Abstract
The biological meaning of uncertain dementia ratings (CDR 0.5) and its treatment implications are unclear. Our study examines the frequency of anti-dementia medication use in individuals with CDR 0.5 and the cognitive, behavioral, and demographic factors associated with memantine and acetylcholinesterase inhibitor (AChEI) use. Subjects were drawn from the National Alzheimer Coordinating Center database, which collects data from 30 Alzheimer Disease Centers. There were 2,512 subjects with the following diagnoses: Normal, 11.8%; Mild cognitive impairment, 44.6%; Alzheimer's disease, 34.9%; and other dementias, 8.7%. Overall, 35% used AChEIs and 13% used memantine. AChEI and memantine use was greater in subjects who were referred by clinics and diagnosed with Alzheimer's disease. AChEI use was associated with being married, younger, male, and more educated while memantine use was associated with less severe apathy and other dementia diagnosis. Non-Hispanic whites were more likely to use AChEI and memantine than non-Hispanic blacks (OR=2.2,2.5). Hispanics were more likely to use AChEI than non-Hispanic blacks. It appears anti-dementia medication use in CDR 0.5 is frequent and represents evidence for extensive off label usage. Diagnosis, severity of impairment, and race, among other variables, affect the likelihood of AChEI and memantine use in this population.
Keywords: acetylcholinesterase inhibitors, Alzheimer's disease, CDR 0.5, disparity, ethnicity, memantine, mild cognitive impairment, off label, race
Introduction
In the United States, usage of memantine and acetylcholinesterase inhibitors (AChEI) in Alzheimer's disease (AD) is subject to indications of the Food and Drug Administration (FDA). However, multiple clinical trials have attempted to demonstrate the utility of these medications in milder AD and non-AD dementias, and extend their use to mild cognitive impairment (MCI). Empirical studies have shown widespread clinical usage in these groups as well and documented the existence of racial disparities in medication use [1-3].
The Clinical Dementia Rating (CDR) scale is a multidimensional rating scale of dementia severity, developed to help clinicians stage dementing illnesses, particularly AD [4]. Within the CDR, uncertain dementia is given a CDR score of 0.5. However, the CDR is not a diagnostic instrument and there is disagreement about the biological meaning of CDR 0.5 and its treatment implications. Leaders in the field disagree as to whether CDR 0.5 represents MCI with a high risk of transformation to AD or very early stage AD or related dementia [5,6]. By definition, individuals with MCI have not yet developed levels of cognitive and functional impairment severe enough to warrant a dementia diagnosis [7].
Little is known about the extent to which patients rated as CDR 0.5 are diagnosed as having MCI or AD, and how diagnosis of these patients affects pharmaceutical treatment. Extant research [1,2] has shown that use of AD medications is directly related to severity of dementia and race/ethnicity.
Using a large database of well-characterized subjects from 30 National Institute on Aging funded Alzheimer Disease Centers (ADCs) accrued between 2005 and 2007, we extend the research on factors related to use of anti-dementia medications to those with uncertain dementia rating of CDR 0.5. Diagnosis, disease course, race, and ethnicity were included as predictors of medication use. We also included variables related to these predictors, including apathy, depression, gender, age, education, and marital status.
Specifically, our aims are to: 1) estimate the relationship of AChEI and memantine use to diagnosis in multivariate models controlling for severity of cognitive impairment, race and ethnicity, and potentially confounding co-morbidities and demographic variables; and 2) estimate the relationship of AChEI and memantine use to race and ethnicity controlling for the other covariates.
Methods
Sample
Subjects were recruited to participate in the research registries at ADCs by a variety of methods. The ADC National Alzheimer's Coordinating Center (NACC) adopted a set of standardized instruments (Uniform Data Set [UDS]) in 2005. During the subjects' first visit to an ADC after UDS adoption, the subjects were characterized using the UDS after providing written informed consent [8].
