Abstract
Existing research shows differences in medication use for Alzheimer's disease (AD) based on demographics such as race, ethnicity, and geographical location. To determine individual and community characteristics associated with differences in acetylcholinesterase inhibitor (AChEI) and memantine use in AD, 3,049 AD subjects were drawn from 30 centers and evaluated using the Uniform data set (UDS). Cases were evaluated at the individual level within the context of 31 communities (one center encompassed two separate geographical regions). Multivariate analysis was used to determine the significance of individual variables on medication use. Compared to non-Hispanic Whites, Blacks were less likely to use AChEI and memantine with odds ratios (OR) of 0.59 (95% CI 0.46-0.76) and 0.43 (95% CI 0.32-0.57), respectively. Compared to non-Hispanic Whites, non-Black Hispanics were less likely to use memantine (OR = 0.69 (95% CI 0.49-0.98)). No association was found between the proportion of Blacks or non-Black Hispanics versus non-Hispanic Whites at an Alzheimer Disease Center and individual use of AChEI or memantine. Other significant variables include gender, age, marital status, dementia severity, source of referral, AChEI use, and education. Education and age somewhat mitigated disparity. Significant racial and ethnic differences in AChEI and memantine use exist at the individual level regardless of the racial and ethnic composition of the individual's community. Research and initiatives at the societal level may be an important consideration toward addressing these differences.
Keywords: acetylcholinesterase inhibitor, Alzheimer's disease, disparity, ethnicity, memantine, race
INTRODUCTION
Two classes of medications are FDA approved for the treatment of Alzheimer's disease (AD), acetylcholinesterase inhibitors (AChEI: donepezil, rivastigmine, galantamine, tacrine) and an NMDA receptor antagonist (memantine). Extant research has shown that race and ethnicity is a factor in the reported use of AD medications [1,2]. However, these have generally been drawn from data encompassing either a single center or geographically limited centers so that national trends and regional variability have been hard to discern. Since minority populations participate to a small degree in AD research, larger samples are necessary to determine if there is differential usage by these groups.
Using the National Alzheimer Coordinating Center (NACC) database of the Uniform data set (UDS) accrued between 2005 and 2007 of well characterized subjects from 30 Alzheimer Disease Centers (ADCs) (29 of which are NIA funded), we examined factors related to use of anti-dementia medications. Because it is anticipated that use of medication may be related to multiple variables, we studied race and ethnicity, gender, age, education, marital status, type of referral, and disease severity as measured by the Mini-Mental Status Examination (MMSE) and Clinical Dementia Rating (CDR) scale. We explore differences in reported AD medication usage related to race, ethnicity, and associated factors not only from the perspective of the individual, but also as individuals within the context of ADC level summary statistics as a reflection of their larger communities from which those individuals are drawn to determine factors related to AD medication use on a broader scale.
Specifically, our aims are to: 1) evaluate the individual and community characteristics that predict AChEI and memantine use; 2) estimate the relationship of AChEI and memantine use to race and ethnicity controlling for the other covariates; and 3) elucidate the factors modifying the association of race and ethnicity on AChEI and memantine use.
METHODS
Sample
NACC adopted and began implementing the UDS in 2005. Subjects were recruited to participate in the research registries at ADCs by a variety of methods. During the subjects' first visit to an ADC after UDS adoption, the subjects were characterized using the UDS after providing written informed consent [3].
