Abstract
Background and objectives: Neutrophil Gelatinase-Associated Lipocalin (NGAL) is rapidly released by renal tubules after injury, potentially allowing early identification of acute kidney injury (AKI) after cardiac surgery. However, the diagnostic performance of NGAL has varied widely in clinical studies, and it remains unknown what factors modify the relationship between NGAL and AKI. We hypothesized the relationship between urinary NGAL and AKI would vary with baseline renal function, allowing a stratified analysis to improve diagnostic performance of this novel biomarker.
Design, setting, participants, & measurements: We performed a prospective observational study in 426 adult cardiac surgical patients. Urinary NGAL was serially determined, commencing preoperatively and continuing 24 hours postoperatively. AKI was defined as increase in serum creatinine from baseline by either >50% or >0.3 mg/dl within 48 hours postoperatively. Patients were stratified by baseline estimated GFR (eGFR). NGAL levels were compared between patients with and without AKI and diagnostic characteristics determined according to baseline eGFR.
Results: In patients with baseline eGFR ≥60 ml/min, urinary NGAL was higher at all postoperative time points in patients who developed AKI compared with those who did not. In patients with baseline eGFR <60 ml/min, urinary NGAL did not differ at any time between those who did and those who did not develop AKI. Postoperative NGAL best identified AKI in patients with baseline eGFR 90 to 120 ml/min.
Conclusions: The relationship between urinary NGAL and AKI after cardiac surgery varies with baseline renal function, with optimal discriminatory performance in patients with normal preoperative function.
Acute Kidney Injury (AKI) is a common complication after cardiac surgery, with reported incidence varying from 20% to 50% depending on the definition used (1–4). Early detection of injury is desirable to facilitate early intervention aimed at limiting associated morbidity and mortality. Neutrophil Gelatinase-Associated Lipocalin (NGAL) is rapidly released by renal tubules in response to injury, and an acute rise in urinary NGAL has been reported to accurately identify evolving AKI in both pediatric and adult populations within 2 to 8 hours of cardiac surgery (5–9). However, other studies have found urinary NGAL to have only modest discriminant ability for AKI after cardiac surgery (3,10). Studies reporting excellent discriminant ability have generally excluded patients with preoperative renal dysfunction, whereas those studies reporting a more modest performance have included patients with a heterogeneous mix of baseline renal function. Although it is unknown whether baseline renal function modifies the relationship between NGAL and AKI, the existence of such a relationship may contribute to the limited predictive ability in these studies. Although NGAL is proposed as a real-time marker of acute renal injury rather than renal function, the nonlinear relationship between GFR and serum creatinine may mean that a relatively larger injury, producing a larger reduction in GFR, is required to cause a rise in serum creatinine sufficient to meet diagnostic criteria for AKI in a patient with normal baseline GFR. Conversely, a much smaller injury (and smaller incremental reduction in GFR) may be sufficient to cause a rise in creatinine that would diagnose AKI in a patient with impaired GFR at baseline. If true, the diagnostic utility of urinary NGAL for a creatinine-based diagnosis of AKI may be enhanced using an approach stratified by baseline renal function. We have previously reported a modest performance of urinary NGAL for early identification of evolving AKI in a large, unselected adult population undergoing cardiac surgery, with a wide range of baseline renal function. In this posthoc analysis we sought to investigate this potential source of effect modification to the relationship between NGAL and postoperative AKI. We hypothesized that the relationship between postoperative urinary NGAL and AKI would vary with baseline renal function, measured by estimated GFR (eGFR). We further hypothesized that the diagnostic performance of NGAL for postoperative AKI would be improved using an analysis stratified by baseline function, allowing the use of different diagnostic thresholds.
Materials and Methods
After Institutional Review Board approval, we performed a prospective observational study in 426 adult cardiac surgical patients, operated on between July 2004 and January 2006. The Institutional Review Board waived the need for individual patient consent in view of the minimal risk represented by collection of urine and routine perioperative data only, in keeping with New York state and US federal regulations. All types of cardiac surgical procedure were included, with preoperative dialysis-dependent renal failure the only exclusion. Data collection included baseline demographic data and Parsonnet score as a measure of perioperative risk for all patients. We further recorded operative procedure, use of aprotinin, and duration of both cardiopulmonary bypass (CPB) and aortic cross-clamp time. Five milliliters of urine was collected after induction of general anesthesia and before surgical incision, on completion of cardiopulmonary bypass and again at 3, 18, and 24 hours later. Samples were processed immediately and stored at −20°C for up to 3 months, a period for which NGAL has been reported to be stable (11). Urinary NGAL level was determined by ELISA assay using a commercially available ELISA kit (Antibodyshop, Gentofte, Denmark). The limit of detection for this assay is 0.5 to 4.0 pg/ml, with intraassay variation in urine of 2.1%. Laboratory investigators were blinded to patient outcomes until study completion. Serum creatinine was measured preoperatively and then twice daily for 48 hours postoperatively by the central laboratory of Columbia-Presbyterian Medical Center. Preoperative eGFR was estimated according to the formula of Cockcroft and Gault (12). In keeping with recommendations of the Acute Kidney Injury Network for stage 1 Acute Kidney Injury (13), AKI was defined as an increase in serum creatinine from preoperative values by either more than 50% or more than 0.3 mg/dl within the first 48 hours after surgery (serum creatinine in mg/dl may be converted into μmol/L by multiplying by 88.4). In view of potential confounding effects from the anticipated high frequency of diuretic use in a cardiac surgical population, urine output was excluded as a criterion for determining AKI in this cohort.
