Abstract
Background and objectives: Mild hyponatremia has traditionally been considered benign, but it may be associated with gait and attention deficits and an increased risk of falls that may result in fracture. A retrospective study was conducted to quantify the association of hyponatremia with fracture occurrence and to examine whether this relationship is independent of osteoporosis.
Design, setting, participants, & measurements: This study analyzed 1408 consecutive female patients who underwent bone mineral density measurement (Lunar IDXA) between September 1, 2006 and April 11, 2007 and who had available laboratory data. Self reported fracture occurrence was confirmed by radiology report or attendance at a fracture clinic. The significance and independence of the association of hyponatremia with fracture was quantified using logistic regression.
Results: The mean (SD) serum sodium ([Na+]) was 140.6 (3.0) mmol/L; 59 (4.2%) had [Na+] < 135 mmol/L. Forty-five percent of subjects were osteoporotic and 18% had a prior fracture. Hyponatremia was present in 8.7% of those with versus 3.2% of those without a confirmed fracture (P < 0.001). On multivariate logistic regression analysis controlling for age, T-score, chronic kidney disease stage, osteoporotic risk factors (amenorrhea, family history, regular steroid use, smoking history, alcohol use, history of liver disease, and low-calcium diet), and osteoporosis treatments (calcium and vitamin D supplements, antiresorptives, and hormonal replacement therapy), [Na+] < 135 versus [Na+] ≥ 135 mmol/L remained significantly and independently associated with fracture occurrence (P < 0.01).
Conclusions: Mild hyponatremia may be a readily identifiable and potentially modifiable risk factor for fracture.
Severe hyponatremia is a well recognized cause of increased patient morbidity and mortality, although milder degrees of hyponatremia (serum sodium levels of 130 to 134 mmol/L) are usually devoid of obvious symptoms (1). However, recent evidence suggests that mild chronic hyponatremia is associated with subtle central nervous system impairment, including gait and attention deficits, which may lead to an increased risk of falling (2,3). Furthermore, two case control studies have reported the association of mild asymptomatic hyponatremia with bone fracture in the ambulatory elderly (4,5), although neither study was able to control for the presence of osteoporosis, a major potential confounder of this relationship, because the prevalence of osteoporosis and hyponatremia increase with older age.
We therefore conducted the following analysis to quantify the presence, strength, and significance of the association of hyponatremia with fracture occurrence, on the basis of the hypothesis that hyponatremia predisposes to falls with an attendant increase in fracture risk, and in particular to assess whether this association is independent of osteoporosis.
Materials and Methods
This is a secondary analysis of data collected for a retrospective study examining the association of chronic kidney disease (CKD) with self reported fracture occurrence (6). Entry criteria for the parent study were female sex, age 18 years or older, an available DXA scan result performed at Cork University Hospital between September 1, 2006 and April 11, 2007, and an available serum creatinine level measured within 1 year of the dual-energy x-ray absorptiometry (DXA) scan (actual median time to measurement was 4 weeks). Subjects who were referred from nephrology services (to avoid referral bias) and those with an estimated GFR (eGFR) >90 ml/min/1.73 m2 (because of concerns regarding the accuracy of the Modification of Diet in Renal Disease equation at these levels) were excluded. For the analyses presented here, we additionally a priori excluded 77 patients who did not have an available serum sodium ([Na+]) level, 203 subjects whose fracture could not be validated (see below), and 11 subjects who had a vertebral collapse fracture, resulting in a sample size of 1408 participants. Patients with vertebral collapse fractures were excluded because they are frequently nontraumatic and our hypothesis centered on hyponatremia leading to an increased risk of falls with resulting fracture.
DXA measurements were performed at the lumbar sacral spine and both hips using a Lunar IDXA scanner (General Electric) and expressed as T-scores indicative of the number of SDs by which the bone mass value deviated from the mean of a group of young normal controls. In keeping with the World Health Organization definition, osteoporosis was defined as a T-score of less than −2.5 (7). Data regarding patient demographics, risk factors, current and prior treatment regimes for osteoporosis, history of prior fracture, and details of coexistent medical conditions were obtained from a standardized self report questionnaire, completed by the subject at the time of the DXA scan.
