Abstract
Background and objectives: The objective of this study was to evaluate epidemiology and outcomes of a large in-center nocturnal hemodialysis (INHD) program.
Design, setting, participants, & measurements: This case-control study compared patients who were on thrice-weekly INHD from 56 Fresenius Medical Care, North America facilities with conventional hemodialysis patients from 244 facilities within the surrounding geographic area. All INHD cases and conventional hemodialysis control subjects who were active as of January 1, 2007, were followed until December 31, 2007, for evaluation of mortality and hospitalization.
Results: As of January 1, 2007, 655 patients had been on INHD for 51 ± 73 d. Patients were younger, there were more male and black patients, and vintage was longer, but they had less diabetes compared with 15,334 control subjects. Unadjusted hazard ratio was 0.59 for mortality and 0.76 for hospitalization. After adjustment for case mix and access type, only hospitalization remained significant. Fewer INHD patients were hospitalized (48 versus 59%) with a normalized rate of 9.6 versus 13.5 hospital days per patient-year. INHD patients had greater interdialytic weight gains but lower BP. At baseline, hemoglobin values were similar, whereas albumin and phosphorus values favored INHD. Mean equilibrated Kt/V was higher in INHD patients related to longer treatment time, despite lower blood and dialysate flow rates.
Conclusions: Patients who were on INHD exhibited excellent quality indicators, with better survival and lower hospitalization rates. The relative contributions of patient selection versus effect of therapy on outcomes remain to be elucidated in prospective clinical trials.
Despite gradual improvement in survival in the past decade, overall death rates of long-term hemodialysis (HD) patients remain high (1). The Hemodialysis (HEMO) Study failed to show a significant benefit with increasing dialysis dosage based solely on greater urea clearance, within the framework of conventional thrice-weekly HD (2). Efforts to optimize renal replacement therapies to improve patient outcomes have spurred interest in more frequent dialysis regimens (3). While results from the Frequent Hemodialysis Network on two randomized trials that evaluated outcomes from short daily in-center and long nightly home dialysis are pending (4), two recent publications renewed interest in the potential impact of longer hemodialysis session length, also referred to as treatment time (TT), to improve survival within the most prevalent practice of a thrice-weekly regimen. The Australian/New Zealand experience and the pooled international (Europe, Japan, and the United States) experience from the Dialysis Outcomes and Practice Patterns Study (DOPPS) both indicated better survival as TT exceeds 4 h (240 min), with the US cohort averaging only 211 to 221 min (approximately 3.5 h) (5,6).
In contrast, patients in Tassin, France, have traditionally been prescribed HD for 8 h thrice weekly, performed as daylong treatments (also overnight), and have long been reported to have excellent outcomes (7). Although overnight HD outside the patient's home may have been performed in the early years of dialysis in North America (8), it was first reported as maintenance therapy in a 10-patient (and two-nurse) program initiated around September 1995 at a hospital-based dialysis unit in Montreal, Canada (9). Using a lower blood flow rate (BFR) of 200 ml/min with dialysate flow rate (DFR) at 500 ml/min, they reported an average single-pool Kt/V of 1.83 and noted potential beneficial effects on hypertension, acidosis, and phosphorus control. From 21 patients who had entered the program between September 1995 and January 1998, the authors concluded that long-duration overnight HD was a valuable therapeutic modality.
The first free-standing in-center nocturnal hemodialysis (INHD) program was started in the United States in April 1999 by Dr. Joe T. Chandler, in collaboration with Fresenius Medical Care, North America (FMCNA). As a result, in part, of Dr. Chandler's enthusiasm in sharing his INHD experience, increased physician interest toward INHD resulted in the program's growth to 56 facilities by year-end 2006. Recent publications indicated improvement in laboratory and patient outcomes upon conversion from conventional HD (CHD) to INHD in small, single-center experiences (10–12). We sought to evaluate the epidemiology and outcomes of prevalent INHD patients from the largest multicenter outpatient INHD program in the world.
