Abstract
Background and objectives: Arteriovenous fistulas (AVFs) remain the preferred vascular access for hemodialysis patients. Dialysis facilities that fail to meet Centers for Medicare & Medicaid Services goals cite patient case-mix as a reason for low AVF prevalence. This study aimed to determine the magnitude of the variability in AVF usage across dialysis facilities and the extent to which patient case-mix explains it.
Design, setting, participants, & measurements: The vascular access used in 10,112 patients dialyzed at 173 Dialysis Clinic Inc. facilities from October 1 to December 31, 2004, was evaluated. The access in use was considered to be an AVF if it was used for >70% of hemodialysis treatments. Mixed-effects models with a random intercept for dialysis facilities evaluated the effect of facilities on AVF usage. Sequentially adjusted multivariate models measured the extent to which patient factors (case-mix) explain variation across facilities in AVF rates.
Results: 3787 patients (38%) were dialyzed using AVFs. There was a significant facility effect: 7.6% of variation in AVF use was attributable to facility. This was reduced to 7.1% after case-mix adjustment. There were no identified specific facility-level factors that explained the interfacility variation.
Conclusions: AVF usage varies across dialysis facilities, and patient case-mix did not reduce this variation. In this study, 92% of the total variation in AVF usage was due to patient factors, but most were not measurable. A combination of patient factors and process indicators should be considered in adjudicating facility performance for this quality indicator.
An arteriovenous fistula (AVF), the vascular access associated with the lowest morbidity, mortality, and cost, is the preferred vascular access for a patient treated with hemodialysis (HD) (1). The Centers for Medicare & Medicaid Services (CMS) Fistula First National Vascular Access Improvement initiative aimed for AVF use by 66% of prevalent HD patients by 2009 (2). CMS' 2008 dialysis facility Clinical Performance Measures include a measure of AVF use, and in the Medicare Improvements for Patients and Providers Act of 2008, the US Congress specified AVF use as one of the performance measures from which CMS must construct a “pay-for-performance” (P4P) index, which will determine reimbursement as early as 2012 (3).
Rates of AVF use vary across countries (4,5) and, within the United States, across ESRD Networks and individual dialysis facilities (6–9). Factors related to the patient, the nephrologist, the dialysis facility staff, and the surgeon may all influence the probability that the patient will eventually be dialyzed using a mature AVF. Facilities and nephrologists determine the time required to make the referral but share with surgeons, radiologists, and surgical facilities responsibility for the time required to coordinate appointments, vessel mapping, AVF surgery, and follow-up visits and imaging, if needed. Community factors, such as the timing of referral of patients with chronic kidney disease, the number and availability of local surgeons and radiologists with expertise in AVF creation and imaging, and the availability of operating room time, all influence the time required before a patient can be dialyzed using an AVF. In the long term, the nephrologist and the dialysis provider, although not individual staff, can arguably influence these. Much less controversially, several patient factors also influence the likelihood of successful AVF creation, including patient age and sex, diabetes, and the extent of vascular disease (6,8–10).
The objective of this study is to examine the extent to which differences among facilities in patient factors (i.e., “case-mix”) explain the variation in AVF use across a large, national not-for-profit dialysis organization. In addition, this study aims to identify facility-specific factors that explain differences in AVF rates between facilities and that persist even after patient case-mix has been accounted for.
Materials and Methods
Patient Population
There were 12,747 patients undergoing HD in 173 Dialysis Clinic Inc. facilities across the United States in the fourth quarter (October 1 to December 31) of 2004. We used the same exclusions as the Clinical Performance Measures Program (3) to identify the study population. We excluded patients younger than 18 years as of October 1, 2004 (n = 53), patients not undergoing thrice weekly in-center treatment (n = 261), patients who died before December 31, 2004 (n = 547), patients who did not have at least one laboratory measurement for hemoglobin, albumin, or Kt/V in the fourth quarter (n = 603), and those who started dialysis between September 1 and December 31, 2004 (n = 839). Dialysis facilities treating fewer than 20 patients (n = 300) and patients without matching CMS claims files (n = 32) were excluded. This left 10,112 patients in the study population. We conducted sensitivity analyses restricted to patients with Medicare as a primary insurer (n = 6915), because the most complete comorbidity information is available on these patients through the CMS claims data files.