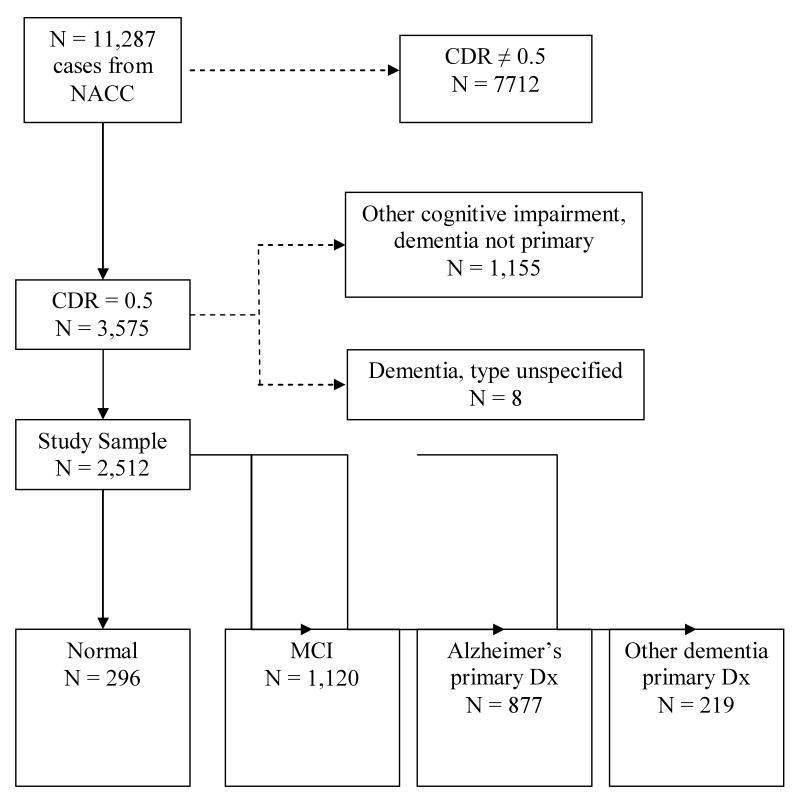
We were granted access to selected variables from the UDS in 2007. The resulting data set contained 11,287 cases and included persons diagnosed as cognitively normal, MCI, dementia, and other cognitive impairments. For this study, we selected only those cases with a CDR of 0.5. Cases with unspecified dementia (n = 8) and cases diagnosed with a cognitive impairment without dementia as a primary condition (n = 1,055) were eliminated from the study sample, resulting in a sample size of 2,512 (Figure 1). This latter group had a number of primary diagnoses coded on the UDS, such as stroke, Parkinson's disease or normal pressure hydrocephalus but had no indication whether cognition was normal or impaired. The number of cases per ADC ranged from 11 to 179 with a mean of 83.7 (SD = 47.4).
Figure 1.
Inclusion and Exclusion Criteria for Study Sample
NACC = National Alzheimer's Coordinating Center; CDR = Clinical Dementia Rating; MCI = Mild Cognitive Impairment; CDR= Clinical Dementia Rating
Measures
Identical UDS questionnaires and forms were used at each ADC and a centralized training session was held for all clinicians involved [8].
Diagnosis
Diagnosis of AD was made in accordance with NINCDS/ADRDA criteria [9]. The criteria for MCI are: 1) cognitive complaint; 2) cognitive decline not normal for age; 3) no dementia; and 4) essentially normal functional activities [7,10]. Based on diagnosis, we placed subjects who met our inclusion criteria into one of four groups: normal, MCI, AD, and other dementia (Figure 1). For use in logistic regression analysis, we created dummy variables for normal, AD, and other dementia, with MCI as the reference group.
Dementia severity
Only subjects with a global CDR (CDR-GLOB) of 0.5 were included and we included the CDR sum of boxes (CDR-SUM) and the Mini-Mental Status Examination (MMSE) [11] in our regression models.
Other instruments
Subjects were rated on severity of apathy as 0 (none), 1 (mild), 2 (moderate), or 3 (severe) on the neuropsychiatric inventory (NPI) [12]. Subjects were evaluated for the presence of depression in the past two years (1 = depression, 0 = no depression). The total score on the short form of the Geriatric Depression Scale (GDS) was also used in the analysis. [13].
Demographics
Information was collected on gender, race, ethnicity (Hispanic/Latino origins, regardless of race), age, marital status, years of education, and type of referral to ADC. Because education was skewed in the direction of higher values, we used the natural log of education in the regression analyses to reduce the skew. Dummy variables were created for gender (1 = male, 0 = female), marital status (1 = married, 0 = other), and clinician referral (1 = clinician/clinic, 0 = other). Based on ethnicity and race, subjects were placed in one of three mutually exclusive groups, coded 1 if a member of the group and 0 otherwise: Hispanic/Latino, non-Hispanic white, and non-Hispanic black or other race. Two of these dummy variables (Hispanic/Latino and non-Hispanic white) were used to represent the three groups in regression analysis, with non-Hispanic blacks and other races as the reference group.