Statistical Analyses.
Continuous variables were assessed for normality and are presented as mean ± SD or median and interquartile range, as appropriate. Categorical variables are expressed as counts and proportions. Patients were initially considered according to preoperative eGFR greater than or less than 60 ml/min before further stratification according to five predefined eGFR groupings (Table 1). Although based on Kidney Disease Outcomes Quality Initiative guidelines, we made minor modifications for the purpose of maintaining equally spaced categories of eGFR, considering eGFR <30 ml/min as one group and eGFR >120 ml/min as a distinct group. Differences in baseline variables between groups were assessed using parametric and nonparametric tests of significance as appropriate. Within each eGFR group, urinary NGAL levels were compared between patients with and without AKI (Mann-Whitney U test), and nonparametric receiver-operator characteristic (ROC) curves were generated. A sensitivity analysis was undertaken to further explore results. Results are presented as comparison of group medians with P value or area under the ROC curve with 95% confidence intervals, as appropriate. A P value <0.05 was considered statistically significant.
Table 1.
Baseline demographics, operative characteristics, and AKI incidence, stratified by preoperative eGFR
| Preoperative eGFR, ml/mina |
P | |||||
|---|---|---|---|---|---|---|
| <30 (n = 21) | 30 to 60 (n = 101) | 60 to 90 (n = 142) | 90 to 120 (n = 109) | >120 (n = 53) | ||
| Baseline patient variables | ||||||
| Women, n (%) | 7 (33) | 45 (45) | 48 (34) | 31 (28) | 13 (25) | 0.07 |
| Age, yr, mean (SD) | 73.4 (13) | 73.6 (10) | 65.6 (13) | 55.5 (14) | 49.2 (12) | 0.01 |
| BMI, kg/m2, median (interquartile range) | 25.2 (21 to 30) | 25.3 (22 to 27) | 26.5 (24 to 29) | 26.6 (24 to 31) | 30 (27 to 34) | 0.01 |
| Preoperative creatinine, mg/dl, mean (SD) | 2.1 (1.21) | 1.3 (0.36) | 1.0 (0.26) | 0.9 (0.19) | 0.9 (0.18) | 0.001 |
| Parsonnet score, mean (SD) | 12.7 (7.6) | 11.9 (9.1) | 9.1 (7.0) | 6.9 (6.2) | 7.7 (6.3) | 0.001 |
| eGFR, mean (SD) | 15.3 (11) | 47.3 (8.5) | 73.7 (8.1) | 102.5 (8.2) | 140 (24.7) | N/A |
| Operative characteristics | ||||||
| OPCABG, n (%) | 2 (10) | 15 (15) | 18(13) | 13 (12) | 5 (9) | 0.88 |
| CABG, n (%) | 3 (14) | 22 (22) | 26 (18) | 18 (17) | 3 (6) | 0.24 |
| Open chamber +/− CABG, n (%) | 13 (62) | 56 (55) | 90 (64) | 73 (67) | 40 (75) | 0.15 |
| Transplant/VAD, n (%) | 3 (14) | 8 (8) | 8 (6) | 3 (3) | 1 (2) | 0.37 |
| Other, n (%) | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 4 (8) | 0.02 |
| Reoperation, n (%) | 2 (10) | 16 (16) | 21 (15) | 10 (9) | 9 (17) | 0.52 |
| CPB time, min, mean (SD) | 120 (52) | 124 (49) | 128 (50) | 123 (45) | 113 (43) | 0.65 |
| AXC time, min, mean (SD) | 90 (28) | 90 (38) | 87 (33) | 83 (35) | 78 (31) | 0.49 |
| Aprotinin, n (%) | 10 (48) | 57 (56) | 76 (54) | 41 (38) | 24 (45) | 0.05 |
| Postoperative characteristics | ||||||
| AKI, n (%)b | 9 (41) | 20 (20) | 35 (25) | 13 (12) | 8 (15) | 0.01 |
OPCABG, off-pump coronary artery bypass graft surgery; CABG, coronary artery bypass graft surgery, on-pump; VAD, ventricular assist device; AXC, aortic cross-clamp duration.
eGFR was calculated by Cockcroft-Gault equation.
AKI was defined by serum creatinine increase >50% or >0.3 mg/dl above baseline within 48 hours of surgery.
Results
Preoperative eGFR was normally distributed with mean 80 ± 34 ml/min and was inversely associated with age, preoperative creatinine, and Parsonnet score (Table 1). Operative characteristics, including use of aprotinin, were not significantly different across eGFR strata. AKI developed in 85 patients (20%), the incidence highest in the group with the lowest preoperative eGFR. Regardless of evolving AKI status, urinary NGAL levels peaked immediately after CPB in all groups except those with preoperative eGFR <30 ml/min, in whom NGAL continued to increase through 24 hours postoperatively, regardless of AKI status (Table 2).
Table 2.
