Abstract
Successful linguistic processing requires efficient encoding of successively-occurring auditory input in a time-constrained manner, especially under noisy conditions. In this study we examined the early neural response dynamics to rapidly-presented successive syllables in schizophrenia participants and healthy comparison subjects, and investigated the effects of noise on these responses. We used magnetoencephalography (MEG) to reveal the time-course of stimulus-locked activity over bilateral auditory cortices during discrimination of syllable pairs that differed either in voice onset time (VOT) or place of articulation (POA), in the presence or absence of noise. We also examined the association of these early neural response patterns to higher-order cognitive functions.
The M100 response, arising from auditory cortex and its immediate environs, showed less attenuation to the second syllable in patients with schizophrenia than healthy comparison subjects during VOT-based discrimination in noise. M100 response amplitudes were similar between groups for the first syllable during all three discrimination conditions, and for the second syllable during VOT-based discrimination in quiet and POA-based discrimination in noise. Across subjects, the lack of M100 attenuation to the second syllable during VOT-based discrimination in noise was associated with poorer task accuracy, lower education and IQ, and lower scores on measures of Verbal Learning and Memory and Global Cognition.
Because the neural response to the first syllable was not significantly different between groups, nor was a schizophrenia-related difference obtained in all discrimination tasks, early linguistic processing dysfunction in schizophrenia does not appear to be due to general sensory input problems. Rather, data suggest that faulty temporal integration occurs during successive syllable processing when the signal-to-noise ratio is low. Further, the neural mechanism by which the second syllable is suppressed during noise-challenged VOT discrimination appears to be important for higher-order cognition and provides a promising target for neuroscience-guided cognitive training approaches to schizophrenia.
Keywords: schizophrenia, MEG, auditory processing, linguistic perception, M100, voice-onset time, place of articulation, second syllable attenuation
INTRODUCTION
Schizophrenia represents one of the quintessential disorders of human language operations. Impairments are found in a range of critical psychophysiologic processes, from those that govern early speech perception to those involved in the generation of meaning; findings have included abnormal neural oscillatory activity to speech sounds (Hirano et al., 2008), aberrant functional lateralization of speech processing (Ngan et al., 2003), and neuroanatomical dissociations during semantic integration (Kuperberg et al., 2008), to name only a few. However, the key components of early linguistic processing in schizophrenia, and their potential relationship to higher-order cognitive dysfunction, have not yet been elucidated.
Although mounting evidence suggests that schizophrenia is associated with deficient sensory processing that interacts with more complex cognition (Chen et al., 2008; Kim et al., 2009; Turetsky et al., 2009; Haenschel et al., 2007; Butler et al., 2005; Leitman et al., 2007), the specific nature of the auditory processing deficits relevant to language function remain unknown. Efficient linguistic processing relies on intact initial auditory encoding, as well as the accurate integration of a rapid succession of critical linguistic information that occurs in a time-constrained fashion. Temporal integration or sequencing dysfunction has long been proposed as a major contributor to language and reading impairment in clinical populations (Tallal and Piercy, 1974). For example, dyslexic children and adults are impaired in their ability to recognize and sequence the elements of rapidly successive speech and non-speech stimuli (Farmer and Klein, 1995; Ahissar et al., 2000; Stein and Walsh, 1997; Hari and Renvall, 2001). Furthermore, the inability of dyslexic subjects to process brief and rapid successive stimuli occurring in a temporal sequence is correlated with abnormal representations of such stimuli at the earliest levels of the auditory pathways (Nagarajan et al., 1999; Duffy et al., 1999; Kujala et al., 2000; Cunningham et al., 2001; Kraus, 2001; Kraus et al., 1996). Given that schizophrenia is characterized by high rates of reading impairment (Revheim et al., 2006) as well as abnormalities in early auditory processing (Force et al., 2008; Javitt et al., 2000; Thonnessen et al., 2008; Blumenfeld and Clementz, 2001; Thoma et al., 2003; Hanlon et al., 2005), it is important to determine whether schizophrenia patients also manifest similarly abnormal early neural representations of rapid successive linguistic stimuli.
The primary objectives of this study were to investigate the key sensory processing defects underlying early linguistic operations in schizophrenia. Specifically, the defect may be one of abnormal initial encoding of speech sounds, resulting in the formation of generally “noisy” or “imprecise” early sensory representations (c.f. Force et al., 2008; Johannesen et al., 2005). Alternatively (or concomitantly), a subtler deficit, related to temporal integration or temporal prediction across speech sound representations, may underlie the linguistic dysfunction of schizophrenia, as suggested by known abnormalities in auditory mismatch negativity and tone matching (Javitt et al., 2000). Further, we asked: how do such early linguistic processing defects relate to functionally important higher-order verbal cognitive operations, such as verbal learning and memory?
We used magnetoencephalography (MEG) to investigate the early neural responses to auditory linguistic stimuli under processing conditions that contain “noise” versus quiet in the sensory information stream, and that require the rapid temporal sequencing of sensory representations versus those that do not; we also related MEG findings to task performance and to higher-order verbal cognitive functions. Neural responses to two successively occurring speech sounds were recorded in 47 well-characterized participants with schizophrenia and in 19 healthy comparison subjects while performing three separate syllable discrimination tasks, providing a time-course of stimulus-locked activity over left and right sensory cortex. The M100, known to arise from the auditory cortex and to provide a sensitive indicator of speech sound representation (e.g. Gage et al., 1998; reviewed in Näätänen et al., 1994), was used as a measure of early auditory activity. While similar to the N100 component reported in studies utilizing electroencephalographic recording to examine auditory processing, M100 activity may be a superior indicator of auditory cortical processing due to its localization primarily in the superior temporal plane (Hari et al., 1980), while sources underlying the N100 appear to arise from both auditory and frontal cortices (reviewed in Näätänen and Picton, 1987; see also Picton et al., 1999).
Rapid linguistic sequencing by auditory cortex was investigated via the neural response to: 1) Syllable processing in noise vs. quiet; 2) Syllable 2 vs. syllable 1; 3) Discrimination of voice onset time (VOT) stimuli vs. place of articulation (POA) stimuli. We hypothesized that, if the primary linguistic processing deficit in schizophrenia is one of impaired initial sensory encoding (“imprecise” formation of auditory representations), abnormal neural responses would be evident in both first and second syllables during syllable pairs, more so in noise versus quiet conditions. However, if the primary deficit arises in temporal precision and integration across successive speech sounds, abnormal neural responses to only syllable 2, more evident in VOT and noise conditions, would occur.
MATERIALS AND METHODS
Subjects
This report describes baseline data from a longitudinal study of MEG findings associated with a randomized controlled trial of neuroplasticity-based cognitive training in schizophrenia, registered at ClinicalTrials.gov NCT00312962. Clinically stable, chronically ill, volunteer schizophrenia subjects were recruited from community mental health clinics. Healthy comparison subjects were recruited from the larger San Francisco community via posted advertisements and were matched to the patient group on age, education and gender. After having study procedures explained, participants gave written informed consent and underwent baseline behavioral assessments over a 2–3 week period, followed by a battery of auditory tasks during MEG recording. A total of 19 healthy subjects and 47 patients with schizophrenia were studied.
All subjects were administered neuropsychological tests recommended by the MATRICS Committee (Measurement and Treatment Research to Improve Cognition in Schizophrenia, Nuechterlein and Green, 2006). Measures were obtained from test publishers, and raw scores were converted to z-scores using normative data, stratified by age, published by the test authors. In this study, we focused on four composite neuropsychological measures related to verbal cognition: Speed of Processing (Trail Making Test Part A, Category Fluency, Symbol Coding), Verbal Working Memory (Letter-Number Span), Verbal Learning and Memory (Hopkins Verbal Learning Test, Immediate and Delayed Recall), and General Cognition (mean cognitive performance across measures). The average z-score was computed across measures defining each composite. IQ was assessed using the Wechsler Abbreviated Scale of Intelligence, two-subtest format (Wechsler, 1999). Schizophrenia participants also received the Positive and Negative Syndrome Scale (Kay et al., 1987).
All 47 patient participants were maintained on their normal medication schedule during the study. Forty-one participants were treated with atypical antipsychotic medications, 3 with conventional antipsychotic medication, and 3 were not taking antipsychotics at the time of the study. The average dosing of the antipsychotic medication in chlorpromazine equivalents is summarized in Table 1. Other psychiatric medications included sedative hypnotics (15), antidepressants (14), mood stabilizers (8), benztropine (5), and busipirone (3). Healthy comparison subjects were free of psychiatric medications. No participant showed hearing loss of greater than 20 dB via pure tone audiometry at 1000, 4000, and 8000 hz prior to MEG recording. This study was approved by the UCSF committee on human research and performed in accordance with the ethical standards specified in the 1964 Declaration of Helsinki. Table 1 summarizes the characteristics of each subject group.