Medication reporting
In accordance with the UDS procedure manual, subject medication use was determined “by the clinician or ADC staff, based on subject/informant report, medical records and/or observation.” We created dummy variables for memantine use (1 = yes; 0 = no) and AChEI use (1 = yes; 0 = no.)
Statistical Analysis
Univariate statistics were used to describe the sample in terms of medication use, diagnosis, severity of cognitive impairment, race and ethnicity, co-morbidities, and the demographic variables previously specified. Binary logistic regression analysis with multiple predictors was used to estimate the impact of diagnosis, race and ethnicity, severity of impairment, co-morbidities, and demographics on medication use. The data set consisted of a cluster sample of cases where each ADC is a cluster. In a cluster sample, the cases within a cluster are more similar to one another than a random sample of cases would be and the standard errors of ordinary regression coefficients are biased downwards. The antidote is to use multilevel models, also called hierarchical linear models and mixed models. We used the MLwiN program [14] to obtain unbiased estimates of the regression coefficients utilizing an iterative version of restricted generalized least squares. In the logistic regression results to be reported next, cases with a missing value on any of these variables that were included in the final models were excluded from the regression analysis (list-wise deletion). Significance tests were based on a two-tailed alpha of 0.05.
Results
Table 1 shows univariate statistics for the variables in our study and the bivariate relationships between diagnosis and the other variables. As shown, 12% of the subjects were diagnosed as normal, 45% as MCI, 35% as Alzheimer's, and 9% as other dementia.
Table 1. Means and standard deviations for study variables by diagnosis.
| Normal (11.8%) |
MCI (44.6%) |
Alzheimer's (34.9%) |
Other dementia (8.7%) |
Total (100%) |
N (2512) |
|
|---|---|---|---|---|---|---|
| Age | 75.8 (9.55) | 76.4 (9.38) | 76.0 (9.12) | 70.3 (10.25) | 75.6 (9.53) | 2512 |
| Education | 15.4 (3.35) | 14.7 (3.65) | 14.3 (3.54) | 14.9 (3.26) | 14.7 (3.56) | 2497 |
| CDR Sum of Boxes | 0.88 (0.66) | 1.3 (0.92) | 2.8 (1.25) | 2.5 (1.19) | 1.9 (1.30) | 2512 |
| MMSE | 28.7 (1.41) | 27.0 (2.68) | 24.0 (4.13) | 24.1 (5.36) | 25.9 (3.85) | 2462 |
| GDS | 1.5 (1.90) | 1.8 (1.92) | 2.4 (2.44) | 3.7 (3.42) | 2.1 (2.34) | 2408 |
| Apathy (%) | 18 | 19 | 41 | 74 | 31 | 2361 |
| Acetylcholinesterase Inhibitors Use (%) | 9 | 24 | 55 | 42 | 35 | 2512 |
| Memantine Use (%) | 2 | 6 | 23 | 20 | 13 | 2512 |
| Depression in last 2 years (%) | 19 | 20 | 36 | 47 | 28 | 2512 |
| Hispanic or Latino (%) | 9 | 7 | 7 | 4 | 7 | 2512 |
| White, non-Hispanic (%) | 79 | 78 | 80 | 86 | 79 | 2512 |
| Black, non-Hispanic (%) | 10 | 13 | 10 | 7 | 11 | 2512 |
| Male sex (%) | 50 | 50 | 47 | 60 | 50 | 2512 |
| Married (%) | 65 | 63 | 67 | 78 | 66 | 2500 |
| Evaluated in year(s) prior to UDS (%) | 64 | 53 | 47 | 42 | 51 | 2512 |
| Referred by clinic or clinician (%) | 25 | 36 | 49 | 58 | 41 | 2512 |
Mean (S.D.); *The means of these variables equal the proportion of cases coded as present. MCI = Mild Cognitive Impairment; CDR = Clinical Dementia Rating; MMSE = Mini-Mental State Examination; GDS = Geriatric Depression Scale; UDS = Uniform Data Set.