Materials and Methods
Patient Population
For index cases, we collated demographic information (age, gender, race, diabetes, vintage, body surface area, and cause of renal disease) from all patients who were treated by INHD as of January 1, 2007, in 56 FMCNA facilities with an active program. The means of all available values for the month of December 2006 were obtained for TT, BFR, DFR, dialyzer type, systolic BP (SBP) measurements before and after treatments, interdialytic weight gains (IDWGs), fluid removed during dialysis (i.e., ultrafiltration [UF]) volume per treatment, and laboratory results (albumin, hemoglobin, pre- and postdialysis urea nitrogen, phosphorus, and transferrin saturation [TSat]). Albumin was determined by bromcresol green method, whereas equilibrated Kt/V (eKt/V) was derived from single-pool Kt/V obtained by urea kinetic modeling on the basis of two-sample blood urea nitrogen variable volume method, previously described (13). Because INHD facilities were not uniformly distributed around the country (Figure 1), we collated concurrent information from all other prevalent CHD patients treated in 244 FMCNA facilities within the surrounding geographic area as the 56 INHD facilities to serve as control subjects.
Figure 1.
Location of FMCNA facilities that provide INHD as of January 1, 2007, in the United States.
All patients were followed up to December 31, 2007, with the two primary outcomes being mortality (a composite of death and withdrawal from dialysis therapy) and hospitalization (i.e., time to first hospitalization event). Patients who were lost to follow-up during the year contributed exposure time until kidney transplantation or the last day before transfer out of the FMCNA system. Secondary outcomes included a comparison of the proportion of patients who were hospitalized and the average number of hospitalization events and hospital days, both normalized to actual exposure years. Because prevalent cases were already on INHD for an average of 51 ± 73 d as of January 1, 2007, laboratory values obtained in December 2006 for albumin, hemoglobin, eKt/V, and phosphorus as well as SBP, IDWG, and UF rate were considered “outcome measures” related to the therapy. Results from SF-36 quality-of-life surveys were available for one in five patients during the period from December 1, 2006, to March 31, 2007, so physical (PCS) and mental component scores (MCS) obtained during this period (all within 33 ± 23 d of January 1, 2007), albeit incomplete, were also reported.
Treatment Parameters
Within each INHD facility, a section was converted each night for nocturnal treatments, with the majority having only either an Monday-Wednesday-Friday or Tuesday-Thursday-Saturday nocturnal schedule because of the need to disinfect and regenerate the water system on off-nights. The average census was 10 to 12 patients per nocturnal shift, staffed by at least one nurse and one to two patient care technicians. Each nocturnal station used the Fresenius 2008H or 2008K hemodialysis machine (Fresenius USA, Walnut Creek, CA) with the sensitivity, volume, and intensity of machine alarms unaltered. Patients underwent dialysis either in a recliner that converts into a near-flat sleeping surface and can be placed in Trendelenburg position for emergencies (e.g., Champion Chairs [Elkhart, IN] or Winco Inc. [Ocala, FL]) or, in a few facilities, twin-size beds. All patients were treated using biocompatible synthetic high-flux dialyzers (Fresenius USA) without dialyzer reuse.
Individual physicians determined rounding schedules and patient-specific HD prescriptions and ordered additional laboratory tests beyond routine monthly blood draws at their discretion. All laboratory results were sent to a single central laboratory (Spectra Laboratory, Rockleigh, NJ). There were no restrictions on the type of vascular access, although a strict policy was enforced to keep accesses uncovered at all times during the treatment. Central venous catheters required the use of Hemosafe safety-lock connectors (Fresenius USA) to prevent accidental disconnection. Patients who were on INHD were followed by the same team of nutritionists and social workers. Lights were usually dimmed (not turned off) about 1 h after all patients had initiated treatment, and most patients slept, with the rare case requiring prescription sleep-inducing medication.
Statistical Analysis
Descriptive data at baseline were presented as means or percentages of the total. Statistical significance was determined on the basis of t tests and χ2 tests, where appropriate. Cox proportional hazard regression models were used to determine survival differences between INHD and CHD groups, by intention to treat in the main analysis. These models were presented as unadjusted, case mix–adjusted (includes age, gender, race, diabetes, vintage, and body surface area), and a case-mix + vascular access type–adjusted models. Laboratory values were considered as surrogate outcomes of the therapy and were not used as adjustors in the models. For SF-36 scores, a logistic regression model was constructed with treatment group as the outcome variable and PCS/MCS as the independent variable. Subsequent models also included adjustment for case mix and case mix plus vascular access. We performed two sensitivity analyses designed to test robustness of the primary Cox models for mortality and hospitalization: (1) Only concurrent patients who were treated by CHD within the same 56 INHD facilities served as controls; and (2) patients were censored upon changing modality when they did not resume baseline therapy within 30 d. All statistical tests were performed using SAS 9.2 (SAS Institute, Cary, NC).
