and peripheral iron indices to identify the erythropoietic response was similar and moderate. Areas under the ROCs were approximately 70% and did not differ from one test to another. The combination of TSAT and ferritin was not superior to each test in predicting response (Figure 3). When examining the accuracy of the central and peripheral iron indices to identify a positive erythropoietic response at different cutoff values, the sensitivity was low, whereas the specificity was high (i.e., nonresponders were better identified by a negative test; Table 2). The posttest PPV and NPV for response of peripheral iron indices were balanced at cutoffs of 15% for TSAT (mean 0.76 [95% Cl, 0.50 to 0.93] and 0.72 [95% Cl, 0.64 to 0.79]) and 75 ng/ml for ferritin (mean 0.74 [95% Cl, 0.46 to 0.92] and 0.70 [95% Cl, 0.62 to 0.77]). In the case of a low macrophage iron score, sensitivity and specificity were >60%, but the PPV was low (mean 0.55 [95% Cl, 0.40 to 0.69]), whereas the NPV was higher (mean 0.74 [95% Cl, 0.60 to 0.85]). The same was true when bone marrow iron stores (deplete versus replete) were examined.
Figure 3.
Sensitivity and specificity of TSAT and serum ferritin (ferritin) and their combination (TSAT + ferritin) and bone marrow iron (BM iron) to identify correctly a positive erythropoietic response (≥1-g/dl increase in Hb [ΔHb]) to intravenous iron in 100 nondialysis patients with CKD (areas under the ROCs). The areas under the ROCs do not differ in the case of TSAT, ferritin, and BM iron, but TSAT + ferritin area under the ROC is significantly lower than the other. *P < 0.05 versus TSAT and ferritin.
Table 2.
Sensitivity, specificity, and the predictive values of peripheral and bone marrow indices to predict the erythropoietic response to intravenous iron in nondialysis patients with CKD
| Parameter | Sensitivity | Specificity | Posttest Predictive Valuea |
|
|---|---|---|---|---|
| For Response if Test Positive | For Nonresponse if Test Negative | |||
| TSAT (%) | ||||
| 10 | 0.31 (0.18 to 0.45) | 0.90 (0.79 to 0.97) | 0.68 (0.36 to 0.90) | 0.66 (0.59 to 0.73) |
| 15 | 0.47 (0.33 to 0.62) | 0.90 (0.79 to 0.97) | 0.76 (0.50 to 0.93) | 0.72 (0.64 to 0.79) |
| 20 | 0.63 (0.48 to 0.77) | 0.80 (0.67 to 0.90) | 0.68 (0.49 to 0.84) | 0.77 (0.66 to 0.85) |
| 25 | 0.76 (0.61 to 0.87) | 0.51 (0.37 to 0.65) | 0.51 (0.39 to 0.62) | 0.76 (0.59 to 0.88) |
| Ferritin (ng/ml) | ||||
| 50 | 0.22 (0.12 to 0.37) | 0.94 (0.84 to 0.99) | 0.72 (0.33 to 0.95) | 0.65 (0.59 to 0.70) |
| 75 | 0.41 (0.27 to 0.56) | 0.90 (0.79 to 0.97) | 0.74 (0.46 to 0.92) | 0.70 (0.62 to 0.77) |
| 100 | 0.49 (0.34 to 0.64) | 0.82 (0.69 to 0.92) | 0.65 (0.43 to 0.83) | 0.71 (0.61 to 0.79) |
| 125 | 0.57 (0.42 to 0.71) | 0.76 (0.63 to 0.87) | 0.62 (0.43 to 0.79) | 0.73 (0.62 to 0.82) |
| 150 | 0.61 (0.46 to 0.75) | 0.69 (0.54 to 0.81) | 0.56 (0.39 to 0.72) | 0.72 (0.59 to 0.82) |
| Bone marrow iron | ||||
| macrophage iron score | ||||
| 1 | 0.63 (0.48 to 0.77) | 0.67 (0.52 to 0.79) | 0.55 (0.40 to 0.69) | 0.74 (0.60 to 0.85) |
| 2 | 0.31 (0.18 to 0.45) | 0.63 (0.48 to 0.76) | 0.35 (0.19 to 0.56) | 0.58 (0.47 to 0.68) |
| ≥3 | 0.06 (0.01 to 0.17) | 0.71 (0.56 to 0.83) | 0.12 (0.02 to 0.39) | 0.53 (0.46 to 0.60) |
| bone marrow iron stores (deplete versus replete) | 0.63 (0.48 to 0.77) | 0.67 (0.52 to 0.79) | 0.56 (0.40 to 0.71) | 0.73 (0.60 to 0.84) |
Data are presented as mean (95% Cl).