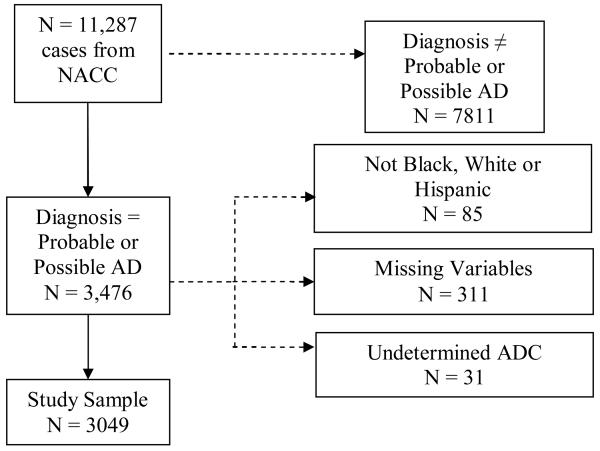
We were granted access to selected variables from the UDS in 2007. The subject selection procedure is shown in Figure 1. From 11,287 cases, we included 3,476 possible or probable AD. All cases that were not White, Black, or Hispanic (n = 85) were removed from the sample as were cases whose de-identified ADCs could not be inferred from zip codes (n = 31). Of the remaining cases, 311 were missing data in at least one of the covariates and 3,049 had complete data. These 3,049 cases were used in our statistical analysis with AChEI or memantine use as the outcome.
Figure 1.
Inclusion and exclusion criteria for study sample. AD= Alzheimer's Disease; NACC = National Alzheimer's Coordinating Center; ADC = Alzheimer's Disease Center.
Measures
Identical UDS questionnaires and forms were used at each ADC. Representatives from each center received centralized training for administering the standardized instruments, such as the global Clinical Dementia Rating (CDRGLOB) and MMSE, and the criteria for making diagnoses [4,5].
Medication reporting
In accordance with the UDS procedure manual, subject medication use was determined “by the clinician or ADC staff, based on subject/informant report, medical records, and/or observation.” The medication database was personally reviewed by one of the authors (AJL) to determine all possible alternative spellings in the database. We created dummy variables for memantine use (1 = yes; 0 = no) and AChEI use (1 = yes; 0 = no.)
Diagnosis
Diagnosis of AD was made in accordance with NINCDS/ADRDA criteria [6] either on the basis of a single clinician's assessment or on the basis of a consensus conference at the ADC.
Dementia severity
The CDRGLOB was completed for each individual and scored according to a formula provided in the UDS procedure manual [4]. The Mini-Mental State Exam (MMSE) was also completed for each individual [5].
Demographics
Information was collected on gender, race, ethnicity (Hispanic/Latino origins, regardless of race), age, marital status, years of education, and type of referral to ADC. Dummy variables were created for gender (1 = male, 0 = female), marital status (1 = married, 0 = other), and clinician referral (1 = clinician/clinic, 0 = other). Based on ethnicity and race, subjects were placed in one of three mutually exclusive groups, coded 1 if a member of the group and 0 otherwise: Black, non-Black Hispanic, and non-Hispanic White. Two of these dummy variables (Black and non-Black Hispanic) were used to represent the three groups in the analysis, with non-Hispanic Whites as the reference group. Based on years of education, subjects were placed into one of three mutually exclusive groups (<12, 12-16, and >16, with <12 as the reference group. Age is included as a continuous covariate as age does not appear to have a nonlinear effect (models with continuous and categorical age were compared with an F-test and found not significant.)
Specific ADC for each subject was de-identified in the UDS to preclude any ability to identify specific subjects based on extant variables, but subject's 3 digit zip code (provided in the UDS) were used to allocate subjects to an ADC community for analysis. One ADC with two locations in different states was split into two groups for a total of 31 communities. The proportion of non-Hispanic Black individuals to non-Hispanic White individuals by ADC is shown in Figure 2. Non-Black Hispanics were not included in the figure because of the small number at each ADC.
Figure 2.
Number of non-Hispanic Black and Non-Hispanic White per community. *ADC source for each subject was de-identified in the dataset.
Statistical analysis
Logistic regression analysis was used to model the association between the subject characteristics and the log-odds of medication use at the subject level. Separate models were fit for AChEI and memantine. Multivariable models were fit in order to obtain adjusted estimates of the associations. We explored the associations between medication use and several ADC-level summary measures of the covariates, but there were no statistically significant findings. The ADC-level summary measures were excluded from the models to simplify the interpretation of the other coefficients.
