Abstract
Background and objectives: To explore the correlation between dietary sodium intake and cardiovascular and overall mortality, and then determine whether this correlation can be explained by protein and energy intake paralleled with sodium intake in dialysis patients.
Design, setting, participants, & measurements: This single-center retrospective cohort study enrolled 305 incident patients who started peritoneal dialysis in our unit from July 2002 to February 2007. All patients were followed until death or until being censored in February 2008. Demographic data were collected at baseline. Biochemical, dietary, and nutrition data were examined at baseline and thereafter at regular intervals to calculate the average values throughout the study.
Results: Participants with the highest average sodium intake were more likely to be younger, male, and overweight. Patients in the high tertile of average sodium intake had higher albumin, prealbumin, and lean body mass levels, and more nutrient intakes paralleling with sodium intake. Low average sodium intake independently predicted the increased risk for overall and cardiovascular death after adjusting for recognized confounders. Further adjustment for dietary protein, energy, and other nutrient intakes individually had minimal impact on the association between average sodium intake and overall death, with hazard ratios varying between 0.35 and 0.44, and cardiovascular death, with hazard ratios varying between 0.06 and 0.11.
Conclusions: This study revealed that low dietary sodium intake independently predicts the high overall and cardiovascular mortality in dialysis patients. This correlation could not be entirely explained by deficient protein and energy intake.
Cardiovascular disease (CVD) has been extensively documented in patients with end-stage renal disease, including those undergoing peritoneal dialysis (PD) and hemodialysis (1,2), and CVD accounts for approximately 50% of the annual mortality in dialysis patients (3). Among the numerous risk factors for CVD, sodium has been a controversial one over the past half-century in the general population and among chronic kidney disease (CKD) patients (4–6). These controversies are partly due to an inconsistent relationship between sodium intake and overall and CVD mortality in the general population (7–12). Compared with the general population, the observational evidence on this correlation in dialysis patients is lacking. A few intervention studies, although indicating the benefit of sodium restriction on left ventricular hypertrophy and cardiac function in dialysis patients (13,14), did not show the actual sodium intake levels during the study period.
Of note, there is a phenomenon of heterogeneity in the correlation between sodium intake, estimated by either dietary intake or urinary removal, and overall and CVD mortality across the healthy, obese, and hypertensive populations (4,7–12). To some extent, the heterogeneity is due to the fact that sodium intake is parallel with protein and energy or other nutrient intakes. Accordingly, the correlation of sodium intake and mortality probably is confounded by the intake of these nutrients. For example, when sodium intake is positively correlated with energy intake (7,9), it predicts improved survival and vice versa (11,12,15). Deficient protein and energy intake and malnutrition are present in approximately 20% to 50% of the maintenance dialysis patients (16,17). Therefore, we hypothesized that dietary sodium intake may be negatively correlated with overall and CVD mortality because low sodium intake could be accompanied by deficient protein and energy intake in this population.
Therefore, we aim to explore the correlation of dietary sodium intake and overall and CVD mortality in dialysis patients, and to further determine whether this correlation can be explained by protein and energy intake through this retrospective cohort study. To our knowledge, this is the first study to address this issue in the dialysis population.
Materials and Methods
Subjects and Follow-Up
Our retrospective cohort study enrolled a total of 305 incident patients who started PD and lived longer than 6 months in our unit from July 2002 to February 2007. All patients could visit a physician at least once every 3 months. Demographic and clinical data collected within the week preceding PD catheter implantation included age, gender, body mass index (BMI), etiology of end-stage renal disease, diabetes (DM), and CVD (18). BMI higher than 23 kg/m2 was defined as overweight according to the Asian standard (19). All patients were followed until death, transfer to hemodialysis, renal transplantation, or February 2008. Both CVD death and overall death were recorded. All patients were delivered lactate-buffered glucose PD solutions and had the twin-bag connection system (Baxter Healthcare, Guangzhou, China). The study was approved by the Medical Ethical Committee of Peking University. Written informed consent was obtained from each patient.
Dietary Variables
A daily intake of no more than 2.3 g per day of sodium, at least 0.8 g/kg protein, and 25 kcal/kg energy have been recommended in terms of our previous data and guidelines from the European Best Practice Guidelines and International Society of Renal Nutrition & Metabolism at the initiation of PD treatment (18,20,21). During the follow-up, all patients completed 3-day dietary records before they visited the dietitian. A dedicated dietitian checked the diary using food models. The dietary records would be invalid if they were recorded in less than 3 days or did not get checked successfully by the dietitian. Daily sodium, protein, energy, carbohydrate, fat, potassium, and fiber were calculated by using a computer software program (PD information Management System, Peritoneal Dialysis Center, Peking University, Beijing, China). The total caloric intakes include intakes from dietary and dialysate sources. Both daily total protein and daily energy intakes were normalized for standard body weight. Sodium intake during the first 3 months was represented as the baseline sodium intake. All of the measurements during the study were averaged.
