Abstract
Background
New intraparenchymal brain injury on MRI is observed in 36–73% of neonates after cardiac surgery with cardiopulmonary bypass (CPB). Brain immaturity in this population is common. We performed brain magnetic resonance imaging (MRI) before and after neonatal cardiac surgery, using a high flow CPB protocol, hypothesizing that MRI brain injury would be associated with brain immaturity.
Methods
CPB protocol included 150 ml/kg/min flows, pH stat management, hematocrit >30%, and high flow antegrade cerebral perfusion. Regional brain oxygen saturation (rSO2) was monitored, with a treatment protocol for rSO2 <50%. Brain MRI, consisting of T1, T2, and diffusion weighted imaging, and MR spectroscopy were performed preoperatively, 7 days postoperatively, and at age 3–6 months.
Results
Twenty-four of 67 patients (36%) had new postoperative white matter injury (WMI), infarction, or hemorrhage, and 16% had new WMI. Associations with preoperative brain injury included low brain maturity score (p=0.002). Postoperative WMI was associated with single ventricle (SV) diagnosis (p=0.02), preoperative WMI (p<0.001) and low brain maturity score (p=0.05). Low brain maturity score was also associated with more severe postoperative brain injury (p=0.01). Forty-five patients had a 3rd scan, with a 27% incidence of new minor lesions, but 58% of previous lesions had partially or completely resolved.
Conclusions
We observed a significant incidence of both pre- and postoperative MRI abnormality, and an association with brain immaturity. Many lesions resolved in the first 6 months after surgery. Timing of delivery and surgery with bypass could affect the risk of brain injury.
Keywords: heart defects, congenital, bypass, brain, surgery, anesthesia
Introduction
Despite current survival rates of greater than 90%1, up to 50% of children aged 5 years or older who underwent cardiac surgery as newborns or young infants have long term neurodevelopmental impairment.2 These children have problems that are similar to those seen in premature infants, including attention deficit hyperactivity disorder, and other cognitive and fine motor deficits.2 Robertson et al3 documented that 26.4% of a cohort of 53 neonates without chromosomal abnormalities who underwent repair of hypoplastic left heart syndrome, transposition of the great vessels, or total anomalous pulmonary venous return had a full scale IQ score of <85 at 5 years of age. Manjemer et al4 documented gross motor delays in 49%, and fine motor delays in 39% of a cohort of 94 patients tested at age 5 years, who had undergone complex congenital cardiac surgery as infants. Magnetic resonance imaging (MRI) has documented a preoperative incidence of white matter injury, or other ischemic lesions of 20–40%.5,6 Associations with poor neurodevelopmental outcomes include chromosomal abnormalities, particularly 22.q11.2 microdeletion, common in tetralogy of Fallot, interrupted aortic arch and truncus arteriosus, resulting in significantly lower mental and physical development.7
Perioperative causes of neurological injury include cerebral hypoxia/ischemia due to cyanosis, surgical or cardiopulmonary bypass techniques including prolonged DHCA,8 cerebral emboli, low cardiac output, and intercurrent events such as cardiac arrests.9 Attempts to reduce acute brain injury have included newer perfusion techniques, such as antegrade cerebral perfusion (ACP), which allow continuous delivery of oxygenated blood to the brain during cardiopulmonary bypass in neonates.10 Monitoring of cerebral oxygenation (rSO2) using near infrared spectroscopy (NIRS), and cerebral blood flow velocity using transcranial Doppler ultrasound (TCD) 11 have also been utilized allow individualized strategies for continuous oxygen delivery to the brain. pH stat blood gas management12 and hematocrit of 25% or greater13 allow for more even brain cooling and provide greater oxygen delivery to the brain, resulting in improved neurological outcomes. In spite of these new intraoperative techniques, MRI studies have revealed a 36–73% incidence of new or worsening white matter or other ischemic brain lesions after neonatal cardiac surgery.5,6,14
Recently, neonates with complex CHD have been discovered to have structural brain immaturity due to delays in myelination, cortical development, and maturation of germinal matrix and glial cell migration.15 Magnetic resonance spectroscopy also documents delayed microcellular maturation in CHD neonates.16 This brain immaturity may predispose these infants to greater risk of brain injury, particularly white matter injury (WMI), which is frequently seen in premature infants with proven immature brain development.17–19
This study was undertaken to determine the incidence and severity of new MRI brain injury with high flow cardiopulmonary bypass (maintaining full 150 ml/kg/min flow and high-flow ACP) and monitoring cerebral oxygenation with NIRS. We also sought to determine the patient and procedural factors that had significant associations with brain injury, particularly brain maturation. Our hypothesis was that patients with structural brain immaturity would be more likely to have pre- and postoperative brain injury.