Urinary NGAL levels at various time points preoperatively and postoperatively, comparing patients who developed AKI with those who did not
| Urine NGAL, ng/ml, |
P | ||
|---|---|---|---|
| No AKI | AKI-Positive | ||
| eGFR 0 to 30 ml/min (n = 21) | |||
| n | 12 | 9 | |
| Preoperative | 20 (11 to 379) | 32 (10 to 76) | 0.91 |
| Immediately after CPB | 300 (146 to 1349) | 295 (87 to 933) | 0.62 |
| 3 h after CPB | 339 (15 to 904) | 329 (112 to 1638) | 0.68 |
| 18 h after CPB | 788 (96 to 3120) | 260 (98 to 2607) | 0.63 |
| 24 h after CPB | 1380 (151 to 5144) | 755 (152 to 813) | 0.37 |
| eGFR 30 to 60 ml/min (n = 101) | |||
| n | 81 | 20 | |
| Preoperative | 10 (3 to 44) | 7 (3 to 83) | 0.96 |
| Immediately after CPB | 731 (39 to 1951) | 630 (130 to 2216) | 0.64 |
| 3 h after CPB | 463 (16 to 1385) | 407 (59 to 1546) | 0.64 |
| 18 h after CPB | 44 (14 to 800) | 88 (32 to 608) | 0.48 |
| 24 h after CPB | 80 (21 to 958) | 291 (28 to 642) | 0.92 |
| eGFR 60 to 90 ml/min (n = 142) | |||
| n | 107 | 35 | |
| Preoperative | 7 (3 to 21) | 8 (3 to 26) | 0.96 |
| Immediately after CPB | 549 (7 to 1993) | 1740 (157 to 4399) | 0.01 |
| 3 h after CPB | 106 (4 to 830) | 1335 (14 to 2714) | 0.001 |
| 18 h after CPB | 37 (11 to 195) | 53 (23 to 483) | 0.13 |
| 24 h after CPB | 44 (14 to 318) | 46 (25 to 464) | 0.40 |
| eGFR 90 to 120 ml/min (n = 109) | |||
| n | 96 | 13 | |
| Preoperative | 5 (2 to 10) | 22 (6 to 38) | 0.03 |
| Immediately after CPB | 43 (5 to 1472) | 1354 (34 to 2674) | 0.06 |
| 3 h after CPB | 17 (3 to 405) | 256 (29 to 3003) | 0.049 |
| 18 h after CPB | 15 (7 to 40) | 148 (39 to 313) | 0.002 |
| 24 h after CPB | 18 (10 to 33) | 283 (72 to 866) | 0.0001 |
| eGFR >120 ml/min (n = 53) | |||
| n | 45 | 8 | |
| Preoperative | 5 (2 to 12) | 5 (2 to 9) | 0.82 |
| Immediately after CPB | 1047 (18 to 2795) | 3 (0 to 1922) | 0.01 |
| 3 h after CPB | 88 (12 to 2195) | 1 (1 to 6) | 0.03 |
| 18 h after CPB | 17 (6 to 55) | 9 (3 to 16) | 0.08 |
| 24 h after CPB | 19 (8 to 99) | 8 (4 to 15) | 0.05 |
Values are given as median, with interquartile range in parentheses. Comparisons are stratified by baseline renal function determined by eGFR (calculated by Cockroft-Gault equation). Comparisons are made using Mann-Whitney U test.
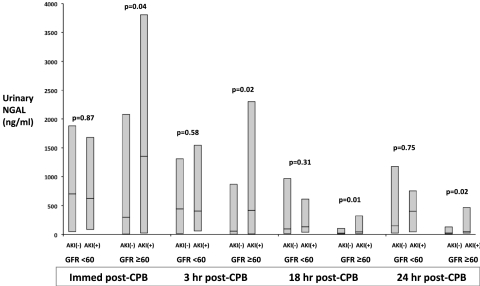
In patients with baseline eGFR ≥60 ml/min, urinary NGAL was higher at all postoperative time points through 24 hours in patients who developed AKI compared with those who did not develop AKI. Conversely, in patients with baseline eGFR <60 ml/min, urinary NGAL did not differ at any postoperative time point between patients who did and who did not develop AKI (Figure 1). When considered according to the five prespecified eGFR groupings, in each of the lowest two eGFR strata (<30 ml/min, 30 to 60 ml/min), urinary NGAL did not differ at any time preoperatively or postoperatively between patients with and without AKI (Table 2). In patients with preoperative eGFR 60 to 90 ml/min, those developing AKI had significantly higher urinary NGAL immediately after CPB and at 3 hours after CPB only. In patients with preoperative eGFR 90 to 120 ml/min, those developing AKI had higher urinary NGAL at all times measured through 24 hours postoperatively. In patients with the highest preoperative eGFR (>120 ml/min), NGAL was paradoxically lower among patients developing AKI, this difference persisting throughout 24 hours postoperatively.
Figure 1.
Urinary NGAL (ng/ml) at serial time points after CPB, comparing patients who did or did not develop AKI. For each comparison, patients are stratified according to preoperative eGFR above or below 60 ml/min. Shaded boxes represent median and interquartile range for NGAL levels. Comparisons are made between patients with and without AKI, within eGFR strata, at each time point using the Mann-Whitney U test with P values annotated.