Several steps were taken to ensure the reliability and stability of dietary sodium intake assessment: (1) each patient was asked to measure the amount of added salt by using a 1-g or 2-g salt spoon and soy sauce using a 5-ml little cup. (2) Patients avoided processed foods and eating out because the amount of hidden salt would be unknown. (3) Patients were taught to check the amount of salt labeled on snack or canned foods. (4) Patients ate foods separately from family members to ascertain how much salt they consumed. (5) Dietitian and primary nurses repeatedly highlighted the importance of recording dietary salt intake.
Measurement of Blood Pressure and Antihypertensive Medications
Systolic and diastolic BPs (SBP and DBP) were measured according to the standard method. Mean arterial pressure (MAP) was calculated. The dose of antihypertensive drugs was quantified by the defined daily dose (DDD) developed by the World Health Organization (22). The SBP, DBP, MAP, and DDD during the first 3 months were averaged as the baseline values. All of the measurements for them were averaged during the study.
Biochemical, Dialysis Adequacy, and Nutrition Variables
Biochemical indices, including hemoglobin (Hb), serum albumin (Alb), prealbumin (PA), blood urea nitrogen (BUN), serum creatinine (Scr), calcium (Ca), phosphate (P), bicarbonate, and LDL were examined using an automatic Hitachi chemistry analyzer at regular intervals. The product of Ca and P was calculated. Estimated GFR was calculated by a Chinese equation before PD catheterization (23). Biochemical indices during the first 3 months were represented as baseline values, and all of the measurements throughout the study were averaged. Serum high-sensitive C-reactive protein (CRP) measured by immune rate nephelometric analysis during the first 3 months was represented as a baseline value. Dialysis adequacy was calculated by collecting dialysate and urine over the course of 24 hours to measure fluid and solute clearances. Weekly total, peritoneal, and renal Kt/V urea; weekly total, peritoneal, and renal creatinine clearance; and residual renal function were calculated using standard methods. Lean body mass (LBM) by creatinine kinetics method was used to reflect muscle protein stores (24,25) and also normalized by the square of height. Total sodium removal was the sum of urinary and dialysate sodium removal (26). The total Kt/V (TKt/V), total creatinine clearance (Tccr), total sodium removal, and LBM during the first 6 months were represented as baseline values. All of the measurements throughout the study were averaged.
Statistical Analyses
Statistical analyses were performed using the SPSS software package (version 13.0; SPSS, Chicago, IL). Variables are expressed in a standard way. Average sodium intake was categorized by tertile based on the distribution among the study population. One-way ANOVA, Kruskal-Wallis, or the χ2 test was used to compare the differences of variables between groups. Partial correlation analysis was used to analyze the correlation of sodium intake to nutritional and dietary variables adjusted for age and gender. Recognized confounders combined with the baseline and average sodium intakes, respectively, were evaluated by the Cox proportional regression model to determine the risk for CVD or overall mortality. When the baseline sodium intake was examined, the covariates included age, gender, BMI, the history of DM or CVD, baseline TKt/V, Tccr, MAP, Alb, Hb, Ca × P, LDL, and CRP; when the average sodium intake was examined, the covariates included age, gender, BMI, the history of DM or CVD, average TKt/V, Tccr, MAP, Alb, Hb, Ca × P, and LDL. Next, to examine the predicting role of average sodium intake in CVD and overall mortality, we included each dietary variable individually as a continuous variable in separate multivariate models to examine their impact on the hazard ratio (HR) estimate for the average sodium intake. Among these dietary variables, dietary energy intake instead of total energy intake was used. The final models contained the variables that remained in the model with a significance level of 0.05. The HRs and their 95% confidence intervals for mortality were shown in the final results. We accepted P < 0.05 as the indicator of statistical significance.
Results
Subject Demographics and Follow-up
We followed 305 incident PD patients (129 men, 176 women), mean age of 59.4 ± 14.2 years (range: 19∼94 years), for 31.4 ± 13.7 months (range: 8∼64 months); 42.3% (129 of 305) were men, 40.3% (123 of 305) had diabetes, and CVD was present in 61.6% (188 of 305).
At the end of study, 187 patients were still being maintained on PD, 74 had died, 16 had transferred to hemodialysis, 24 had undergone renal transplantation, and four had transferred to other hospitals. The causes of death were cardiovascular diseases in 32 patients, systemic infection in 32, severe malnutrition in four, and unknown causes or multiple organ failure in six. A total of 43.2% (32 of 74) of all deaths were due to cardiovascular causes.
