Abstract
Background and objectives: Health-related quality of life (HRQOL) is a measure of the well being of hemodialysis patients and an independent prognostic predictor. Our aim was to determine whether HRQOL among hemodialysis patients has changed over time.
Design, setting, participants, & measurements: We retrospectively analyzed data collected by Dialysis Clinic, Inc. from adult patients starting hemodialysis between January 1, 1997 and May 31, 2006. The primary outcome was HRQOL assessed by Short Form 36, 6 to 18 months after and closest to the 1-year anniversary of starting hemodialysis. Secular trends were analyzed by linear regression for continuous variables and logistic regression for categorical ones. Year of starting dialysis was the predictor. A five-point difference on a 0 to 100 scale was considered clinically significant.
Results: Short Form 36 scores were available for 11,079 patients. Role Physical, General Health, Vitality, Social Functioning, and Physical Component Summary scores were unchanged among patients over the study period. Statistically significant (P < 0.05) but clinically insignificant changes were observed in Physical Functioning (−0.2 points/yr), Bodily Pain (+0.2 points/yr), Mental Health (+0.15 points/yr), and Mental Component Summary scores (+0.13 points/yr). Only Role Emotional showed clinically significant improvement. Trends were unchanged after adjusting for age, gender, race, diabetes, hemoglobin, phosphorous, Kt/V, and albumin.
Conclusions: Most HRQOL domains showed either no statistically significant change or statistically but not clinically significant change over almost a decade. These results suggest that, despite important developments in hemodialysis care since 1997, little progress was made in improving HRQOL of hemodialysis patients.
Health-related quality of life (HRQOL) in patients with ESRD is compromised compared with that of the general population and of kidney transplant recipients (1–4), particularly in physical health domains (5). This diminished level of HRQOL is attributed to medical factors such as comorbidities and lower hemoglobin concentration (2,6), as well as to psychosocial factors related to the major lifestyle changes that dialysis imposes (1,5). Patient-reported HRQOL is an independent predictor of survival (7,8). Our aim was to examine whether self-reported, subjective quality of life of hemodialysis (HD) patients has changed since data on this outcome were first collected on a large scale in this population.
In 1995, routine measurement of responses to the Medical Outcomes Study Short Form 36 Item Health Survey (SF-36) (9) was initiated at clinics operated by Dialysis Clinic, Inc. (DCI), the largest not-for-profit dialysis provider in the United States. This study describes trends in self-perceived HRQOL ∼1 year after starting HD among patients starting dialysis between 1997 (the first year where the percentage of patients with SF-36 scores approached 50%) and 2006.
Dialysis patients' hemoglobin and Kt/V values rose in recent years, and the use of erythropoietin and vitamin D analogs expanded (10). Some of these interventions and trends were observed to have a positive impact on HRQOL (5,11). Clinical practice guidelines for dialysis treatment were promulgated, and the Centers for Medicare and Medicaid Services implemented public reporting of quality indicators (12). At the same time, the proportion of dialysis patients who were diabetic and average patient age both continued to rise (10).
We hypothesized that certain aspects of HD patients' HRQOL improved with advances in treatment and implementation of quality improvement and monitoring activities over the study period. We also speculated that trends in HRQOL could be influenced by demographic changes in the dialysis population over time. To explore these processes, we examine trends over time in patient-reported HRQOL at ∼1 year after staring dialysis, as well as trends in demographic and clinical variables. We discuss the implications of our findings for patient care and health policy.
Materials and Methods
This is a retrospective, observational, cross-sectional study. The study was approved by the Tufts Medical Center Institutional Review Board.
Patient Selection
Patients included in the study were selected from all patients who started dialysis in any DCI outpatient facility from January 1, 1997 through May 31, 2006. DCI is the largest not-for-profit dialysis provider in the United States, with facilities in 27 states. Eligible patients were older than 18 years of age and received chronic HD treatment for at least 6 months. Exclusion criteria included treatment by peritoneal dialysis, death, kidney transplantation or loss to follow-up within 6 months after starting dialysis.