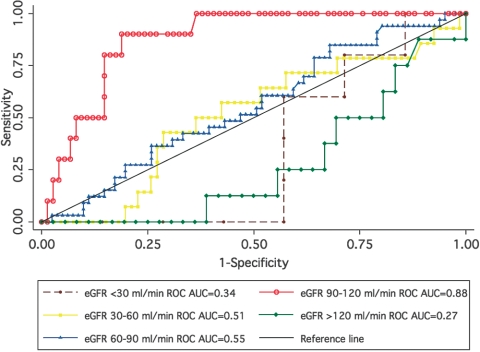
ROC curves were constructed for each strata of baseline renal function to assess the utility of NGAL at each time point to identify patients with AKI (Figure 2). Diagnostic characteristics were further defined by sensitivity, specificity, and likelihood ratios, on the basis of selected cut points for NGAL (Table 3). In patients with baseline eGFR <60 ml/min, NGAL was unable to identify evolving AKI at any time. Performance improved in patients with a mild reduction in baseline eGFR (60 to 90 ml/min), with area under the ROC curve (AUC) of 0.64 to 0.66 in the early postoperative period. However, discriminant ability declined beyond 3 hours postoperatively. Urinary NGAL was best able to identify evolving AKI in patients with normal preoperative eGFR (90 to 120 ml/min). Although the AUC was significant at all time points in this cohort, performance peaked 24 hours postoperatively with an AUC of 0.88 (95% confidence interval, 0.79 to 0.96). The ability of urinary NGAL to identify evolving AKI in patients with preoperative eGFR 90 to 120 ml/min was significantly better than in other patient groups at both 18 and 24 hours postoperatively, but not before this time. At a threshold value for NGAL of 53 ng/ml at 24 hours after CPB, sensitivity and specificity were 90% and 81%, respectively, for the diagnosis of AKI (Table 3). For sensitivity analysis, we examined the effect of redefining AKI as occurring only when serum creatinine increased by more than 50% above baseline over a period of 7 days postoperatively. Using these criteria, 68 patients developed AKI, whereas 40 patients diagnosed with AKI by the original criteria were no longer considered to have had AKI. Again, only in the group with baseline eGFR ≥60 ml/min was urinary NGAL higher in patients who developed AKI. Although some reduction in the AUC was observed among patients with normal preoperative eGFR, discriminant utility of urinary NGAL remained fair to good in patients with baseline eGFR ≥60 ml/min, whereas it was unable to identify AKI at any time point in patients with baseline eGFR <60 ml/min (Tables 4 and 5). On exclusion of patients with BMI ≥30 kg/m2 or <18.5 kg/m2, eGFR remained significantly associated with AKI only in patients with eGFR ≥60 ml/min, with optimal diagnostic performance in patients with normal baseline renal function (Table 6). Further analysis considering patients with eGFR 90 to 120 ml/min and >120 ml/min as a single group produced a null result across all comparisons.
Figure 2.
ROC curve for urinary NGAL at 24 hours postoperatively, stratified by baseline eGFR (ml/min). The AUC is significantly greater for patients with preoperative eGFR 90 to 120 ml/min compared with all other groups (P < 0.003).
Table 3.
Diagnostic characteristics of urinary NGAL for AKI, stratified by preoperative eGFR
| ROC (95% CI)a | Cut pointb | Sensitivity, % | Specificity, % | LR (+)c | LR (−)d | |
|---|---|---|---|---|---|---|
| eGFR <30 ml/min | ||||||
| After CPB | 0.44 (0.17 to 0.70) | 87 | 78 | 17 | 0.93 | 1.33 |
| 3 h | 0.56 (0.28 to 0.83) | 164 | 75 | 45 | 1.38 | 0.55 |
| 18 h | 0.42 (0.04 to 0.79) | 98 | 83 | 33 | 1.25 | 0.50 |
| 24 h | 0.34 (0.00 to 0.69) | 152 | 80 | 29 | 1.12 | 0.70 |
| eGFR 30 to 60 ml/min | ||||||
| After CPB | 0.53 (0.39 to 0.68) | 88 | 80 | 29 | 1.12 | 0.70 |
| 3 h | 0.54 (0.39 to 0.68) | 43 | 83 | 31 | 1.20 | 0.55 |
| 18 h | 0.55 (0.42 to 0.69) | 20 | 83 | 39 | 1.36 | 0.43 |
| 24 h | 0.51 (0.34 to 0.67) | 28 | 79 | 30 | 1.13 | 0.71 |
| eGFR 60 to 90 ml/min | ||||||
| After CPB | 0.64 (0.53 to 0.75) | 157 | 77 | 42 | 1.34 | 0.54 |
| 3 h | 0.66 (0.54 to 0.77) | 13 | 80 | 35 | 1.23 | 0.57 |
| 18 h | 0.59 (0.48 to 0.70) | 23 | 76 | 40 | 1.27 | 0.59 |
| 24 h | 0.55 (0.44 to 0.66) | 25 | 79 | 36 | 1.23 | 0.59 |
| eGFR 90 to 120 ml/min | ||||||
| After CPB | 0.66 (0.52 to 0.80) | 24 | 85 | 44 | 1.50 | 0.35 |
| 3 h | 0.68 (0.52 to 0.83) | 25 | 83 | 55 | 1.85 | 0.30 |
| 18 h | 0.77 (0.62 to 0.92) | 47 | 77 | 77 | 3.32 | 0.32 |
| 24 h | 0.88 (0.79 to 0.96) | 53 | 90 | 81 | 4.76 | 0.12 |
| eGFR >120 ml/min | ||||||
| After CPB | 0.20 (0.00 to 0.42) | 8 | 29 | 9 | 0.31 | 8.03 |
| 3 h | 0.20 (0.00 to 0.47) | 9 | 20 | 21 | 0.25 | 3.73 |
| 18 h | 0.30 (0.12 to 0.48) | 20 | 13 | 50 | 0.25 | 1.75 |
| 24 h | 0.27 (0.10 to 0.44) | 19 | 13 | 44 | 0.23 | 1.97 |
eGFR calculated by Cockroft-Gault equation.