Average Total Sodium Intake and Baseline Characteristics
The average sodium intake was 1.82 g/d (0.76∼5.53 g/d) in our cohort. The baseline characteristics of the study population tertiled by the average sodium intake are given in Table 1. Participants with the highest intake of sodium were more likely to be younger, male, overweight, and have lower CRP levels (P < 0.05). The prevalence of DM and CVD, and baseline estimated GFR, Hb, and Alb levels were not significantly different between groups. The average sodium intake in the high tertile group tended to have higher sodium removal (P < 0.001) and slightly higher DBP (P = 0.07). No significant differences in average SBP, MAP, and DDD levels were observed between groups (Table 2).
Table 1.
Baseline demographic and clinical characteristics of study subjects according to tertile of average sodium intake
| Basic Characteristic | Tertile of Average Sodium Intake |
P | ||
|---|---|---|---|---|
| Low | Middle | High | ||
| Follow-up, mo | 30.59 ± 11.31 | 32.88 ± 13.82 | 30.56 ± 15.63 | 0.38 |
| Age, yr | 63.08 ± 12.8a | 61.07 ± 13.02a | 54.16 ± 15.14 | <0.001b |
| Male gender, % (n) | 20.8 (21/101)a | 42.2 (43/102)c | 63.7 (65/102) | <0.001b |
| BMI, kg/m2 | 22.63 ± 3.59c,d | 23.89 ± 3.71 | 23.68 ± 3.94 | 0.04e |
| Diabetes, % (n) | 33.7 (34/101) | 46.1 (47/102) | 41.2 (42/102) | 0.16 |
| Cardiovascular disease, % (n) | 64.4 (65/101) | 61.8 (63/102) | 58.8 (60/102) | 0.82 |
| Urine volume, ml | 808.97 ± 425.80a | 882.31 ± 435.93c | 1010.85 ± 496.14 | 0.007 |
| eGFR, ml/min per 1.73 m2 | 6.64 (1.8∼21.4) | 6.97 (2.4∼16.7) | 6.32 (1.5∼23.2) | 0.43 |
| Hb, g/L | 100.70 ± 16.82 | 102.75 ± 16.33 | 104.36 ± 16.11 | 0.31 |
| Alb, g/L | 35.82 ± 4.86 | 35.32 ± 4.32 | 35.69 ± 4.48 | 0.58 |
| CRP, mg/L | 4.11 (0.17∼292) | 2.31 (0.17∼90.7) | 2.08 (0.17∼59.78) | 0.008e |
| Bicarbonate, mmol/L | 24.04 ± 2.51 | 24.36 ± 2.32 | 23.75 ± 2.56 | 0.26 |
Values are mean ± SEM, median (minimum to maximum), or absolute numbers with percentages.
P < 0.001 compared to high-tertile group.
P < 0.001 compared between three groups.
P < 0.05 compared to high-tertile group.
P < 0.001 compared to middle-tertile group.
P < 0.05 compared between three groups.
Table 2.
The average sodium intake and removal, BP, and defined daily dose of antihypertensive drugs of study subjects according to tertile of average sodium intake
| Basic Characteristic | Tertile of Average Sodium Intake |
P | ||
|---|---|---|---|---|
| Low | Middle | High | ||
| Sodium intake, g/d | 1.41 ± 0.17a,b | 1.81 ± 0.11c | 2.47 ± 0.54 | <0.001d |
| Sodium removal, g/d | 2.20 ± 1.21a,b | 2.78 ± 1.09 | 3.03 ± 1.11 | <0.001d |
| SBP, mmHg | 136.55 ± 15.88 | 136.90 ± 15.07 | 135.69 ± 15.05 | 0.91 |
| DBP, mmHg | 77.84 ± 10.95c | 78.24 ± 9.17 | 80.91 ± 10.65 | 0.07 |
| MAP, mmHg | 97.41 ± 10.99 | 97.66 ± 9.67 | 99.17 ± 10.27 | 0.42 |
| Defined daily dose | 0.30 (0 to 1.16) | 1.74 (1.17 to 2.40) | 3.52 (2.4 to 10.67) | 0.35 |
Values are mean ± SEM or median (minimum to maximum)
P < 0.001 compared to high-tertile group.
P < 0.001 compared to middle tertile group.
P < 0.05 compared to high-tertile group.
P < 0.001 compared between three groups.