The DXA database was manually crossreferenced against the central laboratory database at Cork University Hospital, using agreement of at least two of three potential patient identifiers (name, date of birth, medical record number) to identify those subjects who had a serum creatinine measured within 1 year of the index DXA scan. Laboratory results obtained during inpatient admissions were not included unless these were similar to previously measured outpatient levels. Serum creatinine was assayed using the modified Jaffe reaction and reported in micromoles per liter. The eGFR (in ml/min/1.73 m2 body surface area) was calculated using the four-variable Modification of Diet in Renal Disease prediction equation (8). Subjects were categorized using a modified National Kidney Foundation staging system with eGFR cutoffs of 75 to 89, 60 to 74, 30 to 59, and <29 ml/min per 1.73 m2. [Na+] concentrations were measured by an ion-selective electrode and reported in millimoles per liter. Hyponatremia was defined as [Na+] < 135mmol/L and mild hyponatremia as a value between 130 to 134 mmol/L.
In an attempt to partially validate fracture occurrence, we crosschecked self reported fracture against radiology reports and/or fracture clinic attendance at Cork University Hospital. Because local patients who were referred to Cork University Hospital for a DXA scan would also be expected to be referred here for fracture care, this approach would be expected to confirm many but certainly not all patients with an actual past history of fracture. Alternative fracture services are also provided at two other regional public hospitals and at a private hospital, the records of which we were not able to crossreference.
Statistical Considerations
Outlying and clinically implausible values were double-checked against the original clinical data. The distribution, central tendency, and variance of each variable were examined using standard tabular and graphical methods. The study population was categorized by [Na+] levels and characteristics were compared using the Mann–Whitney test for continuous variables and χ2 for categorical variables. We examined the presence, strength, significance, and independence of the association of hyponatremia with fracture occurrence using logistic regression, adjusting sequentially for age, osteoporotic risk factors (amenorrhea, family history, regular steroid use, smoking history, alcohol use, history of liver disease, and low-calcium diet), and osteoporosis treatments (calcium and vitamin D supplements, antiresorptives, and hormonal replacement therapy). To examine if there was a relationship between values within or above the normal reference range with fracture occurrence, we additionally repeated the above multivariate logistic regression model, expressing the [Na+] as a six-level categorical variable; namely, <135, 136 to 137, 138 to 140 (reference), 141 to 142, 143 to 145, and >145 mmol/L. All analyses were performed using SPSS Version 12.0 software, (Chicago, IL) with a two-sided type 1 error rate of 0.05. The Clinical Research Ethics Committee of the Cork Teaching Hospitals approved the study protocol.
Results
Clinical Characteristics
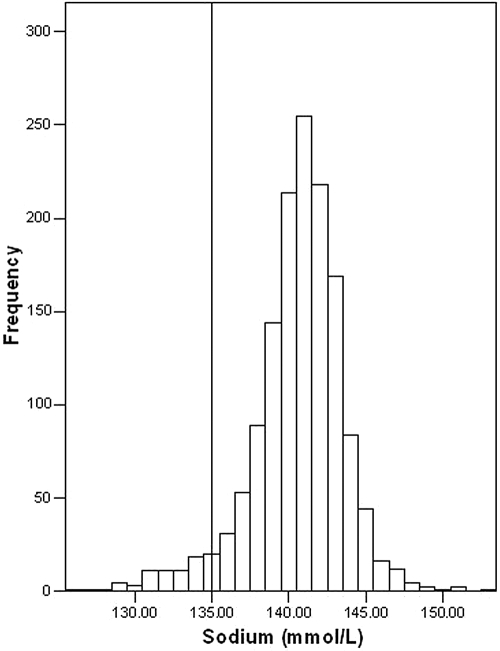
The mean (SD) age of the 1408 subjects analyzed was 61 (11) years and mean (SD) eGFR was 69 (12) ml/min/1.73 m2, Table 1. The mean T-score (SD) was −2.3 (1.1); 45% of subjects had osteoporosis (T-score < −2.5). [Na+] was normally distributed with a mean (SD) of 140.6 (3.0) mmol/L (Figure 1). Hyponatremia was present in 59 patients (4.2%) and was mild in 53 (3.8%) patients.