Mixed effects models were used to control for the correlation in the outcome within the ADCs. Random intercepts were included for each ADC center. According to an analysis of deviance, the models with random intercepts fit the data significantly better than a standard logistic regression model. The exponentiated coefficient of a predictor is interpreted as the relative change in the odds of medication use (AChEI or memantine, depending on the model) associated with a one unit increase in the predictor, controlling for the other covariates and conditional on ADC.
All analyses were performed using R version 2.7.2 (R Foundation, Vienna, Austria).
RESULTS
Table 2 shows multivariate statistics of AChEI and memantine use. Table 3 shows the interaction terms for Black and non-Black Hispanic with other demographic variables for AChEI and memantine.
Table 2.
Odds ratios and 95% confidence intervals from a generalized linear multivariate mixed model with AChEI or memantine use as the outcome and a logit link. The model included random intercepts for each ADC location. The standard deviation of the estimated random effects is 0.50, which is significantly different from 0 (p< 0.001). According to an analysis of deviance, this model fits significantly better than a model without random effects (p< 0.001). MMSE = Mini-Mental State Examination; CDRGLOB = Global Clinical Dementia Rating; AChEI = Acetylcholinesterase Inhibitor.
|
Multivariate AChEI |
Multivariate Memantine |
|
|---|---|---|
| Variable | OR (95% CI) | OR (95% CI) |
| (Intercept) | 6 (2.23, 16.14) | 0.22 (0.09, 0.59) |
| White | 1 | 1 |
| Black | 0.59 (0.46, 0.76) | 0.43 (0.32, 0.57) |
| Non-Black Hispanic | 0.8 (0.58, 1.1) | 0.69 (0.49, 0.98) |
| Male | 1.30 (1.08, 1.56) | 1 (0.83, 1.2) |
| Age | 0.98 (0.97, 0.99) | 0.99 (0.98, 1) |
| Education <12 | 1 | |
| Education 12-16 | 0.97 (0.8, 1.18) | 1.19 (0.98, 1.45) |
| Education >16 | 1 (0.79, 1.27) | 1.38 (1.09, 1.76) |
| Married | 1.21 (1, 1.47) | 1.41 (1.15, 1.74) |
| Clinical Referral | 1.66 (1.38, 1.99) | 1.22 (1.01, 1.46) |
| MMSE | 0.99 (0.98, 1.01) | 0.97 (0.96, 0.99) |
| CDRGLOB | 1.21 (1.02, 1.43) | 1.45 (1.22, 1.72) |
| AChEI | - | 4.58 (3.73, 5.63) |
Table 3.
Model for AChEI and memantine use with interaction terms for Black and Non-Black Hispanic with the other demographic variables. MMSE = Mini-Mental State Examination; CDRGLOB = Global Clinical Dementia Rating; AChEI = Acetylcholinesterase Inhibitor. Significant interactions are noted with grey background.