As shown in the total column of Table 1, four variables (apathy, MMSE, education, and marital status) had fewer than 2,512 valid values, the number of included cases. The amount of missing data was greatest for apathy, followed by MMSE, education, and marital status in that order. A comparison of excluded cases with those included showed several significant differences. Cases with missing values were: 1) less likely to use AChEI or memantine; 2) less likely to be AD or MCI and more likely to be normal; 3) had less severe cognitive impairments on MMSE and CDR-SUM; and 4) older. Although such differences are potential sources of bias in our results, the fact that the excluded cases are only a small percentage of all cases (7.6% in the final memantine equation) mitigates any bias.
All of the variables except medication use were initially included in the logistic regression equations for AChEI and memantine. Variables that were not statistically significant were dropped from the equation except if one of the two dummy variables representing race/ethnicity or one of the three representing diagnosis was not significant. A non-significant race/ethnicity variable or diagnosis variable was retained to maintain non-Hispanic blacks and MCI cases as the reference groups. Variables with marginal statistical significance (i.e., slightly greater than p = 0.05) were also retained.
Table 2 shows results of the final regression analyses of AChEI and memantine use. Normal subjects were less likely to use AChEI than those diagnosed with MCI (odds ratio = 0.362). Subjects with AD were more likely to use AChEI than MCI subjects (odds ratio = 2.7). Non-Hispanic whites were more likely to use AChEI compared to non-Hispanic blacks (odds ratio 2.2). Hispanics were more likely than non-Hispanic blacks to use AChEI, although this association was not significant (odds ratio 1.6; p = 0.08). The likelihood of AChEI use was also greater for those who were younger, male, more educated, married, referred by clinics, and with a higher CDR-SUM. There was a nonlinear relationship between MMSE and AChEI use as indicated by the significance of MMSE squared. As MMSE increased, AChEI use increased at first but then use declined for higher values of MMSE.
Table 2. Regression coefficients and odds ratios for logistic models of AChEI and memantine use.
| Y = AChEI | Y = memantine | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | -0.018 | 0.006 | -3.000 | 0.003 | 0.98 | . | . | . | . | . |
| White | 0.778 | 0.174 | 4.471 | 0.000 | 2.18 | 0.909 | 0.304 | 2.990 | 0.003 | 2.48 |
| Hispanic | 0.493 | 0.281 | 1.754 | 0.079 | 1.64 | 0.081 | 0.502 | 0.161 | 0.872 | 1.08 |
| Male | 0.269 | 0.112 | 2.402 | 0.016 | 1.31 | . | . | . | . | . |
| Loge education | 0.523 | 0.202 | 2.589 | 0.010 | 1.69 | 0.594 | 0.318 | 1.871 | 0.061 | 1.81 |
| Married | 0.514 | 0.126 | 4.079 | 0.000 | 1.67 | 0.689 | 0.187 | 1.000 | 0.317 | 1.99 |
| CDR sum of boxes | 0.294 | 0.050 | 5.880 | 0.000 | 1.34 | 0.266 | 0.068 | 3.912 | 0.000 | 1.31 |
| MMSE | 0.183 | 0.066 | 2.779 | 0.005 | 1.20 | -0.036 | 0.019 | -1.895 | 0.058 | 0.96 |
| MMSE Sq. | -0.005 | 0.002 | -3.333 | 0.001 | 0.99 | . | . | . | . | . |
| Normal diagnosis | -1.017 | 0.244 | -4.168 | 0.000 | 0.36 | -0.509 | 0.461 | -1.103 | 0.270 | 0.60 |
| AD diagnosis | 0.989 | 0.139 | 7.115 | 0.000 | 2.69 | 0.691 | 0.196 | 3.520 | 0.000 | 1.99 |
| Other dementia diagnosis | 0.225 | 0.195 | 1.154 | 0.249 | 1.25 | 0.761 | 0.268 | 2.840 | 0.005 | 2.14 |
| Clinic referral | 0.796 | 0.111 | 7.171 | 0.000 | 2.22 | 0.554 | 0.152 | 3.645 | 0.000 | 1.74 |
| Apathy | . | . | . | . | . | -0.251 | 0.112 | -2.249 | 0.025 | 0.78 |
| AChEI | . | . | . | . | . | 1.718 | 0.167 | 10.294 | 0.000 | 5.57 |
| Constant | -4.828 | 1.024 | -4.715 | 0.000 | . | -6.083 | 0.987 | -6.163 | 0.000 | . |
| N | 2436 | 2322 | ||||||||
CDR = Clinical Dementia Rating; AChEI = Acetylcholinesterase Inhibitor; MMSE = Mini-Mental State Examination; AD = Alzheimer's disease.