Table 1.
Subject characteristics
| Characteristic | Total | [Na+] < 135 mmol/L | [Na+] >135 mmol/L | P |
|---|---|---|---|---|
| Number of patients (%) | 1408 | 59 (4.2) | 1349 (95.8) | |
| Mean (SD) age (years) | 61.4 (10.7) | 67.8 (13.0) | 61.1 (10.5) | <0.001 |
| range | 23 to 97 | 32 to 97 | 23 to 89 | |
| Mean (SD) T-score | −2.3 (1.1) | −2.6 (1.2) | −2.3 (1.1) | 0.03 |
| range | −6.5 to 0.9 | −5.6 to −0.2 | −6.5 to 0.9 | |
| Osteoporosis (%) (T-score < −2.5) | 44.9 | 57.6 | 44.3 | 0.04 |
| Osteoporosis risk factors (%) | ||||
| amenorrhea | 83.9 | 81.4 | 84.0 | 0.59 |
| dairy servings <3/wk | 42.3 | 25.4 | 43.0 | <0.01 |
| alcohol >5 units/wk | 18.0 | 8.5 | 18.5 | 0.05 |
| maintenance steroids | 6.4 | 3.4 | 6.5 | 0.34 |
| ever smoked | 18.3 | 18.6 | 18.3 | 0.95 |
| family history | 10.3 | 5.1 | 10.5 | 0.18 |
| liver disease | 0.7 | 1.7 | 0.7 | 0.36 |
| Treatment for osteoporosis (%) | ||||
| calcium | 33.0 | 39.0 | 32.8 | 0.32 |
| vitamin D | 8.3 | 13.6 | 8.1 | 0.14 |
| antiresorptive | 21.9 | 28.8 | 21.6 | 0.19 |
| hormonal replacement therapy | 9.6 | 11.9 | 9.5 | 0.54 |
| Mean (SD) [Na+] (mmol/L) | 140.6 (3.0) | 132.2 (1.8) | 141.0 (2.4) | <0.001 |
| range | 127 to 153 | 127 to 134 | 135 to 153 | |
| Mean (SD) eGFR (ml/min/1.73 m2) | 68.8 (12.3) | 66.9 (14.4) | 68.9 (12.2) | 0.43 |
| range | 20 to 89 | 29 to 88 | 20 to 89 |
Figure 1.
Distribution of [Na+] values in 1408 women undergoing DXA scanning.
Subjects with, as compared with those without, hyponatremia were significantly older, had a lower bone mineral density, and a higher prevalence of osteoporosis but similar eGFR. The use of antiresorptive agents, calcium and vitamin D, and hormonal replacement therapy was not significantly different between the two groups (Table 1).
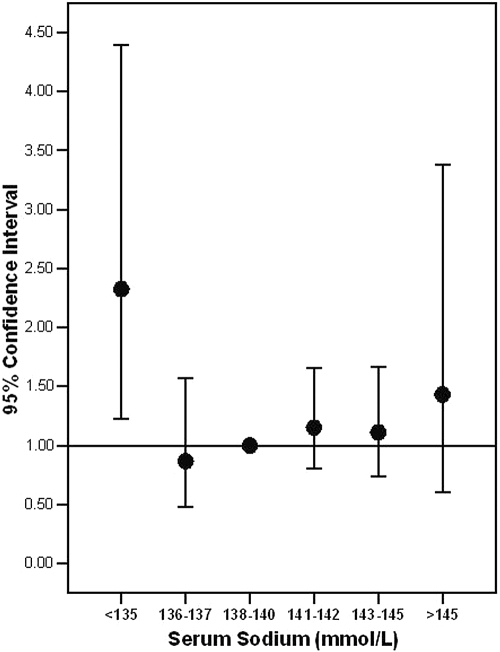
Overall 254 (18.0%) subjects had at least one prior fracture. Hyponatremia was present in 8.7% of those with a fracture versus 3.2% of those without a fracture, P (χ2) <0.001. Patients with hyponatremia had a 2.86-fold increased unadjusted odds ratio (OR) for fracture occurrence as compared with nonhyponatremic subjects, P < 0.001 (Table 2). On adjusting simultaneously for age and T-score, the OR [95% confidence interval (CI)] for fracture occurrence remained significantly elevated at 2.06 (1.14 to 3.65). In the fully adjusted model (simultaneously adjusting for age, T-score, CKD stage, osteoporotic risk factors, and osteoporosis treatments as listed in Table 1) there continued to be a significantly increased OR (95% CI) of fracture occurrence in the hyponatremic group of 2.25 (1.24 to 4.09). When [Na+] was entered into the above multivariate model as a six-level categorical variable and using the 138 to 140 category as the reference group, the <135 category was independently associated with fracture whereas the CIs for categories >135 largely overlapped each other and were not significantly different from the reference group (Figure 2).