Area under the receiver-operator characteristic curve (95% CI).
Threshold value for urine NGAL (ng/ml) for diagnosis of AKI.
Likelihood ratio for a positive test result.
Likelihood ratio for a negative test result.
Table 4.
Urinary NGAL levels (median, interquartile range) at various time-points, comparing patients who developed AKI with those who did not, redefining AKI as an increase in serum creatinine by >50% above baseline within 7 days of surgery
| Urine NGAL, ng/ml |
P | ||
|---|---|---|---|
| No AKI | AKI-Positive | ||
| eGFR 0 to 60 ml/min (n = 122) | |||
| n | 101 | 21 | |
| Preoperative | 11 (4 to 50) | 26 (3 to 83) | 0.67 |
| Immediately after CPB | 690 (48 to 1969) | 534 (172 to 1124) | 0.93 |
| 3 h | 368 (15 to 1458) | 560 (182 to 1312) | 0.29 |
| 18 h | 98 (18 to 712) | 108 (16 to 1138) | 0.56 |
| 24 h | 152 (24 to 958) | 505 (28 to 1380) | 0.51 |
| eGFR >60 ml/min (n = 304) | |||
| n | 257 | 47 | |
| Preoperative | 6 (3 to 18) | 6 (3 to 21) | 0.65 |
| Immediately after CPB | 295 (8 to 2096) | 1314 (87 to 2938) | 0.04 |
| 3 h | 53 (4 to 867) | 844 (11 to 2559) | 0.01 |
| 18 h | 22 (8 to 87) | 117 (19 to 415) | 0.001 |
| 24 h | 25 (10 to 129) | 61 (20 to 479) | 0.01 |
Patients are stratified by baseline renal function determined by eGFR. Comparisons are made using Mann-Whitney U test. Values given are median, with interquartile range in parentheses.
Table 5.
Area under ROC curve (95% CI) of urinary NGAL for AKI, stratified by preoperative eGFR (AKI has been redefined as an increase in serum creatinine by >50% above baseline within 7 days of surgery)
| eGFR <30 ml/min | eGFR 30–60 ml/min | eGFR 60–90 ml/min | eGFR 90–120 ml/min | eGFR >120 ml/min | |
|---|---|---|---|---|---|
| After CPB | 0.23 (0.01 to 0.44) | 0.58 (0.44 to 0.71) | 0.64a (0.52 to 0.76) | 0.64a (0.50 to 0.78) | 0.18 (0.00 to 0.46) |
| 3 h | 0.49 (0.21 to 0.76) | 0.61 (0.48 to 0.75) | 0.68a (0.54 to 0.81) | 0.67a (0.53 to 0.80) | 0.28 (0.00 to 0.73) |
| 18 h | 0.45 (0.05 to 0.85) | 0.55 (0.37 to 0.74) | 0.69a (0.58 to 0.80) | 0.69a (0.53 to 0.85) | 0.37 (0.16 to 0.58) |
| 24 h | 0.65 (0.34 to 0.96) | 0.54 (0.35 to 0.74) | 0.62 (0.49 to 0.73) | 0.71a (0.56 to 0.87) | 0.31 (0.09 to 0.54) |
eGFR calculated by Cockcroft-Gault equation.
P < 0.05.
Table 6.
Area under ROC curve (95% CI) of urinary NGAL for AKI, stratified by preoperative eGFR (patients with BMI <18.5 kg/m2 or ≥30 kg/m2 were excluded from analysis)
| eGFR <30 ml/min (n = 14) | eGFR 30 to 60 ml/min (n = 87) | eGFR 60 to 90 ml/min (n = 107) | eGFR 90 to 120 ml/min (n = 75) | eGFR >120 ml/min (n = 25) | |
|---|---|---|---|---|---|
| After CPB | 0.39 (0.06 to 0.72) | 0.49 (0.34 to 0.64) | 0.61 (0.48 to 0.74) | 0.77 (0.62 to 0.91)a | 0.26 (0.00 to 0.78) |
| 3 h | 0.42 (0.02 to 0.82) | 0.49 (0.33 to 0.65) | 0.65 (0.51 to 0.79)a | 0.69 (0.44 to 0.95) | 0.67 (0.00 to 1.00) |
| 18 h | 0.07 (0.00.25)a | 0.52 (0.38 to 0.67) | 0.55 (0.42 to 0.68) | 0.78 (0.56 to 1.0)a | 0.29 (0.00 to 0.87) |
| 24 h | 0.00 (0.00 to 0.00)a | 0.46 (0.28 to 0.65) | 0.56 (0.41 to 0.70) | 0.89 (0.78 to 1.0)a | 0.44 (0.07 to 0.82) |
eGFR calculated by Cockcroft-Gault equation.