The significant predictors of memantine use (Table 2) differed somewhat from those for AChEI use. Memantine use was more likely in subjects with AD and those with “other dementia” than subjects diagnosed with MCI (odds ratio 2.0 and 2.1, respectively). Non-Hispanic whites were more likely to use memantine than non-Hispanic blacks (odds ratio 2.5) but there was no significant difference between Hispanics and non-Hispanic blacks in memantine use. Subjects who were referred by clinics, had a higher CDR-SUM, and with less severe apathy, were more likely to use memantine
MMSE had a monotonically decreasing relationship with memantine use that differed from its nonlinear relationship with AChEI. We also included AChEI use as a predictor of memantine use. Those using AChEI were 5.6 times more likely to be using memantine. The bivariate relationship between the two variables indicates that 81.6% of those who use memantine also use AChEI.
The significant variance of the constant in both equations indicates that there is between-ADC variance in the constant, which is consistent with significant between-ADC variance in the percentage of subjects using AChEI or memantine
Discussion
Our two major findings are that “off label” usage of anti-dementia medications (both AChEI and memantine) is frequent in individuals with a CDR 0.5 rating, and that multiple factors modify this likelihood, including diagnosis, race, and other demographic factors. These findings extend our previous study showing substantial memantine use in mild dementia (CDR 1.0) [1]. The study also extends our previous findings regarding racial disparities in anti-dementia drug use through use of a large national sample.
There have been several clinical studies of AChEI in MCI, defined as CDR 0.5, most notably by Petersen and colleagues comparing donepezil, vitamin E and placebo on AD conversion rates. Neither this study nor the clinical trial of galantamine in MCI have shown differences in conversion rates to AD, suggesting that benefits on disease course are modest at best in MCI [15,16].
Memantine is FDA approved for moderate to severe AD based on two pivotal trials [17,18] and its use in CDR 0.5, in general, suggests appreciable off-label use in this mildly impaired population. While there have been several clinical trials in “mild to moderate” AD, no studies have been published regarding memantine and MCI akin to the Petersen study mentioned above [19-21]. There have also been extensive clinical trials and case reports in psychiatric disorders, indicating hope that glutaminergic modulation may be important in these disorders [22,23].
Other factors that are believed to be associated with off-label use that cannot be investigated with the UDS data include narrow therapeutic indications, advertising, and lack of other effective therapeutic options. Previous research has also suggested that adverse effects are more common with off-label usage, but the UDS does not include this information [24-28].
Diagnosis and likelihood of treatment
Diagnostic labeling is an important factor in treatment decisions. The issues regarding why an individual subject evaluated at an ADC might receive a diagnosis of MCI or AD is beyond the scope of this paper, and currently under investigation.
Demographics and medication use
Race appears to be more important than ethnicity (Hispanic versus Non-Hispanic); given the small number of Hispanic subjects, this conclusion warrants further study. Family advocacy may be important (more use in married individuals), but the role of socio-economic factors and access to medicines because of insurance coverage cannot be assessed since these variables are not in the UDS.
A number of attempts have been made to isolate how racial and ethnic disparities arise. McGinnis et al. [29] looked at treatment outcomes of dementia patients based on race and ethnic concordance between interventionists and caregivers and found only minor differences between concordant and discordant dyads. Connell and collaborators [30] found that blacks and Hispanics were significantly more likely to perceive AD as a natural aspect of aging than were whites.
Implications of these beliefs could resonate in seeking care and in health outcomes. Looking at differences between African Americans and whites regarding attitudes towards genetic testing for AD, Hipps and colleagues [31] found that African Americans showed less interest in testing, endorsed fewer reasons for pursuing it, and anticipated fewer negative consequences from a positive test result. In a broader context Belle et al. [32] found that across ethnicities, tailoring a multi-component intervention to individual risk profiles increased positive quality of life outcome. Han and Liu [33] also stressed the need for education programs for minorities. In our model, educational attainment was more important for AChEI use than memantine use.
Behavioral symptoms and medication use
While test performance and an indirect activities of daily living (ADL) measure (CDR-SUM) are important variables in medication usage, it is less clear how behavioral variables contribute to variance in medication usage. It is possible that selective use of a new medication occurs in subjects perceived to be healthier or subjects with behavioral problems utilize medical care at different rates. Further studies using all NPI variables would be useful, but should consider the underlying three-factor structure of the NPI in this population, as previous studies have shown [34-36].