Sources of Predictor and Outcome Data.
Laboratory parameters, BP readings, delivered dialysis dose, and details of dialysis treatments, including the vascular access type used, were abstracted from DARWIN, a proprietary electronic medical information system used at all Dialysis Clinic Inc. facilities. We considered the access in use for each individual to be an AVF if an AVF was used for >70% of HD treatments in the last quarter of 2004. The facility % AVF use was defined as the number of patients who used an AVF for 70% of treatments in the quarter divided by the total number of patients in the unit that quarter. Hemoglobin, albumin, Kt/V, calcium, and phosphate were measured at least monthly. The last value of the month was averaged for the 3 months in the last quarter of 2004 for each patient. The study was approved by the Institutional Review Board at Tufts Medical Center.
Comorbidity.
Information about patient comorbidity was on the basis of two sources: (1) the comorbidity checklist on the Medical Evidence Form (Form 2728), which is completed in all Medicare-entitled patients at the start of dialysis, and (2) diagnostic codes accompanying CMS Institutional and Physician/Supplier Claims for the years 2002 to 2004. Standard methods were used to increase the specificity of using claims data to identify comorbid conditions (10,11). Diagnostic codes accompanying institutional claims where the facility was a hospital or rehabilitation facility were included, but diagnoses accompanying laboratory tests or diagnostic imaging studies were excluded because these may have been ordered as screening tests. Those codes accompanying equipment service types were also excluded because these are not entered by physicians. For the remaining physician/supplier claim types, there needed to be three uses of a diagnostic code, each separated by a minimum of 14 days, for the condition to be considered present. Dates of hospitalizations were obtained from DARWIN. Each facility maintains a log, which is reconciled weekly, of missed dialysis treatments and the reasons for the patient absences. This process provides reliable information about dates of hospitalization.
Center Characteristics
Center characteristics for the dialysis facilities were derived from the CMS Form 2744 (Year End ESRD Facility Survey), which is an annual survey completed by all Medicare-approved dialysis providers. The survey captures patient and treatment counts at each facility on an annual basis. Geographical factors, including urban/rural status and median income at facility zip code, were obtained from US Census data for the year 2000. A facility was considered rural if more than 50% of the population in the facility's zip code was defined as rural (12).
Statistical Analyses
Patient and facility characteristics were compared across quartiles of facility % AVF use. ANOVA and χ2 tests were used to compare continuous and categorical variables, respectively, across quartiles of facilities % AVF with trend P values. Nonparametric Kruskal-Wallis test was used to compare facility-level variables between quartiles of facilities % AVF. To assess the magnitude of dialysis facility effect on AVF use, we used a two-level logistic regression model with patients nested within dialysis facilities.
 |
uj is normally distributed with mean 0 and between dialysis facility variance σu2, xij indicates characteristics of the ith patient in the jth dialysis facility.
Three statistical measures were used to quantify dialysis facility variation and were derived from sequentially adjusted models: the patient- and facility-related R2, the median odds ratio (MOR), and plots of each of the observed and predicted proportions of AVF use at each dialysis facility. To partition the variation in fistula use at facility and patient levels, we used an R2 measure derived from a two-level logistic regression with random intercepts (13). We also used the MOR, which quantifies variation between dialysis facilities on the odds ratio scale (14). This measure compares two randomly selected patients with same covariates from two randomly chosen dialysis facilities. If MOR = 1, there is no variation between dialysis facilities. The 95% confidence intervals for the MOR were calculated using bootstrap resampling with 500 simulations. The models above were fitted using the GLIMMIX procedure in SAS (version 9.1).