Methods
This study was approved by the Baylor College of Medicine Institutional Review Board, and patients were enrolled after signed informed consent was obtained from parents. It was a prospective, observational study with a single patient cohort receiving uniform CPB and perioperative treatment protocols.
Patient Population
Neonates (<30 days of age) undergoing cardiac surgery with hypothermic (<30° C) cardiopulmonary bypass for 60 minutes or greater were eligible. Both single ventricle (SV) and two-ventricle (2V) repairs were included. Exclusion criteria were gestational age less than 35 weeks at birth, weight less than 2.0 kg, recognizable dysmorphic syndrome, or preoperative cardiac arrest for greater than 3 minutes.
Preoperative Management
PGE1 infusion was used in patent ductus arteriosus (PDA)-dependent systemic perfusion lesions or for dextrotransposition of the great arteries (TGA) patients with significant cyanosis manifested by prolonged peripheral oxygen saturation (SpO2) <75%. Patients with TGA with intact ventricular septum had balloon atrial septostomy (BAS).
Anesthesia, Surgery and Perfusion Protocol
Anesthetic technique consisted of fentanyl, 100–400 mcg/kg total dose, midazolam 0.25–3 mg/kg total dose, and isoflurane, up to 1% end-tidal concentration before and after cardiopulmonary bypass (CPB), and isoflurane up to 3% inspired concentration in the CPB sweep gas. Vecuronium or pancuronium were used for neuromuscular blockade.
Cardiopulmonary bypass technique consisted of arterial cannulation and bicaval or single atrial venous cannulation. CPB flow rates of 150 ml/kg/min were used at all times, except for periods of DHCA, or ACP. Target mean arterial pressure was 30–35 mm Hg, facilitated if necessary with α-receptor blockade with phenoxybenzamine 0.25–0.5 mg/kg, or phentolamine 0.1–0.3 mg/kg. Cooling on CPB was accomplished over 20 minutes or more. pH stat blood gas management was used throughout the CPB period. One dose of methylpredisolone, 20 mg/kg, was given in the CPB prime. Aprotinin was utilized for the first 55 cases: 60,000 kallekrein inhibiting units (KIU)/kg loading dose followed by infusion of 7000 KIU/kg/hr. A CPB circuit prime of 60,000 KIU/kg of aprotinin was given. After safety concerns about aprotinin in adult cardiac surgery were known, the last 13 cases utilized ε-aminocaproic acid instead of aprotinin, at a dose of 75 mg/kg IV load to the patient, and 75 mg/kg/hr infusion throughout surgery, with a 75 mg/kg CPB prime dose. Hematocrit was maintained at 30–35% during cooling and hypothermic periods, and increased to 40–45% during rewarming. Conventional ultrafiltration was utilized throughout the CPB period; post-CPB modified ultrafiltration was not used.
Antegrade cerebral perfusion was utilized for aortic arch reconstruction.10 A 3.5 mm polytetrafluoroethylene graft was sutured to the right innominate artery as arterial inflow. CPB flow during ACP was adjusted to maintain cerebral blood flow velocity, measured with TCD, to within 10% of full CPB baseline, and right brain rSO2 >90%, as described previously.10
Physiological monitor data (SpO2, intra-arterial pressure, heart rate, temperature, intracardiac pressures), as well as cerebral oximeter data were collected in 1 minute intervals and stored electronically for the pre-, intra- and 72 hour postoperative periods. (Bedmaster, Excel Medical Electronics, Inc., Jupiter, FL; and Somanetics, Inc., Troy, MI).