Conclusion
There are several limitations to this study that may affect the robustness of our findings. The NACC database is not an epidemiological sample nor is it longitudinal data. A substantial number of subjects could not be better classified since we could not discern their cognitive status. The size and comprehensive nature of the NACC database give the best possible picture of treatment patterns as reflected by geographically diverse research centers working in concert. Our multi-level analysis controls for site effects, as the data suggest wide variability in prescription patterns.
The actual source of the medications is unknown, and reflects a combination of academic and community physicians, probably of multiple specialties. We are also dependent on accurate medication reporting by subjects and families and on accurate data submission by the ADCs. While referral source was a significant predictor of both AChEI and memantine use, differences by diagnostic category and demographic characteristics remained when referral source was controlled.
Awareness of racial and demographic treatment patterns and the widespread variability in diagnosis and treatment in individuals with clinical ratings of uncertain dementia is important for spurring further research in these areas.
Acknowledgments
The authors wish to acknowledge Nathaniel Mercaldo, MS and the staff of the National Alzheimer's Coordinating Center (U01 AG016976) for assistance in providing access to the database and preparation of datasets. This work was supported in part by NIA grant P50 AG08012. Dr. Lerner is a paid speaker for Forest Labs and Novartis and receives grant funding from Abbott Labs, Elan/Wyeth, and Neurochem.
Footnotes
Communicated by Marwan Sabbagh
References
- 1.Lerner AJ, McClendon MJ, Sami SA, Ogrocki PK, Adams KB, Smyth KA. Factors affecting usage patterns of memantine in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:137–143. doi: 10.1097/WAD.0b013e31815ccd68. [DOI] [PubMed] [Google Scholar]
- 2.Mehta K, Yin M, Resendez C, Yaffe K. Ethnic differences in acetylcholinesterase inhibitor use for Alzheimer disease. Neurology. 2005;65:159–162. doi: 10.1212/01.wnl.0000167545.38161.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vidal JS, Lacombe JM, Dartigues JF, Pasquier F, Robert P, Tzourio C, Alpérovitch A. Memantine Therapy for Alzheimer disease in real-world practice: An observational study in a large representative sample of French patients. Alzheimer Dis Assoc Disord. 2008;22:125–130. doi: 10.1097/WAD.0b013e31815a9e10. [DOI] [PubMed] [Google Scholar]
- 4.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 6.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62:1160–1163. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- 8.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): Clinical and Cognitive Variables and Descriptive Data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 9.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 13.Yesavage J, Brink TL, Rose T, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 14.Rasbash J, Steele F, Browne W, Prosser R. A User's Guide to MLwiN: Version 2.0. University of London: Centre for Multilevel Modeling; London: 2004. [Google Scholar]
- 15.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 16.Winblad B, Gauthier S, Scinto L, Feldman H, Wilcock GK, Truyen L, Mayorga AJ, Wang D, Brashear HR, Nye JS. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70:2024–2035. doi: 10.1212/01.wnl.0000303815.69777.26. [DOI] [PubMed] [Google Scholar]
- 17.Reisberg B, Doody R, Stoffler A, Schmitt S, Ferris S, Mobius HJ. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 18.Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gerge I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291:317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 19.Pomara N, Ott BR, Peskind E, Resnick EM. Memantine treatment of cognitive symptoms in mild to moderate Alzheimer disease: secondary analyses from a placebo-controlled randomized trial. Alzheimer Dis Assoc Disord. 2007;21:60–64. doi: 10.1097/WAD.0b013e318032cf29. [DOI] [PubMed] [Google Scholar]
- 20.Porsteinsson AP, Grossberg GT, Mintzer J, Olin JT, Memantine MEM-MD-12 Study Group Memantine treatment in patients with mild to moderate Alzheimer's disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008;5:83–89. doi: 10.2174/156720508783884576. [DOI] [PubMed] [Google Scholar]
- 21.Ott BR, Blake LM, Kagan E, Resnick M, for the Memantine MEM-MD-11AB study group Open label, multicenter, 28-week extension study of the safety and tolerability of memantine in patients with mild to moderate Alzheimer's disease. J Neurol. 2007;254:351–358. doi: 10.1007/s00415-006-0374-x. [DOI] [PubMed] [Google Scholar]
- 22.Zdanys K, Tampi R. A systematic review of off-label uses of memantine in psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1362–1374. doi: 10.1016/j.pnpbp.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 24.Barbu C, Ciuna A, Nose M, Levi D, Andretta M, Patten SF, Amaddeo F, Tansella M. Off-label and non-classical prescriptions of antipsychotic agents in ordinary in-patient practice. Acta Psychiatr Scand. 2004;109:275–278. doi: 10.1111/j.1600-0447.2003.00283.x. [DOI] [PubMed] [Google Scholar]
- 25.Datamonitor. Stakeholder Insight: Alzheimer's disease - Prescribing Trends Indicate That Neurologists Are Not Adhering to Guidelines. [November 20, 2008]; http://www.datamonitor.com/industries/research/?pid=DMHC2255&type=Report.