P < 0.05.
Discussion
In this prospective observational study of 426 adult cardiac surgical patients, the relationship between postoperative urinary NGAL and AKI varied with baseline renal function, a positive relationship observed only in patients with preoperative eGFR ≥60 ml/min. Furthermore, the ability of urinary NGAL to provide early and accurate identification of evolving AKI was significantly influenced by baseline eGFR, with optimal performance in patients with normal baseline renal function (eGFR 90 to 120 ml/min).
Previous studies in pediatric and adult cardiac surgical populations report excellent discriminant ability for urinary NGAL to identify AKI within 2 to 6 hours after CPB (5–8). However, in each of these studies, patients with preexisting renal dysfunction were excluded. In contrast, the adult population in the current study represents a heterogeneous mix of baseline renal function. We have previously reported that applied to this population as a whole, urinary NGAL had only modest ability to identify evolving AKI after cardiac surgery, with an AUC of 0.61, peaking 18 hours postoperatively (3). Koyner et al. (10) similarly reported modest diagnostic performance of urinary NGAL for AKI after cardiac surgery in 72 adults with a range of baseline renal function similar to the current study. Despite adjusting for urinary creatinine, they found an AUC of 0.70 but did not explore the effect of stratification by preoperative renal function. However, NGAL is a marker of acute renal injury rather than function (14–16), and we therefore hypothesized that the nonlinear relationship between GFR and serum creatinine may result in differing magnitude of injury (NGAL) being required to produce a rise in serum creatinine, depending on preoperative GFR. In fact, our findings indicated a markedly different relationship between baseline renal function, NGAL, and AKI. Despite having a higher incidence of AKI, consistent with current understanding of post-cardiac surgery renal dysfunction, patients with preoperative eGFR <60 ml/min showed no relationship between postoperative urinary NGAL and development of AKI. Only in patients with normal or mildly impaired preoperative renal function (eGFR 60 to 120 ml/min) was postoperative urinary NGAL significantly higher in patients with evolving AKI. Furthermore, the ability of postoperative urinary NGAL to accurately identify AKI was greatest in patients with normal baseline eGFR (90 to 120 ml/min).
Differential expression of NGAL in AKI according to baseline renal function has not been previously reported, and a number of possible explanations may be considered. A reduced ability to rapidly amplify NGAL expression in response to tubular injury in kidneys with preexisting damage may contribute to our findings. Previous authors have discussed the need for a troponin-like biomarker of AKI that could be interpreted largely in isolation from other clinical variables (14,17). However, unlike the passive leakage of preformed troponin from an injured cardiomyocyte, NGAL release is more complex, involving a sequence of renal tubular injury, gene up-regulation with mRNA expression, and subsequent NGAL synthesis and secretion, all requiring tubular cell function. Although such a hypothesis has not been previously explored, we noted a trend toward higher peak NGAL levels among patients with baseline eGFR ≥60 ml/min compared with those with baseline eGFR <60 ml/min. Furthermore, the later and lower peak NGAL level among patients with baseline eGFR <30 ml/min may be further consistent with this hypothesis. Although serum NGAL has good inverse correlation with GFR in children and adults with chronic kidney disease (18,19), this stable setting may not indicate capacity for rapid response in the context of acute injury associated with cardiac surgery. Second, we observed that in all but the lowest baseline eGFR group, urinary NGAL levels peaked immediately after CPB, declining thereafter, whereas optimal discrimination of AKI by urinary NGAL did not occur until 24 hours postoperatively, suggesting different NGAL elimination as potentially influential in discrimination of AKI. It is unknown whether evolving AKI modifies the diuretic response to furosemide. However, if so, patients without AKI who receive furosemide may have a marked diuresis, diluting urinary NGAL, whereas patients with evolving AKI may have a blunted response with less urinary dilution, thus enhancing the apparent discriminant ability of urinary NGAL for AKI. Although adjusting NGAL values for urinary creatinine may have been instructive in this regard, urinary creatinine was not measured, and several previous authors have reported limited incremental benefit in doing so (8,11). Additionally, data on perioperative diuretic administration or urine volume output were not available to further explore this possibility. Third, extrarenal sources of NGAL have generally been considered of limited significance to urinary NGAL measures. Although other sources may contribute to the plasma pool of NGAL, the 3- to 10-fold difference between NGAL concentrations in urine and plasma has been interpreted as indicating a renal-specific origin for most NGAL present in urine (7,20). However, these reports may not be applicable to an adult cardiac surgery population. NGAL is expressed by atherosclerotic vascular tissue (21,22), which may itself be up-regulated in the early postoperative period, and chronic renal disease is a marker for generalized atherosclerosis, potentially enhancing the contribution of extrarenal NGAL in such patients. Furthermore, although animal models indicate systemic NGAL filtered at the renal glomerulus is rapidly and efficiently reabsorbed by the proximal renal tubule (20), this may not be the case in the setting of chronic renal insufficiency. Combined, these factors may limit the ability of urinary NGAL to accurately discriminate AKI in the setting of preexisting renal disease. Haase-Fielitz et al. (4) reported good discriminant utility of plasma NGAL for AKI in 100 adult cardiac surgery patients with a broad range of baseline renal function (AUC, 0.80, 95% confidence interval, 0.63 to 0.96). Although they found minimal change in the diagnostic characteristics of plasma NGAL when excluding patients with impaired preoperative renal function, this group was small (n = 27), and they did not report the diagnostic performance of plasma NGAL applied to this group alone. Furthermore, the performance of plasma and urine NGAL as diagnostic biomarkers may be affected differently by alterations in baseline renal function.