| Variable |
OR (95% CI) AChEI |
OR (95% CI) Memantine |
|---|---|---|
| (Intercept) | 9.52 (3.11, 29.14) | 0.2 (0.07, 0.58) |
| AChEI | - | 4.39 (3.5, 5.5) |
| Black | 0.14 (0.01, 1.95) | 0.36 (0.01, 9.29) |
| Non-Black Hispanic | 0.08 (0, 1.83) | 4.59 (0.16, 135.3) |
| Male | 1.33 (1.08, 1.64) | 1.02 (0.83, 1.25) |
| Age | 0.97 (0.96, 0.98) | 1 (0.98, 1.01) |
| Education 12-16 | 0.91 (0.73, 1.13) | 1.07 (0.86, 1.33) |
| Education >16 | 0.92 (0.7, 1.2) | 1.36 (1.05, 1.76) |
| Married | 1.18 (0.94, 1.48) | 1.36 (1.08, 1.71) |
| Clinical Referral | 1.66 (1.36, 2.04) | 1.24 (1.02, 1.51) |
| MMSE | 0.99 (0.97, 1.02) | 0.98 (0.96, 1) |
| CDRGLOB | 1.21 (0.99, 1.47) | 1.48 (1.22, 1.79) |
| AChEI:Black | - | 1.06 (0.55, 2.05) |
| AChEI:Non-Black Hispanic | - | 1.91 (0.78, 4.7) |
| Black:Male | 0.99 (0.56, 1.75) | 0.89 (0.45, 1.78) |
| Non-Black Hispanic:Male | 0.58 (0.27, 1.25) | 0.88 (0.37, 2.11) |
| Black:Age | 1.03 (1, 1.06) | 1.01 (0.98, 1.05) |
| Non-Black Hispanic:Age | 1.01 (0.98, 1.05) | 0.96 (0.92, 1) |
| Black:Education 12-16 | 1.3 (0.76, 2.21) | 2.3 (1.21, 4.38) |
| Non-Black Hispanic:Education 12-16 | 1.62 (0.7, 3.78) | 1.67 (0.7, 4.02) |
| Black:Education >16 | 1.2 (0.58, 2.52) | 1.16 (0.48, 2.84) |
| Non-Black Hispanic:Education >16 | 4.01 (1.06, 15.12) | 0.36 (0.09, 1.41) |
| Black:Married | 1.08 (0.63, 1.87) | 1.39 (0.7, 2.76) |
| Non-Black Hispanic:Married | 0.95 (0.46, 1.93) | 1 (0.44, 2.27) |
| Black:Clinical Referral | 0.84 (0.52, 1.37) | 1.06 (0.59, 1.91) |
| Non-Black Hispanic:Clinical Referral | 1.27 (0.67, 2.4) | 0.76 (0.36, 1.62) |
| Black:MMSE | 0.97 (0.92, 1.02) | 0.95 (0.9, 1.01) |
| Non-Black Hispanic:MMSE | 1.04 (0.98, 1.1) | 1.02 (0.95, 1.1) |
| Black:CDRGLOB | 0.8 (0.51, 1.25) | 0.79 (0.47, 1.33) |
| Non-Black Hispanic:CDRGLOB | 1.36 (0.79, 2.34) | 1.19 (0.64, 2.21) |
Black individuals were less likely to take AChEI and memantine than non-Hispanic Whites with odds ratios (OR) of 0.59 (95% CI 0.46-0.76) and 0.43 (95% CI 0.32-0.57), respectively. Non-Black Hispanics were less likely to take memantine relative to non-Hispanic Whites (OR = 0.69 (95% CI 0.49-0.98)). Subjects referred by a clinician or clinics were more likely to take AChEI and memantine than those not referred by a clinician or clinic (OR = 1.66 (95% CI 1.38-1.99), 1.22 (95% CI 1.01-1.66)) and subjects with higher CDRGLOB scores, suggestive of greater severity of AD, were more likely to take AChEI and memantine than those with lower scores (OR = 1.21 (95% CI 1.02-1.43), 1.45 (95% CI 1.22-1.72)). Males relative to females and younger subjects relative to older subjects were more likely to take AChEI (OR = 1.30 (95% CI 1.08-1.56), 0.99 (95% CI 0.98-1.0), respectively).
The strongest predictor of memantine use was treatment with an AChEI, 4.58 (95% CI 3.73-5.63). Memantine treatment was more likely in subjects with more than 16 years of education relative to those with less than 12 years of education (OR = 1.38 (95% CI 1.09-1.76)). Marriage increased the relative rate of treatment with AChEI (OR 1.21 (95% CI 1-1.47)), an effect probably greater with memantine (OR 1.41 (95% CI 1.15-1.74)). Lower MMSE scores increased the likelihood of memantine treatment slightly (OR 0.97 (95% CI 0.96-0.99)).