Table 2.
Crude and adjusted OR for association of hyponatremia with fracture occurrence
| Model | OR | 95% CI | P |
|---|---|---|---|
| 1a | 2.86 | 1.66 to 4.94 | <0.001 |
| 2b | 2.06 | 1.14 to 3.65 | 0.02 |
| 3c | 2.06 | 1.15 to 3.71 | 0.02 |
| 4d | 2.25 | 1.24 to 4.09 | 0.01 |
Unadjusted.
Adjusted for age (years) and T-score.
Adjusted for age (years), T-score, and CKD stage.
Adjusted for age (years), T-score, CKD stage, osteoporotic risk factors (amenorrhea, low dietary calcium intake, high alcohol intake, maintenance steroids, ever having smoked, family history of osteoporosis, and history of liver disease), and osteoporosis treatment (use of calcium, vitamin D, antiresorptive therapy, and hormonal replacement therapy).
Figure 2.
OR (95% CI) of fracture occurrence by [Na+] category, adjusting simultaneously for age (years), T-score, CKD stage, osteoporotic risk factors (amenorrhea, low dietary calcium intake, high alcohol intake, maintenance steroids, ever having smoked, family history of osteoporosis, and history of liver disease), and osteoporosis therapy (use of calcium, vitamin D, antiresorptive therapy, hormonal replacement therapy).
In a sensitivity analysis, we excluded hyponatremic subjects with [Na+] below 130 to examine whether the observed association with fracture was driven by the small number of extreme values. The unadjusted OR (95% CI) for mild hyponatremia (130 to 134) versus those with [Na+] ≥135 was 2.92 (1.65 to 5.18), P < 0.001. This remained significant after sequential models with the adjusted OR (95% CI) as per model 4, Table 2, being 2.2 (1.18 to 4.13) P = 0.014
Discussion
We report that mild hyponatremia, of a degree that is often ignored in clinical subjects, is significantly associated with fracture occurrence independently of bone mineral density. Renneboog et al. have shown that mild chronic hyponatremia is associated with gait and attention deficits and with an increased risk of falls. They also found that hyponatremia caused more attention deficits than did a serum alcohol level of 0.6 g/L in an age- and sex-matched control group (2). In a preliminary report of an additional study, the threshold for gait and attention deficits induced by hyponatremia were 134 and 132 mEq/L, respectively (3). Furthermore, a case control study by Kengne et al. demonstrated that mild asymptomatic hyponatremia (mean [Na+] 131 mmol/L) was associated with bone fracture after incidental fall in the ambulatory elderly with an adjusted OR of 4.16 (95% CI 2.24 to 7.71) (4) and a similar significant association reported in a recent additional study (5). A limitation of both of these studies was the inability to fully adjust for bone mineral density, which as demonstrated in our analysis partially—although incompletely—attenuates the association seen in unadjusted analyses. Advanced CKD is associated with a range of qualitative abnormalities of bone turnover. However, most of the subjects in our analysis had well preserved renal function (mean eGFR 69 ml/min/1.73 m2), whereas the association of hyponatremia with fracture was independent of the presence of CKD. In addition to predisposing to falls through disturbance of balance and gait, hyponatremia may also theoretically contribute to poor underlying bone health by a direct effect on bone turnover. Unfortunately we were unable to examine this issue in this retrospective study.