Cerebral Oxygenation Monitoring and Treatment Protocol
Regional cerebral oxygen saturation (rSO2) was monitored with a sensor on the right forehead. (Somanetics 5100A, Somanetics, Inc., Troy, Michigan). If the regional cerebral oxygen saturation (rSO2) was less than 50%, attempts were made to increase oxygen delivery to the brain, or decrease oxygen consumption.11 This protocol was used throughout the preoperative, intraoperative, and 72 hour postoperative study periods. Suggested interventions included:
Increasing systemic cardiac output with volume infusions, afterload reduction, or inotropic support.
Increasing hematocrit with blood transfusion, or hemofiltration on CPB to 40% or greater.
Treating fever, with target rectal temperature <36.5° C, and cooling further while on CPB.
Increasing FiO2, intubating the trachea and ventilating the patient to decrease work of breathing and increase oxygen delivery, increase the PaCO2 to 45–55 mm Hg to increase cerebral blood flow.
Sedation or neuromuscular blockade to decrease oxygen consumption.
Increasing cardiopulmonary bypass flow or perfusion pressure.
Increasing temperature-corrected PaCO2 on CPB.
The threshhold of rSO2 <50% for intervention was chosen because of previously published data suggesting that prolonged rSO2 <45% in the postoperative period is associated with new postoperative MRI brain injury in neonates undergoing the Stage I palliation for hypoplastic left heart syndrome.5 The priority and sequence of these interventions was left to the discretion of the attending anesthesiologist and surgeon during surgery. In the intensive care unit, the bedside nurse notified the responsible physician for rSO2 values <50%, and intervention was undertaken according to the physician’s discretion using the algorithm listed above. No attempt was made to record the number of interventions, and the response to interventions.
Brain Magnetic Resonance Imaging Protocol
Brain MRI was obtained immediately before surgery, with general endotracheal anesthesia. MR imaging was performed on a 1.5T Intera scanner (Philips Medical Systems, Best, the Netherlands), including standard T1 and T2 weighted images, diffusion weighted imaging, and MR spectroscopy.20 The MRI protocols are detailed in Appendix I. Postoperative MRI was obtained when the patient was clinically stable, with a goal of 7 days postoperatively. Patients were sedated with intravenous pentobarbital, 5–10 mg/kg. A third MRI was obtained at age 3–6 months, immediately before a second stage surgery with general endotracheal anesthesia, or with intravenous sedation with pentobarbital or propofol.
MRI Analysis
All MRI were evaluated by a board certified neuroradiologist (JVH), unaware of diagnosis or surgery. Grading of the T1,T2, and diffusion weighted images was done according a modification of the scoring system devised by Barkovich, McQuillen et al.14,20 (See Appendix II). Abnormalities were classified in 9 categories: white matter injury (WMI), infarction or ischemic stroke, intraparenchymal hemorrhage or hemorrhagic stroke, punctate lesions, elevated lactate on MR spectroscopy, subdural hemorrhage (SDH), dural sinovenous thrombosis (DSVT), intraventricular hemorrhage (IVH), and congenital malformations. Abnormalities in each category were scored 0 for none, 1 for mild, 2 for moderate, 3 for severe. The score in each category was multiplied by a proposed outcome significance multiplier: 3 for WMI, infarction or IP hemorrhage, 2 for punctate lesions or lactate, and 1 for SDH, DVST, IVH, or congenital malformations. A total injury score of 0 signified no injury, a score of 1–5 a mild injury, 6–10 a moderate injury, and >10 a severe injury. Developmental maturity of the brain was assessed from T1 and T2-weighted images using the scoring system of Childs et al, grading myelination, cortical infolding, involution of the germinal matrix, and presence of bands of migrating glial cells to produce a Total Maturity Score (TMS).15,21 (See Appendix III).
Appendix II.