Results
The study cohort included 655 patients who were treated by INHD for an average of 55 ± 73 d as of January 1, 2007, along with 15,334 control subjects who were treated by CHD, with patient characteristics shown in Table 1. Patients who were on INHD were younger (51.2 versus 62.4 yr) but with longer vintage (4.8 versus 3.6 yr), were more likely to be male (69 versus 53%) and black (52 versus 45%), had larger body surface area (2.01 versus 1.82 m2) and proportionately more fistulas (51 versus 42%), and were less likely to have diabetes (42 versus 51%; all P < 0.0001).
Table 1.
Characteristics of Patients Treated by INHD and control subjects on CHD as of January 1, 2007
| Characteristics at Baseline | In-Center Long-Term Dialysis |
|
|---|---|---|
| INHDa | CHD | |
| Patients (n [%]) | 655 (4) | 15,334 (96) |
| Age (yr; mean ± SD) | 51.2 ± 12.7 | 62.4 ± 15.0 |
| Male gender (%) | 69.2 | 52.9 |
| Race (%) | ||
| black | 52.4 | 44.6 |
| white | 44.1 | 49.0 |
| other | 3.5 | 6.4 |
| Any diabetes (%) | 42.3 | 51.3 |
| Vintage (yr; mean ± SD) | 4.8 ± 4.5 | 3.6 ± 3.7 |
| Body surface area (m2; mean ± SD) | 2.01 ± 0.34 | 1.82 ± 0.28 |
| Cause of ESRD (%) | ||
| diabetes | 31.0 | 41.3 |
| hypertension | 36.6 | 34.7 |
| glomerulonephritis | 16.6 | 8.9 |
| hereditary/cystic | 3.5 | 2.3 |
| other (includes unknown) | 12.3 | 12.8 |
| Vascular access (%) | ||
| fistula | 50.8 | 41.6 |
| graft | 23.7 | 25.5 |
| catheter | 25.5 | 32.7 |
| unknown | 0.0 | 0.2 |
Compared with CHD, all significant at P < 0.0001.
Patients who were on INHD exhibited favorable quality-of-care indicators (Table 2) compared with control subjects, such as higher albumin (3.95 versus 3.81 g/dl; P < 0.0001) and lower phosphorus (5.3 versus 5.5 mg/dl; P = 0.003) values. Hemoglobin was similar (12.2 versus 12.1 g/dl; P = 0.3), although INHD patients had slightly lower TSat (25.4 versus 27.2%; P < 0.0001). The mean eKt/V was higher (2.21 versus 1.46) related to longer TT (470 versus 222 min) in INHD patients, despite lower BFR (306 versus 414 ml/min), lower DFR (496 versus 682 ml/min), and greater use of smaller (membrane) surface area dialyzers (73 versus 44% dialyzer membrane surface area of 1.5 m2; all P < 0.0001). The extended TT allowed for slower UF rate (6.0 versus 8.8 ml/h per kg; P < 0.0001) despite greater IDWG (4.0 versus 2.8 kg; P < 0.0001), requiring larger UF volumes in INHD; however, as a percentage of body weight, IDWG was only slightly greater in INHD patients (4.4 versus 3.7%; P < 0.0001), reflecting larger body size of patients who were on INHD.
Table 2.
