Abstract
Background and objectives: Kidney biopsy (KB), to date the only tool for the evaluation of renal fibrosis, carries specific risks, and its relevance is limited by the small size of renal parenchyma assessed. Thus, a noninvasive alternative to KB is required. Collagen type III amino-terminal propeptide (PIIINP) is a degradation product of collagen type III, the increase of which may reflect an ongoing fibrotic process.
Design, setting, participants, & measurements: In a prospective study including 199 patients with various stages of chronic kidney disease (CKD), the association between urinary PIIINP/creatinine ratio (UPIIINP/Cr), patients' characteristics, and renal fibrosis was assessed.
Results: A total of 118 of the patients had UPIIINP/Cr measured simultaneously with the performance of a KB. In patients, median UPIIINP/Cr was 290 ng/mmol versus 93.7 ng/mmol in controls. In univariate analysis, UPIIINP/Cr was correlated with serum creatinine, estimated GFR, CKD stage, presence of coronary artery disease, and nephropathy type (glomerulonephritis versus other types). In multivariate analysis, only estimated GFR and nephropathy type were correlated with UPIIINP/Cr. UPIIINP/Cr was closely correlated with the extent of interstitial fibrosis in KB assessed using two different methods. Moreover, UPIIINP/Cr >800 ng/mmol had a negative predictive value of 94% to detect patients in whom KB will eventually prove “noninformative” (KB leading neither to a definite diagnosis of nephropathy nor to specific treatment).
Conclusions: UPIIINP/Cr is a promising fibro-test for the kidney and may alleviate the need for KB in some patients with CKD. Its predictive value for CKD progression deserves evaluation in prospective studies.
Glomerular and interstitial fibrosis are the hallmark of progressive chronic kidney diseases (CKDs). Fibrosis is due to the increased deposition of various extracellular matrix components, such as fibronectin, decorin, and several types of collagen, including collagen type I and type III. In the normal kidney, small amounts of collagen type III are expressed in the interstitium, but this type of collagen remains undetectable in glomeruli (1). In contrast, in scarred kidneys, the expression of collagen type III is increased in the interstitium during the earliest steps of fibrosis, and PIIINP eventually accumulates in sclerotic glomeruli. Collagen type III is synthesized as a procollagen with amino-terminal propeptides (PIIINPs) present at both extremities of the molecule. During the processing of the procollagen before its deposition in the extracellular matrix, some of the PIIINPs are cleaved and released in the extracellular matrix and fluids, including blood and urine. The PIIINP molecule consists of three identical polypeptide chains, has a molecular weight of 42 kD, and has a short half-life (1 hour). It is degraded mainly in the liver into high- and low-molecular weight fragments (2).
Circulating and fluid PIIINP levels have been shown to reflect the fibrotic process occurring during various pathologic conditions, such as acute lung injury (3,4), viral and nonviral liver diseases (5,6), systemic sclerosis (7,8), and vascular remodeling (9). Besides, two previous studies have suggested that serum and urinary PIIINP levels may prove useful in the assessment of renal fibrosis in the native as well as in the transplanted kidney (1,10).
We undertook a prospective study to assess the association between urinary PIIINP levels and patients' characteristics and the use of urinary PIIINP as a noninvasive marker of renal fibrosis.
Materials and Methods
This prospective study was undertaken in a university hospital, Hôpital Necker, Paris, France, between January 2006 and June 2008.
Included in the study:
(1) All patients admitted to the Nephrology Department in Hospital Necker for a kidney biopsy (KB).
(2) Outpatients seen by one nephrologist (F.F.) during a 3-month period.
Excluded from the study:
(1) Patients with significant impairment of liver function, because hepatic dysfunction interferes with PIIINP metabolism.
(2) Patients with extrarenal fibrotic diseases, such as systemic sclerosis and pulmonary fibrosis.
(3) Patients with acute renal failure.
(4) Patients with a transplanted kidney.
(5) Patients with a presumptive diagnosis of the type of nephropathy not documented by a KB.
For all patients, relevant clinical and biologic data were collected.