Results
Study Population
A total of 3787 patients (38%) were dialyzed using AVFs. In 25% of facilities, fewer than 28% of patients were dialyzed by an AVF, and in another 25% of facilities, more than 46% of patients were dialyzed using an AVF. The median proportion of patients dialyzing with an AVF per facility was 38%, and the interquartile range was 29% to 46%.
Patient and Facility Characteristics across Quartiles of Facility % AVF Use
As shown in Table 1, the proportion of women and African Americans decreases with decreasing quartile of facility AVF use. With decreasing quartile of facility AVF use, there was also a higher proportion of patients with diabetes and hypertension as the cause of ESRD, a higher proportion of patients with a history of cerebrovascular disease, and a trend toward a higher proportion of patients with peripheral vascular disease, although the latter was not statistically significant. Increasing facility size and a higher median household income were associated with increasing quartile of facility AVF use. No other facility-level characteristics were significantly different across quartiles of facility AVF use.
Table 1.
Patient and dialysis facility characteristics across quartiles (Q) of facility % AVF use
| Q1 % AVF 3.6% to 28.6% (n = 2518) | Q2 % AVF 28.6% to 38.0% (n = 2563) | Q3 % AVF 38.0% to 46.2% (n = 2481) | Q4 % AVF 46.2% to 70.0% (n = 2550) | Trend P | |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age, yr, mean ± SD | 59.7 ± 15.1 | 60.1 ± 15.2 | 62.3 ± 15.3 | 61.5 ± 15.1 | <0.001 |
| Female gender, % | 49 | 47 | 47 | 45 | 0.006 |
| Race, % | <0.001 | ||||
| White, | 35 | 41 | 53 | 49 | |
| African American, | 62 | 54 | 35 | 33 | |
| Hispanic, | 2 | 2 | 6 | 11 | |
| Other, | 1 | 2 | 7 | 7 | |
| Cause of ESRD, % | <0.001 | ||||
| Glomerulonephritis | 12 | 11 | 12 | 15 | |
| Hypertension | 31 | 33 | 25 | 24 | |
| Diabetes | 40 | 39 | 44 | 42 | |
| Other | 14 | 14 | 16 | 15 | |
| PKD | 3 | 3 | 3 | 4 | |
| Dialysis vintage, % | 0.0001 | ||||
| <3 mo | 4 | 4 | 5 | 4 | |
| 3 mo to 1 yr | 15 | 16 | 18 | 18 | |
| 1 to 3 yr | 33 | 33 | 35 | 35 | |
| >3 yr | 48 | 47 | 43 | 43 | |
| BMI categories, % | 0.25 | ||||
| ≤18.5 kg/m2 | 6 | 5 | 6 | 5 | |
| 18.5 to ≤24.9 kg/m2 | 38 | 40 | 37 | 38 | |
| 24.9 to ≤29.9 kg/m2 | 28 | 27 | 29 | 29 | |
| >29.9 kg/m2 | 28 | 28 | 28 | 29 | |
| Predialysis systolic BP, % | <0.0001 | ||||
| <120 | 5 | 7 | 9 | 9 | |
| 120 to <140 | 21 | 21 | 25 | 26 | |
| 140 to <160 | 35 | 37 | 36 | 36 | |
| ≥160 | 39 | 35 | 30 | 30 | |
| Comorbidities, % | |||||
| History of CVA/TIA | 24 | 25 | 23 | 22 | 0.03 |
| Diabetes mellitus | 59 | 57 | 61 | 59 | 0.22 |
| History of PVD | 26 | 26 | 27 | 24 | 0.15 |
| History of IHD | 46 | 48 | 51 | 47 | 0.18 |
| Laboratory parameters | |||||
| Albumin, g/dl, mean ± SD | 3.8 ± 0.4 | 3.8 ± 0.4 | 3.8 ± 0.4 | 3.8 ± 0.4 | 0.05 |
| Facility characteristics, median (interquartile range) | |||||
| Total patients per facility, n | 50 (36 to 71) | 56 (43 to 86) | 55 (39 to 76) | 63 (48 to 99) | 0.05 |
| Total patient care staff per 100 patients, n | 18 (15 to 21) | 18 (15 to 21) | 18 (16 to 20) | 17 (14 to 19) | 0.35 |
| Percent of employed patients | 6 (3 to 10) | 5 (1 to 10) | 5 (2 to 9) | 8 (3 to 12) | 0.58 |
| Percent receiving a kidney transplant within the past year | 4 (1 to 8) | 4 (2 to 8) | 5 (3 to 7) | 5 (3 to 8) | 0.22 |
| Median household income, $ | 32,419 (27,190 to 40,663) | 32,838 (26,648 to 37,254) | 35,353 (30,721 to 37,778) | 36,850 (30,655 to 45,878) | 0.03 |
BMI, body mass index; CVA/TIA, cerebrovascular accident/transient ischemic attack; PVD, peripheral vascular disease; IHD, ischemic heart disease. Comorbidities are derived using data from the Form 2728 and Medicare claims for the years 2002 to 2004. See text for details. Total patient care staff includes registered nurses, licensed practical nurses, and patient care technicians.