We found no evidence to suggest an association between the proportion of Blacks versus non-Hispanic Whites at an ADC and whether or not an individual uses AChEI or memantine. We found no evidence to suggest an association between the proportion of non-Black Hispanic versus non-Hispanic Whites as an ADC and whether or not an individual uses AChEI or memantine.
There are two significant interactions in predicting AChEI use. The disparity between Black and non-Hispanic White AChEI use decreases as age increases, and the non-black Hispanic versus non-Hispanic White disparity is less among those with 16 or more years of education. There was one significant interaction in predicting memantine use; the disparity between Black and non-Hispanic White is less among those with 12-16 years of education.
DISCUSSION
We found that AChEI and memantine usage was substantially associated with an individual's race and memantine usage was associated with an individual's ethnicity. Blacks were substantially less likely to receive AChEI or memantine compared to non-Hispanic Whites, after controlling for several demographic variables.
We found younger age to be weakly associated with increased likelihood of anti-dementia drug usage, and higher education increased the likelihood of memantine usage, probably due to a recency effect (introduced into US in 2003). Mehta and colleagues found that minority AD patients from Alzheimer's Disease Research Centers in California were less likely to use AChEI than white AD patients [2]. In their study, subjects with higher MMSE, younger, and more educated had higher AChEI usage, whereas we found lower MMSE to be weakly associated with AChEI use. Johnell et al. [7] in a large survey from Sweden found that higher educational attainment was associated with increased likelihood of medication usage, also most pronounced for memantine which was used by 0.4% of their sample of 600,000 individuals, compared to 3% usage rate for AChEI. An allied study of Haider and collaborators [8] found the persistence of education related to inequalities in the prescription of newly marketed drugs similar to our findings. A much smaller English study by Matthews et al. [9] also found increased usage with higher education and higher social class. The increased usage of medication by married subjects suggests the possible role of family advocacy.
Studies in many other disease states have reported racial and ethnic differences in medication use, in particular with regards to mental health, but this is one of the first studies in a large heterogeneous American AD sample. Mallinger and coworkers found Blacks were less likely than Whites to receive second-generation antipsychotics for schizophrenia [10]. This is line with our finding of even greater racial disparity in newer drugs (memantine versus AChEI.) Similarly Kreyenbuhl et al. [11] found Blacks were significantly less likely to receive new-generation antipsychotic medications compared to Whites despite being more likely to receive depot antipsychotic medications. Han and Liu [12] found Blacks and Hispanics relative to Whites were less likely to use psychiatric drugs even when controlling for underlying health status and Copeland and colleagues [13] found Black and Hispanic Veterans used less atypical antipsychotics was than Whites with schizophrenia. Briesacher, Limcangco, and Gaskin reported racial and ethnic disparity in prescription drug use for diabetes, hypertension, and heart disease that remained when controlling for illness and insurance coverage [14] and Tamayo-Sarver et al. [15] found emergency department physicians less likely to prescribe opioid analgesics to Blacks than to Whites in a national sample.
This disparity in medication use persisted in our study after accounting for gender, age, education, marital status, clinical referral, severity (MMSE, CDRGLOB), and racial composition of the community; however, variables we did not explicitly account for that have previously been studied include insurance coverage, income, and transportation. Numerous studies have looked at racial and ethnic disparity among Medicare and Medicaid recipients. The Dartmouth Atlas Project reports disparities among Medicare beneficiaries in leg amputation, mammography screening, diabetes testing, and hospital usage not only within regions by race, but across regions [16]. Zito et al. [17] found that among youth with Medicaid coverage, Blacks showed markedly lower rates of psychopharmacological treatment than Whites across multiple diagnostic categories; Ray, Hall, and Meador [18] found considerable less antidepressant treatment of Blacks with severe mood disorders as compared to Whites. Among dually eligible beneficiaries Schore, Brown, and Lavin [19] found that controlling for chronic illness, Blacks had significantly fewer prescriptions filled than Whites. In regards to income, a study by Rao and collaborators [20] found that income level is associated with worse outcomes for acute coronary syndrome patients. Mamdani et al. [21] in a study of more than 120,000 Canadian elderly suggested persistent income related differences in drug prescription. In regards to transportation, possibly a proxy for access to care, Allman and collaborators [22] found significantly lower baseline mobility among older Black adults relative to older White adults although decline was more likely in the White population. However, this latter study showed greater decline in Whites in mobility scores with different factors associating with each race (e.g., dementia for Blacks, low education for Whites).