All patients gave an informed consent, and the study was approved by the hospital Ethics Committee.
Hypertension was defined by a systolic BP >140 mmHg or a diastolic BP >90 mmHg on two consecutive visits, or the need for antihypertensive therapy. Renal function was assessed by estimated GFR (eGFR), estimated using the Modification of Diet in Renal Disease study four-item equation, which is validated in the French population (11). Measurement of serum creatinine was carried out using a modified kinetic Jaffe colorimetric method.
CKD stages were defined and classified according to the Kidney Disease Outcomes Quality Initiative criteria (12). Etiologic diagnosis of CKD was classified as vascular disease, diabetic nephropathy, glomerulonephritis, and tubulointerstitial disease. Hereditary renal diseases were classified according to their predominant renal histopathologic lesion (glomerular, tubulointerstitial, or vascular).
UPIIINP/Cr Measurement
Patients' urine samples (first urine of the morning) were collected during routine urine analysis by dipstick and stored at −20°C until assay. Samples were stored for less than 1 month before the assay was performed. Urine was also collected from 37 healthy volunteers without kidney disease and with normal eGFR (mean: 105 ml/min ± 15 ml/min).
PIIINP urinary level was measured using a commercialized RIA kit (RIA-Gnost PIIINP; CIS Bio International, Gif sur Yvette, France). This RIA test uses a human PIIINP peptide and a mouse anti-human PIIINP monoclonal antibody and is carried out using a pH buffer. It detects higher-molecular weight forms of PIIINP but not smaller degradation products. The intraassay and interassay coefficients of variation were 3.2% and 9.5%, respectively. Selected samples were tested and then stored for 2 months and retested. Results of the second assay varied by less than 5% compared with the initial assay. The detection limit of the test is 100 ng/ml. Creatinine level was measured in the same urine samples using a modified kinetic Jaffe colorimetric method, and urinary PIIINP concentrations were normalized for urinary creatinine concentration (UPIIINP/Cr).
Renal Fibrosis Assessment
Renal fibrosis was assessed using two distinct methods:
BANFF Classification.
Renal sections (fixed in Bouin solution, embedded in paraffin, sectioned at 2 mm, and stained with light green trichrome) were blindly reviewed by an expert pathologist (L.H.N.), and fibrosis was quantified using the BANFF criteria (13) adapted to the native kidney with a semiquantitative image analysis (grade 0: <10%, grade I: 10% to 25%, grade II: 25% to 50%, and grade III >50%). Glomerular fibrosis was estimated as the percentage of sclerotic glomeruli noted in a kidney section.
Color Segmentation Image Analysis Software.
Masson trichrome-stained kidney sections were analyzed using a previously described color segmentation image analysis software that quantifies interstitial fibrosis (14). Briefly, for each biopsy, a cortical section was imaged using a color video camera (Nikon DXM1200) mounted on a light microscope (Nikon Eclipse E1000M; magnification, ×40). Images of the entire cortex area of the biopsy were obtained. The medulla was excluded from analysis at the acquisition phase or during analysis by the observer. Images were analyzed using a color segmentation image analysis software on the basis of color image quantization and clustering to extract green pixels.
The program automatically extracts green color areas characteristic of interstitial fibrosis. Renal capsule, tubular basement membranes, and sclerotic glomeruli are recognized and automatically excluded from analysis. The proportion of green to nongreen pixels in the image is calculated and used as an index of interstitial fibrosis. An index of surface interstitial fibrosis was defined as the ratio between the surface area of green pixels to the total number of pixels in the original biopsy. It takes approximately 5 to 10 minutes to capture the image of each section and less than 1 minute to process the images.
Informative versus Noninformative KB
KBs were classified as “noninformative” if the extent of renal fibrosis precluded any definite diagnosis of the type of the nephropathy and/or did not lead to any treatment other than the supportive therapy that would have been instituted regardless of the results of the KB.
Statistical Analyses
Results are expressed as frequencies and percentages for categoric variables and as a mean (±SD) or median [range] for continuous variables. Because UPIIINP/Cr was not normally distributed, we used its log transformation in the whole analysis.