Average Sodium Intake, Dietary, and Nutritional Variables
The comparison of nutritional and dietary variables showed that patients in the high tertile of average sodium intake have significantly higher Alb, PA, LBM, and LBM/height (27) levels (P < 0.001∼0.05), as well as higher protein, energy, fat, carbohydrate, fiber, and potassium intake compared with patients in low tertile or both low and middle tertile groups (Table 3). Partial correlation analysis revealed that average sodium intake was significantly correlated with LBM, dietary protein, energy, fat, carbohydrate, potassium, and fiber intake, with r values of 0.13 (P = 0.02), 0.3 (P < 0.001), 0.29 (P < 0.001), 0.31 (P < 0.001), 0.21 (P < 0.001), and 0.19 (P = 0.001) respectively. There was no correlation between average sodium intake and Hb, Alb, and PA levels.
Table 3.
The average levels of nutritional, dietary, and related clinical variables of study subjects according to tertile of average sodium intake
| Variables | Tertile of Average Sodium Intake |
P | ||
|---|---|---|---|---|
| Low | Middle | High | ||
| Hb, g/L | 106.53 ± 14.47a | 107.59 ± 13.16 | 110.65 ± 14.76 | 0.10 |
| Alb, g/L | 35.45 ± 3.40a | 36.11 ± 4.66 | 36.94 ± 4.26 | 0.04b |
| PA, mg/dl | 266.32 ± 129.02c | 288.40 ± 89.24 | 304.70 ± 87.61 | 0.03b |
| LBM, kg | 32.22 ± 7.38c,d | 36.11 ± 7.95c | 41.36 ± 9.57 | <0.001e |
| LBM/height2, kg/m2 | 12.7 ± 2.42c,d | 13.64 ± 2.53c | 15.06 ± 2.89 | <0.001e |
| DPI, g/kg per d | 0.75 ± 0.14c,d | 0.84 ± 0.15 | 0.87 ± 0.16 | <0.001e |
| DEI, kcal/kg per d | 26.23 ± 3.74c,f | 28.47 ± 3.67 | 29.33 ± 4.55 | <0.001e |
| Total protein intake, g/d | 40.26 ± 8.38c,f | 47.73 ± 8.44a | 51.89 ± 10.46 | <0.001e |
| Total energy intake, kcal/d | 1145.79 ± 229.35c,f | 1341.19 ± 203.39c | 1469.19 ± 305.27 | <0.001e |
| Fat intake, g/d | 43.17 ± 8.44c,f | 52.10 ± 10.16c | 58.36 ± 16.59 | <0.001e |
| Carbohydrate intake, g/d | 161.87 ± 36.81c,d | 180.81 ± 33.19a | 199.67 ± 54.19 | <0.001e |
| Potassium intake, g/d | 1.14 ± 0.29c,d | 1.33 ± 0.27 | 1.37 ± 0.32 | <0.001e |
| Fiber intake, g/d | 6.19 ± 2.05c,f | 7.45 ± 2.35 | 7.32 ± 3.24 | 0.001 |
| Bicarbonate, mmol/L | 25.32 ± 2.21 | 25.45 ± 1.77 | 24.99 ± 2.09 | 0.27 |
| TKt/V | 1.80 ± 0.43 | 1.85 ± 0.47 | 1.91 ± 0.42 | 0.20 |
| Tccr, L/wk per 1.73 m2 | 62.89 ± 22.67 | 67.88 ± 30.64 | 68.59 ± 22.14 | 0.22 |
| Residul renal function, ml/min | 0.72 (0 to 1.33) | 2.22 (1.34 to 3.12) | 4.64 (3.13 to 18.67) | 0.18 |
Values are mean ± SEM or median (minimum-maximum). DPI, daily protein intake; DEI, daily energy intake.
P < 0.05 compared between to high-tertile group.
P < 0.05 compared between three groups.
P < 0.001 compared to high-tertile group.
P < 0.01 compared to middle-tertile group.
P < 0.001 compared between three groups.
P < 0.001 compared to middle-tertile group.
Predictive Value of Sodium Intake for Overall and CVD Mortality
The relationship between baseline or average sodium intake and mortality was analyzed respectively. The baseline sodium intake was significantly associated with overall mortality after adjusting for age, gender, BMI, DM and CVD history, MAP, Hb, Alb, Ca × P, LDL, Kt/V, Tccr, and CRP, with an HR of 0.45 (0.23 to 0.90) (P = 0.02), and showed a trend to be correlated with CVD mortality, with an HR of 0.33 (0.10 to 1.10) (P = 0.07) (Table 4). Similarly, average sodium intake was correlated with overall mortality, with an HR of 0.44 (0.20 to 0.95) (P = 0.04), and CVD mortality, with an HR of 0.11 (0.03 to 0.48) (P = 0.003), after adjusting for above covariates besides CRP (Table 5).
Table 4.