Beyond socioeconomic differences, cultural bound explanatory models of disease may also contribute to our findings. Blacks and Hispanics have a greater tendency to perceive AD as a natural part of aging and less of a cause for concern [23,24]. In the general community, these differences may be compounded by factors associated with minority status such as insurance adequacy, pharmacy access to AChEI or memantine, availability and adequacy of dementia assessment resources, including reduced access to primary care physicians.
More subtle biases that may be operative in our subjects include community health care systems under-diagnosing dementia and exhibiting bias against prescribing newer medications to minority patients. Minority patients may also be wary of newer medications reflecting a need for better patient education. Media may also play a role, reflecting and propagating barriers, as seen in the low prioritization of pharmaceutical advertising in Black versus White oriented magazines [25].
Limitations in our study include the cross-sectional nature of the database, which limits our ability to do more comprehensive analysis of medication use patterns. The specialized nature of ADCs and their patient sample may not generalize to patients seen in primary care or to the general population of their “community”.
Our results on Hispanics and anti-dementia medication use are consistent with previous research [2]. Since our sample included relatively few Hispanics and they were more likely to have missing data, additional study in this group may be warranted. Superimposed on this are issues of definitions of race and ethnicity, the effect of race and ethnicity on test performance, and associated socio-economic factors. However, the analysis of interactions showed only modest interaction effects.
The UDS does not capture socioeconomic variables, such as income or income adequacy. Further studies with prospectively defined epidemiologically representative samples may help elucidate the sources of variability and disparity in dementia medication usage.
Community-based research and education initiatives can have a beneficial impact reducing ethnic and racial healthcare disparities. The Robert Wood Johnson Foundation commissioned the Dartmouth Atlas Project report and has pledged significant allocation of resources toward community-focused programs, under “Aligning Forces for Quality”, based on their similar findings [13,26]. Community-based approaches such as the Urban Safety Net System and the Comprehensive Community Mental Health Services for Children and their Families Program have shown success in reducing racial, ethnic, and socioeconomic disparity in such measures as cancer screening, blood pressure control, diabetes management, and mental health [27,28]. Therefore, research and programs to reduce health disparities in AD should be considered as well.
Table 1.
Descriptive statistics comparing subjects who have complete data to those missing at least one of the covariates. Entries are percentages for categorical variables and means with standard deviations for continuous variables. MMSE = Mini-Mental State Examination; CDRGLOB = Global Clinical Dementia Rating.
| No missing data, n = 3049 |
Some missing data, n = 311 |
|||
|---|---|---|---|---|
| Variable | Value |
Percent/ Mean (sd) |
Percent/ Mean (sd) |
p-value |
| AChEI | No | 36.67% | 43.09% | 0.0301 |
| Yes | 63.33% | 56.91% | ||
| Memantine | No | 64.25% | 63.99% | 0.9759 |
| Yes | 35.75% | 36.01% | ||
| Race/Ethnicity | White | 77.63% | 72.73% | 0.0034 |
| Black | 14.56% | 13.8% | ||
| Non-Black Hispanic | 7.81% | 13.47% | ||
| Gender | Female | 57.33% | 59.81% | 0.4344 |
| Male | 42.67% | 40.19% | ||
| Age | - | 77.27 (9.12) | 78.14 (10.25) | 0.1530 |
| Education | <12 | 43.26% | 45.26% | 0.1739 |
| 12-16 | 37.03% | 31.75% | ||
| >16 | 19.71% | 22.99% | ||
| Marital Status | Not Married | 36.93% | 40.28% | 0.2893 |
| Married | 63.07% | 59.72% | ||
| Referral | Other | 46.84% | 25.24% | < 0.0001 |
| Clinic/Clinician | 53.16% | 74.76% | ||
| MMSE | - | 18.95 (7.16) | 18.75 (7.85) | 0.7544 |
| CDRGLOB | - | 1.25 (0.75) | 1.8 (0.96) | < 0.0001 |
ACKNOWLEDGMENTS
The authors wish to acknowledge Nathaniel Mercaldo, MS and the staff of the National Alzheimer's Coordinating Center (U01 AG016976) for assistance in providing access to the database and preparation of datasets. This work was supported in part by NIA grant P50 AG08012.