Treatment parameters and intermediate outcomes for patients who were treated by INHD and matched control subjects who were on CHD as of January 1, 2007 (includes mean of last recorded values obtained from the previous month)
| Parameter | In-Center Long-Term Hemodialysis |
|
|---|---|---|
| INHD | CHD | |
| Treatment information | ||
| time (min; mean ± SD) | 470 ± 29 | 222 ± 28 |
| BFR (ml/min; mean ± SD) | 306 ± 58 | 414 ± 57 |
| DFR (ml/min; mean ± SD) | 496 ± 118 | 682 ± 129 |
| dialyzer surface area (%) | ||
| 1.5 m2 | 72.8 | 44.0 |
| 1.8 m2 | 24.9 | 47.9 |
| 2.0 m2 | 2.1 | 7.5 |
| other dialyzers | 0.2 | 0.6 |
| SF-36 quality-of-life scores (mean ± SD)a | ||
| PCS | 37.5 ± 11.7 | 33.1 ± 10.6 |
| MCS | 49.0 ± 10.6b | 47.9 ± 11.2 |
| Laboratory variables (mean ± SD) | ||
| eKt/V | 2.21 ± 0.56 | 1.46 ± 0.32 |
| hemoglobin (g/dl) | 12.16 ± 1.43b | 12.11 ± 1.40 |
| albumin (g/dl) | 3.95 ± 0.36 | 3.81 ± 0.42 |
| phosphorus (mg/dl) | 5.31 ± 1.54c | 5.50 ± 1.64 |
| TSat (%) | 25.4 ± 9.9 | 27.2 ± 11.2 |
| Interdialytic weight gain (kg; mean ± SD) | 4.0 ± 1.5 | 2.8 ± 1.2 |
| Interdialytic weight gain (% of weight; mean ± SD) | 4.4 ± 1.6 | 3.7 ± 1.4 |
| UF volume (L; mean ± SD) | 4.0 ± 1.4 | 2.5 ± 1.1 |
| UF rate (ml/h per kg; mean ± SD) | 6.0 ± 2.0 | 8.8 ± 2.9 |
| Predialysis SBP (mmHg; mean ± SD) | 147.7 ± 21.1d | 149.9 ± 21.5 |
| Postdialysis SBP (mmHg; mean ± SD) | 130.4 ± 19.4 | 135.3 ± 18.9 |
Only one in five patients with SF-36 scores in both groups (20.5% for INHD and 23.4% for CHD).
P = 0.3,
P = 0.003,
P = 0.009 versus CHD (all others at P < 0.0001).
The distribution of mean UF volume in INHD patients was 4.0 ± 1.4 L, matching that for IDWG at exactly 4.0 ± 1.4 kg, likely reflecting appropriate fluid balance. In contrast, the distribution of mean UF volume in control subjects was 2.5 ± 1.0 L, whereas mean IDWG was 2.8 ± 1.2 kg, reflecting mismatches between fluid gain and fluid removal. In addition, SBPs were lower by 2 mmHg before dialysis (P = 0.009) and by 5 mmHg after dialysis (P < 0.0001) in the INHD group, although comparative use of antihypertensive medications was not available. Results from SF-36 surveys were available for only 20.5% of patients and 23.4% of CHD control subjects, with mean PCS of 37.5 ± 11.7 compared with 33.1 ± 10.6 (P < 0.0001), respectively. Although INHD patients' PCS scores were approximately 4 points higher than those of control subjects, adjustment for case mix and vascular access type reduced the difference to 2.7 points (P = 0.003), still favoring INHD. MCS scores were not significantly different between groups.
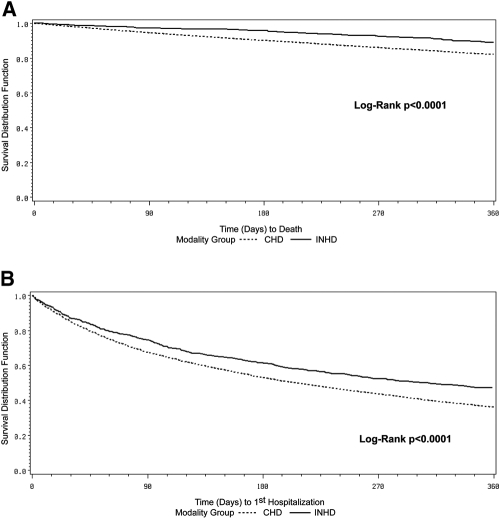
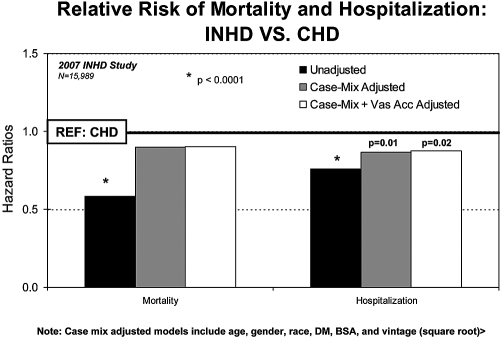
Overall exposure time at risk was 570 patient-years on INHD and 13,014 patient-years on CHD. Unadjusted 1-yr survival and hospitalization-free survival, both of which were significantly better for INHD relative to CHD (P < 0.0001), are depicted using the Kaplan-Meier method in Figure 2. The unadjusted hazard ratio (HR) was 0.59 (95% confidence interval [CI] 0.46 to 0.75) for mortality and 0.76 (95% CI 0.68 to 0.85) for hospitalization (both P < 0.0001). Adjustment for case mix and vascular access type indicated mortality HR of 0.90, although the difference with control subjects was no longer statistically significant (P = 0.4); however, the difference in hospitalization risk remained significant after adjustment for case mix and access type (HR 0.88; 95% CI 0.78 to 0.98; P < 0.02; Figure 3). Fewer INHD patients were hospitalized (48 versus 59%) during the year, with fewer normalized hospitalization events at 1.26 versus 1.74 hospitalization events per patient-year and a lower normalized rate of 9.6 versus 13.5 hospital days per patient-year (all P < 0.0001). The sensitivity analysis using only the 3720 CHD patients who were treated within the same 56 INHD facilities as control subjects yielded results very similar to the primary analysis (data not shown). A second sensitivity analysis censored patients upon switching modality when they did not resume it within 30 d, and results indicated slightly improved HR for INHD compared with those in the primary analysis, although the study conclusions were unchanged (data not shown).