MRI Abnormality Scoring System
| Category* | Subcategory | Score | Outcome Significance Multiplier | Definition | Size (total mm, largest diameter, add all lesions) | Rule/example | MR sequences |
|---|---|---|---|---|---|---|---|
| Focal acquired | WMI | 0 | 3 | none | 0 | ↑T1, ±↓Dav | T1, Dav |
| WMI | 1 | 3 | mild: <= 3, < 2mm | 1 to 5 mm | McQuillen et al Stroke 2007;113: 280; Fig 1E–F (ref 12) | ||
| WMI | 2 | 3 | moderate: >3, > 2mm | 6 to 15 mm | |||
| WMI | 3 | 3 | severe: 10% white matter | >15 mm | |||
| Infarction (stroke—ischemic) | 0 | 3 | none | 0 | ↑T2, ↓Dav (Includes watershed) | T2, Dav | |
| Infarction | 1 | 3 | < 1/3 of vascular territory of ACA, MCA, or PCA in one hemisphere | 1 to 5 mm | |||
| Infarction | 2 | 3 | 1/3–2/3 vasc territory | 6 to 15 mm | McQuillen et al Stroke 2007;113: 280; Fig 1A–D (ref 12) | ||
| Infarction | 3 | 3 | > 2/3 vasc territory | >15 mm | |||
| IP hemorrhage (stroke-- hemorrhagic) | 0 | 3 | 0 | ↑T1, ↓T2, ↓SWI | T1, T2, SWI | ||
| IP hemorrhage | 1 | 3 | 1 to 5 mm | ||||
| IP hemorrhage | 2 | 3 | 6 to 15 mm | ||||
| IP hemorrhage | 3 | 3 | >15 mm | ||||
| Punctate lesions | 0 | 2 | none | 0 | 0–2 mm discrete, isolated lesions; emboli from platelet/fibrin clot, air, other particulate material ↓T2, ↓SWI | T2, SWI | |
| Punctate lesions | 1 | 2 | 1–3 lesions | all ≤ 2 mm | |||
| Punctate lesions | 2 | 2 | 4–6 lesions | all ≤ 2 mm | |||
| Punctate lesions | 3 | 2 | >6 lesions | all ≤ 2 mm | |||
| ↑Lactate on MRS | 0 | 2 | None to Lac/Cr ratio of 0.15 | NA | |||
| ↑Lactate on MRS | 1 | 2 | Lac/Cr ratio of 0.16–0.5 | Lactate doublet peak at 1.33 PPM | Short TE MR spec, post-processing LC model | ||
| ↑Lactate on MRS | 2 | 2 | Lac/Cr 0.5–1 | ||||
| ↑Lactate on MRS | 3 | 2 | Lac/Cr >1 | ||||
| IVH | 0 | 1 | 0 | ↑T1, ↓T2, ↓SWI | T1, T2, SWI | ||
| IVH | 1 | 1 | subependymal/germinal matrix hemorrhage/choroid plexus hemorrhage | 1 to 5 mm | |||
| IVH | 2 | 1 | IVH-isolated | 6 to 15 mm | |||
| IVH | 3 | 1 | IVH with ventricular dilation | >15 mm | |||
| SDH | 0 | 1 | subdural blood above tentorium; minimal SDH below tentorium frequently 2° birth process and not considered abnormal | ↑T1, ↓T2, | T1, T2, SWI | ||
| SDH | 1 | 1 | minimal just above tentorium | ||||
| SDH | 2 | 1 | Spread to interhemispheric fissure in occipital area | ||||
| SDH | 3 | 1 | Larger hemorrhage; interhemispheric to parietal or frontal area; any mass effect | ||||
| DVST | 0 | 1 | Flow voids in dural venous sinuses, confirmed by MR venogram | 0 | T1, T2, venogram | ||
| DVST | 1 | 1 | R or L transverse alone | ||||
| DVST | 2 | 1 | Bilateral R and L | ||||
| DVST | 3 | 1 | Straight and/or Sagittal sinus | ||||
| Congenital Malformation | None | 0 | 1 | Absent CC, Dandy-Walker, Venous malformation, cortical malformation | Semiobjective severity | T1, T2 | |
| 1 | 1 | ||||||
| 2 | 1 | ||||||
| 3 | 1 | ||||||
| Developmental | See Total Maturity Score | Not included in total injury score. | T1, T2 | ||||
| Total Injury Score | All categories | None | Total score 0 | Multiply score in each of 9 categories by outcome significance mulitplier | T1, T2, DWI, Dav, SWI, MR spec | ||
| Mild | Total Score 1–5 | Sum all 9 subscores for total score | |||||
| Moderate | Total Score 6–10 | Range of scores: 0 to 51. | |||||
| Severe | Total Score >10 |
Abbreviations: WMI, white matter injury; DWI, diffusion-weighted imaging; Dav, averaged diffusion coefficient maps; ACA, anterior cerebral artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; SWI, susceptibility-weighted imaging; IP, intraparenchymal; MRS, magnetic resonance spectroscopy; IVH, intraventricular hemorrhage; SDH, subdural hemorrhage; DVST, dural sinovenous thrombosis; CC, corpus callosum; Lac, lactate; Cr, creatine
Appendix III.
