Abstract
Background and objectives: Ambulatory blood pressure (BP) monitoring is commonly used to assess the circadian pattern of BP. Circadian BP pattern is influenced by physical activity and sleep cycle. The effect of BP monitoring itself on the level of physical activity and sleep remains unknown. If BP monitoring affects these parameters, then monitoring itself may influence the circadian BP pattern.
Design, setting, participants, & measurements: To assess the effect of ambulatory BP monitoring on sleep duration, sleep efficiency, and daytime activity, we measured physical activity using wrist actigraphy in 103 veterans with chronic kidney disease. After 6 to 7 days of continuous activity monitoring, participants underwent ambulatory BP monitoring with simultaneous actigraphy. The above experiment was repeated after 1 mo.
Results: Among the top tertile of patients (most sleep), when wearing ambulatory BP patients spent less time in bed at night (−92 min, P < 0.0001), were less asleep during those hours (−98 min, P < 0.0001), and had reduced sleep efficiency (82% versus 77%, −5% P = 0.02). On the day of ambulatory BP monitoring, patients were more sedentary during waking hours (+27 minutes, P = 0.002). During ambulatory BP monitoring, waking after sleep onset more than median was associated with greater odds for nondipping (odds ratio 10.5, P = 0.008).
Conclusions: Ambulatory BP monitoring is associated with disturbed sleep and reduced physical activity, characteristics that influence dipping. Ambulatory BP monitoring may itself induce nondipping and may thus mitigate the prognostic significance of the dipping phenomenon.
Blood pressure (BP) in healthy people follows a circadian pattern that is influenced by the level of activity during the day and depth of sleep at night (1). Sleeping systolic BP, which should at least be 10% lower than awake systolic BP, is often measured by 24-hour ambulatory BP monitoring. This fall in BP during sleep—dipping—is a determinant of prognosis (2). Nondipping occurs in a variety of conditions and diseases, such as in patients with chronic kidney disease (CKD), sleep apnea, volume overload, nocturia, and those with sympathetic activation (3); nondipping is associated with left ventricular hypertrophy (4) and increased cardiovascular risk (5).
Davies et al. (6) have reported that sensory stimulation as may occur during cuff inflation may cause cortical arousal, disturb sleep, and raise BP—a phenomenon they characterized in the sleep laboratory among six normal volunteers. However, it remains unknown to what extent ambulatory BP recording can impair nighttime sleep and whether it can also affect daytime activity.
The purpose of this study was to assess the effect of ambulatory BP monitoring on sleep duration, sleep efficiency, and daytime activity. We hypothesized that ambulatory BP monitoring will reduce sleep efficiency and reduce daytime activity. Further, we hypothesized that those patients who have the maximal sleep disturbance will have the least dipping at night. If so, it would suggest that ambulatory BP monitoring may induce nondipping per se. These findings would have implications for assessing the independent prognostic value of dipping.
Materials and Methods
Participants
We studied veterans between the ages of 18 and 90 years with CKD defined as an estimated GFR (eGFR) of <60 ml/min/1.73 m2, or presence of proteinuria (spot urine/protein ratio of >200 mg/g creatinine), or the presence of structural kidney disease (e.g., adult polycystic kidney disease). We excluded those patients who met any of the following characteristics: morbid obesity (body mass index ≥40 kg/m2), eGFR ≤15 ml/min/1.73 m2, hospitalization within the prior 2 mo, seated clinic BP ≥140/90 mmHg, or substantial cardiac arrhythmia (defined as ≥6 beats/min).
Study Protocol
The study protocol was approved by the institutional review boards and the VA Research and Development Committee. Patients were recruited between June 2007 and May 2009 after written informed consent and studied on two occasions 1 mo apart with ambulatory BP monitoring and simultaneous actigraphy as described below.
Ambulatory BP Monitoring
Ambulatory BP monitoring (ABPM) was performed in the nondominant arm for 24 hours using the SpaceLab 90207 monitor (SpaceLabs Medical Inc., Redmond, WA) with cuff inflations every 20 minutes during the day (6:00 a.m. to 10:00 p.m.) and every 30 minutes during the night. Accuracy of ambulatory BP recordings was confirmed against auscultated BP. Hourly averages were calculated and the average of these averages represented the mean systolic and diastolic BP. Patients were asked to record the sleep and wake times during this recording. A sleep-to-wake systolic BP ratio of <0.9 was taken as evidence for dipping.