Table 1.
Subject characteristics (mean, SD). Ethnicity is coded as Cauc = Caucasian non-Hispanic, AfrAm = African-American, Latino = Caucasian Hispanic, Asian = Asian, NatAm = Native American, Other = unspecified/declined to answer.
| Healthy comparison subjects (N=19) | Schizophrenia subjects (N=47) | |
|---|---|---|
| Average Subject Age | 42.4 (11.3) | 38.7 (11.1) |
| Gender (Female/Male) | 5/14 | 10/37 |
| Ethnicity | Cauc(11), AfrAm(5), Latino(1), Asian(2), NatAm(0), Other(0) | Cauc(26), AfrAm(8), Latino(3), Asian(8), NatAm(1), Other(1) |
| Average Years Education | 14.2 (1.7) | 13.1 (2.1) |
| Mean IQ | 112.4 (11.0) | 102.6 (15.7) |
| Age of Onset | 21.5 (8.4) | |
| Average PANNS Score at Baseline | 2.4 (.6) | |
| Global Assessment of Functioning (GAF) | 43.6 (12.7) | |
| Chlorpromazine Equivalents | 387.7 (443.2) | |
| Mean Verbal Learning Memory Score (normalized) | −1.0 (.9) | −2.0 (1.3) |
| Mean General Cognition Score (normalized) | −.5 (.8) | −1.0 (.7) |
Task Parameters
Subjects performed three different successive syllable discrimination tasks in the presence and absence of simultaneous ongoing noise presented at 69.8 dB sPL. Noise signal was obtained from the International Collegium for Rehabilitative Audiology (ICRA, Dreschler et al. 2001) collection, providing a well-specified set of speech-like noises with spectra shaped according to gender and vocal effort, with different amounts of speech modulation that simulates one or more speakers. ICRA noise in these tasks used 3-band speech-modulated noise having 6 persons babble, and included both male- and female-weighted spectrum. Each task utilized different syllable and/or noise parameters: (1) Ba-Pa during Quiet, where “/ba//pa/” or “/pa//ba/” served as the successive syllable pair on a trial and differ only in their voice onset time (VOT), (2) Ba-Pa during Noise, or (3) Ba-Da during Noise, during which subjects discriminated between “/ba//da/” or “/da//ba/” successive syllable pairs, which differ only in their spectral features (i.e., their place of articulation, POA). Individual syllables were recorded from a single male speaker. From several tokens, three exemplars were selected and digitally normalized to have identical intensity and durations (of 400 ms). Syllable sequences were then synthesized by inserting a silent gap of 100 ms between each syllable.
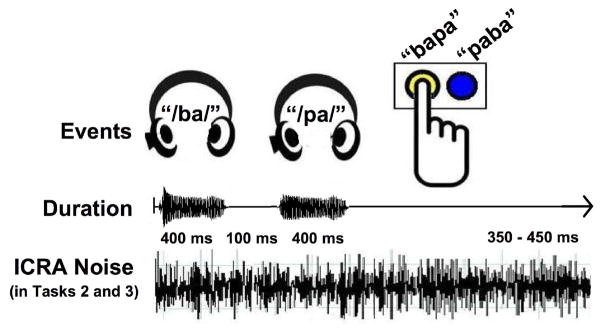
Subjects were instructed to listen to successive syllable pairs delivered binaurally through eartubes (ear tone 3A made by Etymotic E-A-RTONE, Indianapolis), and indicate which syllable was presented first by pressing a button with their dominant hand. A screen projecting simple task instructions and providing response feedback on each trial was located approximately 18 inches away from the subject’s face and adjusted to suit individual comfort levels. Buttons were arranged such that the “/ba/”-as-first response was always performed on the left button. Responses were recorded as valid when they occurred after a visual signal appeared on the screen, 100 ms after offset of the second syllable. Each syllable lasted 400 ms in duration (61.8 dB SPL), with an intervening 100 ms period. Thus, the first syllable occurred at trial start (“Syllable 1” 0–400 ms) and the second syllable 500 ms later (“Syllable 2” 500 – 900 ms). Each subsequent trial began after the previous trial response, with a random delay between 350 and 450 ms. A standard task run included 80 trials of each syllable discrimination task, although a few subjects (N=1 Ba-Pa Noise, N=2 Ba-Pa Quiet, N=2 Ba-Da Noise) experienced multiple blocks for a particular task due to technical issues. Figure 1 illustrates the task events.
Figure 1.
Timeline of events in Syllable Identification task. The events for Task 2 (Ba-Pa Noise) illustrate the general timeline of stimulus events during an experimental session. Participants heard two successive syllables, delivered binaurally through eartubes, corresponding to/ba/or/pa/(/ba/or/da/in Task 3) and subsequently pressed a button to indicate whether the order of syllables corresponded to “bapa” or “paba” (“bada” or “daba” in Task 3). Simultaneously-presented ICRA noise was present throughout a recording session during Tasks 2 and 3, but not during Task 1 (Ba-Pa Quiet).
Subjects were instructed to remain still throughout task recording, including during breaks. Breaks were offered every 20 trials, with subjects pressing a button to continue with the experiment. Practice trials were given prior to the MEG recording of each task to ensure that they understood the events of the task and response instructions. Within a session, each of the three syllable identification tasks was presented in its entirety prior to switching to a different syllable discrimination task. Tasks were pseudo-counterbalanced across subjects for order of presentation with respect to which noise task came first, however Ba-Pa in Quiet always followed Ba-Pa in Noise.
MEG recording and analysis
Acquisition
MEG was recorded from a 275-channel sensor array during task performance within a magnetically-shielded room, using the Omega 2000 Whole-cortex system (CTF Systems Inc./VSM MedTech, Ltd. Port Coquitlam, BC, Canada; see also Hämäläinen et al., 1993; Vrba and Robinson, 2001). Twenty-nine reference sensors were used to correct distant magnetic field disturbance by calculating a synthetic 3rd order gradiometer (Weinburg et al., 1984; Vrba and Robinson, 2001). Participants were lying supine, with their head supported near the center of the sensor array. Head location was measured at the start and end of each task run using three magnetic coils attached to the fiduciary landmarks (Hämäläinen et al., 1993). All participants included in analyses of MEG sensor data had < 5 mm translation in head movement. MEG data was sampled at 1200 Hz and acquired under a bandpass filter of 0.001–300Hz.
Data processing
Trial epochs for each participant’s data were defined as −500 to 1000 msec relative to onset of the first syllable pair, and band pass-filtered from 6 to 40 Hz using a 4th order Butterworth filter applied forwards and backwards. Artifacts were defined as magnetic flux exceeding 2.5 pT at 9 frontal sensors. Epochs were rejected from further analysis if they contained artifacts or participant responses outside a window of 0.8 to 3 s post-trial onset.
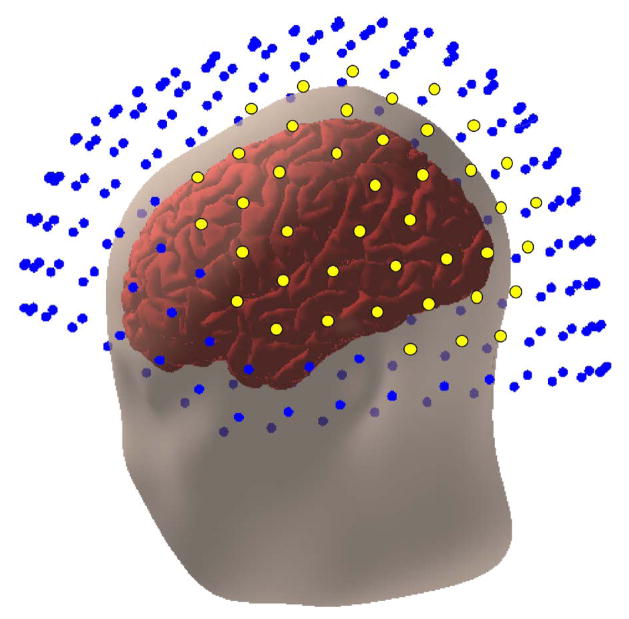
Activity (for each artifact-rejected trial) was extracted from 42 sensors concentrated over auditory cortex of each hemisphere (see Figure 2), a linear detrend performed on each extracted epoch, and removal of trials for any given channel that exceeded 2 standard deviations from the mean activity obtained across time, trials, and channels. Resulting data was used to calculate the root-mean-square (RMS) activity at each time point across the selected sensors for each trial. These data were denoised relative to a 200 ms period prior to the onset of the first syllable pair using the SEFA algorithm (Zumer et al., 2007) available in the NUTMEG (Dalal et al., 2004) toolbox for MATLAB (Mathworks, Inc. Natick, MA) and averaged across trials.
Figure 2.