Footnotes
Authors' disclosures available online (http://www.j-alz.com/disclosures/view.php?id=139).
REFERENCES
- 1.Lerner AJ, McClendon MJ, Sami S, Ogrocki PK, Adams KB, Smyth KA. Factors affecting usage patterns of Memantine in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:137–143. doi: 10.1097/WAD.0b013e31815ccd68. [DOI] [PubMed] [Google Scholar]
- 2.Mehta K, Yin M, Resendez C, Yaffe K. Ethnic differences in Acetylcholinesterase inhibitor use for Alzheimer disease. Neurology. 2005;65:159–162. doi: 10.1212/01.wnl.0000167545.38161.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): Clinical and Cognitive Variables and Descriptive Data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 4.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 5.Folstein MF, Folstein SE, McHugh PR. "Mini-Mental State": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 6.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 7.Johnell K, Ringback Weitoft G, Fastbom J. Education and use of dementia drugs: A register-based study of over 600,000 older people. Dementia Getriatr Cogn Disord. 2008;25:54–59. doi: 10.1159/000111534. [DOI] [PubMed] [Google Scholar]
- 8.Haider S, Johnell K, Weitoft GR, Thorslund M, Fastbom J. The influence of educational level on polypharmacy and inappropriate drug use: a register-based study of more than 600,000 older people. J Am Geriatr Soc. 2009;57:62–69. doi: 10.1111/j.1532-5415.2008.02040.x. [DOI] [PubMed] [Google Scholar]
- 9.Matthews FE, McKeith I, Bond J, Brayne C. reaching the population with dementia drugs: what are the challenges? Int J Geriatr Psychiatr. 2007;22:627–631. doi: 10.1002/gps.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallinger J, Fisher S, Brown T, Lamberti JS. Racial disparities in the use of second-generation antipsychotics for the treatment of schizophrenia. Psychiat Serv. 2006;57:133–136. doi: 10.1176/appi.ps.57.1.133. [DOI] [PubMed] [Google Scholar]
- 11.Kreyenbuhl J, Zito J, Buchanan RW, Soeken ML, Lehman AF. Racial disparity in the pharmacological management of schizophrenia. Schizophr Bull. 2003;29:183–193. doi: 10.1093/oxfordjournals.schbul.a006996. [DOI] [PubMed] [Google Scholar]
- 12.Han E, Liu G. Racial disparities in prescription drug use for mental illness among populations in US. J Ment Health Policy Econ. 2005;8:131–143. [PubMed] [Google Scholar]
- 13.Copeland L, Zeber J, Valenstein M, Blow FC. Racial disparity in the use of atypical antipsychotic medications among veterans. Am J Psychiatry. 2003;160:1817–1822. doi: 10.1176/appi.ajp.160.10.1817. [DOI] [PubMed] [Google Scholar]
- 14.Briesacher B, Limcangco R, Gaskin D. Racial and ethnic disparities in prescription coverage and medication use. Health Care Financ Rev. 2003;25:63–76. [PMC free article] [PubMed] [Google Scholar]
- 15.Tamayo-Sarver J, Hinze S, Cydulka R, Baker DW. Racial and ethnic disparities in emergency department analgesic prescription. Am J Public Health. 2003;93:2067–2073. doi: 10.2105/ajph.93.12.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher ES, Goodman DC, Chandra A. Disparities in Health and Health Care among Medicare Beneficiaries: A Brief Report of the Dartmouth Atlas Project. 2008 http://www.dartmouthatlas.org/af4q.shtm, Accessed July 4, 2008. [PubMed]