Measurement of HRQOL
HRQOL was assessed using the Medical Outcomes Study SF-36. The SF-36 consists of eight domains (Physical Functioning, Role-Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role-Emotional, Mental Health), together totaling 36 questions. Two summary scores, the physical and mental composite score (PCS and MCS, respectively), were calculated from the eight domains using standard methods (13,14). The SF-36 scale score data are presented on a transformed 0 to 100 scale. During the period studied, DCI facilities administered the SF-36 two to four times per year. Facility staff chose the frequency of SF-36 administration and were prompted by staff of DCI's Outcomes Monitoring Program to offer it to all patients dialyzing at the facility. Patients with decreased visual acuity were interviewed by dialysis unit staff.
No systematic effort was made to obtain later responses from patients who were not present in the facility at the time of SF-36 administration, whether because of schedule changes, travel, or hospitalization. The SF-36 was offered in English, Spanish, French, German, Italian, Portuguese, Chinese, Vietnamese, and Korean. These analyses are based on SF-36 forms completed within 180 days of the 1-year anniversary of starting dialysis. The 1-year anniversary was chosen as a reference point with the intent to observe HRQOL after stabilization of clinical status. Where more than one form was completed during this time frame, the one closest to the 1-year anniversary of starting dialysis was chosen for analysis.
Clinical and Demographic Data
Patient age, race (black, white, Native American, Pacific Islander, Asian, other), presence of diabetes, laboratory data (hemoglobin, serum albumin and phosphorous concentrations, and single pool Kt/V), and primary cause of ESRD were entered by dialysis facility staff into DARWIN, DCI's electronic medical information system, for all DCI patients. These data were abstracted for this analysis. Laboratory data were those obtained on the date closest to SF-36 completion.
Statistical Analysis
Characteristics of patients with and without a completed SF-36 within 1 (±0.5) year of starting dialysis were compared using Student's t test for continuous variables and the χ2 test for categorical variables. Each SF-36 domain score and the PCS and MCS were described by a mean and SD. Changes in SF-36 scores over time were analyzed using linear regression with year as the predictor. Subsequent analyses incorporated age, gender, race, presence of diabetes, and hemoglobin, albumin, and phosphorous levels in a multivariate regression model. The multiple imputation method was used to account for missing data. Trends in binary outcomes (e.g., presence of diabetes over time) were determined using logistic regression and the two-sided Cochran-Armitage test for trend.
Results
We identified 39,045 patients who started HD in a DCI facility between January 1, 1997 and May 31, 2006. Patients who died, received a kidney transplant, or transferred out of a DCI facility or were censored for an unknown reason before completing 6 months of dialysis were excluded (n = 12,756), as were patients <18 years of age (n = 263) and patients treated by peritoneal dialysis (n = 1076), leaving 24,950 eligible patients. The proportion of excluded patients varied from 32.2 to 38.0% per year, with a statistically significant increase over the years (odds ratio = 1.01, P = 0.003). The rate of transplant as a cause of not completing 6 months of treatment did not significantly change over the time period studied, whereas the rate of death as a cause declined slightly (data not shown). Of the 24,950 eligible patients, 11,079 (44.4%) had completed an SF-36 between 6 and 18 months after starting dialysis.
Patient Characteristics
Over the 10-year period, the cohorts of patients who completed SF-36 questionnaires were similar across the years with respect to race and the proportion with diabetes and the mortality rate for the period between 6 and 18 months after starting dialysis (Table 1). The average age of the cohorts increased by 0.3 yr/yr, and the proportion of men increased by 2%/yr on average over the 10-year period. The time of SF-36 completion relative to starting dialysis was shortened by 6.7 days over the decade. Similar to the population included in the analysis, among patients who did not have a SF-36 score, there was a 0.2 yr/yr increase in age (P < 0.0001).