Figure 2.
Kaplan-Meier unadjusted 1-yr survival curves comparing patients on INHD (solid line) with patients on CHD (dotted line) in terms of mortality (A) and hospitalization (B).
Figure 3.
Results from Cox proportional hazard models comparing time to death and time to first hospitalization from patients who were treated by INHD with patients who were on CHD. Case mix–adjusted models included age, gender, race, diabetes, body surface area, and vintage (square root).
During the year-long study period, patients spent an average of 263 ± 126 d (median 365 d) on INHD therapy. From 655 patients at baseline, 381 (58.2%) were still on INHD at the end of follow-up. Of these, 334 (51%) were on INHD throughout, whereas 47 (7.2%) discontinued INHD temporarily then resumed therapy by year-end. Other than deaths, 63 (9.6%) patients transferred out of FMCNA and 45 (6.9%) patients underwent transplantation. Only 481 survivors were actively undergoing dialysis (from the original INHD cohort) on December 31, 2007, with 381 of them still being treated by INHD, indicating 1-yr technique survival rate of 79.2%. Among patients who abandoned INHD during the study period, only five switched to peritoneal dialysis and two to home HD, with the rest opting for CHD. Their median time to discontinuing INHD was 136 d (mean 147 ± 95 d).
Discussion
To our knowledge, this study is the first to evaluate mortality and hospitalization outcomes in INHD patients relative to patients on CHD and is the largest study of INHD patients reported to date. Patients who were on INHD exhibited 41% lower HR for mortality and 24% lower HR for hospitalization compared with CHD control subjects; however, favorable survival factors related to younger age, longer vintage, less diabetes, more black patients, and larger body size were notable in INHD patients. Thus, although a 10% mortality advantage remained after adjustment for case mix and vascular access, mortality was no longer statistically significant (but hospitalization risk remained significant lower by 12.4% after similar adjustment). The study was underpowered to detect a significant survival difference of only 10%, when considering the great imbalance in demographic characteristics between patients and control subjects and only a 1-yr follow-up period, on the basis of previous power calculation (14). This was further accentuated by a death rate in the control group of only 17%, much lower than the 22.5% rate reported by US Renal Data System for the US HD population (1).
Nevertheless, all laboratory-based quality-of-care indicators that we examined favored INHD (13). Because of doubling of TT, dialysis dosage was markedly increased beyond the differences attained in the HEMO Study (2). Longer treatments eclipsed the impact of lower BFR, lower DFR, and preponderant use of dialyzers with the smallest membrane surface area. Perhaps improved clearance with or without contribution from slower UF rate played a role in subsequent results for albumin, phosphorus, and hemoglobin, all of which tended to improve (albeit not always statistically significantly) in association with INHD in previous reports (10–12). The difference in mean albumin observed between patients and control subjects was only approximately 0.15 g/dl; however, this difference may be clinically significant, because we previously noted that even a 0.2-g/dl differential in albumin levels between groups was associated with marked difference in mortality and hospitalization risk (14). Phosphorus was approximately 0.2 mg/dl lower, even with greater dietary intake, consistent with the higher albumin levels and approximately 1.2-kg heavier IDWG. These results were not surprising considering that extensive phosphorus clearance has been a key advantage of nightly home HD in the setting of improved dietary intake and to a lesser extent also been noted in daylong thrice-weekly HD (7,16). Hemoglobin levels were similar between patients and control subjects, because it was actively managed by careful titration of erythropoietin dosage and maintenance of iron stores. The group means for TSat were clearly above the minimum target of 20%, although the average TSat was slightly lower in INHD patients versus control subjects. This finding may reflect more efficient use of iron for hematopoiesis, a potential benefit of more effective clearance of uremic toxins (17,18).