Pretest probability of response 0.4.
Inflammation had a low influence on both iron indices and response. Neither median CRP nor the proportion of patients with CRP ≥10 mg/L was different in responders as compared with nonresponders. No correlation was found between ferritin and CRP, and the proportions of responders were similar when evaluated at different CRP cutoffs (data not shown).
In multivariable logistic regression analysis, the final model included only TSAT and ferritin and predicted 19% of the variability of the erythropoietic response (Table 3). Only TSAT made a significant contribution (i.e., the chances of response increased with 7% for each 1% decrease in TSAT).
Table 3.
Relationship between the index test (a >1-g/dl increase in Hb) and the reference tests in a model of multivariable binary logistic regression analysis
| Variablea | B | SE | Wald | df | P | Exp(B) (95% CI) |
|---|---|---|---|---|---|---|
| TSAT | −0.08 | 0.03 | 8.21 | 1 | 0.00 | 0.93 (0.88 to 0.98) |
| Ferritin | −0.01 | 0.01 | 3.06 | 1 | 0.08 | 0.99 (0.99 to 1.00) |
| Constant | 2.29 | 0.62 | 13.45 | 1 | 0.00 | 9.85 |
Cox and Snell R2 = 0.19 (χ2 = 21.32; dif = 2; P < 0.001).
Variables entered on step 1: Gender, age, Hb, CRP, TSAT, ferritin, GFR, and bone marrow iron (deplete or replete).
Discussion
This is one of the largest studies to evaluate the accuracy of central and peripheral iron indices to predict the response to intravenous iron in nondialysis patients with CKD. It confirms that bone marrow iron stores are depleted in an important proportion of the nondialysis patients with CKD and anemia and brings further support to the therapeutic intravenous iron test, because the predictive values of iron indices are only moderate.
Few data are available about the prevalence of iron deficiency in nondialysis patients with CKD, because the bone marrow examination—the “gold standard” in iron deficiency diagnosis—is seldom performed. On the basis of bone marrow examination, 48% of our patients had deplete iron stores, a lower percentage than reported by Silverberg and colleagues (9) (90%) but higher than in data reported by Rahman et al. (8) (23%). Part of this wide variation could be attributed to the method of examination. In the study by Silverberg and colleagues (9), the bone marrow iron evaluation was not detailed, Rahman et al. (8) used only the number of macrophage siderotic particles, and we used a more complex examination, involving not only macrophage iron but also sideroblasts; however, our data support a significant prevalence of deplete bone marrow iron stores in nondialysis patients with CKD in advanced stages, not very different from reports of HD patients who were on epoetin therapy (40 to 63%) (4,26).
Bone marrow iron is almost never used for clinical decision in day-to-day practice, and decisions are instead based only on peripheral iron indices. Typical markers of iron deficiency and cutoff values used in HD patients are a TSAT <20% and a ferritin <100 ng/ml. Kidney Disease Outcomes Quality Initiative (KDOQI) recommend these levels also for nondialysis patients with CKD (1). The proportions of patients who were below these cutoffs in our study were 33, 31, and 17% for the combination of TSAT and ferritin, respectively. By comparison, in Third National Health and Nutrition Examination Survey (NHANES III) cohort, in nondialysis patients with CKD and anemia (GFR <30 ml), only 35% of women and 33% of men had both a TSAT <20% and a ferritin <100 ng/ml (27). The higher proportions in NHANES III cohort, despite a lower percentage of patients with stage 5 CKD, could indicate that these cutoffs are not appropriate and may falsely identify too many patients as being iron deficient (27). Given these uncertainties, we initiated iron therapy in all patients, irrespective of their bone marrow iron status or peripheral iron indices. We chose to give iron intravenously, not orally, because studies (11,28) suggested a benefit of the intravenous route in patients with characteristics similar to our cohort (initial low Hb and GFR and lack of epoetin therapy). In addition, a recent meta-analysis showed a small but significant difference in Hb level favoring the intravenous iron route (29).