Associations of patients' clinical and biologic features at inclusion and UPIIINP/Cr measurement were tested by t test, ANOVA, or estimation of the Pearson correlation coefficient (or the Spearman correlation coefficient when appropriate). All variables with P value less than or equal to 0.10 in univariate analysis were included in a multivariate linear regression model. A backward stepwise linear regression analysis was used to identify the clinical factors independently associated with UPIIINP/Cr measurement. All statistical analyses were performed using R, version 2.5.0 (http://cran.r-project.org).
Results
During the study period, 199 patients had a UPIIINP/Cr measurement. Among the 199 remaining patients, 118 had a UPIIINP/Cr measurement simultaneously (within 1 month) with the KB.
Patients' characteristics at the time of UPIIINP/Cr measurement are summarized in Table 1. Most patients included presented with moderate CKD, with a median eGFR of 48.9 ml/min per 1.73 m2 (range: 3.4 to 203.1 ml/min per 1.73 m2), and a quarter of the patients (23%) had stage 4 or 5 CKD. Glomerulonephritis represented the main type of nephropathy (62%). Fifty-two percent of patients had hypertension and 42% received angiotensin-converting enzyme inhibitors and/or angiotensin II receptor blockers. In the 199 included patients, median UPIIINP/Cr was 290 ng/mmol (range: 2 to 5690 ng/mmol) versus 93.7 ng/ml (range: 2 to 110 ng/mmol) in healthy controls.
Table 1.
Patients' characteristics at inclusion in the study
| Variable | Value |
|---|---|
| At inclusion (n = 199) | |
| Age, yr, mean ± SD | 46 ± 17 |
| Male gender, n (%) | 96 (48) |
| Caucasian patients, n (%) | 175 (88) |
| BMI, kg/m2, mean ± SD (n = 99) | 24.2 ± 4.8 |
| Creatinine, μmol/L, median [range] | 123 [38 to 1186] |
| eGFR, ml/min per 1.73 m2, median [range] | 48.9 [3.4 to 203.1] |
| CKD stages, n (%) | |
| eGFR ≥ 90 ml/min per 1.73 m2 | 41 (21) |
| < 60 ≤ eGFR < 90 ml/min per 1.73 m2 | 44 (22) |
| 30 ≤ eGFR < 60 ml/min per 1.73 m2 | 68 (34) |
| 15 ≤ eGFR < 30 ml/min per 1.73 m2 | 29 (15) |
| eGFR < 15 ml/min per 1.73 m2 | 17 (8) |
| Etiology of CKD, n (%) | |
| Vascular disease | 20 (10) |
| Diabetic nephropathy | 13 (7) |
| Interstitial/obstructive nephropathy | 37 (19) |
| Glomerular disease | 124 (62) |
| Unknown cause | 5 (2) |
| Blood pressure: diastolic, mmHg, mean ± SD | 134 ± 21 |
| Blood pressure: systolic, mmHg, mean ± SD | 79 ± 12 |
| Proteinuria, g/24 h, median [range] | 1.0 [0 to 16.2] |
| Hypertension, n (%) | 103 (52) |
| ACEI/ARB, n (%) | 83 (42) |
| Diabetes mellitus, n (%) | 31 (16) |
| Coronary artery disease, n (%) | 16 (8) |
| Left ventricular hypertrophy, n (%) | 30 (18) |
| Liver disease, n (%) | 13 (7) |
| Chronic dialysis, n (%) | 22 (11) |
| At 1 yr of follow-up (n = 102) | |
| Creatinine, μmol/L, median [range] | 115 [38 to 1062] |
| Proteinuria, g/24 h, median [range] | 0.35 [0 to 7.7] |
| eGFR, ml/min per 1.73 m2, median [range] | 51.5 [6 to 203] |
| eGFR slope, ml/min per 1.73 m2 per yr, mean ± SD | 0.08 ± 1.48 |
| At the end of follow-up (n = 115) | |
| Creatinine, μmol/L, median [range] | 124 [38 to 1098] |
| eGFR, ml/min per 1.73 m2, median [range] | 50.0 [5 to 203] |
| Follow-up, mo, median [range] | 19.6 [9.7 to 29.7] |
| eGFR slope, ml/min per 1.73 m2 per yr, mean ± SD | −0.01 ± 1.26 |
BMI, body mass index; eGFR, estimated glomerular filtration rate estimated using the Modification of Diet in Renal Disease simplified formula; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin II receptor blockers.