- 17.Zito J, Safer D, dosReis S, Riddle MA. Racial disparity in psychotropic medications prescribed for youths with Medicaid insurance in Maryland. J Am Acad Child Adolesc Psychiatry. 1998;37:179–184. doi: 10.1097/00004583-199802000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Ray W, Hall K, Meador K. Racial differences in antidepressant treatment preceding suicide in a Medicaid population. Psychiatr Serv. 2007;58:1317–1323. doi: 10.1176/ps.2007.58.10.1317. [DOI] [PubMed] [Google Scholar]
- 19.Schore J, Brown R, Lavin B. Racial disparities in prescription drug use among dually eligible beneficiaries. Health Care Financ Rev. 2003;25:77–90. [PMC free article] [PubMed] [Google Scholar]
- 20.Rao S, Kaul P, Newby L, Lincoff AM, Hochman J, Harrington RA, Mark DB, Peterson ED. Poverty, process of care, and outcome in acute coronary syndromes. J Am Coll Cardiol. 2003;41:1948–1954. doi: 10.1016/s0735-1097(03)00402-9. [DOI] [PubMed] [Google Scholar]
- 21.Mamdani MM, Tu K, Austin PC, Alter DA. Influence of socioeconomic status on drug selection for the elderly in Canada. Ann Pharmacother. 2002;36:804–808. doi: 10.1345/aph.1A044. [DOI] [PubMed] [Google Scholar]
- 22.Allman R, Baker P, Maisiak R, Sims RV, Roseman JM. Racial similarities and differences in predictors of mobility change over eighteen months. J Gen Intern Med. 2004;19:1118–11126. doi: 10.1111/j.1525-1497.2004.30239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connell CM, Scott Roberts J, McLaughlin SJ. Public opinion about Alzheimer disease among blacks, hispanics, and whites: results from a national survey. Alzheimer Dis Assoc Disord. 2007;21:232–240. doi: 10.1097/WAD.0b013e3181461740. [DOI] [PubMed] [Google Scholar]
- 24.Hipps YG, Roberts JS, Farrer LA, Green RC. Differences between African Americans and Whites in their attitudes toward genetic testing for Alzheimer's disease. Genet Test. 2003;7:39–44. doi: 10.1089/109065703321560921. [DOI] [PubMed] [Google Scholar]
- 25.Omonuwa SC. Health disparity in black women: lack of pharmaceutical advertising in black vs. white-oriented magazines. J Natl Med Assoc. 2001;93:263–266. [PMC free article] [PubMed] [Google Scholar]
- 26.Aligning Forces for Quality Press Release. Robert Wood Johnson Foundation Announces $300 Million Commitment To Dramatically Improve Quality of U.S. Health Care. Updated June 5, 2008. http://www.rwjf.org/files/research/nationalpressrelease.pdf. Accessed July 4, 2008.
- 27.Eisert SL, Mehler PS, Gabow PA. Can America's Urban Safety Net Systems be a Solution to Unequal Treatment? J Urban Health. 2008;85:766–778. doi: 10.1007/s11524-008-9296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miech R, Azur M, Dusablon T, Jowers K, Goldstein AB, Stuart EA, Walrath C, Leaf PJ. The potential to reduce mental health disparities through the comprehensive community mental health services for children and their families program. J Behav Health Serv Res. 2008;35:253–264. doi: 10.1007/s11414-008-9123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]