After intravenous iron sucrose administration, a significant hematologic response was noted in the whole cohort: 48% had a ≥1-g/dl increase in Hb, and 26% of patients attained ≥11 g/dl Hb. A ≥1-g/dl increase in Hb after intravenous iron is a useful predictive test for a positive response to iron therapy, recommended by KDOQI (1) in case of discordant iron indices, a position sustained by the Dialysis Patients' Response to IV Iron with Elevated Ferritin (DRIVE) study results in HD patients (30). In nondialysis patients with CKD, the reported proportion of responders only to intravenous iron were 68% (9), 44% (10), 38% (11), and 39% (12). Thus, a 40% prevalence of responders in this population would be reasonable and could be safely used for the evaluation of posttest probability of response.
Although at first look both peripheral and central iron indices could differentiate responders from nonresponders (Figure 2, Table 1), the areas under the ROCs were approximately 70%, reflecting a moderate clinical utility (Figure 3). Of note, even 30% of patients with adequate iron stores responded. Furthermore, when predictive values were examined at different cutoffs, only a TSAT <15% and a ferritin <75 ng/ml performed clinically better, predicting a positive response in >70% of cases and excluding a negative response in the case of a negative test with a probability of approximately 70%. These cutoff values are closer to limits of nondialysis patients with CKD than to those of HD patients, in accordance with Fishbane's (31) assumption. In logistic regression, the utility of peripheral and bone marrow iron indices was also moderate; because only TSAT and ferritin were retained in the final model, a 1% lower TSAT increased the response chances by 7%. This is in line with a recent analysis of NHANES data, in which the percentage of nondialysis patients with CKD and anemia had a decreasing trend in TSAT but not in serum ferritin quartiles (32). In other studies that investigated the relationship between iron indices and response in nondialysis patients with CKD, the prognostic value of TSAT and serum ferritin for the erythropoietic response was also of marginal clinical utility. In a study by Van Wyck et al. (11), these tests proved unhelpful, but patients had a higher eGFR, the anemia was less severe, TSAT was lower, and ferritin was higher than in our cohort, which can partially explain the differences. Similarly, Silverberg and colleagues (9) found no correlation among TSAT, ferritin, and the erythropoietic response, although 90% of patients were iron deplete, but the number of patients was low. More data are needed to clarify this issue.
A ≥1-g/dl increase in Hb was noted in one third of our patients without deplete bone marrow iron stores. This is similar to data from HD patients in the DRIVE study (30), in which peripheral iron indices were also not reliable predictors of response. Given the reduced prognostic utility of peripheral iron indices—and even of the bone marrow stores—for a positive response, a therapeutic trial with 1000 mg of intravenous iron seems to be more useful, but its practice should be carefully weighed against the potential deleterious effect of iron (13,14).
What are the limits of this study? The assessment of bone marrow iron stores was made by a qualitative method on an aspirate, and a recent study that used a quantitative method proved a strong relationship between iron stores and ferritin in HD patients (33). To overcome these, not only macrophage iron was considered but also the sideroblasts, and a sufficient number of slides were examined (17). Moreover, relationships were found between iron stores and response.
The number of participants, also one of the highest reported, still may be too small, and the composition of patients is different from the other reported series by the low proportion of patients with diabetes, the distribution skewed toward advanced CKD stages, more severe anemia, and highly prevalent inflammation. The high prevalence of inflammation (60% with a CRP >10 mg/L) seems not unusual in patients with anemia and advanced CKD, because recent reports gave similar proportions (74%) (34). In other studies, a correlation was found between CRP and ferritin, because ferritin is an acute-phase reactant and its utility in guiding iron therapy was questioned (4,30,35–37). An influence of inflammation on the erythropoietic response is not sustained by our data, because no correlation was found between CRP and iron indices, and, similar to HD patients (3), neither in univariable analysis nor in the logistic regression did the CRP level have any influence on the response. The initial Hb level was linked to the response to intravenous iron (7,9), a relationship that was not found in our study, probably because of the severity of anemia in our patients. Nevertheless, all these characteristics are commonly found in day-to-day practice, and even if they could generate some statistical biases, they should be considered in the clinical decision.