As shown in Table 2, in univariate analysis, UPIIINP/Cr displayed close positive correlation with serum creatinine (r = 0.36; P < 0.0001) as well as close negative correlation with eGFR (r = −0.38; P < 0.0001). UPIIINP/Cr was also significantly correlated with age and the presence of coronary artery disease. UPIIINP/Cr also increased with CKD stage. Mean UPIIINP/Cr was significantly lower in patients with glomerulonephritis versus other types of nephropathies, this difference being most pronounced between glomerulonephritis (470 ± 740) versus tubulointerstitial/obstructive nephropathies (1070 ± 1340). Conversely, there was no correlation between UPIIINP/Cr and gender, body mass index, ethnicity, hypertension, diabetes mellitus, liver disease, albuminemia, or proteinuria.
Table 2.
Relationship between UPIIINP/Cr and patients' clinical and biologic features at inclusion
| Variable | UPIIINP/Cr |
|
|---|---|---|
| Value | P | |
| Age, yr, r | 0.20 | 0.004 |
| Male/female, ng/mmol, mean ± SD | 660 ± 1020/650 ± 880 | 0.42 |
| Caucasian/black, ng/mmol, mean ± SD | 630 ± 910/830 ± 1210 | 0.49 |
| BMI, kg/m2, r (n = 99) | −0.04 | 0.66 |
| Creatinine, μmol/L, r | 0.36 | <0.0001 |
| eGFR, ml/min per 1.73 m2, r | −0.38 | <0.0001 |
| CKD stages, ng/mmol mean ± SD | ||
| eGFR ≥ 60 ml/min per 1.73 m2 | 500 ± 860 | |
| 30 ≤ eGFR < 60 ml/min per 1.73 m2 | 540 ± 720 | |
| 15 ≤ eGFR < 30 ml/min per 1.73 m2 | 730 ± 670 | |
| eGFR < 15 ml/min per 1.73 m2 | 1770 ± 1680 | |
| CKD etiology, ng/mmol, mean ± SDa | ||
| Glomerular disease | 470 ± 740 | |
| Vascular disease | 980 ± 1220 | 0.022 |
| Diabetic nephropathy | 770 ± 570 | 0.046 |
| Interstitial/obstructive nephropathy | 1070 ± 1340 | 0.0011 |
| Unknown etiology | 500 ± 530 | 0.92 |
| Proteinuria, g/24 h, r | 0.08 | 0.24 |
| Hypertension −/+, ng/mmol, mean ± SD | 640 ± 1000/670 ± 1000 | 0.92 |
| ACEI/ARB −/+, ng/mmol, mean ± SD | 790 ± 1530/470 ± 500 | 0.13 |
| Blood pressure systolic/diastolic, r | 0.12/0.14 | 0.10/0.06 |
| Diabetes mellitus −/+, ng/mmol, mean ± SD | 670 ± 1010/590 ± 470 | 0.39 |
| Ischemic heart disease −/+, ng/mmol, mean ± SD | 610 ± 850/1240 ± 1630 | 0.023 |
| Liver disease −/+, ng/mmol, mean ± SD | 630 ± 910/1080 ± 1380 | 0.26 |
BMI, body mass index; eGFR, estimated glomerular filtration rate estimated using the Modification of Diet in Renal Disease simplified formula; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin II receptor blockers.
Glomerular disease versus other.
A multivariate logistic regression model analysis was set up to identify independent factors correlated with UPIIINP/Cr. Models tested were limited to four potential factors: age, eGFR, CKD etiology, and coronary artery disease. After backward stepwise regression, age and coronary artery disease could be excluded, and the multivariate model was finally reduced to two independent factors correlated with UPIIINP/Cr: glomerular diseases and eGFR (Table 3).