As in daylong HD treatments, improved fluid management leading to more effective BP control has also been reported as a major benefit of long-duration HD (7,19). Troidle et al. (10) showed increased IDWG and subsequent UF in patients who converted from CHD to INHD; overall mean UF rate also declined from 10.3 to 5.9 ml/h per kg. There was an associated decline in post-HD SBP from 136 to 128 mmHg in that study. We confirmed similar differences between patients and control subjects in this study. Despite much greater IDWG, INHD patients were able to balance larger weight gains with appropriately greater fluid removal. In addition, UF occurred gently, at slower normalized UF rates, than in CHD control subjects. Finally, even in this setting of greater IDWG, we determined that both pre- and post-HD SBP were lower in patients than in control subjects.
The growth of INHD therapy is remarkable considering the barriers to implementing INHD in the United States: (1) Criticism that historical outcome data were skewed by highly selected patients; (2) competitive “modality choice” because many patients who are eligible for INHD are often also eligible for home dialysis options; (3) logistical issues facing clinic managers from accommodating longer TT for patients in outpatient dialysis units that are filled to capacity; (4) the constant struggle to convince patients to stay longer for in-center treatments, even by just a few minutes; (5) local staffing issues as a result of a variable supply of dialysis nurses and patient care technicians who are willing to do a night shift; and (6) increased cost of providing therapy without additional reimbursement, becoming apparent when patient participation falls below critical mass. INHD is clearly not for all patients with ESRD (20), but it seems to have a niche among options for renal replacement therapy. Of note, there were proportionately more black patients on INHD, unlike home nocturnal HD programs that were predominantly white (21). Future research is needed to explore the causes and implications of this observation, particularly with continued growth of the program.
The study has several limitations. First, the observational design delineates associations but does not prove causation. A second limitation may be the lack of adjustment for patient comorbidity beyond diabetes; however, recent findings indicated that the contribution of comorbidity in survival studies may be less than expected (22). Third, we studied a prevalent sample of patients, affected by lead time/survival biases; however, mortality was not significantly different between groups in this study, negating the largest potential bias. Furthermore, the bias that is associated with longer vintage tends to narrow potential differences in hospitalization because the cumulative risk for hospitalization and subsequent cumulative incidence inevitably increases over time in surviving dialysis patients. Finally, there remains unmeasured residual confounding because patients who are on INHD are a selected (or self-selected) group. Clearly, this initial evaluation provides an overview of INHD therapy in comparison with CHD but is more hypothesis generating than conclusive, supporting the need for additional studies.
Conclusions
A cross-sectional evaluation of patients who were on INHD exhibited excellent quality indicators with improved fluid balance and slightly lower BP, better survival, and lower hospitalization rates relative to CHD patients within the same geographic area. Characteristics of patients who opt for INHD are not representative of the general CHD population, such that the relative contributions of patient selection versus effect of therapy on outcomes remain to be elucidated. Prospective studies are needed to evaluate this novel, rapidly expanding therapeutic option.
Disclosure
All authors are employees of Fresenius Medical Care, North America.
Acknowledgments
Part of this material was presented in abstract form at the annual meeting of the American Society of Nephrology; San Diego, CA; October 27 through November 1, 2009.