Factors Associated with AVF Use
Table 2shows patient and facility factors associated with AVF use in the final model adjusted for the random facility intercept and the fixed patient and facility characteristics. Decreasing age, white race, and male sex were associated with a higher likelihood of fistula use. A longer period since beginning dialysis treatment (dialysis “vintage”) was also associated with a higher likelihood of fistula use. This effect, however, was attenuated in patients who had been on dialysis for more than 3 years. Fewer comorbidities were associated with a higher likelihood of AVF use. AVF use was not associated with facility size, staffing ratios, or urban/rural status.
Table 2.
Variables significantly associated with AVF use in the final multivariate model
| Variable | OR [95% CI] | F Value Statistic, Df, P |
|---|---|---|
| Patient demographics | ||
| Age (per 10 yr older) | 0.91 [0.88, 0.95] | 23.6, 1, <0.001 |
| Patient race/ethnicity | 12.2, 4, <0.001 | |
| Caucasian (reference) | 1.00 | |
| African American | 0.69 [0.61, 0.79] | |
| Hispanic | 1.11 [0.87, 1.41] | |
| Other | 0.91 [0.69, 1.20] | |
| Patient gender | 313.5, 1, <0.001 | |
| Female | 0.40 [0.37, 0.45] | |
| Patient dialysis vintage | 23.5, 4, <0.001 | |
| 30 to 90 d | 0.34 [0.25, 0.45] | |
| 90 d to 1 yr | 0.69 [0.60, 0.92] | |
| 1 to 3 yr (reference) | 1.00 | |
| More than 3 yr | 0.81 [0.62, 1.07] | |
| Patient characteristics | ||
| History of CVA or TIA | 0.86 [0.74, 0.99] | 3.9, 1, 0.04 |
| Presence of diabetes mellitus | 0.83 [0.72, 0.95] | 7.5, 1, 0.006 |
| Dementia | 0.70 [0.56, 0.88] | 9.3, 1, 0.002 |
| Inability to ambulate/transfer | 0.64 [0.51, 0.79] | 16.5, 1, <0.001 |
| BMI categories | ||
| ≤18.5 kg/m2 | 0.83 [0.66, 1.04] | 4.0, 4, 0.008 |
| 18.5 to ≤24.9 kg/m2 (reference) | 1.00 | |
| 24.9 to ≤29.9 kg/m2 | 0.89 [0.79, 1.00] | |
| >29.9 kg/m2 | 0.81 [0.72, 0.92] | |
| Albumin, per 0.5 g/dl | 1.34 [1.25, .44] | 64.4, 1, <0.001 |
OR, odds ratio; Df, degrees of freedom; CVA, cerebrovascular accident; TIA, transient ischemic attack. Patient-level covariates that were tested but were not significant: etiology of kidney disease, predialysis systolic BP, and the following comorbidities based on Form 2728 plus claims in past 3 years: hypertension, ischemic heart disease, acute coronary syndrome in the past 6 months, arrhythmia, cardiac arrest, congestive heart failure requiring hospitalization in the past 6 months, history of peripheral vascular disease, osteomyelitis in the past 6 months, limb amputation, decubitus ulcer, smoking status, alcohol abuse, HIV, solid or hematologic malignancy, anemia due to hereditary disease or associated with malignancy, end-stage liver disease, chronic lung disease, and medication noncompliance. Facility-level factors that were tested but not significant: patients per center, patient care staff to patient ratio, proportion of patients that had undergone transplantation, proportion of employed patients, median income, and urban/rural status.