Baseline sodium intake and adjusted variables associated with overall and cardiovascular mortality in Cox proportional-hazard regression
| Overall Mortality |
Cardiovascular Mortality |
|||||
|---|---|---|---|---|---|---|
| β | P | HR (95% CI) | β | P | HR (95% CI) | |
| Age-, gender- and BMI-adjusted | ||||||
| Sodium intake, g/d | −0.14 | 0.36 | 0.87 (0.65∼1.17) | −0.43 | 0.09 | 0.65 (0.39∼1.08) |
| Age, yr | 0.04 | 0.001 | 1.04 (1.02∼1.06) | |||
| Multivariate-adjusteda | ||||||
| Sodium intake,g/d | −0.79 | 0.02 | 0.45 (0.23∼0.90) | −1.12 | 0.07 | 0.33 (0.10∼1.10) |
| DM | 1.28 | 0.005 | 3.59 (1.45∼8.71) | 1.43 | 0.04 | 4.18 (1.06∼16.4) |
| Alb,g/dl | −0.15 | 0.03 | 0.86 (0.76∼0.98) | |||
| CRP | 0.03 | 0.05 | 1.03 (1.00∼1.05) | |||
CI, confidence interval.
Adjusted for age, gender, BMI, DM, and CVD history, baseline MAP, Hb, Alb, Ca × P, LDL, Kt/V, Tccr, and CRP.
Table 5.
Average sodium intake and adjusted averaged variables associated with overall and cardiovascular mortality in Cox proportional-hazard regression
| Overall Mortality |
Cardiovascular Mortality |
|||||
|---|---|---|---|---|---|---|
| β | P | HR (95% CI) | β | P | HR (95% CI) | |
| Age-, gender- and BMI-adjusted | ||||||
| Sodium intake, g/d | −0.69 | 0.03 | 0.49 (0.27∼0.93) | −1.18 | 0.03 | 0.31 (0.11∼0.87) |
| Age, yr | 0.03 | 0.001 | 1.04 (1.01∼1.06) | |||
| Multivariate-adjusteda | ||||||
| Sodium intake, g/d | −0.83 | 0.04 | 0.44 (0.20∼0.95) | −2.19−1.30 | 0.003 | 0.11 (0.03∼0.48) |
| MAP | −0.29 | 0.05 | 0.97 (0.95∼0.99) | −0.31 | 0.05 | 0.32 (0.11∼0.84) |
| Alb, g/dl | −0.27 | <0.001 | 0.76 (0.69∼0.83) | <0.001 | 0.73 (0.64∼0.84) | |
| Hb, g/l | −0.03 | 0.003 | 0.97 (0.95∼0.99) | |||
CI, confidence interval.
Adjusted for age, gender, BMI, DM, and CVD history, average MAP, Hb, Alb, Ca × P, LDL, Kt/V, and creatinine clearancer
Predictive Value of Sodium Intake for Overall and CVD Mortality and the Impact of Adjustment for Dietary Nutrients
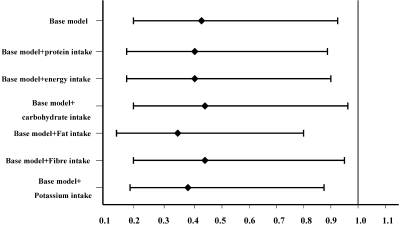
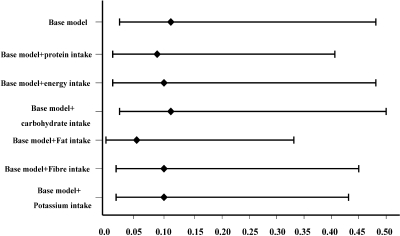
When we further included dietary protein, energy, carbohydrate, fat, fiber, and potassium intakes individually into separate multivariate models, there was no material impact on the HR estimate for average sodium intake for overall mortality, with HRs of 0.41 (P = 0.03), 0.41 (P = 0.03), 0.44 (P = 0.04), 0.35 (P = 0.01), 0.44 (P = 0.04), and 0.39 (P = 0.02), respectively (Figure 1), or for CVD mortality, with HRs of 0.09 (P = 0.002), 0.10 (P = 0.004), 0.11 (P = 0.004), 0.06 (P = 0.001), 0.10 (P = 0.003), and 0.10 (P = 0.002), respectively (Figure 2).
Figure 1.
Multivariate hazard ratio of average dietary sodium intake for all-cause mortality and the impact of adjustment for dietary nutrients. Base model was adjusted for age, gender, body mass index, DM, the history of CVD, averaged variables including mean arterial pressure, Ca × P, hemoglobin, albumin, LDL, TKt/V, and Tccr. Other models were adjusted for covariates included in base model, sequentially added dietary nutrients including dietary protein, energy, carbohydrate, fat, fiber, and potassium intake. The P values for HRs of average dietary sodium intake for all-cause mortality in these models were less than 0.05.