We thank Dr. Joe T. Chandler for providing historical information and reviewing the manuscript. We are also grateful to Sandra MacGregor for compiling information about the FMCNA nocturnal in-center programs. Finally, we thank clinical staff who work the night shift and maintain excellent quality of care for patients who are on INHD therapy.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.US Renal Data System: USRDS Annual Data Report: Atlas of Chronic Kidney and End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008 [Google Scholar]
- 2.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R: Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Lacson E, Jr, Diaz-Buxo JA: Daily and nocturnal hemodialysis: How do they stack up? Am J Kidney Dis 38: 225–239, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Suri RS, Garg AX, Chertow GM, Levin NW, Rocco MV, Greene T, Beck GJ, Gassman JJ, Eggers PW, Star RA, Ornt DB, Kliger AS: Frequent Hemodialysis Network (FHN) randomized trials: Study design. Kidney Int 71: 349–359, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Saran R, Bragg-Gresham JL, Levin NW, Twardowski ZJ, Wizemann V, Saito A, Kimata N, Gillespie BW, Combe C, Bommer J, Akiba T, Mapes DL, Young EW, Port FK: Longer treatment time and slower ultrafiltration in hemodialysis: Associations with reduced mortality in the DOPPS. Kidney Int 69: 1222–1228, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Marshall MR, Byrne BG, Kerr PG, McDonald SP: Associations of hemodialysis dose and session length with mortality risk in Australian and New Zealand patients. Kidney Int 69: 1229–1236, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Charra B, Chazot C, Jean G, Hurot JM, Vanel T, Terrat JC, VoVan C: Long 3 x 8 hr dialysis: A three-decade summary. J Nephrol 16[Suppl 7]: S64–S69, 2003 [PubMed] [Google Scholar]
- 8.Shaldon S: First use of nocturnal hemodialysis. Kidney Int 76: 230, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Deziel C, Bichet D, Cartier P, Charbonneau R, Daigneault B, Madore F, Querin S: In-center long duration (8h) overnight hemodialysis (LDHD): Sept. 95–May 1999 [Abstract]. J Am Soc Nephrol 10: 190A, 1999 [Google Scholar]
- 10.Troidle L, Hotchkiss M, Finkelstein F: A thrice weekly in-center nocturnal hemodialysis program. Adv Chronic Kidney Dis 14: 244–248, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Bugeja A, Dacouris N, Thomas A, Marticorena R, McFarlane P, Donnelly S, Goldstein M: In-center nocturnal hemodialysis: Another option in the management of chronic kidney disease. Clin J Am Soc Nephrol 4: 778–783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell JR, Oluwaseun O, Woo YM, Padmanabhan N, Narasinghan E, Latta C, Tortolano J, Jardine AG, Geddes CC: Ten years experience of in-center thrice weekly long overnight hemodialysis. Clin J Am Soc Nephrol 4: 1097–1101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacson E, Jr, Wang W, Hakim RM, Teng M, Lazarus JM: Associates of mortality and hospitalization in hemodialysis: Potentially actionable laboratory variables and vascular access. Am J Kidney Dis 53: 79–90, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Greene T, Beck GJ, Gassman JJ, Gotch FA, Kusek JW, Levey AS, Levin NW, Schulman G, Eknoyan G: Design and statistical issues of the hemodialysis (HEMO) study. Control Clin Trials 21: 502–525, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Lacson E, Jr, Ikizler TA, Lazarus JM, Teng M, Hakim RM: Potential impact of nutritional intervention on end-stage renal disease hospitalization, death, and treatment costs. J Ren Nutr 17: 363–371, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Mucsi I, Hercz G, Uldall R, Ouwendyk M, Francoeur R, Pierratos A: Control of serum phosphate without any phosphate binders in patients treated with nocturnal hemodialysis. Kidney Int 53: 1399–1404, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Saito A, Suzuki I, Chung TG, Okamoto T, Hotta T: Separation of an inhibitor of erythropoiesis in “middle molecules” from hemodialysate from patients with chronic renal failure. Clin Chem 32: 1938–1941, 1986 [PubMed] [Google Scholar]
- 18.Ifudu O, Feldman J, Friedman EA: The intensity of hemodialysis and the response to erythropoietin in patients with end-stage renal disease. N Engl J Med 334: 420–425, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Pierratos A: Daily (quotidian) nocturnal home hemodialysis: Nine years later. Hemodial Int 8: 45–50, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Ramkumar N, Beddhu S, Eggers P, Pappas LM, Cheung AK: Patient preferences for in-center intense hemodialysis. Hemodial Int 9: 281–295, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Lockridge RS, Jr, Spencer M, Craft V, Pipkin M, Campbell D, McPhatter L, Albert J, Anderson H, Jennings F, Barger T: Nocturnal home hemodialysis in North America. Adv Ren Replace Ther 8: 250–256, 2001 [DOI] [PubMed] [Google Scholar]
- 22.van Manen JG, van Dijk PC, Stel VS, Dekker FW, Cleries M, Conte F, Feest T, Kramar R, Leivestad T, Briggs JD, Stengel B, Jager KJ: Confounding effect of comorbidity in survival studies in patients on renal replacement therapy. Nephrol Dial Transplant 22: 187–195, 2007 [DOI] [PubMed] [Google Scholar]