MRI Brain Development/Maturity Scoring System
| Category* | Designation/Score | Definition |
|---|---|---|
| Myelination(M) | M1 | Myelination evident in the brainstem, cerebellar peduncle, inferior colliculus, cerebellar vermis |
| M2 | M1 + Subthalamic nucleus, globus pallidus, ventrolateral thalamus | |
| M3 | M2 + Caudal portion of the Posterior Limb of the Internal Capsule (PLIC) | |
| M4 | M3 + Complete PLIC | |
| M5 | M4 + optic radiation | |
| M6 | M5 + Corona radiata | |
| M7 | M6 + anterior limb of internal capsule | |
| Cortical Infolding [C] | C1 | Frontal and occipital cortex completely smooth, insula wide open; thin bright cortical rim on T1, generally low-intensity white matter (WM) on T1 |
| C2 | Frontal cortex still very smooth, some sulci evident in occipital cortex; insula still wide with almost smooth internal surface; WM low intensity on T1 | |
| C3 | Frontal and occipital cortex similar number of convolutions; frontal sulci still quite shallow; internal surface of insula more convoluted; WM still somewhat low intensity on T1 | |
| C4 | Frontal and occipital ctex folded and rich in sulci; frontal sulci obvious along interhemispheric fissure; occipital WM separated into strands by deeper sulci; insula completely infolded; WM still distinguishable from gray matter on T1 | |
| C5 | Frontal and occipital WM separated into strands by deeper sulci; insula completely infolded; WM still distinguishable from gray matter on T1 | |
| C6 | As above but WM now isointense with gray matter on T1 | |
| Germinal Matrix (GM) | GM1 | Matrix seen in posterior horn, caudothalamic notch (CTN) and anterior horns of the lateral ventricles |
| GM2 | Matrix seen at CTN and anterior horns of the lateral ventricles | |
| GM3 | Matrix seen in the anterior horns only | |
| GM4 | No Germinal matrix evident | |
| Bands of Migrating Glial Cells (B) | B1 | Broad band with additional narrower bands evident |
| B2 | Broad band alone | |
| B3 | Narrow band alone | |
| B4 | No bands seen |
From Childs AM AJNR 2001;22:1577 (ref 19)
Statistical Analysis
The primary outcome variable was the incidence of new intraparenchymal lesions, including white matter injury, infarction, or intraparenchymal hemorrhage on postoperative brain MRI. Using chi-square analysis with Yates correction, a sample size of 57 patients was needed to detect a 40% incidence of new postoperative lesions with a power of 0.90 and α-level of 0.05. This assumed a baseline incidence of WMI, infarction, or intraparenchymal hemorrhage of 10% and a postoperative incidence of 50%, based on previous studies5,6,14. For detailed analysis, patients were divided into single ventricle (SV), and two ventricle (2V) groups. Data were analyzed using paired T test or Mann Whitney Rank Sum Test, or Fisher Exact Test or χ-square analysis as appropriate. (SPSS Statistics 14.0, SPSS Corporation, Chicago IL).