Figure 2.
Multivariate hazard ratio of average dietary sodium intake for CVD mortality and the impact of adjustment for dietary nutrients. Base model was adjusted for age, gender, body mass index, DM, the history of CVD, averaged variables including mean arterial pressure, Ca × P, hemoglobin, albumin, LDL, TKt/V, and Tccr. Other models were adjusted for covariates included in base model, sequentially added dietary nutrients including dietary protein, energy, carbohydrate, fat, fiber, and potassium intake. The P values for HRs of average dietary sodium intake for CVD mortality in these models were less than 0.05.
Discussion
Our study showed that participants with the highest average sodium intake were more likely to be younger, male, and overweight according to Asian standards (19), and they had better muscle protein stores as estimated by LBM and higher nutrient intakes. The average low sodium intake was a significantly independent predictor of high overall and CVD mortality, even with adjustment for dietary protein, energy, and other nutrient intakes individually. The baseline low sodium intake also independently predicted the high overall mortality as well as showed a trend to predict high CVD mortality.
These data further support our hypothesis that sodium intake per se is closely correlated with protein and energy intake, and the correlation of sodium intake and mortality is confounded by these nutrient intakes. Indeed, low sodium intake also is linked to lower nutrient intakes in the general population. In the National Health and Nutrition Examination Survey (NHANES) I study, subjects in the lowest quartile of sodium intake had the lowest calorie intake (7). In the NHANES II study, subjects with a lower sodium intake also had lower dietary calories and potassium levels (9). Both studies showed the inverse correlations between sodium intake and cardiovascular outcome similar to ours. By contrast, a statistically significant direct association of sodium with CVD and overall mortality was observed in a Finnish community sample (12) and the overweight subgroup of the NHANES I study (11), with stroke in a community sample in Japan (15). Among these three studies, subjects with the lowest sodium intake or sodium-to-energy ratio had relatively high BMI (12), adequate protein, and/or energy intake (11,15).
Our finding provided another example of “reversal epidemiologic” phenomenon, which has already existed in the relationship between a couple of CVD risk factors and outcome in dialysis population (28). In fact, a “U”- or “J”-shaped curve with a horizontal axis of sodium intake and a vertical axis of overall or cardiovascular mortality can well describe their relationship in the general population (29). In our study, low and middle tertiles of average sodium intake (that is, 1.41 g/d and 1.8 g/d) might fall in the down slope of this curve. Therefore, in the case of dialysis patients, harm may outweigh benefit if low-sodium diet came from anorexia and deficient nutrient intakes. We need to cautiously recommend sodium restriction if patients on dialysis treatment already suffer from deficient nutritional intake and protein-energy malnutrition.
Of interest, low sodium intake is significantly related to overall and CVD mortality despite adjusting for protein, energy, and other nutrient intakes in our cohort. This result indicates that the other potential mechanisms linking low dietary sodium to poor outcome are unknown. Sodium restriction did generate undesirable effects in previous studies, including increased insulin resistance (30), activation of the renin-angiotensin system (23), and increased sympathetic nerve activity (32). On the other hand, the positive correlation of sodium intake and mortality is supposed to be built on the direct link of sodium intake to hypertension, which did not exist in our specific population. One possible reason is that patients with higher sodium intake also had higher sodium removal and numerically higher DDD. In summary, the health effects of low sodium intake in this cohort ultimately depend on the sum of these recognized (nutrient deficiency, worse nutrition, activation of endocrinal hormone, no advantage in BP) and probably other unrecognized intermediate effects (4).
Dietary sodium intake was often estimated by a 24-hour dietary recall, food frequency questionnaire, or 24-hour urine sodium output. However, 24-hour urine sodium output cannot reflect the actual sodium intake in CKD and chronic heart failure patients (33), although it was thought a gold standard for measuring sodium intake in healthy subjects. In the dialysis population, sodium removal consists of urine and dialysate sodium output. Sodium removal through drained dialysate depends on the convection through the peritoneal membrane; therefore, it also cannot reflect the actual sodium intake in PD patients even though they lose the urine (26). Of interest, although we tried to achieve a precise estimation by repeatedly educating and training patients, we finally found the sodium intake was actually less than the sodium removal in our patients. So far, only three studies simultaneously present sodium intake and sodium removal data in PD patients showing the similar results as we do; namely, sodium removal higher than sodium intake (34–36). Obviously, a well-designed sodium balance study need to be done in the dialysis population to figure out the gap between sodium intake and sodium removal. On the other hand, the actual sodium intake in this study is markedly lower than the average levels of 6 g/d for the general population in Beijing, northern China (37). The energy and protein intake are even low in the highest tertile of sodium intake. We cannot preclude that some degree of underreporting may have occurred even if subjects were well-educated. Memory can be faulty, estimates of portion size can be mistaken, and diet can change from day to day. However, to the extent that such variation was random, it would tend to mute the relation of exposure (sodium) to outcome (death).