Actigraphy
Concomitant activity monitoring was performed using an actigraph (Actiwatch 64, Mini Mitter, Bend, OR), a watch-sized device worn on the dominant wrist for 7 to 8 days that also included the day of ABPM. This procedure was performed at baseline and repeated at 1 mo. The internal clocks of the ABPM and actigraph were synchronized and activity was assessed in 15-second epochs throughout the 24-hour period. Data were exported to a custom-designed relational database.
Actigraphy-Assisted Sleep Assessment
The following definitions were used to assess sleep:
Duration in bed: Minutes of the rest interval reported by the patient on each day recorded in the diary.
Scored total sleep time: Duration in minutes in which the patient was asleep judged by the actigraphic counts. When a weighted average of the epoch of activity in question and 16 surrounding epochs had a total activity count of <20, the patient was said to be asleep. When total activity counts exceeded ≥20, the patient was said to be awake.
Total awake time: Duration in minutes in which the patient was labeled as awake during the rest interval. Percent of time awake was calculated as the fraction of total awake time to duration in bed ×100%.
Wake after sleep onset (WASO): Number of minutes between sleep onset and wake time was scored as wake.
Sleep efficiency: The percent of scored total sleep time to the duration in bed.
Activity Classification
To determine the level of activity, we first calculated the total duration of the waking period during a 24-hour period starting from midnight. The level of activity counts of <80 were computed, and duration of any count <80 was taken as resting (level 0). Duration of activity between 80 and 160 counts/epoch (level 1), 160 to 320 counts/epoch (level 2), and >320 counts/epoch (level 3) were taken as evidence of increasingly vigorous activity. Visual inspection of a wheelchair-bound patient for each day for 2 wk revealed nearly all counts per epoch to be <160, suggesting a generally sedentary existence. Similarly, inspection of plots of activity in patients who exercised in a gym frequently showed bursts of activity >320 count/epoch, suggesting more vigorous activity.
Data Analyses
Categorical mixed models that account for repeated measurements within subjects to model outcome variables for sleep included duration in bed, scored total sleep time, percent awake time, WASO, and sleep efficiency (7). Specifically, the following model was fitted: yijk = β0 + μ0j + μ0jk + β1(ABPM visit) + rij, with maximal likelihood estimates in which yijk is the outcome variable for sleep on day i for subject j on occasion k (where k could be 12 occasions without ambulatory monitoring or 2 occasions with ambulatory monitoring), β0 is the mean intercept number of y (e.g., sleep efficiency %) (fixed intercept), μ0j is the random intercept for subject j, μ0jk is the random intercept for occasion k in subject j, β1 represents change in y (e.g., sleep efficiency) for the ABPM visits, and rijk is the residual term.
A similar model was used to assess the levels of activity during the day. In a mixed model, we used the number of minutes of activity as a dependent variable nested within participants and visits with the level of activity, ABPM status, and their interactions as independent factors. Specifically, we fitted the following model: yijk = β0 + μ0j + μ0jk + β1(activity level 1) + β2(activity level 2) + β3(activity level 3) + β4(ABPM + activity level 0) + β5(ABPM + activity level 1) + β6(ABPM + activity level 2) + β7(ABPM + activity level 3) + rijk, with maximal likelihood estimates in which yijk is the number of minutes for a specific level of activity on day i for subject j and occasion k; β0 is the mean intercept number of minutes of activity at the lowest level on non-ABPM days (fixed intercept); μ0j is the random intercept for subject j; μ0jk is the random intercept for occasion k in subject j; β1 through β3 represent change in activity in minutes among non-ABPM days for various levels of activity from β0; β4 through β7 represent change in activity in minutes for ABPM days for various levels of activity from β0 to β3, respectively; and rijk is the residual term.
The severity of sleep disturbance assessed by WASO was then related to odds for nondipping by a random intercept logistic regression model. In this model, WASO was dichotomized at median. The dichotomization at median for WASO was performed separately for (1) when patients were not wearing the ambulatory BP monitor and (2) when they were wearing the monitor. This was done to assess the independent effect of WASO at baseline and that induced by ambulatory monitoring on the odds of dipping.