Positioning of the head within the MEG sensor array. Left hemisphere sensor locations submitted to analysis are highlighted in yellow to provide an example of head positioning within the sensor array. These sensor locations were chosen to provide broad coverage over the auditory cortical regions. An analogous set of sensors covering the right hemisphere was also used.
Of the original 19 healthy control subjects and 47 patients with schizophrenia that participated in the MEG session, 6 participants (4 healthy, 2 schizophrenia) did not undergo the Ba-Pa Quiet task, while the Ba-Pa Noise task was not recorded in 1 participant (schizophrenia group). The Ba-Da Noise task was recorded in all subjects. Subsequent to recording, data obtained from 4 participants during the entire recording session (all schizophrenia patients), and 1 healthy control during the Ba-Da task, could not successfully undergo denoising using the SEFA algorithm, and thus were not included in analyses. Therefore, a total of 15 and 39 were available for analysis of the Ba-Pa Quiet task, 19 and 42 for the Ba-Pa Noise task, and 18 and 43 for the Ba-Da task. For these subjects, the number of trials submitted to statistical analyses was generally >80% of the total collected in each task (Average number of trials [sd]: Ba-Pa Noise = 75 [11], Ba-Pa Quiet = 80 [18], Ba-Da = 79 [11]), with only 1 subject having less than 55 trials in any task.
Statistical analyses
To facilitate statistical comparisons of activity across tasks and groups, denoised RMS waveforms for each participant were z-normalized using the mean and standard deviation of activity from the entire trial (0 to 1000 ms post-trial onset). For each syllable pair, time windows for the M100 response (50–150 ms from trial onset for Syllable 1and 550–650 from trial onset for Syllable 2) and late-bin response (150 – 350 ms for Syllable 1 and 650–850 ms for Syllable 2) were identified and the average amplitude of activity in each time bin was extracted for each syllable.
Measures of interest were submitted separately to statistical analyses for behavioral, M100, and late-bin responses via repeated-measures Analysis of Variance (ANOVA, SPSS Inc., Chicago, IL). Physiological analyses included factors for Syllable Pair (First, Second), Hemisphere (Left, Right), and Diagnosis (Healthy, Schizophrenia Patients). When direct comparisons between tasks were desired, an additional factor of Task (Ba-Pa Quiet, Ba-Pa Noise, Ba-Da Noise) was added to the model, with pairwise comparisons of main effects performed using the t distribution and adjusted for multiple comparisons via Bonferroni correction. Additional repeated-measures ANOVA were conducted, post-hoc, to further characterize significant interactions within the full model. Separate Analyses of Covariance (ANCOVA, SPSS Inc., Chicago, IL) were performed to assess relationships between task performance and physiological measures. A Greenhouse-Geisser correction was applied to all reported p-values when Mauchly’s Test of Sphericity indicated a significant violation of the sphericity assumption. Bivariate correlation using Pearson’s r (two-tailed) assessed relationships between MEG-derived responses, task performance, and neuropsychological measures.
RESULTS
Task performance
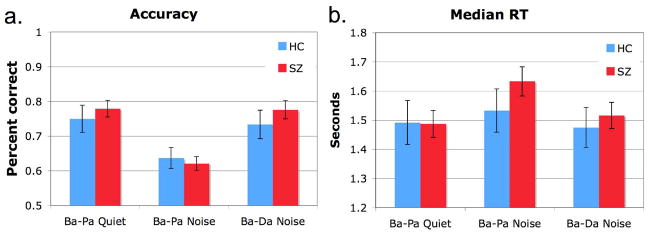
For the subset of subjects included in analyses of MEG data, patients were as accurate and responded as quickly as healthy controls, although differences in accuracy between tasks were found (Accuracy: Task, F[2,110]=29.654, p=.000). Pairwise comparisons revealed decreased accuracy in the Ba-Pa Noise condition relative both to Ba-Pa Quiet (mean difference = −.111, p<.001) and to Ba-Da in Noise (mean difference = −.131, p<.001). Accuracy and median reaction times (RT) for each group are presented in Figure 3.
Figure 3.
Accuracy and median reaction time data. Panel a. presents the overall accuracy data (+/− one standard error bar) for each task and subject group. Panel b. shows the average median reaction time (+/− one standard error bar) for each task and subject group. No significant difference between groups was obtained for either behavioral measure.
Group RMS normalized waveform data
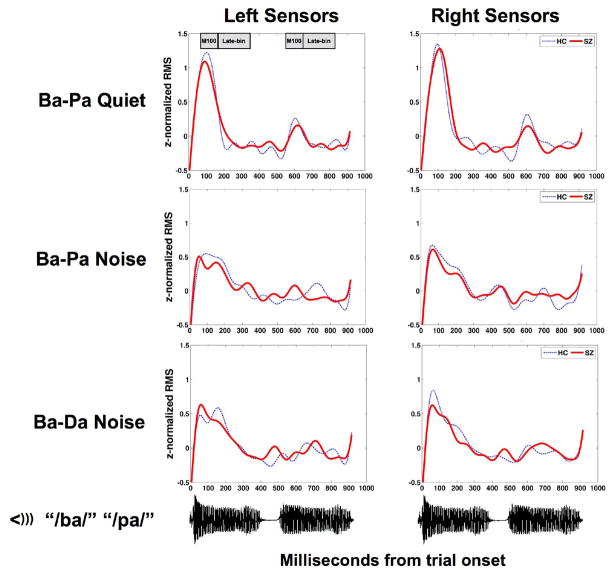
Overall activity for the patient and control groups is shown in Figure 4, with the early (M100) and later analysis windows during both first and second Syllables indicated in the top panel.
Figure 4.
Grandaverage RMS activity during the trial. For each of the three tasks, the grandaverage of RMS activity obtained from patients with schizophrenia (red solid waveforms) and healthy comparison subjects (blue dashed waveforms) are presented for the left and right sensor locations. Analysis windows for the M100 (50–150 ms) and late-bin (150–350 ms) periods are indicated by shaded grey rectangles in the top left panel for each of the successive syllable presentations. For visualization purposes only, data has been low-pass filtered at 10 hertz.
Statistical effects on M100 response to the first and second syllables
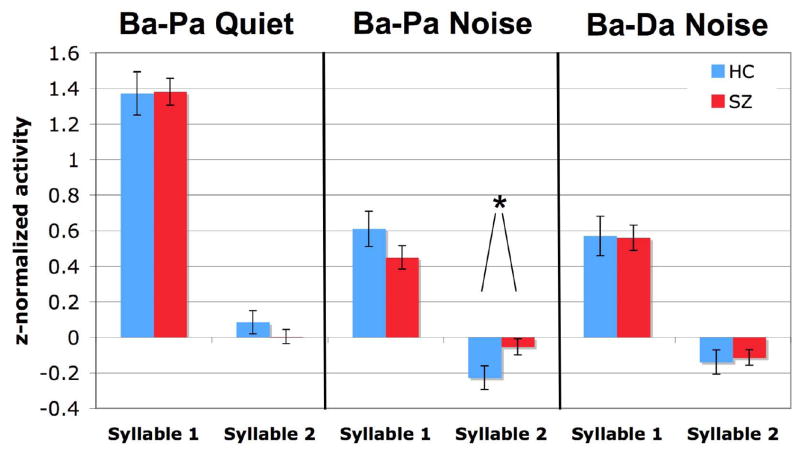
To examine initial linguistic processing in patients with Schizophrenia relative to healthy control subjects, activity during the M100 window obtained in the three syllable identification tasks was assessed for interaction with Diagnosis. Significant differences in the M100 response were obtained between patients with schizophrenia and controls during the Ba-Pa Noise task, but not during Ba-Pa Quiet or Ba-Da Noise tasks (Ba-Pa Noise: Syllable X Diagnosis F[1,59]=4.984, p=.029; Ba-Pa Quiet: all interactions with Diagnosis ns; Ba-Da Noise: all interactions with Diagnosis ns). For the subset of subjects that underwent all three tasks (n=14, 38), direct comparison of activity produced the same result: significant differences in M100 response between patients with schizophrenia and controls during the Ba-Pa Noise task, but not during Ba-Pa Quiet or Ba-Da Noise (Task X Syllable X Diagnosis: F[2, 100]= 3.308, p =.041; posthoc examining Syllable X Diagnosis during Ba-Pa Noise: F[1,50] = 8.193, p=.006, Ba-Pa Quiet ns, Ba-Da Noise ns). No effect of hemisphere was found, indicating a lack of M100 lateralization during each task. The relative group and task differences are shown in Figure 5, averaged over the activity obtained at Left and Right hemisphere sensor locations.
Figure 5.
Average M100 amplitude of each syllable collapsed across hemisphere. Grandaverage amplitudes (+/− one standard error) obtained during 50–150 ms post-syllable onset for each group is presented for each task. The sole significant difference in amplitude between patients with schizophrenia and healthy comparison subjects was obtained during syllable 2 presentation for the Ba-Pa Noise task.