Table 1.
Characteristics of patients with SF-36 data 6 to 18 months after initiation of HD (n = 11,079)
| Year | Patients with SF-36 Completed 6 to 18 Months of Starting HD [N (% of total cohort)] | Mean Interval (days) Between Starting HD and SF-36 Administration (SD) | Mean Age (SD) | Male | Diabetes as the Cause of ESRD | Nonwhitea | Annual Mortality Rate of the Cohortb | SMR of All Hemodialysis Patients in DCI |
|---|---|---|---|---|---|---|---|---|
| 1997 | 999 (41.8%) | 328.2 (96.2) | 59.8 (15.3) | 50.0% | 48.4% | 45.1% | 7.3% | 0.90 |
| 1998 | 1103 (45.1%) | 323.7 (97.4) | 58.9 (15.3) | 53.8% | 51.7% | 46.3% | 7.1% | 0.95 |
| 1999 | 1186 (47.0%) | 312.8 (91.5) | 59.6 (15.6) | 55.7% | 48.4% | 42.3% | 8.4% | 0.98 |
| 2000 | 1152 (42.1%) | 297.1 (80.9) | 61.1 (14.8) | 52.3% | 51.0% | 39.1% | 10.6.% | 0.97 |
| 2001 | 954 (34.9%) | 370.0 (102.6) | 60.6 (14.8) | 52.1% | 55.1% | 42.7% | 6.6% | 0.96 |
| 2002 | 1383 (48.4%) | 319.5 (91.9) | 60.6 (14.8) | 54.4% | 50.0% | 42.1% | 8.3% | 0.94 |
| 2003 | 1318 (48.2%) | 318.5 (93.1) | 61.5 (15.1) | 53.9% | 50.2% | 44.1% | 6.4% | 0.96 |
| 2004 | 1245 (46.4%) | 323.6 (92.1) | 61.4 (15.1) | 56.6% | 53.9% | 40.6% | 5.2% | 0.93 |
| 2005 | 1296 (48.8%) | 323.1 (92.1) | 61.5 (15.1) | 55.7% | 48.5% | 43.7% | 8.2% | 0.95 |
| 2006c | 443 (37.4%) | 288.7 (70.1) | 60.9 (15.3) | 53.7% | 50.8% | 45.4% | 8.6% | 0.92 |
| Annual changed | Change in likelihood of completing an SF-36 within 6–18 months per increasing calendar year: OR (95% CI) = 0.99/yr (0.98–1.00); P = 0.002e | Change in the number of days between starting HD and SF-36 administration, per increasing calendar year: β coefficient (SE) = −0.67 days/yr (0.33) P = 0.045 | Change in age per increasing calendar year: β coefficient (SE) = +0.3 yr/yr (0.05) P < 0.0001f | Change in likelihood of male gender per increasing calendar year: OR (95% CI) = 1.02/yr (1.00–1.03) P = 0.02 | Change in likelihood of diabetes as the cause of ESRD per increasing calendar year: OR (95% CI) = 1.0/yr (0.99–1.02); P = 0.5 | Change in likelihood of non-white race per increasing calendar year: OR (95% CI) = 0.99/yr (0.98–1.01); P = 0.4 | Change in one-year mortality rate per increasing calendar year: OR (95% CI) = 0.98 (0.96–1.01), P = 0.3 |
Missing data (41 patients missing race).
Mortality rate calculated for the 1-year period between 6 and 18 months after the initiation of HD.
January to May.
Calculated through linear regression (for age, mean time between starting dialysis and SF-36 score) and logistic regression (for the other parameters) with calendar year as the predictor variable.
An incident patient was 0.99 times as likely to complete a SF-36 with each subsequent year after 1997.
The mean age of the population increased by 0.3 per year between 1997 and 2006.
OR, odds ratio; CI, confidence interval; SF-36, short form-36; SMR, standardized mortality ratio.