Facility Effect Analysis
Table 3 shows the effects of successively adding patient and facility-level characteristics on the variation in AVF use across patients (column 1), which is partitioned into “within-facility” (column 2) variance (i.e., patient effect) and “between-facility” (column 3) variance (i.e., facility effect). In the base model adjusted for facility only, facility accounts for 7.6% of the variation in AVF usage; the remaining 92.4% is due to patient factors. Successive adjustment for patient factors in models 1 through 4 accounts for 14.1% (unexplained variance decreases from 92.4% to 78.3%) of the within-facility variance but only 0.4% (unexplained variance decreases from 7.6% to 7.2%) of the between-facility variance. Model 5 shows the effects of adding the facility-level factors from Table 1, which minimally decreases the unexplained between-facility variance from 7.2% to 6.4%.
Table 3.
Measures of facility effect after sequentially adjusting for patient and facility characteristics
| Parameter | Total Variance in AVF use (Explained), % | Within-Facility Variance in AVF Use (Unexplained), % | Between-Facility Variance in AVF Use (Unexplained), % | Median Odds Ratio [95% CI]a |
|---|---|---|---|---|
| Model 1: random facility intercept | 0 | 92.4 | 7.6 | 1.65 [1.52 to 1.78] |
| Adjusting for patient characteristics | ||||
| Model 2: model 1 + patient demographics | 10.3 | 82.6 | 7.1 | 1.66 [1.54 to 1.79] |
| Model 3: model 2 + patient comorbid conditions | 11.3 | 81.6 | 7.1 | 1.66 [1.53 to 1.79] |
| Model 4: model 3 + “other” patient case-mix factors | 14.5 | 78.3 | 7.2 | 1.69 [1.57 to 1.82] |
| Adjusting for facility characteristics | ||||
| Model 5: model 4 + facility characteristics | 14.7 | 78.9 | 6.4 | 1.64 [1.46 to 1.76] |
Interpretation of the median odds ratio: for two randomly selected patients with the same case-mix but dialyzed in two different, randomly selected facilities, the ratio of the odds that one patient will have a fistula to the odds that the second will have a fistula.
The Magnitude of the Facility Effect
The last column of Table 3 provides another measure of the magnitude of the facility effect. The MOR quantifies the variation in fistula use between facilities by comparing two randomly selected patients having the same values for all covariates but dialyzed in two different, randomly selected facilities. The MOR of the fully adjusted model is 1.64. This means that a patient with a certain set of characteristics is 64% more likely to be dialyzing with an AVF as another patient with the same characteristics at another randomly selected facility. Adjustment for facility-level characteristics resulted in only a trivial change in the MOR.