Further analyses of data obtained during the Noise task indicated that patients with schizophrenia showed more activity during Syllable 2 relative to activity across the trial than their healthy counterparts (Diagnosis: Syllable 1, ns; Syllable 2, F(1,59)=4.608, p=.036), likely reflecting less-than-normal attenuation of the neural response to Syllable 2 relative to the response to Syllable 2.
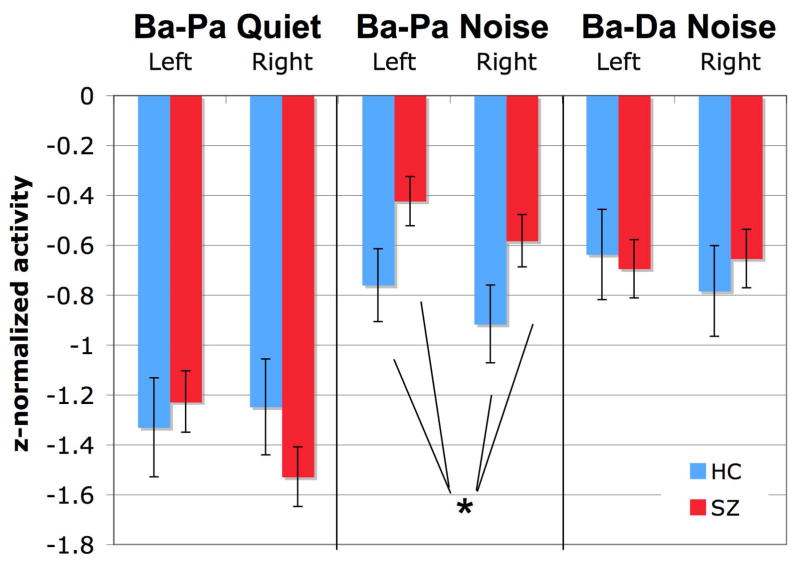
To better examine this ‘attenuation effect’ as a change in the amplitude of the M100 response during presentation of the two syllables in each pair, the average M100 amplitude within a subject for Syllable 1 was subtracted from the M100 amplitude for Syllable 2 in each pair. In Figure 6, greater attenuation of Syllable 2 M100 activity relative to Syllable 1 appears as larger negative amplitudes. This attenuation measure was then submitted to analysis for diagnosis-related differences, confirming a reduced attenuation to Syllable 2 in patients with schizophrenia during the noise task (Ba-Pa Noise: Diagnosis F(1,59)=4.984, p =.029; Ba-Pa Quiet: ns; Ba-Da: ns).
Figure 6.
Attenuation of the second syllable M100 response. An attenuation measure for each task and hemisphere location was calculated by subtracting activity during syllable 1 from the activity during syllable 2 within a subject. Larger negative values indicate more second syllable attenuation, while positive values indicate second syllable enhancement. All subject groups showed second syllable attenuation during all three tasks, however during Ba-Pa in Noise the patient group showed significantly less attenuation than the healthy comparison subjects.
The late window response (150–350 ms from syllable onset)
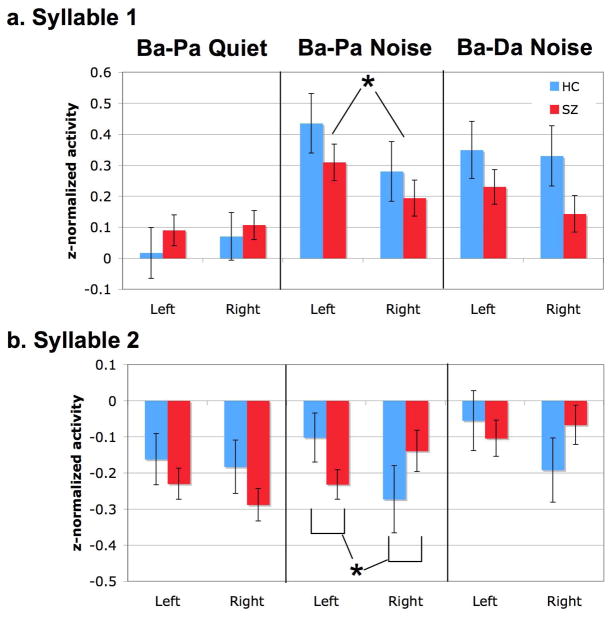
Figure 7 presents the average amplitude values obtained during the late bin (150 – 350 ms post-syllable onset) for each hemisphere and syllable. Direct comparison across the three tasks indicate that late-bin activity differed based on both the task and successive syllable presented (Task x Syllable: F[2, 100]= 3.303, p=.041), and was generally greater at Left sensor locations as compared to Right locations (Hemi: F[1, 51]=4.496, p=.039).
Figure 7.
Activity during the late-bin (150–350) response to Syllable 1 and Syllable 2. Panel a. shows the normalized activity during the late-bin response for Syllable 1 for each task, hemisphere, and subject group. A significant hemisphere effect, occurring across subject group, was found during Syllable 1 of the Ba-Pa Noise task. Panel b. illustrates activity obtained during the late-bin of Syllable 2. An interaction between hemisphere and subject group during Ba-Pa Noise task indicates that patients showed less activity in the left hemisphere than the healthy comparison subjects.
Because the M100 analysis found schizophrenia-related deficits specific to processing the second syllable in only the Ba-Pa task with Noise, we further investigated late-bin activity separately for each task and presentation of each syllable. No significant effects were found in any factors during the Ba-Pa Quiet or Ba-Da Noise tasks for either Syllable 1 or Syllable 2. In contrast, the Ba-Pa Noise task showed a significant increase in left sensor activity relative to right sensors during the first syllable (Hemi: F[1, 51]= 4.649, p=.036). During the second syllable, this asymmetry significantly interacted with diagnosis such that, relative to healthy comparison subjects, patients with schizophrenia showed a relative decrease in activity over the left hemisphere (Hemi X Diagnosis: F[1, 51]= 6.022, p=.018). Thus the physiological mechanism for later, perhaps higher-order, operations related to linguistic discrimination, as reflected in late-bin activity, differed between groups in the presence of noise during the Ba-Pa discrimination task.
The reduced z-normalized RMS values obtained during Ba-Pa Quiet relative to Ba-Pa Noise late in the stimulus presentation period may be due to the increased level of auditory stimulation when ICRA noise is present. Alternatively, the relative reduction in normalized Ba-Pa Quiet activity may be due to the effect of higher overall response amplitude levels (obtained during early periods of Ba-Pa Quiet stimulus presentation) on the whole-trial-based z normalization calculations.
Relationship between M100 and late-bin responses and task performance
Subjects’ accuracy and median RT were submitted as separate covariates in an ANCOVA to examine the correspondence between Syllable 2 attenuation and performance during each task. Attenuation of the M100 was significantly related to accuracy only during the Noise Task (Ba-Pa Noise Accuracy: F[1,58]= 8.218, p=.006), such that a greater attenuation effect across hemispheres corresponded to higher accuracy. Significant covariance between performance measures and the physiological response was not found during the Ba-Pa Quiet or Ba-Da tasks, nor with Syllable 2 attenuation of Late–bin activity in either Ba-Pa task.
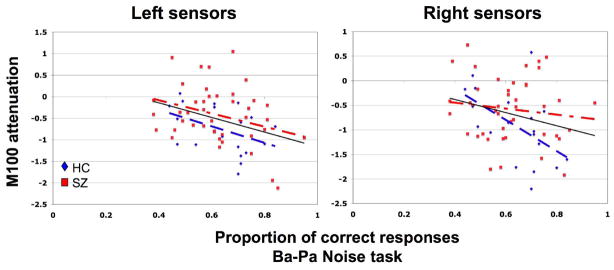
Correlation analyses between Accuracy and the M100 attenuation measure obtained during Ba-Pa Noise confirmed the significant relationship between performance and attenuation across the entire cohort (See Figure 8, n=61: Across Hemisphere r=−.354, p=.005; Left Hemisphere r=−.343, p=.007; Right Hemisphere r=−.252, p=.050). While accuracy did not significantly interact with sensor hemisphere in the ANCOVA-based analyses across subjects, correlation analyses suggest a different asymmetry in patients with schizophrenia versus healthy controls for the relationship between attenuation and performance. The level of second syllable attenuation during the Ba-Pa Noise task over Right hemisphere better predicted accuracy in healthy comparison subjects (Across Hemisphere: r=−.539, p=.017; Left Hemisphere: r=−.421, p=.073; Right Hemisphere: r=−.529, p=.020), while levels of attenuation over Left hemisphere were significantly predictive of accuracy in patients with schizophrenia. (Across Hemisphere: r=−.275, p=.078; Left Hemisphere: −.313, p=.043, Right Hemisphere: ns.)