Thirty-five percent to 49% of patients each year completed the SF-36, 6 to 18 months after starting dialysis. Not unexpectedly, patients who did not complete an SF-36 had a higher mortality rate between 6 and 18 months after starting dialysis (19.3 versus 7.6%) compared with those who completed it (Table 2). The two populations were similar with respect to age, gender, and diabetes. There were slightly more nonwhite patients among those who did not have SF-36 data compared with those who did.
Table 2.
Comparison of patients with (n = 11,079) and without (n = 13,871) a completed SF-36 within 6 to 18 months after initiation of HD
| Patients Who Completed an SF-36 Within 6 to 18 Months After Initiation of HDa | Patients Who Did Not Complete an SF-36 Within 6 to 18 Months After Initiation of HDb | P Value | |
|---|---|---|---|
| Age (SD) | 60.7 ± 14.9 | 61.0 ± 15.6 | P = 0.16 |
| Percent diabetics | 50.2% | 49.7% | P = 0.43 |
| Percent men | 55.6% | 55.6% | P = 0.95 |
| Percent nonwhite | 42.9% | 44.3% | P = 0.03 |
| Annual mortality rate (6 to 18 months after starting dialysis) | 7.6% | 19.3% | P < 0.0001 |
Missing data: race missing for 41 patients.
Missing data: race missing for 154 patients and gender missing for 6 patients.
Trends in Clinical Characteristics by Year
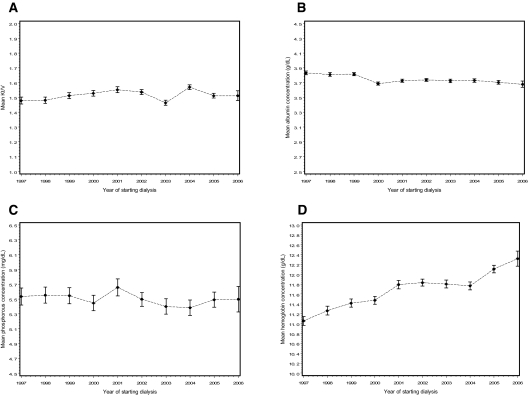
Linear regression analysis showed that the mean hemoglobin rose from 11.1 in 1997 to 12.3 in 2006 by an average of 0.1 g/dl (SE = 0.005) per year (P < 0.0001; Figure 1). Serum albumin concentration exhibited a very small, albeit statistically significant (P < 0.0001), decrease from 3.8 in 1997 to 3.7 in 2006. Phosphorous concentration decreased slightly by 0.01 mg/dl per year (P = 0.04), and Kt/V values rose by only 0.003/yr (P = 0.007) over the 10-year span.
Figure 1.
Secular trends (1/1997 to 5/2006) in mean (±SEM) laboratory values of patients with SF-36 data between 6 and 18 months after starting hemodialysis (n = 11,079): Kt/V (1,004 missing values) (A), serum albumin concentration (1,373 missing values) (B), phosphorous concentration (1,270 missing values) (C), and hemoglobin concentration (1,214 missing values) (D). The values chosen were those closest in time to the date of SF-36 questionnaire administration.