Among patients with the highest preoperative eGFR (>120 ml/min), the inverse relationship between urinary NGAL and AKI is previously unreported. The null result when analyzed together with the eGFR 90 to 120 ml/min group is not surprising, given the directly opposing results within the two groups. However, previous studies demonstrate a clear relationship between NGAL and AKI in patients with normal renal function, supporting our current findings in that group. Supranormal GFR is described in association with both early diabetes and mild hypertension and may be related to afferent arteriolar vasodilation (23–25). However, whether this has any pathophysiologic implications in cardiac surgery remains unknown, and the possibility of sampling error in this subgroup cannot be discounted.
Although we used a standardized definition of AKI, it is possible that the inclusion of absolute increases in creatinine as small as 0.3 mg/dl or restricting creatinine rise to within 48 hours postoperatively may have contributed to the observed impact of preoperative eGFR on the NGAL-AKI relationship. However, large studies confirm the clinical significance of small absolute increases in serum creatinine after cardiac surgery (26,27). Waikar and Bonventre (28) further demonstrated that the rate of the rise of absolute serum creatinine after acute reduction in GFR is relatively constant across a range of baseline renal function, making misclassification bias in patients with impaired baseline function unlikely. Modification of our AKI definition, allowing 7 days for a 50% increase in creatinine (consistent with RIFLE criteria), had minimal impact on our results. The slight reduction in AUC that this produced potentially reflects inclusion of some renal injury occurring beyond 24 hours postoperatively and therefore not detected by perioperative NGAL. We chose the Acute Kidney Injury Network criteria to define our AKI cohort because the 48-hour window for diagnosis limits detected renal injury to that which occurred intraoperatively or in the very early postoperative period, and it is this injury that we would hope to detect with the early use of NGAL.
We used the formula described by Cockcroft and Gault to estimate creatinine clearance, and thus GFR (12). Although the precision of this formula has been questioned, it is reported to suffer similar or less systematic bias than the alternative Modification of Diet in Renal Disease formula across normal and modest degrees of renal impairment (29). Although it is less accurate than measuring creatinine clearance over 24 hours, it presents a practical and reproducible estimate of GFR. The Cockcroft-Gault formula may be further criticized for its sensitivity to body weight and for providing an estimate of GFR not indexed to body surface area (29). However, with the exception of the eGFR >120 ml/min group, BMI did not differ between other eGFR strata and excluding from analysis patients outside a predefined range of BMI (BMI <18.5 kg/m2 or ≥30 kg/m2) did not meaningfully alter results. Increasing magnitude of error in self-reported height and weight has been found in patients older than 65 years of age (30,31), and small errors in estimates of these parameters may be further amplified in the process of indexing eGFR to body surface area. Furthermore, some investigators have questioned the usefulness of routinely indexing GFR to body surface area (32). Although the Modification of Diet in Renal Disease formula may have been a better method to estimate GFR in this context, we did not have the necessary data for this calculation, and future studies should determine whether our findings can be replicated with other methods of eGFR assessment. We assumed preoperative eGFR to be a measure of baseline renal function. However, it is possible that the renal function of some patients may have already been in a state of flux due to recent contrast angiography, acute changes in cardiac function, or adjustment to pharmacotherapy in the preoperative period. The biology of NGAL and its association with AKI are incompletely understood, with numerous other potential sources of variation unexplored. However, this analysis was not intended as an exhaustive investigation of variables that may be associated with urinary NGAL, but rather a hypothesis-driven examination of one factor, baseline renal function, that may have an impact on the relationship between NGAL and AKI in cardiac surgery, and to determine the value of a stratified analysis to improve diagnostic utility. This is the largest study to date of NGAL in an adult cardiac surgery population. However, the current analysis does represent a posthoc subgroup analysis, not part of the original design, and as such our results must be interpreted with caution. Our results should be confirmed in appropriately powered a priori studies. Finally, the diagnosis of AKI in this study relies on an increase in serum creatinine, itself recognized as both a late and insensitive marker of renal function, and therefore not a true gold standard. The diagnostic utility of NGAL may be better assessed by determining its predictive utility for clinical outcomes such as mortality or progression to dialysis-dependent renal failure. However, larger studies are required to address this question.