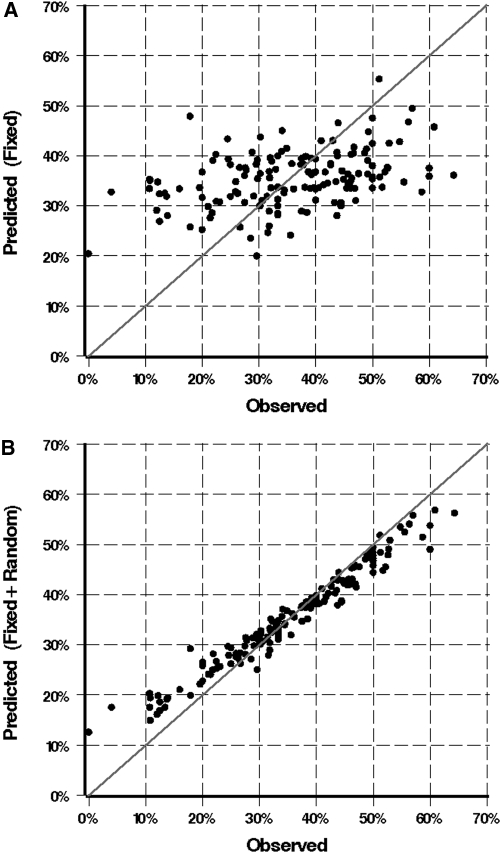
Figure 1 shows the predicted versus observed dialysis facility % AVF use. In Figure 1A, predicted rates include fixed patient and facility characteristics only, and in Figure 1B, predicted rates include fixed patient and facility characteristics and the predicted random facility intercept. Each point corresponds to one dialysis facility, and in a perfect model, all points would fall on the line of identity. The model fit is greatly improved when facility is added. These results show graphically the significant “facility effect” in AVF use in this population.
Figure 1.
Facilities predicted versus observed AVF use. Predicted rates were derived from the final multivariable model. (A) Predicted rates include fixed patient and facility characteristics only. (B) Predicted rates include fixed patient and facility characteristics and the predicted random facility intercept. The improved model fit of model 1B illustrates the significant effect of facility on AVF use.
Sensitivity Analysis
In a sensitivity analysis restricted to Medicare patients only (n = 6915), we found no difference in patient or facility factors associated with fistula use or in the influence of case-mix in explaining the between-facility variation in AVF use. The between-facility variation was 8.1% of the total variation in AVF use across patients and after full patient case-mix adjustment, decreased to 6.5% of the variation in AVF use.
Discussion
The proportion of patients dialyzed using AVFs continues to vary widely across US dialysis facilities. In the present analyses, dialysis facility accounts for 7.6% the variation in AVF use. The effect of facility is nearly as important as the combination of age, sex, race, and vintage, which together explain 10.3% of the variance in AVF rates. Although dialysis facilities may attribute their low AVF rates to the patient case-mix, we find no diminishment in the facility effect after accounting for a gamut of patient case-mix factors, including detailed comorbidity information. Despite quality improvement efforts, there continues to be variation in facility-specific practices that affect AVF usage. The results support the need for ongoing quality improvement efforts and also have implications for monitoring quality and for reimbursement under P4P, as will be discussed.
Our study is the first to quantify the facility effect on AVF use in a US dialysis population. Our estimate of facility-to-facility variation in AVF use (expressed as an intraclass correlation or P = 0.08) is comparable to that reported by past studies for hemoglobin values (P = 0.05) (15) and is less than that seen for dialysis adequacy (P = 0.14) (7). Compared with anemia management and dialysis adequacy, having a working AVF depends on a greater number of processes and structure-related factors, some of which may be considered out of the control of the dialysis unit (e.g., access to interventional radiology/surgeons, surgical expertise, etc.). That there is facility variation, however, implies that these factors are very much under the control of the dialysis facility. In fact, a recent study from the Dialysis Outcomes and Practice Patterns Study also showed a large effect of facility on patient catheter use that was unexplained by patient factors. Numerous patient-level factors explained only a small amount of the variance associated with patient catheter use (R2 = 0.07), whereas the addition of facility to the same model increased the R2 to 0.23 (4). Further research is needed to identify facility-specific practices that affect AVF usage, because these factors are more likely to be modifiable than patient factors.