This study has several strengths. To our knowledge, this is the first study to verify that both the baseline and average sodium intakes can predict the overall and CVD mortality. Of note, all of the observational studies assessed exposure to dietary sodium only once at baseline throughout the long follow-up periods, the longest being up to 20 years (4). We realized a single baseline assessment of dietary sodium is inappropriate in the dialysis population because that population's sodium intake probably changed over time as the result of salt-restricted education, or it fluctuated because of intermittent gastrointestinal symptoms. In addition, the patients were thoroughly examined with nutritional and dietary variables during the follow up, which gave us the unique chance to determine the impact of nutrients on the relationship between sodium intake and mortality.
We realize the limitations of this study. The dietary recall of sodium intake was probably underestimated even if subjects were well-educated, as discussed above. We cannot preclude an unrecognized factor confounds the observed associations by being associated with both exposure and outcome. Accordingly, we cannot further speculate on the potential mechanisms of increased death risk of low sodium intake because of the lack of information on insulin resistance, renin-angiotensin system, sympathetic nerve activity, or other aspects.
Conclusions
Our study revealed that low sodium intake was closely correlated with nutrient deficits and poor muscle protein stores. Both the baseline and average low sodium intakes were significantly independent predictors of high overall and CVD mortality despite adjusting for recognized confounders and dietary nutrient intake. The potential mechanisms on the independent impact of sodium intake on survival are to be determined. Although our observational data cannot change therapeutic recommendations for dialysis patients (27,38), we reveal for the first time that low sodium intake is not necessarily a good thing if it comes from anorexia and protein-energy malnutrition in dialysis patients. How to prescribe the low-sodium diet for patients with various degrees of malnutrition is challenging.
Disclosures
None.
Acknowledgments
The authors express their appreciation to the patients and staff of the peritoneal dialysis center of First Hospital, Peking University, for their participation in the study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Elsayed EF, Tighiouart H, Griffith J, Kurth T, Levey AS, Salem D, Sarnak MJ, Weiner DE: Cardiovascular disease and subsequent kidney disease. Arch Intern Med 167: 1130–1136, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW; American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 42: 1050–1065, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Alderman MH: Evidence relating dietary sodium to cardiovascular disease. J Am Coll Nutr 25: 256S–261S, 2006 [DOI] [PubMed] [Google Scholar]
- 5.De Nicola L, Minutolo R, Bellizzi V, Zoccali C, Cianciaruso B, Andreucci VE, Fuiano G, Conte G: Achievement of target blood pressure levels in chronic kidney disease: A salty question? Am J Kidney Dis 43: 782–795, 2004 [DOI] [PubMed] [Google Scholar]
- 6.McCarron DA: Dietary sodium and cardiovascular and renal disease risk factors: Dark horse or phantom entry? Nephrol Dial Transplant 23: 2133–2137, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alderman MH, Cohen H, Madhavan S: Dietary sodium intake and mortality: The National Health and Nutrition Examination Survey (NHANES I). Lancet 351: 781–785, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Alderman MH, Madhavan S, Cohen H, Sealey JE, Laragh JH: Low urinary sodium is associated with greater risk of myocardial infarction among treated hypertensive men. Hypertension 25: 1144–1152, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Cohen HW, Hailpern SM, Fang J, Alderman MH: Sodium intake and mortality in the NHANES II follow-up study. Am J Med 119: 275–e214, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Geleijnse JM, Witteman JC, Stijnen T, Kloos MW, Hofman A, Grobbee DE: Sodium and potassium intake and risk of cardiovascular events and all-cause mortality: The Rotterdam Study. Eur J Epidemiol 22: 763–770, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He J, Ogden LG, Vupputuri S, Bazzano LA, Loria C, Whelton PK: Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA 282: 2027–2034, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Tuomilehto J, Jousilahti P, Rastenyte D, Moltchanov V, Tanskanen A, Pietinen P, Nissinen A: Urinary sodium excretion and cardiovascular mortality in Finland: A prospective study. Lancet 357: 848–851, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Asci G, Ozkahya M, Duman S, Toz H, Erten S, Ok E: Volume control associated with better cardiac function in long-term peritoneal dialysis patients. Perit Dial Int 26: 85–88, 2006 [PubMed] [Google Scholar]