Figure 8.
Relationship between second syllable attenuation of the M100 response and performance during the Ba-Pa Noise task. Each subject’s relationship between the amount of second syllable attenuation during the M100 response and overall accuracy during the Ba-Pa Noise task is represented for left and right sensor locations. Regression lines illustrate the overall relationship among patients with schizophrenia (red squares, dash-dot regression line), healthy comparison subjects (blue triangles, dashed regression line), and all subjects considered together (solid black regression line). Across subject groups, attenuation in both hemispheres predicted accuracy such that greater second syllable attenuation corresponded with better task performance. Separate analysis of patient and comparison groups differed in this asymmetry, however. In patients, greater second syllable attenuation at left hemisphere sensors corresponded to better performance, while for healthy comparison subjects the correlation between attenuation and performance reached significance for right hemisphere activity.
Relationship of neuropsychological measures to attenuation of the M100 response
To determine the relationship between attenuation of the second syllable response and general cognitive functioning, physiological and neuropsychological measures were submitted to pairwise correlation analyses across the subject groups (n=59; 17 HC and 42 SZ). Attenuation of right sensor M100 responses to Ba-Pa Noise was significantly related to level of education obtained (r = −.297, p=.020, n=61; 19 HC and 42 SZ), to IQ (r=−.319, p=.014), and to composite measures of Verbal Learning and Memory (r=−.335, p=.010) as well as Global Cognition (r=−.328, p=.011). In contrast the Left sensor M100 attenuation measure did not significantly correlate with any neuropsychological measures. There was no association of either attenuation measure with PANSS Total Score, or Positive or Negative Symptoms Subscores.
DISCUSSION
Summary of Findings
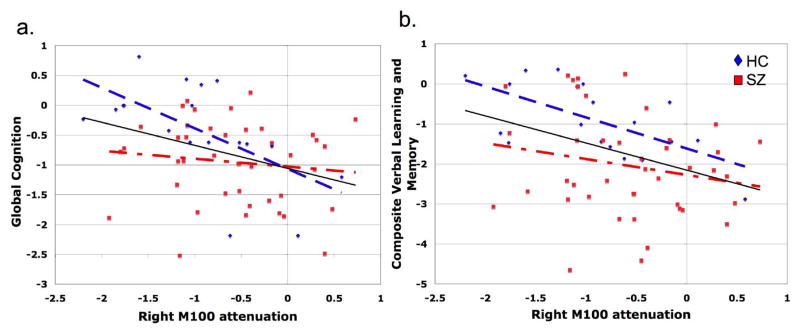
To the best of our knowledge, this is the first study to utilize MEG in a well-characterized and large sample of patients with schizophrenia to determine the neural dysfunction underlying early linguistic processing. Despite large literatures detailing both early auditory and language dysfunction in schizophrenia, the nature of the specific neural deficit underlying initial auditory linguistic processing has remained unclear. The current study reveals that these initial linguistic processing deficits in schizophrenia are characterized by an inability to efficiently integrate aspects of early auditory information in a time-constrained manner when challenged by noise. Specifically, analysis of the M100 response to successive syllable presentation in patients with schizophrenia, relative to healthy comparison subjects, revealed a bilateral increase in second syllable activity during discrimination of VOT in Noise, that did not occur during discrimination of either POA in Noise or VOT in Quiet (Figure 5). This increase in Syllable 2 activity resulted in less overall attenuation of Syllable 2 relative to Syllable 1 in patients versus healthy comparison subjects (Figure 6). In the right hemisphere, the lack of attenuation was significantly correlated with task performance, and also with education, IQ, and verbal cognitive function, across subjects despite a lack of group-related difference in performance (Figures 8 and 9).
Figure 9.
Relationship between neuropsychological measures and attenuation of the right second syllable M100 response during the Ba-Pa Noise task. Each subject’s relationship between the second syllable attenuation of the M100 response during the Ba-Pa Noise task and measures of Global Cognition and Verbal Learning and Memory are presented in Panels a. and b., respectively. Regression lines illustrate the overall relationship across patients with schizophrenia (red squares, dash-dot regression line), healthy comparison subjects (blue triangles, dashed regression line), and all subjects considered together (solid black regression line). Over all subjects, right hemisphere attenuation was associated with both Global Cognition and Verbal Learning and Memory such that greater second syllable attenuation in the right M100 response corresponded with a better assessment on these neuropsychological measures.
Later in the stimulus presentation during VOT-based discrimination in Noise, an overall increase in Left hemisphere activity occurred during Syllable 1, an effect that differed between patients and healthy comparison subjects during Syllable 2 (Figure 7). Generally, during the late period of Syllable 2 patients showed relatively equivalent activity at Left and Right sensors, indicating a reduction of left hemisphere activity and enhancement of right hemisphere activity relative to the left hemisphere asymmetry observed in healthy comparison subjects. This finding indicates that, in patients with schizophrenia, there is a failure in the normal lateralization of language-based information (see also Ngan et al., 2003; Bleich-Cohen et al., 2009; Hirano et al., 2008; Sommer et al., 2001). Unlike the early auditory response, inter-subject variability associated with this later activity was neither related to task performance nor to neuropsychological measures.
Processing Speech Sounds in Noise
Understanding speech in noise is a fundamental characteristic of human language processing and requires the listener to integrate auditory information across temporal dips in background noise (see Alcantara et al., 2004; Groen et al., 2009). Vercammen and colleagues (2008) have recently shown that, as compared to healthy subjects, patients with schizophrenia have higher perceptual thresholds when discriminating pure tones presented in white noise, and lower perceptual sensitivity to speech discrimination when masked by noise. Consistent with these difficulties, the current study finds that neural differences between healthy comparison subjects and patients occur only under the challenge of noise, and the early neural responses exhibit a significant relationship with task performance and cognitive function. This suggests that the presence of noise in the incoming sensory stream, which places additional demands for the accurate integration of auditory information across time, specifically unmasks underlying weaknesses in this aspect of cortical processing in schizophrenia.
Deficits of speech processing in noise have also been reported in dyslexic subject populations (Cunningham et al., 2001; Wible et al., 2002), and signal fidelity in noise has been correlated with composite auditory processing abilities across control and experimental groups (Wible et al., 2002). However, in the current study, only one of the noise conditions produced significant neural response differences between patients and healthy comparison subjects, indicating that overcoming noise alone is not the sole challenge to be addressed during early auditory cortical processing in schizophrenia.
VOT Stimuli vs. POA Stimuli
To further assess the nature of early linguistic processing deficits in schizophrenia, neural responses obtained under noise during discrimination based on place of articulation (POA) were contrasted with those differing only in their voice onset time (VOT). VOT is a central feature of phonetic discrimination that is dependent on very rapid temporal processing in sensory cortex (Steinschneider et al., 1999), while POA relies on rapid spectrotemporal processing of formant transitions across multiple frequency bands (Sussman, 1991; see also Adlard and Hazan, 1998). If all aspects of initial sensory encoding are affected in schizophrenia in a noisy environment, then stimuli requiring detailed spectral representation should show abnormal neural responses similar to those of stimuli requiring rapid temporal processing. Instead, our results indicate that the primary deficit is one of temporal integration, because the neural responses in auditory cortex elicited when VOT-based information is relevant for stimulus discrimination show greater abnormalities than responses during POA-based discrimination in similarly noisy conditions. It is possible that multiple cues are used to identify and discriminate between the syllables. In particular, the initial stop burst for/pa/may be used to identify the syllable/pa/in the/ba//pa/contrast, particularly during the quiet condition, but a less likely factor in the/ba//da/contrast and during the noise conditions. While our findings are consistent with faulty temporal integration in schizophrenia patients, it is also possible that other impairments of acoustic-phonetic processing are occurring. Further work is necessary to elaborate on additional aspects of abnormal acoustic-phonetic processing in this population.