Secular Trends in HRQOL
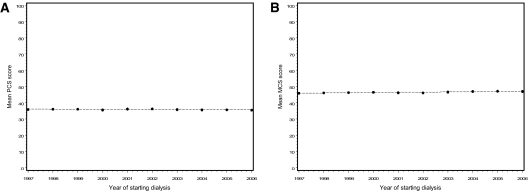
The minimum and maximum SF-36 scale scores observed between 1997 and 2006 for each of the scales were as follows: Physical Functioning, 40.9 to 45.0; Role Physical, 33.3 to 37.0; Bodily Pain, 33.1 to 36.6; General Health, 60.2 to 61.1; Vitality, 57.8 to 58.8; Social Functioning, 48.5 to 49.4; Role Emotional, 58.6 to 64.9; Mental Health, 61.2 to 62.9; PCS score, 35.4 to 36.2; MCS, 45.8 to 47.1. Among the eight basic SF-36 scale scores, statistically significant changes over the years were seen in Physical Functioning (−0.2 points/yr from a baseline of 43.0), Role-Emotional (+0.6 points/yr from a baseline of 58.6), Bodily Pain (+0.2 points/yr from a baseline of 33.1), and Mental Health (+0.15 points/yr from a baseline of 61.2). The change over 10 years for these domains ranged from 1.5 to 6 points. General Health, Role Physical, Social Functioning, and Vitality did not exhibit a significant trend over the years. The PCS score showed a very small (−0.04 points/yr), nonsignificant decline over the years (P = 0.09), whereas the MCS score showed a small (+0.13 points/yr) but statistically significant (P = 0.002) increase over this period (Figure 2). After adjustment for age, gender, nonwhite race, diabetes, hemoglobin, albumin, phosphorous, and Kt/V, the effects of year on Bodily Pain, Role Emotional, Mental Health, and MCS remained significant (P < 0.05). The deterioration in Physical Functioning was, however, no longer associated with year after adjustment (P = 0.89).
Figure 2.
Secular trends (1/1997 to 5/2006), in mean HRQOL component summary scores, assessed by the SF-36 scores between 6 and 18 months after and closest to the 1-year anniversary of starting hemodialysis (n = 11,079): Physical Component summary score (A) and Mental Component summary score (B). Mean scores (●) and regression line are shown.
In evaluating differences in SF-36 scores among groups, a difference of five points is generally considered clinically and socially relevant (14). The six-point increase in Role Emotional over 10 years is the only scale in which the change met this criterion. However, in the linear regression analyses, the R2 value for every model, including Role-Emotional, was <0.01, indicating that >99% of the change over time was caused by random variation or covariates other than the calendar year of starting dialysis.
Discussion
We found that U.S. HD patients' self-reported HRQOL ∼1 year after starting dialysis, as measured by the most widely used generic measure of HRQOL, changed very little between 1997 and 2006. Over this decade, there was no change in the SF-36 domains of General Health, Role Physical, Social Functioning, and Vitality. Of the four domains with statistically significant changes (Physical Functioning, Bodily Pain, Mental Health, and Role Emotional), the change in Role-Emotional was the only one that could be considered clinically relevant. However, a six-point change in a parameter with an SD of >40 points might not qualify as even a small change. A similar absence of a clinically relevant change in composite scores (the PCS and MCS) was seen over the decade.
The SF-36 scores found in our cohort are consistent with previous studies that showed significantly lower scores among dialysis patients compared with the general population. The reduction in PCS scores in the HD population (36.9 versus 50 in the general population) is greater than that observed among patients with cancer, congestive heart failure, chronic lung disease, or limitation in the use of limbs. The decline in MCS among dialysis patients is very small in absolute terms (48.7 versus 50) but greater than in most chronic diseases, except for depression (15). Thus, the small trend of improvement in MCS observed in our study might be more meaningful when interpreted in this context.
It is difficult to determine the reasons for the lack of substantial improvement in HRQOL observed in our population. Over the past decade the average age of the cohorts studied here has increased, and albumin has declined, suggesting that sicker patients were being treated. At the same time, hemoglobin has increased, Kt/V rose very slightly, and serum phosphorous has decreased slightly. Overall, by conventional laboratory metrics, the quality of care may have improved or deteriorated slightly: the interpretation depends on one's beliefs regarding the clinical significance of hemoglobin concentration in ESRD. The effect of dialysis efficacy measured by Kt/V on reducing mortality may be confounded by covariates such as gender and body size (16). Kt/V was found to be poorly correlated with HRQOL (5).