Conclusions
Half of nondialysis patients with CKD responded with a >1-g/dl increase in Hb one month after 1000 mg of intravenous iron. Because even one third of patients with iron-replete bone marrow stores responded, whereas iron indices had a moderate accuracy in predicting the response, the therapeutic intravenous iron trial seems to be a useful tool in the management of anemia in nondialysis patients with CKD.
Disclosures
None.
Acknowledgments
Part of this research was supported by a grant from Anemia Working Group, Romania.
Part of this work was presented as a poster at World Congress of Nephrology; May 22 through 26, 2009; Milan, Italy.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis 50: 471–530, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Fishbane S, Kowalski EA, Imbriano LJ, Maesaka JK: The evaluation of iron status in hemodialysis patients J Am Soc Nephrol 7: 2654–2657, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Tessitore N, Solero GP, Lippi G, Bassi A, Faccini GB, Bedogna V, Gammaro L, Brocco G, Restivo G, Bernich P, Lupo A, Maschio G: The role of iron status markers in predicting response to intravenous iron in haemodialysis patients on maintenance erythropoietin. Nephrol Dial Transplant 16: 1416–1423, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Hoffken B, Wunsch H, Fink H, Kleiner M, Luft EC: Diagnosis of iron deficiency anemia in renal failure patients during the post-erythropoietin era. Am J Kidney Dis 26: 292–299, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Kalantar-Zadeh K, Lee GH: The fascinating but deceptive ferritin: To measure it or not to measure it in chronic kidney disease? Clin J Am Soc Nephrol 1: S9–S18, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Hörl WH: Adjunctive therapy in anaemia management. Nephrol Dial Transplant 17: 56–59, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Silverberg DS, MD, Iaina A, Peer G, Kaplan E, Levi BA, Frank N, Steinbruch S, Blum M: Intravenous iron supplementation for the treatment of the anemia of moderate to severe chronic renal failure patients not receiving dialysis. Am J Kidney Dis 27: 234–238, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Rahman MM, Dutta PK, Hoque M, Khan MIH, Banik D, Dutta AK, Hasan MU, Yunus EB, Yunus ABM, Rahman MJ: Evaluation of iron status by bone marrow iron stain and correlation with serum profile in chronic kidney disease (CKD). Journal of Bangladesh College of Physicians and Surgeons 25: 117–120, 2007 [Google Scholar]
- 9.Gotloib L, Silverberg D, Shostak A: Iron deficiency is a very common cause of anemia in chronic kidney insufficiency and can often be corrected with IV iron. J Nephrol 19: 161–167, 2006 [PubMed] [Google Scholar]
- 10.Mircescu G, Gârneaţă L, Căpuçsă C, Ursea N: Intravenous iron supplementation for the treatment of anemia in pre-dialyzed chronic renal failure patients. Nephrol Dial Transplant 21: 120–124, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Van Wyck DB, Roppolo MO, Martinez CO, Mazey RM, McMurray S: A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int 68: 2846–2856, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Spinowitz BS, Kausz AT, Baptista J, Noble SD, Sothinathan R, Bernardo MV, Brenner L, Pereira BJ: Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol 19: 1599–1605, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zager RA, Johnson AC, Hanson SY, Wasse H: Parenteral iron formulations: A comparative toxicologic analysis and mechanisms of cell injury. Am J Kidney Dis 40: 90–103, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Agarwal R, Vasavada N, Sachs NG, Chase S: Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int 65: 2279–2289, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Greene T, Kusek JW: A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract]. J Am Soc Nephrol 11: A0828, 2000 [Google Scholar]
- 16.Peritoneal Dialysis Study Group: Adequacy of dialysis and nutrition in continuous peritoneal dialysis: Association with clinical outcomes. Canada-USA (CANUSA). J Am Soc Nephrol 7: 198–207, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Hughes DA, Stuart-Smith SE, Bain BJ: How should stainable iron in bone marrow films be assessed? J Clin Pathol 57: 1038–1040, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rath CE, Finch CA: Sternal marrow hemosiderin: A method for the determination of available iron stores in man. J Lab Clin Med 33: 81–86, 1948 [PubMed] [Google Scholar]