The finding that patient case-mix does not diminish the facility effect might suggest that case-mix does not need to be considered when adjudicating facility performance for this quality indicator. Indeed, this is not the case. First, although an extensive list of patient factors was tested, our most complete model explains only 14.7% of the variation in AVF use across patients. It is possible that patient factors still account for a large part of the between-facility variation but were not captured in this study. Second, this study demonstrates a strong association of a number of patient factors with AVF use (as per Table 2). In small facilities or facilities with atypical populations, these factors are likely to be overrepresented or underrepresented, in which case failure to adjust for case-mix will lead to unfair scrutiny or reward (depending on the direction of influence of the case-mix factor). At the same time, case-mix adjusting has the potential to create adverse incentives. Incorporating processes of care in adjudicating this quality indicator reduces the potential for negative incentives that may result from case-mix adjusting. For example, in patient subgroups in whom it is more difficult to obtain a functioning AVF (as identified in Table 2), attempts to create an AVF may be acceptable or, in the patient who has had multiple failed attempts at an AVF, an arteriovenous graft may be acceptable for full reimbursement. The combination of patient case-mix factors and a process measure(s) also reduces the need to identify the factors that account for the large amount of unexplained within-facility variation that remained despite extensive case-mix adjustment in this study. It is critical that the assessment of care under a P4P program be fair. In a recent survey of nephrologists, dialysis nurses, and key opinion leaders, 75% reported that dialysis units “cherry pick” their patients “frequently” or “often.” With a 2% cut in payment legislated by Congress for 2011 (2,16) and with additional reductions for failing to meet quality indicators under P4P, facilities will be even more selective if adequate resources are not provided to care for patients in whom it is more difficult to achieve these quality indicators.
The strengths of our analysis are that we used a large patient population of 10,112 prevalent HD patients across 173 facilities with geographic variation across the United States. We also performed extensive patient case-mix adjustment using comorbidity, demographics, laboratory, and clinical data. Finally, we incorporated facility-level variables in our regression models to confirm a true facility effect and to quantify the extent of facility variation in fistula use.
Our analysis has some limitations: All of our dialysis facilities were from a single not-for-profit provider, and therefore we were not able to capture the effect of dialysis ownership characteristics on outcomes. Second, we studied a prevalent HD cohort as our study population. We did this to be consistent with methods used by the Clinical Performance Measures Program, on which dialysis facility performance is currently graded (3). Nonetheless, we may have misclassified incident patients with maturing AVFs as well as prevalent patients with multiple failed AVFs. We did, however, perform a sensitivity analysis excluding patients who had been on dialysis less than 6 months and found the facility effect to be even stronger (data not shown). The study takes into consideration more extensive patient case-mix information than have past studies, but it is possible that the true facility effect is less than we have reported, if unmeasured patient factors are operating. Finally, we looked at a small number of facility-specific factors. We did not have information on medical director or nephrology provider preferences, the presence of vascular access coordinators, and whether a regular access monitoring program was in place. These may have explained more of the facility effect than we were able to with the facility-level variables available in this study (17–19).
In conclusion, we found that fistula use varies across dialysis facilities and that the facility effect persists even after accounting for differences in patient characteristics across facilities. Future work should explore reasons for the facility effect and should focus on facility-level interventions to improve patient outcomes. This study identifies patient factors that are associated with the presence of functioning AVFs yet, despite extensive patient case-mix adjustment, only a small percentage of the total explained variance in AVF usage is accounted for. We propose that a combination of patient factors and process indicators be used to adjudicate this quality indicator.
Disclosures
None.
Acknowledgments
This work was previously presented in abstract format at the annual meeting of the American Society of Nephrology in 2008 in Philadelphia, Pennsylvania. N.T. was supported by a KRESCENT Post-Doctoral Fellowship (Kidney Foundation of Canada, Canadian Society of Nephrology, and the Canadian Institutes of Health Research). D.M. received salary support from Dialysis Clinics Inc. and National Institutes of Health grant 5 K23 DK066273.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Manns B, Tonelli M, Yilmaz S, Lee H, Laupland K, Klarenbach S, Radkevich V, Murphy B: Establishment and maintenance of vascular access in incident hemodialysis patients: A prospective cost analysis. J Am Soc Nephrol 16: 201–209, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Center for Medicare Services: End Stage Renal Disease Center Available at http://www.cms.hhs.gov/center/esrd.asp Accessed March 17, 2009
- 3.CMS Clinical Performance Measures Project Annual Report. [Accessed March 17, 2009]. Available at http://www.cms.hhs.gov/CPMProject/Downloads/ESRDCPMYear2007Report.pdf.