Thus, although certain intermediate outcomes such as hemoglobin levels did improve, patients' perceptions of their HRQOL changed little. Many therapies other than erythropoietin (11), such as exercise (17), androgens (18), intradialytic parenteral nutrition (19), and growth hormones (20), have been studied but yielded variable effects on physical functioning or self-perceived physical health in HD patients. Some of the largest improvements in HRQOL to date have been seen with more frequent dialysis (21), but it requires greater effort on the part of the patient, and its cost effectiveness is unclear (22). Higher dialysis dose and the use of high flux membranes produced no more than a very limited effect on quality of life (23). Addressing the poor HRQOL of dialysis patients remains a formidable challenge.
According to the United States Renal Data Registry (USRDS) data, the cost of treatment for ESRD (most of which is related to HD), both overall and per patient has risen consistently from 1991 to 2006. Cost for a year of HD in the United States has risen from approximately $54,000 per patient in 1997 to more than $70,000 in 2006 (10). The rise in costs associated with HD in the United States is consistent across causes of ESRD, race, gender, and modality, but is particularly high among patients who have been on dialysis <2 years (10). During this same time period, the annual mortality rate for HD patients who reached ESRD within the prior 2 years has declined very slightly from 23.6% in 1997 to 23.2% in 2006 (10). Much of the rise in overall cost of HD can be attributed to increased use of, and spending on, expensive medications, particularly intravenous vitamin D supplementation, intravenous iron, and erythropoietin injections over this time period (although spending on erythropoietin seems to have reached a plateau after the early 2000s) (10). Other determinants of increased expenditures include the rise in the cost of insurance and hospitalization. Overall, this increased use of resources seems to be associated with a decrease in patient mortality (although somewhat less so in the first 2 years of HD) and improvements of certain physiologic indices, such as mean hemoglobin concentration (10), but not with improved HRQOL of this population. Interventions directly targeting HRQOL in this patient population need to be identified and included in future clinical guidelines and resource allocation schemes.
This study has some limitations. The patient population is a series of nonrandomly selected patient groups by annual cohorts who survive at least the first 6 months of dialysis treatment. Fewer than 50% of the population of patients starting dialysis is represented each year. Completing an SF-36 requires a minimum of functional capacity. Responder bias may be a significant confounder in the interpretation of HRQOL data (24,25). Our findings, particularly the lower mortality rate in SF-36 responders, indicate that responders tend to be healthier than nonresponders (Table 2). Although not representative of the entire dialysis population, what is critical for evaluating secular trends is that the populations represented each year are comparable, which is the case (Table 1). Nonetheless, we cannot discount the possibility that, in the nonresponders, a different trend in HRQOL might have been seen over time than that reported here. In the absence of comorbidity data, we also cannot entirely exclude the possibility that an improvement in HRQOL was present but went undetected because the population has gotten sicker over the years, offsetting any improvement in HRQOL that may be present. On the other hand, there has been no change in the proportion of diabetics, the proportion with a serum albumin <3 g/dl, or the annual mortality rate across the years.
Another limitation is that we looked at HRQOL at one point in time. Changes in HRQOL at longer-term follow-up may not have been the same as those in the first year after starting dialysis. Center characteristics, and related variables such as time of travel to dialysis, were also not analyzed (26).
We used a generic, rather than a disease-specific, instrument to measure HRQOL in the dialysis population. The SF-36 has been validated and widely studied in the dialysis population (5,27). Although disease-specific instruments have been developed (28), their superiority to the SF-36 is debatable. The SF-36 was by far the most widely used instrument in the period studied and therefore the most suitable to examine our hypothesis.
Some authors have argued that the MCS scoring method of the SF-36 may overestimate mental health (29). Finally, the collection of data during dialysis treatment might pose a limitation because patients' cognitive ability may be impaired in these circumstances.