- 19.Hansen H, Weinfeld A: Hemosiderin estimations and sideroblasts counts in the differential diagnosis of iron deficiency and other anemias. Acta Med Scand 165: 333–356, 1959 [DOI] [PubMed] [Google Scholar]
- 20.Altman DG, Bland JM: Diagnostic tests 1: Sensitivity and specificity. BMJ 308: 1552, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman DG, Bland JM: Diagnostic tests 2: Predictive values. BMJ 309: 102, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman DG, Bland JM: Diagnostic tests 3: Receiver-operating characteristics plots. BMJ 309: 188, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tripepi G, Jager KJ, Dekker FW, Zoccali C: Diagnostic methods 2: Receiver operating characteristic (ROC) curves. Kidney Int 76: 252–256, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Zweig MH, Campbell G: Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin Chem 39: 561–577, 1993 [PubMed] [Google Scholar]
- 25.van Stralen KJ, Stel1 VS, Reitsma JB, Dekker FD, Zoccali C, Jager KJ: Diagnostic methods I: Sensitivity, specificity, and other measures of accuracy. Kidney Int 75: 1257–1263, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Fernández-Rodríguez AM, Guindeo-Casasús MC, Molero-Labarta T, Dominguez-Cabrera C, Hortal-Cascon L, Perez-Borges P, Vega-Dýaz N, Saavedra-Santana P, Palop-Cubillo L: Diagnosis of iron deficiency in chronic renal failure. Am J Kidney Dis 34: 508–513, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Hsu C-Y, McCulloch CE, Curham GC: Iron status and hemoglobin level in chronic renal insufficiency. J Am Soc Nephrol 13: 2783–2786, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Panesar A, Agarwal R: Safety and efficacy of sodium ferric gluconate complex in patients with chronic kidney disease. Am J Kidney Dis 40: 924–931, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Rozen-Zvi B, Gafter-Gvili A, Paul M, Leibovici L, Shpilberg O, Gafter U: Intravenous versus oral iron supplementation for the treatment of anemia in CKD: Systematic review and meta-analysis. Am J Kidney Dis 52: 897–906, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Daniel W, Coyne DW, Kapoian T, Suki W, Singh AK, Moran JE, Dahl NV, Rizkala ARDRIVE Study Group: Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: Results of the Dialysis Patients' Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol 18: 975–984, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Fishbane S: Iron management in nondialysis-dependent CKD. Am J Kidney Dis 49: 736–743, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Fishbane S, Pollack S, Feldman HI, Joffe MM: Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey 1988–2004. Clin J Am Soc Nephrol 4: 57–61, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocha LA, Barreto DV, Barreto FC, Dias CB, Moysés R, Silva MR, Moura LA, Draibe SA, Jorgetti V, Carvalho AB, Canzianiet ME: Serum ferritin level remains a reliable marker of bone marrow iron stores evaluated by histomorphometry in hemodialysis patients. Clin J Am Soc Nephrol 4: 105–109, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chonchol M, Lippi G, Montagnana M, Muggeo M, Targher G: Association of inflammation with anaemia in patients with chronic kidney disease not requiring chronic dialysis. Nephrol Dial Transplant 23: 2879–2883, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Kalantar-Zadeh K, Rodriguez RA, Humphreys MH: Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol Dial Transplant 19: 141–149, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Wish JB: Assessing iron status: Beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol 1[ Suppl 1]: S4–S8, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Ford BA, Coyne DW, Eby CS, Scott MG: Variability of ferritin measurements in chronic kidney disease: Implications for iron management. Kidney Int 75: 104–110, 2008 [DOI] [PubMed] [Google Scholar]