Table 3.
Final multivariate regression model for associated factors with UPIIINP/Cr dosage
| Variable | Multivariate Analysis |
|
|---|---|---|
| Coefficient (SD) | P | |
| eGFR, ml/min per 1.73 m2 | −0.47 (0.11) | 0.002 |
| CKD etiology | ||
| Glomerular disease | 1 | |
| Other | 0.52 (0.16) | < 0.0001 |
eGFR, estimated glomerular filtration rate estimated using the Modification of Diet in Renal Disease simplified formula.
UPIIINP/Cr and Renal Fibrosis
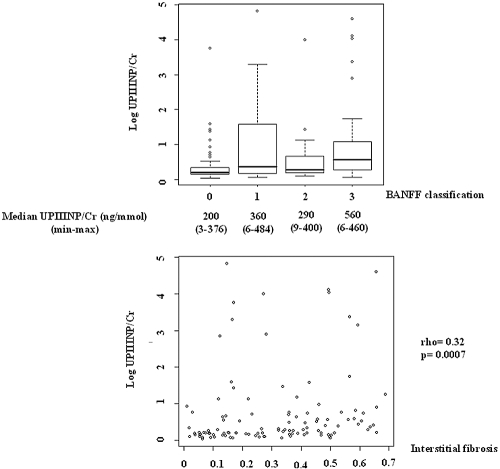
UPIIINP/Cr was highly associated with interstitial fibrosis assessed using the BANFF criteria (P = 0.005) (Figure 1) or the color segmentation image analysis software (r = 0.32; P = 0.0007). Conversely, PIIINP/Cr was not correlated with glomerular fibrosis (r = 0.12; P = 0.31).
Figure 1.
Upper panel: Evolution of urinary PIIINP/creatinine ratio according to BANFF classification. Lower panel: Correlation between urinary PIIINP/creatinine ratio and renal interstitial fibrosis estimated using color segmentation image analysis software. min, minimum; max, maximum.
In the 47 patients with an eGFR <45 ml/min per 1.73 m2, we estimated the sensitivity and specificity of UPIIINP/Cr in predicting the relevance of KB; i.e., “informative” versus “noninformative” KB. The threshold of an eGFR of 45 ml/min per 1.73 m2 was chosen because clinicians clearly need an assessment of the benefits-risk ratio of a KB in this particular subset of patients with significant renal function impairment. A UPIIINP/Cr threshold of 800 ng/mmol gave the best sensitivity and specificity. If we consider as a positive test a UPIIINP/Cr <800 ng/mmol, we obtained a sensitivity and specificity of 94% and 55%, respectively. For a UPIIINP/Cr ≥800 ng/mmol, the probability that a KB proves noninformative is 94% (negative predictive value). Conversely, for a UPIIINP/Cr <800 ng/mmol, the probability that a KB is informative is 94% (positive predictive value).
Discussion
To date, KB remains the only available tool for the evaluation of renal fibrosis. It requires preliminary tests and hospitalization and carries potential risks, including hematoma and hematuria. Moreover, its relevance may be limited because of the relatively small size of renal parenchyma assessed. The analysis of a limited area of the renal parenchyma does not necessarily reflect the ongoing fibrotic process occurring in other parts of the kidney. Thus, an alternative to KB for the assessment of renal fibrosis is required. Such noninvasive tests are already widely used as an alternative to biopsy for the evaluation of fibrosis in a major organ; namely, the liver. For instance, the “fibro-test,” a simple tool relying on easily available blood tests, is widely used as a first-line procedure for the staging of liver fibrosis (15,16).