The term “symptomatic hyponatremia” has been used to refer to the presence of overt complications of hyponatremia, typically gastrointestinal and neuropsychiatric in nature. This is occasionally associated with seizures, and indeed the initial report of hyponatremia-associated fracture occurred in a subgroup of postmenopausal women with severe hyponatremia who presented with fracture after seizure activity (9). Mild chronic hyponatremia, which lacks these overt symptoms, is typically considered asymptomatic; however, subtle alterations in gait and balance are unlikely to be volunteered by patients and may often be attributed to concomitant conditions such as old age. Whether the range of complications associated with mild chronic hyponatremia is limited to disturbances of gait and balance or is more extensive is unknown, as is its effect on quality of life. In view of these uncertainties the suitability of the term “asymtomatic hyponatremia” is open to question.
Fragility fractures, occurring after minor trauma or falls, are a major public health concern because of their attendant morbidity and mortality (10). Recurrent incidental falls result in bone fracture in 4% to 6% of patients, with up to 2% of these patients dying (11). Effective treatment and preventative strategies for recurrent falls remains highly limited (12). Mild chronic hyponatremia is a common electrolyte imbalance with a reported prevalence of 2% to 4% in the general population, rising to 7% to 11% in the ambulatory elderly (13,14) and to 42% in hospitalized subjects (14). The potential importance of the association of mild hyponatremia with fracture relates to this high prevalence of the condition, especially in groups that have a high risk of fracture after falls, such as the elderly. In addition, hyponatremia commonly complicates advanced cardiac and liver failure, and in these conditions it has well recognized prognostic import, (15,16) although the hyponatremia per se is usually considered to be asymptomatic. It is of interest that advanced cardiac failure has been recently associated with an increased incidence of fracture, (17) and it has been speculated that this may be mediated in part through the development of hyponatremia (18).
Two common reversible causes of hyponatremia are the use of thiazide-type diuretics (19) and selective serotonin reuptake inhibitor (SSRI) (20) antidepressants, both of which are in widespread clinical use. Thiazide diuretics are widely used as first-line agents in the treatment of hypertension but are complicated in up to 14% of subjects by hyponatremia (21,22). Among subjects aged 65 years and older resident in a nationally representative sample of U.S. nursing homes, thiazide use was associated with increased fracture risk over 1 year of follow-up (23). However, in addition to their antihypertensive effect, thiazide-type diuretics also decrease urinary calcium excretion and promote a positive calcium balance, thereby helping to maintain and improve bone mineral density (24–26). It is possible that the potential uses of thiazide diuretics for this indication may have led to their selective use in subjects with preexisting osteoporosis and thus confound the association of thiazide-induced hyponatremia with fracture in ours and in other studies examining this relationship. Against this, thiazide-type diuretics have not been widely used in this indication in Irish clinical practice (Professor M.G. Molloy, Professor of Rheumatology, Cork University Hospital, personal communication). Pending further studies, our results would urge caution in the use of thiazide-type diuretics for bone protection in those subjects who develop hyponatremia, because the benefits of a positive calcium balance may be offset by the potential increase in risk of falls. In addition, it may be prudent to routinely screen [Na+] levels after initiation of thiazide diuretics, especially in those at high risk for developing hyponatremia, such as elderly women.
Depression and the use of centrally acting agents are recognized as being associated with increased risk of falls and of fracture. Available evidence suggests that some of this increased risk is due to the agents independently of the presence of depression (27). Of the studied agents, the highest risk of falls has been associated with SSRIs, (28) with peak fracture risk occurring in the first 2 weeks after initiation of therapy. This risk of fracture mirrors the increased risk of hyponatremia in SSRIs as compared with alternative agents and the time course in which hyponatremia typically occurs (27,29); however, the potential role of hyponatremia in predisposing to fracture in this population remains unstudied.