- 14.Ozkahya M, Ok E, Cirit M, Aydin S, Akcicek F, Basci A, Dorhout Mees EJ: Regression of left ventricular hypertrophy in haemodialysis patients by ultrafiltration and reduced salt intake without antihypertensive drugs. Nephrol Dial Transplant 13: 1489–1493, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Nagata C, Takatsuka N, Shimizu N, Shimizu H: Sodium intake and risk of death from stroke in Japanese men and women. Stroke 35: 1543–1547, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Dong J, Fan M, Qi H, Gan H, Liu H, Wang H: Clinical study on malnutrition and low take of protein and energy in peritoneal dialysis patients [in Chinese]. Zhonghua Yi Xue Za Zhi 82: 61–65, 2002 [PubMed] [Google Scholar]
- 17.Ikizler TA, Hakim RM: Nutrition in end-stage renal disease. Kidney Int 50: 343–357, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Dong J, Wang T, Wang HY: The impact of new comorbidities on nutritional status in continuous ambulatory peritoneal dialysis patients. Blood Purif 24: 517–5232006, [DOI] [PubMed] [Google Scholar]
- 19.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363: 157–163, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Trevino-Becerra A, Wanner C: A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73: 391–398, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Fouque D, Vennegoor M, ter Wee P, Wanner C, Basci A, Canaud B, Haage P, Konner K, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall J, Tordoir J, Vanholder R: EBPG guideline on nutrition. Nephrol Dial Transplant 22 [Suppl 2]: ii45–ii87, 2007 [DOI] [PubMed] [Google Scholar]
- 22.WHO Collaborating Centre for Drug Statistics Methodology: ATC/DDD system. [Accessed March 2008]. Last updated September 29, 2009. http://www.whocc.no/atcddd/.
- 23.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY: Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 17: 2937–2944, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Blake PG: A review of the DOQI recommendations for peritoneal dialysis. Dialysis Outcome Quality Initiative, National Kidney Foundation Perit Dial Int 18: 247–251, 1998 [PubMed] [Google Scholar]
- 25.Dong J, Li YJ, Lu XH, Gan HP, Zuo L, Wang HY: Correlations of lean body mass with nutritional indicators and mortality in patients on peritoneal dialysis. Kidney Int 73: 334–340, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Cheng LT, Wang T: Changes in total sodium intake do not lead to proportionate changes in total sodium removal in CAPD patients. Perit Dial Int 26: 218–223, 2006 [PubMed] [Google Scholar]
- 27.Hemodialysis Adequacy 2006 Work Group: Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis 48 [Suppl 1]: S2–S90, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kopple JD: The phenomenon of altered risk factor patterns or reverse epidemiology in persons with advanced chronic kidney failure. Am J Clin Nutr 81: 1257–1266, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Alderman MH: Presidential Address: 21st Scientific Meeting of the International Society of Hypertension: Dietary sodium and cardiovascular disease: The ‘J'-shaped relation. J Hypertens 25: 903–907, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Petrie JR, Morris AD, Minamisawa K, Hilditch TE, Elliott HL, Small M, McConnell J: Dietary sodium restriction impairs insulin sensitivity in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 83: 1552–1557, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Alderman MH, Madhavan S, Ooi WL, Cohen H, Sealey JE, Laragh JH: Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med 324: 1098–1104, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Grassi G, Dell'Oro R, Seravalle G, Foglia G, Trevano FQ, Mancia G: Short- and long-term neuroadrenergic effects of moderate dietary sodium restriction in essential hypertension. Circulation 106: 1957–1961, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Shemin D, Dworkin LD: Sodium balance in renal failure. Curr Opin Nephrol Hypertens 6: 128–132, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Asghar RB, Green S, Engel B, Davies SJ: Relationship of demographic, dietary, and clinical factors to the hydration status of patients on peritoneal dialysis. Perit Dial Int 24: 231–239, 2004 [PubMed] [Google Scholar]
- 35.Avila-Diaz M, Ventura MD, Valle D, Vicente-Martinez M, Garcia-Gonzalez Z, Cisneros A, Furlong MD, Gomez AM, Prado-Uribe MD, Amato D, Paniagua R: Inflammation and extracellular volume expansion are related to sodium and water removal in patients on peritoneal dialysis. Perit Dial Int 26: 574–580, 2006 [PubMed] [Google Scholar]
- 36.Fine A, Fontaine B, Ma M: Commonly prescribed salt intake in continuous ambulatory peritoneal dialysis patients is too restrictive: results of a double-blind crossover study. J Am Soc Nephrol 8: 1311–1314, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Brown IJ, Tzoulaki I, Candeias V, Elliott P: Salt intakes around the world: implications for public health. Int J Epidemiol 38: 791–813, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Lo WK, Bargman JM, Burkart J, Krediet RT, Pollock C, Kawanishi H, Blake PG: Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis. Perit Dial Int 26: 520–522, 2006 [PubMed] [Google Scholar]