Statistical analyses were performed using Stata 11 (Stata Corporation, College Station, TX) and nominal level of statistical significance was set at a two-sided P value of <0.05.
Results
Of the 103 patients, 97% were men, 83% were white, and the mean body mass index was 30 ± 4.7 kg/m2. Etiology of CKD included hypertensive nephrosclerosis (33%), diabetes mellitus (30%), ischemic nephropathy (14%), polycystic kidney disease (5%), GN (9%), obstructive uropathy (4%), and other causes (6%). Mean hemoglobin was 12.9 ± 1.8 g/dl, serum albumin 4.3 ± 0.4 g/dl, and eGFR 38.8 ± 15.4 ml/min/m2. Seated clinic BP was 122/59 mmHg, and the patients took on average 3.2 ± 1.4 antihypertensive drugs.
The sleep parameters for days with and without ABPM are shown in Table 1. Patients were divided by tertiles for each of the sleep categories. For example, self-reported, diary-recorded duration of sleep among patients in the lowest tertile was 406 minutes, 489 minutes in the next tertile, and 568 minutes in the highest tertile. When wearing an ambulatory BP monitor, patients who slept the most also spent the least time in bed at night (−92 minutes, P < 0.0001). Actigraphically measured sleep time among those in the highest tertile was 453 minutes; these patients had a 98-minute reduction in sleep (P < 0.0001). The reduction in sleep time followed a linear trend; lesser perturbations in sleep time were seen among those who slept less well. Patients who were least awake at baseline were awakened the most when wearing the ambulatory monitor. WASO in the soundest sleepers was 41 minutes. This increased by 24 minutes (P < 0.0001) when wearing the ambulatory monitor. Sleep efficiency was reduced by 5% in those with the best sleep (P = 0.02).
Table 1.
| Sleep Parameters | Tertile 1 | Tertile 2 | Tertile 3 |
|---|---|---|---|
| Stated duration of sleep (min) | 406 (8.7) | 489 (8.7) | 568 (8.6) |
| CFB | 38 (14.2) | −72 (18.3) | −92 (18.1) |
| P for change | 0.004 | <0.0001 | <0.0001 |
| Total sleep assessed (min) | 289 (7.1) | 377 (6.9) | 453 (7) |
| CFB | 14 (11.8) | −22 (15.8) | −98 (16.3) |
| P for change | 0.1 | 0.1 | <0.0001 |
| Percent awake (%) | 16 (1.1) | 20 (1.1) | 35 (1.1) |
| CFB | 6 (1.4) | 1 (1.6) | 3 (1.5) |
| P for change | <0.0001 | 0.3 | 0.04 |
| Wake after sleep onset (min) | 41 (4.2) | 62 (4.3) | 105 (4.1) |
| CFB | 24 (5.3) | 0 (6.5) | −11 (5.8) |
| P for change | <0.0001 | 0.5 | 0.04 |
| Sleep efficiency (%) | 58 (1.3) | 74 (1.3) | 82 (1.4) |
| CFB | 3 (1.7) | −2 (2.1) | −5 (2.2) |
| P for change | 0.03 | 0.1 | 0.02 |
The heterogenity in mean CFB between tertiles was <0.0001 for all parameters.
Values in parentheses are SEM.
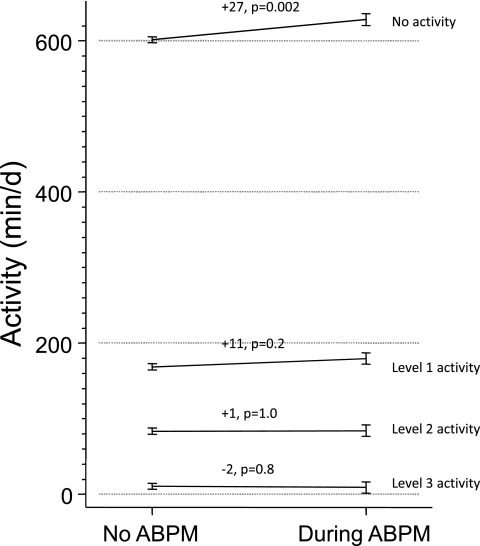
Figure 1 shows the levels of activity by ABPM. Although the number of minutes in activity levels 1, 2, or 3 were not meaningfully different, there was significant heterogeneity observed among the mean change from baseline in levels of activity (P = 0.02). Specifically, on the day of ABPM patients were more sedentary (+27 minutes, P = 0.002).