The mainstay of treatment for persistent hyponatremia, when the syndrome is irreversible or the iatrogenic cause cannot be readily altered, centers on fluid restriction, the severity of which depends on the degree of the diluting defect; however, this is difficult to maintain and patient adherence is often incomplete. As a result, the goal of such therapy is often to limit the severity of the dysnatremia rather than to fully correct it, mild hyponatremia being seen as an acceptable clinical compromise between the dangers of severe hyponatremia and the inconvenience of aggressive therapy. Thus the perception of mild hyponatremia as being devoid of clinical significance in many cases tempers the aggressiveness of therapy. Of the available medical adjuncts to fluid restriction, loop diuretics are of limited efficacy, oral urea is unpalatable, and demeclocycline is potentially nephrotoxic (30). The recent development of selective oral vasopressin V2 receptor antagonists may facilitate the more complete correction of hyponatremia in patients with persistent hyponatremia, (31) but whether such therapy would reduce the occurrence of falls and low-impact fractures in susceptible individuals is to date unknown (32). In acute hyponatremia, as may occur in marathon runners, the provision of 100 cc of hypertonic 3% saline with the aim of raising the [Na+] by 2 to 3 mEq/L, as originally proposed by Ayus and colleagues, is safe and efficacious (33). In chronic hyponatremia its use is restricted to euvolemic patients with moderate or severe symptoms.
Several limitations apply to our analysis. [Na+] measurement was not available at the actual time of fracture but was instead related to the bone density measurement. Although this may have resulted in a degree of nondifferential misclassification, this would tend to reduce the observed association toward the null hypothesis and attenuate the effect size of any association. Indeed, the magnitude of the association we found is less than that described by other authors, which may relate in part to different populations examined; ours was relatively young and may therefore be less severely affected by mild gait disturbances as compared with older subjects. In addition, our effect size is also partially attenuated as it is adjusted for bone mineral density. A major limitation on the inferences that can be drawn from this study is that, as with all observational research, they can at best only provide support for the above hypothesis but cannot prove it. Unmeasured confounders such as unreported comorbid conditions, sedative drug use, or the presence of malignancy might affect the described relationship of hyponatremia with fracture. Nevertheless, there exists a plausible mechanism to explain this putative association and, as in our work, the observed relationship has been independent of a wide range of relevant confounders, including the presence of osteoporosis. Nevertheless, controlled interventional trials are essential to reliably estimate the true attributable risk of hyponatremia with falls and fracture occurrence.
In conclusion, mild chronic hyponatremia is a significant independent risk factor for bone fracture. In keeping with recent studies, our data suggest that mild chronic hyponatremia is neither a benign nor an inconsequential condition. If confirmed in prospective studies, the prevention, identification, and effective management of this relatively common condition may provide an important opportunity to reduce the risk of recurrent falls and repeated fractures and lead to alterations in a wide range of current clinical practices.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Bone Disease as a New Complication of Hyponatremia: Moving Beyond Brain Injury,” on pages 167–168.
References
- 1.Brenner BM: Brenner and Rector's the Kidney, Philadelphia, Saunders, 2004 [Google Scholar]
- 2.Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G: Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med 119: 71e71–e78, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Renneboog BSL, Decaux G: Determination of threshold for attention and gait deficits encountered in chronic hyponatremia [Abstract]. J Am Soc Nephrol 17: 37A, 2006 [Google Scholar]
- 4.Gankam Kengne F, Andres C, Sattar L, Melot C, Decaux G: Mild hyponatremia and risk of fracture in the ambulatory elderly. QJM 101: 583–588, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Sandhu HS, Gilles E, DeVita MV, Panagopoulos G, Michelis MF: Hyponatremia associated with large-bone fracture in elderly patients. Int Urol Nephrol 41: 733–737, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Kinsella S, Chavrimootoo S, Molloy MG, Eustace JA: The association of MDRD estimated glomerular filtration rates (eGFR) with bone mineral density, osteoporosis and fracture occurrence. [Abstract]. J Am Soc Nephrol 17: 830A, 2007 [Google Scholar]
- 7.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy: Osteoporosis prevention, diagnosis, and therapy. JAMA 285: 785–795, 2001. 11176917 [Google Scholar]