- 26.Jonville-Bera AP, Bera F, Autret-Leca E. Are incorrectly used drugs more frequently involved in adverse drug reactions? A prospective study. Eur J Clin Pharmacol. 2005;61:231–236. doi: 10.1007/s00228-004-0881-6. [DOI] [PubMed] [Google Scholar]
- 27.Win HK, Caldera AE, Maresh K, Lopez J, Rihal CS, Parikh MA, Granada JF, Marulkar S, Nassif D, Cohen DJ, Kleiman NS. Clinical outcomes and stent thrombosis following off-label use of drug-eluting stents. JAMA. 2007;297:2001–2009. doi: 10.1001/jama.297.18.2001. [DOI] [PubMed] [Google Scholar]
- 28.Lurie P, Tran T, Wolfe SM, Goodman R. Violations of exhibiting and FDA rules at an American Psychiatric Association Annual Meeting. J Public Health Policy. 2005;26:389–399. doi: 10.1057/palgrave.jphp.3200049. [DOI] [PubMed] [Google Scholar]
- 29.McGinnis KA, Schulz R, Stone RA, Klinger J, Mercurio R. Concordance of race or ethnicity of interventionists and caregivers of dementia patients: relationship to attrition and treatment outcomes in the REACH study. Gerontologist. 2006;46:449–455. doi: 10.1093/geront/46.4.449. [DOI] [PubMed] [Google Scholar]
- 30.Connell CM, Scott Roberts J, Mclaughlin SJ. Public opinion about Alzheimer disease among blacks, Hispanics, and whites: results from a national survey. Alzheimer Dis Assoc Disord. 2007;21:232–240. doi: 10.1097/WAD.0b013e3181461740. [DOI] [PubMed] [Google Scholar]
- 31.Hipps YG, Roberts JS, Farrer LA, Green RC. Differences between African Americans and Whites in their attitudes toward genetic testing for Alzheimer's disease. Genet Test. 2003;7:39–44. doi: 10.1089/109065703321560921. [DOI] [PubMed] [Google Scholar]
- 32.Belle SH, Burgio L, Burns R, Coon D, Czaja SJ, Gallagher-Thompson D, Gitlin LN, Klinger J, Kopeke KM, Lee CC, Martindale-Adams J, Nichols L, Schulz R, Stahl S, Stevens A, Winter L, Zhang S. Resources for Enhancing Alzheimer's Caregiver Health (REACH) II Investigators. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized, controlled trial. Ann Intern Med. 2006;145:727–738. doi: 10.7326/0003-4819-145-10-200611210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han E, Liu G. Racial disparities in prescription drug use for mental illness among populations in US. J Ment Health Policy Econ. 2005;8:131–143. [PubMed] [Google Scholar]
- 34.Cummings JL, McRae T, Zhang R, Donepezil-Sertraline study group Effects of donepezil on neuropsychiatric symptoms in patients with dementia and severe behavioral disorders. Am J Geriatr Psychiatry. 2006;14:605–612. doi: 10.1097/01.JGP.0000221293.91312.d3. [DOI] [PubMed] [Google Scholar]
- 35.Lerner AJ, Strauss M, Sami SA. Recognizing apathy in Alzheimer's disease. Geriatrics. 2007;62:14–17. [PubMed] [Google Scholar]
- 36.Petrovic M, Hurt C, Collins D, Burns A, Camus V, Liperoti R, Marriott A, Nobili F, Robert P, Tsolaki M, Vellas B, Verhey F, Byrne EJ. Clustering of behavioural and psychological symptoms in dementia (BPSD): a European Alzheimer's disease consortium (EADC) study. Acta Clin Belg. 2007;62:426–432. doi: 10.1179/acb.2007.062. [DOI] [PubMed] [Google Scholar]