The major strength of this study is that we have a decade of HRQOL scores in a large, representative HD population. The large sample size increases precision of estimates and confidence around the results. Analysis of the basic covariates shows that the population of patients with SF-36 scale scores in our study is similar to the U.S. population in age, gender, and race distribution and the prevalence of diabetes over the years. The proportion of nonwhite patients among those with SF-36 scores was slightly lower compared with those without. Nevertheless, the percentage of nonwhite patients in the cohort with HRQOL data were similar to that in the USRDS data (49.3% in 1996, 43.5% in 2006). This allows for generalization of results.
In conclusion, analysis of secular trends in HRQOL of patients starting HD shows small or nonsignificant changes in most domain scores and composite scores. These findings suggest that recent developments in patient care, increased expenditures on new therapies, and the implementation of treatment guidelines have been associated with little change in HRQOL. From the societal perspective, they suggest a need to reevaluate the optimal strategy for allocating efforts and resources to improve the HRQOL of HD patients. It may be that the clinical syndrome of ESRD and the psycho-social reality of dialysis dependence have such a profound inherent impact on HRQOL that improved care and novel therapies of the last decade have had a minor impact on this outcome in these patients.
Disclosures
None.
Acknowledgments
Parts of this work were presented in abstract form as a poster in the American Society of Nephrology meeting; San Diego, CA; October 27 through November 1, 2009.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Liem YS, Bosch JL, Arends LR, Heijenbrok-Kal MH, Hunink MG: Quality of life assessed with the Medical Outcomes Study Short Form 36-Item Health Survey of patients on renal replacement therapy: A systematic review and meta-analysis. Value Health 10: 390–397, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Wight JP, Edwards L, Brazier J, Walters S, Payne JN, Brown CB: The SF36 as an outcome measure of services for end stage renal failure. Qual Health Care 7: 209–221, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merkus MP, Jager KJ, Dekker FW, de Haan RJ, Boeschoten EW, Krediet RT: Physical symptoms and quality of life in patients on chronic dialysis: Results of The Netherlands Cooperative Study on Adequacy of Dialysis (NECOSAD). Nephrol Dial Transplant 14: 1163–1170, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Lopes AA, Bragg-Gresham JL, Satayathum S, McCullough K, Pifer T, Goodkin DA, Mapes DL, Young EW, Wolfe RA, Held PJ, Port FK: Health-related quality of life and associated outcomes among hemodialysis patients of different ethnicities in the United States: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 41: 605–615, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Spiegel BM, Melmed G, Robbins S, Esrailian E: Biomarkers and health-related quality of life in end-stage renal disease: A systematic review. Clin J Am Soc Nephrol 3: 1759–1768, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akizawa T, Pisoni RL, Akiba T, Saito A, Fukuhara S, Asano Y, Hasegawa T, Port FK, Kurokawa K: Japanese haemodialysis anaemia management practices and outcomes (1999–2006): Results from the DOPPS. Nephrol Dial Transplant 23: 3643–3653, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowrie EG, Curtin RB, LePain N, Schatell D: Medical outcomes study short form-36: A consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis 41: 1286–1292, 2003 [DOI] [PubMed] [Google Scholar]
- 8.DeOreo PB: Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis 30: 204–212, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Ware JE, Jr, Sherbourne CD: The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30: 473–483, 1992 [PubMed] [Google Scholar]
- 10.U.S. Renal Data System: USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008 [Google Scholar]
- 11.Moreno F, Sanz-Guajardo D, Lopez-Gomez JM, Jofre R, Valderrabano F: Increasing the hematocrit has a beneficial effect on quality of life and is safe in selected hemodialysis patients. Spanish Cooperative Renal Patients Quality of Life Study Group of the Spanish Society of Nephrology. J Am Soc Nephrol 11: 335–342, 2000 [DOI] [PubMed] [Google Scholar]