We undertook a prospective study to assess the correlation of UPIIINP/Cr with patients' characteristics and renal fibrosis and the relevance of UPIIIN/Cr as a fibro-test for the nephrologist. Our results clearly show that UPIIINP/Cr is highly correlated with the extent of interstitial fibrosis assessed using two distinct methods. The relevance of PIIINP for the assessment of renal fibrosis in native kidneys has been previously evaluated, mainly in a single study conducted by Soylemezoglu et al. (1). However, this previous study included only 40 patients—mainly with primary glomerulonephritis—of whom 10 had already reached end-stage renal disease. In contrast, our study included 199 patients with a wide range of types of nephropathies and variable severity of CKD. Moreover, two distinct methods were used for the assessment of renal fibrosis, including recently developed color segmentation image analysis software (14). Thus, our report definitely documents the close correlation between UPIIINP/Cr and renal fibrosis. Besides, in contrast to the previous study by Soylemezoglu et al. (1), our data show a close correlation between UPIIINP/Cr and eGFR, underlying the fact that renal fibrosis is a major determinant of renal function.
Clearance of PIIINP takes place mainly in the liver, and to a lesser extent in the kidney (2). These data may suggest that urinary PIIINP could reflect, at least partially, the amount of circulating PIIINP filtrated and/or degraded by the kidney. However, it is usually assumed that only the smaller fragments of PIIINP are extracted by the kidney, and these fragments are not detected by the RIA kit used here. This is corroborated by our finding that UPIIINP/Cr did not correlate with proteinuria. Thus, UPIIINP/Cr is most likely to reflect the intrarenal synthesis of this peptide.
Our results also suggest that UPIIINP/Cr used as a fibro-test for the kidney may help the nephrologist avoid unnecessary KBs. Nephrologists frequently face the clinical dilemma of performing or not performing a KB in a patient with CKD, moderately atrophic kidneys, and nonactive urinary sediment. Two types of patients exemplify such dilemma: (1) patients presenting with significant renal impairment (eGFR <45 ml/min per 1.73 m2) in the absence of any previous assessment of renal function; and (2) patients with documented stable CKD and presenting with sudden and rapid degradation of their renal function, a pattern compatible both with the natural history of CKD and with the occurrence of superimposed acute damage to the kidney. The benefits of a KB in this setting (establish a diagnosis of nephropathy, decision to use or not use steroids and/or immunosuppressive agents) are to be weighed against the risks of such procedures in patients with CKD, reduced-size kidneys, and an increased risk for bleeding after KB. Nonetheless, the KB remains indicated in patients with nephrotic syndrome, active urine sediment, and/or extrarenal manifestations.
We believe that UPIIINP/Cr may prove helpful for the clinician facing such a dilemma. The excellent negative predictive value (around 95%) of UPIIINP/Cr for the relevance of KB noted here pleads for the use of UPIIINP/Cr as a first-line assessment of renal fibrosis. A UPIIINP/Cr ≥800 ng/mmol probably pleads against the performance of a KB which will prove most probably noninformative and risky. Similarly to the fibro-test used in hepatology, an optimal fibro-test for the nephrologist will probably have to include several parameters besides UPIIINP/Cr. TGFβ and fibronectin may be candidates for inclusion into such a compound score (17).
Another major potential role for UPIIINP/Cr in nephrology patients is the monitoring of the progression of CKD and the effect of therapeutic interventions, thus alleviating the need for iterative KBs. The limited follow-up of the patients included in our study (<2 years), and thus the absence of significant modification of eGFR during follow-up (Table 1), precluded any analysis of the predictive value of UPIIINP/Cr for the decline in renal function. However, in a study of renal transplant recipients (10), UPIIINP/Cr correlated with the evolution of eGFR. During a mean follow-up of roughly 4 years, GFR increased or remained unchanged in patients with a UPIIINP/Cr <100 ng/mmol and decreased in 42% of patients with a UPIIINP/Cr >100 ng/mmol. Finally, several therapeutic agents widely used in nephrology patients affect the expression of UPIIINP. For instance, steroids and cyclophosphamide decrease the synthesis of PIIINP (18–20). Whether the effect of these treatments on PIIINP accounts, at least in part, for their nephroprotective effect in various nephropathies deserves further evaluation.