Failure in the Temporal Integration of Successive Auditory Stimuli
In schizophrenia, various experimental paradigms have shown abnormalities in the early neural response to an “unexpected” or “unpredicted” auditory stimulus that follows a previous stimuli set, such as occurs during mismatch negativity tasks (e.g., Näätänen and Kähkönen, 2009; Javitt et al., 2000; Baldeweg et al., 2004; Todd et al., 2008; Turetsky et al., 2009). Indeed, Vaz Pato, Jones, and colleagues (2002 Indeed, Vaz Pato, Jones, and colleagues (1999) have suggested that the mismatch process is involved in the extraction of sequential information and the detection of change in sound patterns. Likewise, in the auditory paired-click paradigm, patients with schizophrenia show a “failure to attenuate” the very early neural response to the second click stimulus, which has been interpreted as a failure of sensory gating (Thoma et al., 2003; Thoma et al., 2005; Thoma et al., 2008; Freedman et al., 2003; but see also Blumenfeld and Clementz, 2001; Johannesen et al., 2005 for its possible relation to integrative sensory disturbance to the first stimulus). Taken together, these disparate findings in schizophrenia can also be seen as consistent with a failure of cortex to temporally integrate or “bind together” successive auditory events. Thus, in schizophrenia, the deviant stimulus in a stream of auditory stimuli, or the second click in a paired-click paradigm, are processed by the cortex as if it had no prior exposure to salient auditory stimuli. Conceptually, it is as if the cortex has difficulty using the previous sensory event to accurately prepare for and “predict” the next event in an auditory sequence—resulting in an abnormal response to deviant events in the mismatch paradigm, as well as a possible failure to show the normal attenuation of the neural response to the second click in a paired-click paradigm. Our data are consistent with this general conceptualization, as we find an abnormal M100 response only to the second syllable of the pair, and only under conditions which actively require the cortex to temporally integrate auditory information under low signal-to-noise conditions. While the SOA of 500 ms used in the current study is longer than SOA durations typically used in paired-click paradigms, successively-presented 400 ms syllables at a 500 ms SOA only provide for 100 ms during which first syllable processes can resolve before sensory cortex is challenged by the onset of the second syllable. An ISI of 100 ms is within the temporal integration window of sub-segmental (i.e. syllabic) linguistic information processing and hence can indeed be considered “rapid” successive signal processing.
Interestingly, a body of work has demonstrated that a reduced mismatch negativity response is associated with lower verbal learning and memory performance and with poorer psychosocial functioning in healthy subjects (Light et al., 2007). In schizophrenia, it is associated with impaired verbal memory (Kawakubo et al., 2006), with the inability to decode semantic and emotional aspects of speech (Leitman et al., 2007), with language-related symptoms (Turetsky et al., 2009), and with poor functional status (Javitt et al., 2007; Light et al., 2005). The abnormal M50 S2/S1 response to the paired-click paradigm found in schizophrenia has been shown to correlate with negative symptoms (Thoma et al., 2005), as well as with measures of attention and working memory in patients (Thoma et al., 2003). Similar to these studies, we found a significant association between verbal cognitive functions and the lack of attenuation to the second syllable during the VOT condition in noise, across subject groups. Thus, it appears that impaired temporal integration of auditory sensory information has significant functional consequences. While more prominent in schizophrenia subject samples, it appears to be a cognitive finding that is present in healthy subject samples as well, with implications for IQ, educational attainment, and verbal cognition.
Lack of Hemispheric Lateralization During Later Stimulus Processing
In this study, we also found group differences in the left hemisphere response to the later stimulus processing of Syllable 2 during VOT discrimination in noise. Our data reveals the time course of lateralization of activity during the successive syllable discrimination task, and are consistent with observations that auditory cortex initially responds bilaterally to linguistic stimuli, with further lateralization occurring in higher-order sensory, parietal, and frontal areas (reviewed in Poeppel et al., 2008). We observed no significant lateralization in the M100 responses, but significant left-lateralization in the 150 to 350 ms response to the first syllable in both patients and comparison subjects. Our data further reveal the temporal course of reduction in asymmetry observed in schizophrenia patients occurs late in Syllable 2 processing, 650 to 850 ms post trial-onset, with no difference in the asymmetry of responses between patients and comparison subjects in the analysis periods prior to 650 ms.
While, our results are consistent with a general finding of reduced asymmetry in schizophrenia when processing linguistic information (Bleich-Cohen et al., 2009; Sommer et al., 2001, 2003), these previous fMRI-based studies found reduction in asymmetry due to an increase in the overall activity of selected right hemisphere areas in patients (Sommer et al., 2001, 2003), rather than a decrease in left hemisphere activity. Sommer et al (2001, 2003) propose that increased right hemisphere activity indicated a failure to inhibit the nondominant language areas, as opposed to hypoactivity in left hemisphere language-dominant regions. These discrepancies may be due to the regions included in the calculation of asymmetry in prior studies versus the primarily temporal regions represented in the current analysis (but see Limitations section below), and/or the inability of fMRI to measure potential changes in the temporal evolution of left-right asymmetry at an adequate resolution, as well as use of different types of linguistic tasks. The time course of the current results reveal that differences in asymmetry between patients and controls arise dynamically due to the functional requirements of the task, rather than result from an anomalous decrease or increase in hemispheric function in patients with schizophrenia that produces reduced asymmetry. Patients exhibited differences in asymmetry only in the late portion of the trial, primarily due to decreased left hemisphere activity, corresponding to a faulty transfer of information to left hemisphere regions when a discrimination decision and response is imminent, as opposed to substandard left hemisphere functioning during the perception and encoding of linguistic information.
Limitations of the Current Study
The current study utilized a large cohort of patients with schizophrenia maintained on their individual medication schedules. Thus, the current study is unable to address neural activity during early linguistic processing in patients with schizophrenia in the absence of pharmacological intervention, nor can we rule out the possibility that differences in neural activity between patients and comparison subjects may arise from the differential modulation of neurotransmitter systems in medicated versus unmedicated subjects. Further, the sample of patients reported here were self-selected to participate in the research and required to schedule and attend multiple interviews to obtain neuropsychological and MEG measures, thus may be more motivated or higher-functioning than the schizophrenic population at large. Finally, while the activity reported here is expected to originate in auditory cortex, particularly during the time period of the well-characterized M100 (e.g. Gage et al., 1998; Hanlon et al., 2005), the MEG sensors chosen for analysis in this study may reflect parietal and inferior frontal contributions to measures of RMS activity. MEG recording has been used in studies of schizophrenia to examine auditory dysfunction during non-linguistic paradigms related to sensory gating (Thoma et al., 2003; Thoma et al., 2005; Thoma et al., 2008; Hanlon et al, 2005), stimulus suppression (Blumenfeld and Clementz, 2001), and mismatch negativity (Näätänen and Kähkönen, 2009; Thonnessen et al, 2008), largely using equivalent current dipole methods to examine activity attributed to sources within auditory cortex. RMS measures used in the current study are more likely to reflect activity arising from a broader cortical region than dipole-derived sources, thus strong linguistic responses that occur in sensory and higher-order areas are likely to be observed while focal activity may be less discernable. Particularly during the 150–350ms analysis window, potential inclusion of activity arising from frontal regions associated with established language areas, such as Broca’s Area in the left hemisphere, may contribute to lateralization differences between the patient and comparison groups.
Conclusions
The current study finds that early linguistic processing deficits in schizophrenia are related to the faulty temporal integration of successive speech stimuli, particularly when stimulus representations are of low fidelity. In a sense, timing is everything: this impairment in the ability to “bind together” relevant auditory stimuli in the incoming sensory stream would have deleterious downstream consequences in task performance. More importantly, subjects who manifest this impairment are of lower IQ, have less educational attainment, and have worse verbal learning and memory and general cognition. Thus, our data indicate that a cognitive endophenotype relevant to schizophrenia may be represented by deficits in the cortical neuronal mechanisms that represent rapidly changing or temporally-constrained processing of successive sensory events. It is interesting in this light to note that schizophrenia has been associated in several studies with a faulty “central clock” mechanism (e.g. Davalos et al., 2002; Carroll et al., 2008; for neural mechanisms underlying a central clock see Gibbon et al., 1997; Meck et al., 2008), while Jones (2002) has speculated on the relationship between the mismatch process, the internal auditory clock, and altered consciousness.
The current findings are of more than theoretical interest—they are of strong practical import, as it is known that the cortex can be “trained” to segment in a more rapid and efficient manner the representations of rapidly changing or successive events. In other words, the basic effectiveness with which the cortex can derive accurate sensory representations across time is subject to powerful plasticity effects (Merzenich et al., 1996; Karni and Sagi, 1991). Advances in the basic science of learning-induced neuroplasticity have led to the development of human cognitive training programs that aim to address impairments in early sensory processes in order to generate improvement in “downstream” cognitive operations such as verbal learning and memory (Mahncke et al., 2006; Fisher et al., in press). To that end, a precise knowledge of the nature of the functionally relevant early auditory processing deficits in schizophrenia is essential to develop the most optimally effective approaches to cognitive training. That is, computerized cognitive training exercises for schizophrenia could be designed to improve the fidelity and efficiency of all aspects of early auditory encoding or, alternatively, they could focus specifically on increasing the efficiency of temporal integration during successively-presented stimuli. At this point in time, our data suggest that the latter approach has the highest likelihood of showing generalization to the functionally critical domains of verbal learning and memory, and thus yielding the greatest real-world benefit to individuals with schizophrenia.