- 4.Pisoni RL, Arrington CJ, Albert JM, Ethier J, Kimata N, Krishnan M, Rayner HC, Saito A, Sands JJ, Saran R, Gillespie B, Wolfe RA, Port FK: Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: An instrumental variable analysis. Am J Kidney Dis 53: 475–491, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, Wolfe RA, Goodkin DA, Held PJ: Vascular access use in Europe and the United States: Results from the DOPPS. Kidney Int 61: 305–316, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Hirth RA, Turenne MN, Woods JD, Young EW, Port FK, Pauly MV, Held PJ: Predictors of type of vascular access in hemodialysis patients. JAMA 276: 1303–1308, 1996 [PubMed] [Google Scholar]
- 7.Fink JC, Armistead N, Turner M, Gardner J, Light P: Hemodialysis adequacy in Network 5: Disparity between states and the role of center effects. Am J Kidney Dis 33: 97–104, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Allon M, Ornt DB, Schwab SJ, Rasmussen C, Delmez JA, Greene T, Kusek JW, Martin AA, Minda S: Factors associated with the prevalence of arteriovenous fistulas in hemodialysis patients in the HEMO study. Hemodialysis (HEMO) Study Group Kidney Int 58: 2178–2185, 2000 [DOI] [PubMed] [Google Scholar]
- 9.O'Hare AM, Dudley RA, Hynes DM, McCulloch CE, Navarro D, Colin P, Stroupe K, Rapp J, Johansen KL: Impact of surgeon and surgical center characteristics on choice of permanent vascular access. Kidney Int 64: 681–689, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Klabunde CN, Potosky AL, Legler JM, Warren JL: Development of a comorbidity index using physician claims data. J Clin Epidemiol 53: 1258–1267, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Klabunde CN, Warren JL, Legler JM: Assessing comorbidity using claims data: An overview. Med Care 40: IV-26–35, 2002 [DOI] [PubMed] [Google Scholar]
- 12.US Census Bureau: Census 2000 Datasets Available at: http://www.census.gov/main/www/cen2000.html Accessed March 17, 2009
- 13.Snijders TAB, Bosker RJ: Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modelling, London, Sage, 1999 [Google Scholar]
- 14.Larsen K, Merlo J: Appropriate assessment of neighborhood effects on individual health: Integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol 161: 81–88, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Fink JC, Hsu VD, Zhan M, Walker LD, Mullins CD, Jones-Burton C, Langenberg P, Seliger SL: Center effects in anemia management of dialysis patients. J Am Soc Nephrol 18: 646–653, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Leavitt MO: Secretary of Health and Human Services Report to Congress: A Design for a Bundled End-Stage Renal Disease Prospective Payment System. Available at: http://www.cms.hhs.gov/ESRDGeneralInformation/downloads/ESRDReportToCongress.pdf Accessed March 17, 2009
- 17.McGill RL, Marcus RJ, Healy DA, Brouwer DJ, Smith BC, Sandroni SE: AV fistula rates: Changing the culture of vascular access. J Vasc Access 6: 13–17, 2005 [DOI] [PubMed] [Google Scholar]
- 18.McGill RL, Marcus RJ, Healy DA, Nye S, Brouwer DJ, Smith BC, Sandroni SE: Long-term outcomes of a fistula initiative: Sustaining “fistula culture”. J Vasc Access 7: 83–86, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Polkinghorne KR, Seneviratne M, Kerr PG: Effect of a vascular access nurse coordinator to reduce central venous catheter use in incident hemodialysis patients: A quality improvement report. Am J Kidney Dis 53: 99–106, 2009 [DOI] [PubMed] [Google Scholar]