- 12.McClellan WM, Frankenfield DL, Frederick PR, Helgerson SD, Wish JB, Sugarman JR: Improving the care of ESRD patients: A success story. Health Care Financ Rev 24: 89–100, 2003 [PMC free article] [PubMed] [Google Scholar]
- 13.McHorney CA, Ware JE, Jr, Rogers W, Raczek AE, Lu JF: The validity and relative precision of MOS short- and long-form health status scales and Dartmouth COOP charts. Results from the Medical Outcomes Study. Med Care 30: MS253–MS265, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Ware J, Kosinski, Keller, SK: Physical and Mental Health Summary Scales, A User's Manual, Boston, The Health Institue, 1994 [Google Scholar]
- 15.Mittal SK, Ahern L, Flaster E, Maesaka JK, Fishbane S: Self-assessed physical and mental function of haemodialysis patients. Nephrol Dial Transplant 16: 1387–1394, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Leypoldt JK, Cheung AK: Revisiting the hemodialysis dose. Semin Dial 19: 96–101, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Fabbian F, Manfredini F, Malagoni AM, Malacarne F, Russo G, Soffritti S, Molino C, Catizone L: Exercise training in peripheral vascular arterial disease in hemodialysis patients: A case report and a review. J Nephrol 19: 144–149, 2006 [PubMed] [Google Scholar]
- 18.Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T: Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: A randomized, controlled trial. J Am Soc Nephrol 17: 2307–2314, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Hiroshige K, Iwamoto M, Kabashima N, Mutoh Y, Yuu K, Ohtani A: Prolonged use of intradialysis parenteral nutrition in elderly malnourished chronic haemodialysis patients. Nephrol Dial Transplant 13: 2081–2087, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Feldt-Rasmussen B, Lange M, Sulowicz W, Gafter U, Lai KN, Wiedemann J, Christiansen JS, El Nahas M: Growth hormone treatment during hemodialysis in a randomized trial improves nutrition, quality of life, and cardiovascular risk. J Am Soc Nephrol 18: 2161–2171, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Locatelli F, Buoncristiani U, Canaud B, Kohler H, Petitclerc T, Zucchelli P: Dialysis dose and frequency. Nephrol Dial Transplant 20: 285–296, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Anantharaman P, Moss AH: Should the medicare ESRD program pay for daily dialysis? An ethical analysis. Adv Chronic Kidney Dis 14: 290–296, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Unruh M, Benz R, Greene T, Yan G, Beddhu S, DeVita M, Dwyer JT, Kimmel PL, Kusek JW, Martin A, Rehm-McGillicuddy J, Teehan BP, Meyer KB: Effects of hemodialysis dose and membrane flux on health-related quality of life in the HEMO Study. Kidney Int 66: 355–366, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Bardwell WA, Ancoli-Israel S, Dimsdale JE: Response bias influences mental health symptom reporting in patients with obstructive sleep apnea. Ann Behav Med 23: 313–317, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Sales AE, Plomondon ME, Magid DJ, Spertus JA, Rumsfeld JS: Assessing response bias from missing quality of life data: The Heckman method. Health Qual Life Outcomes 2: 49, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moist LM, Bragg-Gresham JL, Pisoni RL, Saran R, Akiba T, Jacobson SH, Fukuhara S, Mapes DL, Rayner HC, Saito A, Port FK: Travel time to dialysis as a predictor of health-related quality of life, adherence, and mortality: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 51: 641–650, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Johansen KL, Painter P, Kent-Braun JA, Ng AV, Carey S, Da Silva M, Chertow GM: Validation of questionnaires to estimate physical activity and functioning in end-stage renal disease. Kidney Int 59: 1121–1127, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Carmichael P, Popoola J, John I, Stevens PE, Carmichael AR: Assessment of quality of life in a single centre dialysis population using the KDQOL-SF questionnaire. Qual Life Res 9: 195–205, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Nortvedt MW, Riise T, Myhr KM, Nyland HI: Performance of the SF-36, SF-12, and RAND-36 summary scales in a multiple sclerosis population. Med Care 38: 1022–1028, 2000 [DOI] [PubMed] [Google Scholar]