In a posthoc analysis, we found the relationship between postoperative urinary NGAL and AKI after cardiac surgery varies with baseline renal function assessed by eGFR. Only in patients with baseline eGFR ≥60 ml/min was AKI associated with elevated postoperative urinary NGAL levels with optimal diagnostic utility in patients with normal preoperative eGFR (90 to 120 ml/min). Further studies are required to confirm this relationship and explore the underlying mechanism, helping to clarify both the potential role and limitations of NGAL as an acute kidney injury biomarker.
Disclosures
None.
Acknowledgments
This work was supported by Intramural funding from the Department of Anesthesiology, Columbia University, New York, New York.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Haase M, Haase-Fielitz A, Bagshaw SM, Read MC, Morgera S, Seevenayagam S, Matalanis G, Buxton B, Doolan L, Bellomo R: Phase II, randomized, controlled trial of high-dose N-acetylcysteine in high-risk cardiac surgery patients. Crit Care Med 35: 1324–1331, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Stafford-Smith M, Podgoreanu M, Swaminathan M, Phillips-Bute B, Mathew JP, Hauser EH, Winn MP, Milano C, Nielson DM, Smith M, Morris R, Newman MF, Schwinn DA: Association of genetic polymorphisms with risk of renal injury after coronary bypass graft surgery. Am J Kidney Dis 45: 519–530, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT: Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Am J Kidney Dis 52: 425–433, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Dragun D, Haase M: Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery–A prospective cohort study. Crit Care Med 37: 553–560, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P: Urine NGAL predicts severity of acute kidney injury after cardiac surgery: A prospective study. Clin J Am Soc Nephrol 3: 665–673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, Devarajan P: Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: A prospective uncontrolled cohort study. Crit Care (London, England) 11: R127, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Xin C, Yulong X, Yu C, Changchun C, Feng Z, Xinwei M: Urine neutrophil gelatinase-associated lipocalin and interleukin-18 predict acute kidney injury after cardiac surgery. Ren Fail 30: 904–913, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Tuladhar SM, Puntmann VO, Soni M, Punjabi PP, Bogle RG: Rapid detection of acute kidney injury by plasma and urinary neutrophil gelatinase-associated lipocalin after cardiopulmonary bypass. J Cardiovasc Pharmacol 53: 261–266, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, Kasza KE, O'Connor MF, Konczal DJ, Trevino S, Devarajan P, Murray PT: Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int 74: 1059–1069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han W, Wagener G, Zhu Y, Wang S, Lee H: Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol 4: 873–882, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cockroft D, Gault M: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 13.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A: Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care (London, England) 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coca SG, Yalavarthy R, Concato J, Parikh CR: Biomarkers for the diagnosis and risk stratification of acute kidney injury: A systematic review. Kidney Int 73: 1008–1016, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Mori K, Nakao K: Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int 71: 967–970, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT: Increased incidence of acute kidney injury with aprotinin use during cardiac surgery detected with urinary NGAL. Am J Nephrol 28: 576–582, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Parikh CR, Devarajan P: New biomarkers of acute kidney injury. Crit Care Med 36: S159–S165, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Mitsnefes MM, Kathman TS, Mishra J, Kartal J, Khoury PR, Nickolas TL, Barasch J, Devarajan P: Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol 22: 101–108, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, Nicosia G, Buemi M: Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol 4: 337–344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D'Agati V, Devarajan P, Barasch J: Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 115: 610–621, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemdahl AL, Gabrielsen A, Zhu C, Eriksson P, Hedin U, Kastrup J, Thoren P, Hansson GK: Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol 26: 136–142, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Ohlsson S, Wieslander J, Segelmark M: Increased circulating levels of proteinase 3 in patients with anti-neutrophilic cytoplasmic autoantibodies-associated systemic vasculitis in remission. Clin Exp Immunol 131: 528–535, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones SL, Viberti G: Renal functional reserve in subjects with diabetes mellitus. Semin Nephrol 15: 475–481, 1995 [PubMed] [Google Scholar]
- 24.Odutola TA, Ositelu SB, D'Almeida EA, Okeiyi JC: Supra-normal creatinine clearance in black mild hypertensive patients in Nigeria. J Hum Hypertens 2: 133–134, 1988 [PubMed] [Google Scholar]
- 25.ter Wee PM, van Ballegooie E, Rosman JB, Meijer S, Donker AJ: Renal reserve filtration capacity in patients with type 1 (insulin-dependent) diabetes mellitus. Nephrol Dial Transplant 2: 504–509, 1987 [PubMed] [Google Scholar]
- 26.Lassnigg A, Schmid ER, Hiesmayr M, Falk C, Druml W, Bauer P, Schmidlin D: Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: Do we have to revise current definitions of acute renal failure? Crit Care Med 36: 1129–1137, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M: Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol 15: 1597–1605, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Waikar SS, Bonventre JV: Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 20: 672–679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P: Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol 16: 763–773, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Craig BM, Adams AK: Accuracy of body mass index categories based on self-reported height and weight among women in the United States. Matern Child Health J 13: 489–496, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elgar FJ, Stewart JM: Validity of self-report screening for overweight and obesity. Evidence from the Canadian Community Health Survey. Can J Public Health 99: 423–427, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner ST, Reilly SL: Fallacy of indexing renal and systemic hemodynamic measurements for body surface area. Am J Physiol 268: R978–R988, 1995 [DOI] [PubMed] [Google Scholar]