Acknowledgments
We would like to thank Leighton Hinckley, Alex B. Herman, Kasra Khatibi, and Darren Weber for their help with the analysis and presentation of this research. This project was supported with resources and the use of facilities at the San Francisco VA Medical Center, San Francisco, CA, and by NIH RO1 grant MH068725 to S.V. and NIH R01 DC4855 and DC6435 to S.S.N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlard A, Hazan V. Speech Perception in Children with specific reading difficulties. Q J Exp Psychology. 1998;51:153–177. doi: 10.1080/713755750. [DOI] [PubMed] [Google Scholar]
- Ahissar M, Protopapas A, Reid M, Merzenich MM. Auditory processing parallels reading abilities in adults. Proc Natl Acad Sci. 2000;97:6832–6837. doi: 10.1073/pnas.97.12.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldeweg T, Klugman A, Gruzelier J, Hirsch SR. Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophr Res. 2004;69:203–217. doi: 10.1016/j.schres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Alcantara JI, Weisblatt EJ, Moore BC, Bolton PF. Speech-in-noise perception in high-functioning individuals with autism or Asperger’s syndrome. J Child Psychol Psychiatry. 2004;45:1107–1114. doi: 10.1111/j.1469-7610.2004.t01-1-00303.x. [DOI] [PubMed] [Google Scholar]
- Bleich-Cohen M, Hendler T, Kotler M, Strous RD. Reduced language lateralization in first-episode schizophrenia: An fMRI index of functional asymmetry. Psychiatry Research: Neuroimaging. 2009;171:28–93. doi: 10.1016/j.pscychresns.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Blumenfeld LD, Clementz BA. Response to the first stimulus determines reduced auditory evoked response suppression in schizophrenia: single trials analysis using MEG. Clin Neurophysiol. 2001;112:1650–1659. doi: 10.1016/s1388-2457(01)00604-6. [DOI] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CA, Boggs J, O’Donnell BF, Shekhar A, Hetrick WP. Temporal processing dysfunction in schizophrenia. Brain Cogn. 2008;67:150–161. doi: 10.1016/j.bandc.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Grossman ED, Bidwell LC, Yurgelun-Todd D, Gruber SA, Levy DL, Nakayama K, Holzman PS. Differential activation patterns of occipital and prefrontal cortices during motion processing: evidence from normal and schizophrenic brains. Cogn Affect Behav Neurosci. 2008;8:293–303. doi: 10.3758/cabn.8.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham J, Nicol T, Zecker SG, Bradlow A, Kraus N. Neurobiologic responses to speech in noise in children with learning problems: deficits and strategies for improvement. Clin Neurophysiol. 2001;112:758–767. doi: 10.1016/s1388-2457(01)00465-5. [DOI] [PubMed] [Google Scholar]
- Dalal SS, Zumer JM, Agrawal V, Hild KE, Sekihara K, Nagarajan SS. NUTMEG: a neuromagnetic source reconstruction toolbox. Neurol Clin Neurophysiol. 2004;2004:52. [PMC free article] [PubMed] [Google Scholar]
- Davalos DB, Kisley MA, Ross RG. Deficits in auditory and visual temporal perception in schizophrenia. Cogn Neuropsychiatry. 2002;7:273–282. doi: 10.1080/13546800143000230. [DOI] [PubMed] [Google Scholar]
- Dreschler WA, Verschuure H, Ludvigsen C, Westermann S. ICRA Noises: Artificial noise signals with speech-like spectral and temporal properties for hearing aid assessment. Audiology. 2001;40:148–157. [PubMed] [Google Scholar]
- Duffy FH, McAnulty GB, Waber DP. Auditory evoked responses to single tones and closely spaced tone pairs in children grouped by reading or matrices abilities. Clin Electroencephalogr. 1999;30:84–93. doi: 10.1177/155005949903000303. [DOI] [PubMed] [Google Scholar]
- Farmer ME, Klein R. The evidence for a temporal processing deficit linked to dyslexia: a review. Psychonomic Bull Rev. 1995;2:460–493. doi: 10.3758/BF03210983. [DOI] [PubMed] [Google Scholar]
- Fisher M, Holland C, Merzenich M, Vinogradov S. Neuroplasticity-Based Auditory Training Improves Verbal Memory in Schizophrenia. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2009.08050757. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force RB, Venables NC, Sponheim SR. An auditory processing abnormality specific to liability for schizophrenia. Schizophr Res. 2008;103:298–310. doi: 10.1016/j.schres.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Ross RG, Waldo MC, Stevens KE, Adler LE, Leonard S. The genetics of sensory gating deficits in schizophrenia. Curr Psychiatry Rep. 2003;5:155–161. doi: 10.1007/s11920-003-0032-2. [DOI] [PubMed] [Google Scholar]
- Gage N, Poeppel D, Roberts TPL, Hickok G. Auditory evoked M100 reflects onset acoustics of speech sounds. Brain Research. 1998;814:236–239. doi: 10.1016/s0006-8993(98)01058-0. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Malapani C, Dale CL, Gallistel CR. Toward a neurobiology of temporal cognition: advances and challenges. Current Opinion in Neurobiology. 1997;7:170–184. doi: 10.1016/s0959-4388(97)80005-0. [DOI] [PubMed] [Google Scholar]
- Groen WB, van Orsouw L, Huurne NT, Swinkels S, van der Gaag RJ, Buitelaar JK, Zwiers MP. Intact Spectral but Abnormal Temporal Processing of Auditory Stimuli in Autism. J Autism Dev Disord. 2009 Jan 16; doi: 10.1007/s10803-008-0682-3. epub. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Haertling F, Rotarska-Jagiela A, Maurer K, Singer W, Linden DE. Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia: a study with event-related potentials and functional magnetic resonance imaging. Arch Gen Psychiatry. 2007;64:1229–1240. doi: 10.1001/archpsyc.64.11.1229. [DOI] [PubMed] [Google Scholar]
- Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV. Magnetoencephalography—theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys. 1993;65:413–497. [Google Scholar]
- Hanlon FM, Miller GA, Thoma RJ, Irwin J, Jones A, Moses SN, Huang M, Weisend MP, Paulson KM, Edgar JC, Adler LE, Canive JM. Distinct M50 and M100 auditory gating deficits in schizophrenia. Psychophysiology. 2005;42:417–427. doi: 10.1111/j.1469-8986.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- Hari R, Aittoniemi K, Järvinen ML, Katila T, Varpula T. Auditory evoked transient and sustained magnetic fields of the human brain. Localization of neural generators. Experimental Brain Research. 1980;40:237–240. doi: 10.1007/BF00237543. [DOI] [PubMed] [Google Scholar]
- Hari R, Renvall H. Impaired processing of rapid stimulus sequences in dyslexia. Trends Cogn Sci. 2001;5:525–532. doi: 10.1016/s1364-6613(00)01801-5. [DOI] [PubMed] [Google Scholar]
- Hirano S, Hirano Y, Maekawa T, Obayashi C, Oribe N, Kuroki T, Kanba S, Onitsuka T. Abnormal neural oscillatory activity to speech sounds in schizophrenia: a magnetoencephalography study. J Neurosci. 2008;28:4897–4903. doi: 10.1523/JNEUROSCI.5031-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Shelley A, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol. 2000;111:1733–7. doi: 10.1016/s1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Rabinowicz E, Silipo G, Dias EC. Encoding vs. retention: differential effects of cue manipulation on working memory performance in schizophrenia. Schizophr Res. 2007;91:159–168. doi: 10.1016/j.schres.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesen JK, Kieffaber PD, O’Donnell BF, Shekhar A, Evans JD, Hetrick WP. Contributions of subtype and spectral frequency analyses to the study of P50 ERP amplitude and suppression in schizophrenia. Schizophr Res. 2005;78:269–284. doi: 10.1016/j.schres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Jones SJ. The internal auditory clock: what can evoked potentials reveal about the analysis of temporal sound patterns, and abnormal states of consciousness? Neurophysiol Clin. 2002;32:241–253. doi: 10.1016/s0987-7053(02)00309-x. [DOI] [PubMed] [Google Scholar]
- Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc Natl Acad Sci. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakubo Y, Kasai K, Kudo N, Rogers MA, Nakagome K, Itoh K, Kato N. Phonetic mismatch negativity predicts verbal memory deficits in schizophrenia. Neuroreport. 2006;17:1043–1046. doi: 10.1097/01.wnr.0000221828.10846.ba. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scal (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kim DI, Mathalon DH, Ford JM, Mannell M, Turner JA, Brown GG, Belger A, Gollub R, Lauriello J, Wible C, O’Leary D, Lim K, Toga A, Potkin SG, Birn F, Calhoun VD. Auditory oddball deficits in schizophrenia: an independent component analysis of the fMRI multisite function BIRN study. Schizophr Bull. 2009;35:67–81. doi: 10.1093/schbul/sbn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus N, McGee TJ, Carrell TD, Zecker SG, Nicol TG, Koch DB. Auditory neurophysiologic responses and discrimination deficits in children with learning problems. Science. 1996;273:971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- Kraus N. Auditory pathway encoding and neural plasticity in children with learning problems. Audiol Neurootol. 2001;6:221–227. doi: 10.1159/000046837. [DOI] [PubMed] [Google Scholar]