Figure 1.
Levels of activity by ABPM. Although the number of minutes in activity levels 1, 2, or 3 was not significantly different, there was significant heterogeneity observed among mean change from baseline in levels of activity (P = 0.02). On the day of ABPM, patients were more sedentary (+27 minutes, P = 0.002).
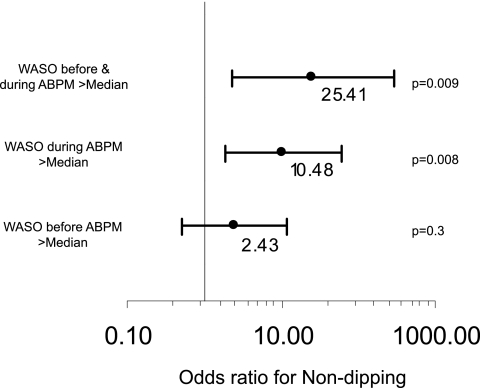
Figure 2 shows the odds of nondipping when patients were waking after sleep onset when not wearing the ambulatory monitor versus when wearing ambulatory monitor. WASO was defined as above median (47 minutes) when ambulatory BP was not being performed. WASO recorded when ABPM was not being performed was not predictive of nondipping (P = 0.3). Upon ABPM, WASO increased to median of 56 minutes. WASO above median during ABPM was associated with nondipping (odds ratio 10.48, P = 0.008). The additive interaction between WASO at baseline and during ABPM was significant (odds ratio 25.4, P = 0.009). Thus, when patients were more awake after sleep onset at baseline and this state worsened with ABPM, they experienced greater nondipping.
Figure 2.
WASO when ABPM was not being performed was not associated with nondipping (P = 0.3). WASO during ABPM was associated with nondipping (P = 0.008). When WASO was more than median before and during ABPM, the odds or nondipping were much higher (P = 0.009). Model χ2 = 7.85, P = 0.02.
Discussion
Earlier research using polysomnography and continuous noninvasive BP monitoring (using the Finapres device) has demonstrated that ambulatory BP machines cause a rise in simultaneously recorded beat-to-beat systolic and diastolic BP measurements and that the rise is often associated with electroencephalographic arousal from sleep (6). In fact, BP can rise during measurement even in the absence of electroencephalographic arousal from sleep. Such a rise leads to an overestimate of the true systolic BP during sleep, which varies among subjects and stages of sleep. This research discounted the findings of earlier work that detected no change in ambulatory BP despite disturbed sleep (8); however, even this report was limited to six healthy volunteers (6).
Our research extends these findings to a larger group of patients in their natural environment and in whom the detection of dipping is highly relevant because of its absence. Our study shows that ABPM results in less time spent in bed at night (by approximately 1.5 hours in those who have the longest sleep duration), with more of the time spent awake in bed (16% versus 22%, 6% P < 0.0001). Patients were more awake after sleep onset and they had reduced sleep efficiency when undergoing ABPM. We found that on the day of APBM, patients were more sedentary (+27 minutes) than when not wearing the ambulatory BP monitor. The slight but statistically insignificant increments in level 1 and 2 activity may be related to the fact that patients had to visit the hospital twice during a 24-hour period to have the ambulatory BP monitor placed and removed. This would require travel and perhaps result in greater than usual activity in this mostly sedentary population of older men. Reduced activity during the day and disturbed sleep during the night together with increased nocturnal physical activity would be expected to blunt the dipping pattern. Indeed, we found that during ABPM, waking after sleep onset was associated with a nearly 10-fold increase in odds of nondipping.
The results of our study are relevant for interpretation of ambulatory BP dipping patterns. Manning et al. (9) reported that perceived quality of sleep measured with a patient-completed questionnaire was associated with nondipping. Participants who reported greater perceived sleep disturbance were less likely to dip. Our study extends their findings by directly measuring the quality of sleep. Verdecchia et al. (10) reported that perceived quantity of sleep during overnight ambulatory BP recording influenced the dipping pattern. Overall, 30% of the patients reported sleep duration <2 hours less than usual, 10% between 2 and 4 hours less than usual, and 4% >4 hours less than usual. The independent prognostic value of nighttime BP for total cardiovascular end points and all-cause mortality was lost in the 14% of the patients who had sleep disturbance of >2 hours. Given that ABPM is a cause of sleep disturbance, it is possible that a less intrusive method of ABPM may better assess the prognostic value of ABPM.