- 8.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–266, 2002 [PubMed] [Google Scholar]
- 9.Ayus JC, Arieff AI: Chronic hyponatremic encephalopathy in postmenopausal women: Association of therapies with morbidity and mortality. JAMA 281: 2299–2304, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Cole ZA, Dennison EM, Cooper C: Osteoporosis epidemiology update. Curr Rheumatol Rep 10: 92–96, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Sattin RW: Falls among older persons: A public health perspective. Annu Rev Public Health 13: 489–508, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Gillespie LD, Robertson MC, Gillespie WJ, Lamb SJ, Gates S, Cumming RG, Rowe BH: Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev: CD007146, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Miller M, Hecker MS, Friedlander DA, Carter JM: Apparent idiopathic hyponatremia in an ambulatory geriatric population. J Am Geriatr Soc 44: 404–408, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Hawkins RC: Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta 337: 169–172, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Gheorghiade M, Rossi JS, Cotts W, Shin DD, Hellkamp AS, Pina IL, Fonarow GC, DeMarco T, Pauly DF, Rogers J, DiSalvo TG, Butler J, Hare JM, Francis GS, Stough WG, O'Connor CM: Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE trial. Arch Intern Med 167: 1998–2005, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Angeli P, Wong F, Watson H, Gines P: Hyponatremia in cirrhosis: Results of a patient population survey. Hepatology 44: 1535–1542, 2006 [DOI] [PubMed] [Google Scholar]
- 17.van Diepen S, Majumdar SR, Bakal JA, McAlister FA, Ezekowitz JA: Heart failure is a risk factor for orthopedic fracture: A population-based analysis of 16,294 patients. Circulation 118: 1946–1952, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Reeder DN, Anderson SD: Letter by Reeder and Anderson regarding article, “Heart failure is a risk factor for orthopedic fracture: a population-based analysis of 16 294 patients”. Circulation 120: e11; author reply e12, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Spital A: Diuretic-induced hyponatremia. Am J Nephrol 19: 447–452, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson TJ, Begg EJ, Winter AC, Sainsbury R: Incidence and risk factors for hyponatraemia following treatment with fluoxetine or paroxetine in elderly people. Br J Clin Pharmacol 47: 211–217, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann SJ: The silent epidemic of thiazide-induced hyponatremia. J Clin Hypertens (Greenwich) 10: 477–484, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP: Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol 61: 87–95, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spector W, Shaffer T, Potter DE, Correa-de-Araujo R, Rhona Limcangco M: Risk factors associated with the occurrence of fractures in U.S. nursing homes: Resident and facility characteristics and prescription medications. J Am Geriatr Soc 55: 327–333, 2007 [DOI] [PubMed] [Google Scholar]
- 24.LaCroix AZ, Ott SM, Ichikawa L, Scholes D, Barlow WE: Low-dose hydrochlorothiazide and preservation of bone mineral density in older adults. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 133: 516–526, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Giusti A, Barone A, Pioli G, Girasole G, Siccarchi V, Palummeri E, Bianchi G: Alendronate and indapamide alone or in combination in the management of hypercalciuria associated with osteoporosis: A randomized controlled trial of two drugs and three treatments. Nephrol Dial Transplant 24: 1472–1477, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Bolland MJ, Ames RW, Horne AM, Orr-Walker BJ, Gamble GD, Reid IR: The effect of treatment with a thiazide diuretic for 4 years on bone density in normal postmenopausal women. Osteoporos Int 18: 479–486, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Vestergaard P: Fracture risks of antidepressants. Expert Rev Neurother 9: 137–141, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Richards JB, Papaioannou A, Adachi JD, Joseph L, Whitson HE, Prior JC, Goltzman D: Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med 167: 188–194, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Jacob S, Spinler SA: Hyponatremia associated with selective serotonin-reuptake inhibitors in older adults. Ann Pharmacother 40: 1618–1622, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Ellison DH, Berl T: Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med 356: 2064–2072, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C: Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 355: 2099–2112, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Miller M: Role of arginine vasopressin receptor antagonists in hyponatremia in the elderly. Geriatrics 62: 20–26, 2007 [PubMed] [Google Scholar]
- 33.Ayus JC, Arieff A, Moritz ML: Hyponatremia in Marathon Runners. N Engl J Med 353: 427–428, 2005 [PubMed] [Google Scholar]