- Kujala T, Myllyviita K, Tervaniemi M, Alho K, Kallio J, Näätänen R. Basic auditory dysfunction in dyslexia as demonstrated by brain activity measurements. Psychophysiology. 2000;37:262–266. [PubMed] [Google Scholar]
- Kuperberg GR, West WC, Lakshmanan BM, Goff D. Functional magnetic resonance imaging reveals neuroanatomical dissociations during semantic integration in schizophrenia. Biol Psychiatry. 2008;64:407–418. doi: 10.1016/j.biopsych.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Hoptman MJ, Foxe JJ, Saccente E, Wylie GR, Nierenberg J, Jalbrzikowski M, Lim KO, Javitt DC. The neural substrates of impaired prosodic detection in schizophrenia and its sensorial antecedents. Am J Psychiatry. 2007;164:474–482. doi: 10.1176/ajp.2007.164.3.474. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005;62:127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Braff DL. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J Cogn Neurosci. 2007;19:1624–1632. doi: 10.1162/jocn.2007.19.10.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog Brain Res. 2006;157:81–109. doi: 10.1016/S0079-6123(06)57006-2. [DOI] [PubMed] [Google Scholar]
- Meck WH, Penney TB, Pouthas V. Cortico-striatal representation of time in animals and humans. Current Opinion in Neurobiology. 2008;18:145–152. doi: 10.1016/j.conb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Merzenich M, Wright B, Jenkins W, Xerri C, Byl N, Miller S, Tallal P. Cortical plasticity underlying perceptual, motor, and cognitive skill development: implications for neurorehabilitation. Cold Spring Harb Symp Quant Biol. 1996;61:1–8. [PubMed] [Google Scholar]
- Näätänen R, Ilmoniemi RJ, Alho K. Magnetoencephalography in the studies of human cognitive brain function. Trends in Neuroscience. 1994;17:389–395. doi: 10.1016/0166-2236(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Picton TW. The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Kähkönen S. Central auditory dysfunction in schizophrenia as revealed by the mismatch negativity (MMN) and its magnetic equivalent MMNm: a review. Int J Neuropsychopharmacol. 2009;12:125–135. doi: 10.1017/S1461145708009322. [DOI] [PubMed] [Google Scholar]
- Nagarajan S, Mahncke H, Salz T, Tallal P, Roberts T, Merzenich MM. Cortical auditory signal processing in poor readers. Proc Natl Acad Sci. 1999;96:6483–6488. doi: 10.1073/pnas.96.11.6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngan ET, Vouloumanos A, Cairo TA, Laurens KR, Bates AT, Anderson CM, Werker JF, Liddle PF. Abnormal processing of speech during oddball target detection in schizophrenia. Neuroimage. 2003;20:889–897. doi: 10.1016/S1053-8119(03)00385-9. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF. MATRICS Consensus Cognitive Battery Manual. Los Angeles, CA: MATRICS Assessment, Inc; 2006. [Google Scholar]
- Picton TW, Alain C, Woods DL, John MS, Scherg M, Valdes-Sosa P, Bosch-Bayard J, Trujillo NJ. Intracerebral sources of auditory evoked potentials. Audiology and Neuro-otology. 1999;4:64–79. doi: 10.1159/000013823. [DOI] [PubMed] [Google Scholar]
- Poeppel D, Idsardi WJ, van Wassenhove V. Speech perception at the interface of neurobiology and linguistics. Phil Trans Royal Soc B. 2008;363:1071–1086. doi: 10.1098/rstb.2007.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revheim N, Butler PD, Schechter I, Jalbrzikowski M, Silipo G, Javitt DC. Reading impairment and visual processing deficits in schizophrenia. Schizophrenia Res. 2006;87:238–245. doi: 10.1016/j.schres.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Kah RS. Language lateralization in schizophrenia, an fMRI study. Schizophr Res. 2001;52:57–67. doi: 10.1016/s0920-9964(00)00180-8. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Mandl RC, Kahn RS. Language lateralization in female patients with schizophrenia: an fMRI study. Schizophr Res. 2003;60:183–190. doi: 10.1016/s0920-9964(02)00300-6. [DOI] [PubMed] [Google Scholar]
- Stein J, Walsh V. To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci. 1997;20:147–152. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- Steinschneider M, Volkov IO, Noh MD, Garell PC, Howard MA., 3rd Temporal encoding of the voice onset time phonetic parameter by field potentials recorded directly from human auditory cortex. J Neurophysiol. 1999;82:2346–2357. doi: 10.1152/jn.1999.82.5.2346. [DOI] [PubMed] [Google Scholar]
- Sussman HM. The representation of stop consonants in three-dimensional acoustic space. Phonetica. 1991;48:18–31. doi: 10.1159/000261869. [DOI] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Developmental aphasia: rate of auditory processing and selective impairment of consonant perception. Neuropsychologia. 1974;12:83–93. doi: 10.1016/0028-3932(74)90030-x. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Hanlon FM, Moses SN, Edgar JC, Huang M, Weisend MP, Irwin J, Sherwood A, Paulson KM, Bustillo J, Adler LE, Miller GA, Canive JM. Lateralization of auditory sensory gating and neuropsychological dysfunction in schizophrenia. Am J Psychiatry. 2003;160:1595–1605. doi: 10.1176/appi.ajp.160.9.1595. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Hanlon FM, Moses SN, Ricker D, Huang M, Edgar JC, Irwin J, Torres F, Weisend MP, Adler LE, Miller GA, Canive JM. M50 sensory gating predicts negative symptoms in schizophrenia. Schizophr Res. 2005;73:311–318. doi: 10.1016/j.schres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Hanlon FM, Miller GA, Moses SN, Smith A, Parks L, Lundy SL, Sanchez NM, Jones A, Huang M, Weisend MP, Canive JM. Schizophrenia diagnosis and anterior hippocampal volume make separate contributions to sensory gating. Psychophysiology. 2008;45:926–935. doi: 10.1111/j.1469-8986.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonnessen H, Zvyagintsev M, Harke KC, Boers F, Dammers J, Norra Ch, Mathiak K. Optimized mismatch negativity paradigm reflects deficits in schizophrenia patients. A combined EEG and MEG study. Biol Psychol. 2008;77:205–216. doi: 10.1016/j.biopsycho.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Todd J, Michie PT, Schall U, Karayanidis F, Yabe H, Näätänen R. Deviant matters: duration, frequency, and intensity deviants reveal different patterns of mismatch negativity reduction in early and late schizophrenia. Biol Psychiatry. 2008;63:58–64. doi: 10.1016/j.biopsych.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Bilker WB, Siegel SJ, Kohler CG, Gur RE. Profile of auditory information-processing deficits in schizophrenia. Psychiatry Res. 2009;165:27–37. doi: 10.1016/j.psychres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz Pato M, Jones SJ. Cortical processing of complex tone stimuli: mismatch negativity at the end of a period of rapid pitch modulation. Cogn Brain Res. 1999;7:295–306. doi: 10.1016/s0926-6410(98)00032-9. [DOI] [PubMed] [Google Scholar]
- Vaz Pato M, Jones SJ, Perez N, Sprague L. Mismatch negativity to single and multiple pitch-deviant tones in regular and pseudo-random complex tone sequences. Clin Neurophysiol. 2002;113:519–527. doi: 10.1016/s1388-2457(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Vercammen A, de Haan EGF, Aleman A. Hearing a voice in the noise: auditory hallucinations and speech perception. Psychol Med. 2008;38:1177–1184. doi: 10.1017/S0033291707002437. [DOI] [PubMed] [Google Scholar]
- Vrba J, Robinson SE. Signal processing in magnetoencephalography. Methods. 2001;25:249–271. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Weinberg H, Brickett PA, Vrba J, Fife AA, Burbank MB. The use of a squid third order spatial gradiometer to measure magnetic fields of the brain. Ann N Y Acad Sci. 1984;425:743–5. doi: 10.1111/j.1749-6632.1984.tb23597.x. [DOI] [PubMed] [Google Scholar]
- Wible B, Nicol T, Kraus N. Abnormal neural encoding of repeated speech stimuli in noise in children with learning problems. Clin Neurophysiol. 2002;113:485–494. doi: 10.1016/s1388-2457(02)00017-2. [DOI] [PubMed] [Google Scholar]
- Zumer JM, Attias HT, Sekihara K, Nagarajan SS. A probabilistic algorithm integrating source localization and noise suppression for MEG and EEG data. Neuroimage. 2007;37:102–15. doi: 10.1016/j.neuroimage.2007.04.054. [DOI] [PubMed] [Google Scholar]