The results of our study may help to generate normative data for ambulatory BP during sleep and activity. If actimetry is performed during days when ABPM is not performed and when it is performed, it may help guide the extent of sleep disturbance attributable to ambulatory monitoring. Normative data can then be generated from among those in whom sleep is not disturbed and then among those in whom sleep is disturbed. These data would have implications for the classification of patients into categories of masked hypertension and white-coat hypertension (11). For example, masked hypertension diagnosed with the criterion standard of nocturnal ambulatory BP was reported to be extremely common among participants in the African-American Study of Kidney Disease (12). Actigraphy when concomitantly performed with ABPM can clarify the nocturnal drop in BP during sleep, especially in conditions associated with nocturnal awakening such as heart failure, sleep apnea, prostatism, and CKD.
Because our study was limited to mostly older veterans who are generally retired, we had few younger people who may be physically more active. In the latter individuals, who have better sleep characteristics, ABPM may evoke the most marked disturbance in sleep. Thus the results of our findings may be particularly applicable to individuals who are more active and those who sleep better. Although we excluded patients with morbid obesity, we did not perform polysomnography, therefore we cannot comment on the role of sleep apnea on dipping or lack thereof.
Nondipping BP has numerous causes. Among patients with CKD, who have the highest prevalence of nondipping (13), the causes of nondipping include increased nocturnal physical activity (1), nocturia (14), increased sympathetic activation (15), and sodium sensitivity (16). ABPM-induced sleep disturbance should now be added to the list of causes of nondipping.
Disclosures
This study was supported by a grant from the VA Merit Review.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Agarwal R, Light RP: Physical activity and hemodynamic reactivity in chronic kidney disease. Clin J Am Soc Nephrol 3: 1660–1668, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muxfeldt ES, Cardoso CR, Salles GF: Prognostic value of nocturnal blood pressure reduction in resistant hypertension. Arch Intern Med 169: 874–880, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R, Peixoto AJ, Santos SF, Zoccali C: Out-of-office blood pressure monitoring in chronic kidney disease. Blood Press Monit 14: 2–11, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F, Porcellati C: Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation 81: 528–536, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Verdecchia P, Angeli F, Cavallini C: Ambulatory blood pressure for cardiovascular risk stratification. Circulation 115: 2091–2093, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Davies RJ, Jenkins NE, Stradling JR: Effect of measuring ambulatory blood pressure on sleep and on blood pressure during sleep. BMJ 308: 820–823, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holden JE, Kelley K, Agarwal R: Analyzing change: A primer on multilevel models with applications to nephrology. Am J Nephrol 28: 792–801, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwan A, Eriksson G: Effect on sleep—but not on blood pressure—of nocturnal non-invasive blood pressure monitoring. J Hypertens 10: 189–194, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Manning G, Rushton L, Donnelly R, Millar-Craig MW: Variability of diurnal changes in ambulatory blood pressure and nocturnal dipping status in untreated hypertensive and normotensive subjects. Am J Hypertens 13: 1035–1038, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Verdecchia P, Angeli F, Borgioni C, Gattobigio R, Reboldi G: Ambulatory blood pressure and cardiovascular outcome in relation to perceived sleep deprivation. Hypertension 49: 777–783, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Bangash F, Agarwal R: Masked hypertension and white-coat hypertension in chronic kidney disease: A meta-analysis. Clin J Am Soc Nephrol 4: 656–664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pogue V, Rahman M, Lipkowitz M, Toto R, Miller E, Faulkner M, Rostand S, Hiremath L, Sika M, Kendrick C, Hu B, Greene T, Appel L, Phillips RA: Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 53: 20–27, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Agarwal R, Light RP: GFR, proteinuria and circadian blood pressure. Nephrol Dial Transplant 24: 2400–2406, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Agarwal R, Light RP, Bills JE, Hummel LA: Nocturia, nocturnal activity, and nondipping. Hypertension 54: 646–651, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Converse RL, Jr, Jacobsen TN, Toto RD, Jost CMT, Cosentino F, Fouad-Tarazi F, Victor RG: Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 327: 1912–1918, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B: Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med 346: 913–923, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.