In all, UPIIINP/Cr is probably a useful fibro-test for the kidney and may alleviate the need for a KB in some patients with CKD. Its clinical relevance remains to be confirmed in a prospective, multicentric study. Its predictive value for the progression of CKD and the effect of therapeutic agents deserve evaluation in prospective studies.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Soylemezoglu O, Wild G, Dalley AJ: Urinary and serum type III collagen: Markers of renal fibrosis. Nephrol Dial Transplant 12: 1883–1889, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Jensen LT, Henriksen JH, Risteli J: Fate of circulating amino-terminal propeptide of type III procollagen in conscious pigs. Am J Physiol 265: R139–R145, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Cinaz P, Buyan N, Gokcora N: Changes in serum type I and III procollagen levels in children with chronic renal failure. Nephron 72: 189–191, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Chesnutt AN, Matthay MA, Tibayan FA: Early detection of type III procollagen peptide in acute lung injury. Pathogenetic and prognostic significance. Am J Respir Crit Care Med 156: 840–845, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Teare JP, Sherman D, Greenfield SM: Comparison of serum procollagen III peptide concentrations and PGA index for assessment of hepatic fibrosis. Lancet 342: 895–898, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Zarski JP, Bedossa P, Bronowicki JP: Non invasive markers using for the assessment of fibrosis in hepatitis C [in French]. Gastroenterol Clin Biol 31: 4S34–4S39, 2007 [PubMed] [Google Scholar]
- 7.Nagy Z, Czirjak L: Increased levels of amino terminal propeptide of type III procollagen are an unfavourable predictor of survival in systemic sclerosis. Clin Exp Rheumatol 23: 165–172, 2005 [PubMed] [Google Scholar]
- 8.Scheja A, Wildt M, Wollheim FA: Circulating collagen metabolites in systemic sclerosis. Differences between limited and diffuse form and relationship with pulmonary involvement. Rheumatology (Oxford) 39: 1110–1113, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Lin YH, Ho YL, Wang TD: The relation of amino-terminal propeptide of type III procollagen and severity of coronary artery disease in patients without myocardial infarction or hibernation. Clin Biochem 39: 861–866, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Teppo AM, Tornroth T, Honkanen E: Urinary amino-terminal propeptide of type III procollagen (PIIINP) as a marker of interstitial fibrosis in renal transplant recipients. Transplantation 75: 2113–2119, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J, Greene T: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Glassock RJ, Winearls C: Screening for CKD with eGFR: Doubts and dangers. Clin J Am Soc Nephrol 3: 1563–1568, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Racusen LC, Halloran PF, Solez K: Banff 2003 meeting report: New diagnostic insights and standards. Am J Transplant 4: 1562–1566, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Servais A, Meas-Yedid V, Buchler M: Quantification of interstitial fibrosis by image analysis on routine renal biopsy in patients receiving cyclosporine. Transplantation 84: 1595–1601, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Shaheen AA, Wan AF, Myers RP: FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: A systematic review of diagnostic test accuracy. Am J Gastroenterol 102: 2589–2600, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Munteanu M, Ratziu V, Morra R: Noninvasive biomarkers for the screening of fibrosis, steatosis and steatohepatitis in patients with metabolic risk factors: FibroTest-FibroMax experience. J Gastrointestin Liver Dis 17: 187–191, 2008 [PubMed] [Google Scholar]
- 17.Deelman L, Sharma K: Mechanisms of kidney fibrosis and the role of antifibrotic therapies. Curr Opin Nephrol Hypertens 18: 85–90, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Heickendorff L, Zachariae H, Bjerring P: The use of serologic markers for collagen synthesis and degradation in systemic sclerosis. J Am Acad Dermatol 32: 584–588, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Nuutinen P, Riekki R, Parikka M: Modulation of collagen synthesis and mRNA by continuous and intermittent use of topical hydrocortisone in human skin. Br J Dermatol 148: 39–45, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Saarela T, Risteli J, Koivisto M: Effects of short-term dexamethasone treatment on collagen synthesis and degradation markers in preterm infants with developing lung disease. Acta Paediatr 92: 588–594, 2003 [DOI] [PubMed] [Google Scholar]