The following preoperative variables were analyzed for association with preoperative brain injury using bivariate non-parametric Spearman correlation: SV vs. 2V, birth weight, gestational age, Apgar scores, balloon atrial septostomy, lowest sustained (≥10 minutes total in 24 hours) preoperative SpO2, preoperative minutes of rSO2 <45%, and brain TMS. The following intra- and postoperative variables were analyzed for association with postoperative brain injury: SV vs. 2V, gestational age, CPB time, aortic crossclamp time, ACP time, DHCA time, total minutes of rSO2 <45%, mean rSO2 intraoperatively, and on postoperative day 1,2, and 3; lowest sustained postoperative systolic and diastolic blood pressure and SpO2 (≥30 minutes total in 72 hours); presence of a preoperative brain injury, and brain TMS (on preoperative MRI). In addition, gestational age, birth weight, TMS, brain injury on postoperative MRI, lowest sustained systolic and diastolic blood pressure, and total time at rSO2 <45% were tested for association with late death. All analyses of physiologic and cerebral oximeter parameters were performed using data stored digitally in Excel (Microsoft Corp., Redmond WA) spreadsheet files and transferred into appropriate statistical applications.
Linear regression analysis by block entry model determined association of clinical variables with preoperative and postoperative brain injury, and late death, which were found on bivariate Spearman analysis to have R > 0.2, after screening for multicollinearity. Final model multivariable analysis clinical variables with P<0.05 were considered to be significantly associated with brain injury. A B value with 95% confidence intervals, and percentage variance for explanation of the dependent variable with the final model are also reported.
Results
Sixty-Eight patients were enrolled in the study from November 2005 until August 2008. Data from the pre-, intra-, and postoperative periods are presented in Tables 1 and 2. The two ventricle patients had a higher birthweight, lower 1 minute Apgar scores, lower preoperative SpO2, and more patients undergoing balloon atrial septostomy. Significant differences in bypass management included fewer patients receiving antegrade cerebral perfusion (ACP) in the two ventricle group. Postoperatively, two-ventricle patients had almost no rSO2 <45%, versus a significant incidence and duration of low rSO2 in the single ventricle patients. Sixty-six of 68 patients were 30-day inhospital survivors (97.1%). One SV patient required postoperative extracorporeal membrane oxygenation and died 8 days postoperatively. Another SV patient died of sudden cardiorespiratory arrest in hospital 19 days postoperatively after apparent excellent full surgical recovery. There were 6 additional late deaths, 5 SV patients who died before their second stage surgery between 2 and 6 months of age, of cardiorespiratory arrest suffered in hospital after readmission (two) or at an outside hospital (three), and one SV patient who died in hospital after bidirectional cavopulmonary connection surgery of P. aeruginosa septic shock.
Table 1.
Preoperative Patient Data
| Parameter | Single Ventricle, n=36 | Two Ventricle, n=32 | P value |
|---|---|---|---|
| Anatomic Diagnosis: | |||
| HLHS | 35 | NA | NA |
| TGA, DILV | 1 | NA | NA |
| TGA | NA | 21 | NA |
| Truncus arteriosus | NA | 5 | NA |
| IAA, VSD | NA | 6 | NA |
| Birthweight (kg) | 3.01 ± 0.33 | 3.27 ± 0.54 | 0.02* |
| Gestational age (weeks) | 38.3 ± 1.2 | 38.8 ± 1.3 | 0.10 |
| Head circumference (cm.) | 33.3 ± 1.6 | 33.8± 1.3 | 0.11 |
| Prenatal diagnosis, no., (%) | 17 (47) | 13 (44) | 0.97 |
| Cesarean delivery, no., (%) | 13(36) | 13 (41) | 0.90 |
| 1 minute Apgar score | 7.9 ± 1.3 | 7.2 ± 1.5 | 0.05* |
| 5 minute Apgar score | 8.8 ± 0.5 | 8.3 ± 1.0 | 0.01* |
| Balloon atrial septostomy, no., (%) | 1 (3) | 18 (56) | <0.001* |
| Lowest SpO2 (sustained 10 min) | 88 ± 4 | 81 ± 10 | <0.001* |
| Lowest rSO2 (sustained 10 min) | 53 ± 8 | 51 ± 12 | 0.25 |
| Preoperative rSO2 minutes <45% | 28 ± 69 | 78 ± 207 | 0.18 |
| Preoperative rSO2 minutes ≥45% | 918 ± 347 | 783 ± 272 | 0.09 |
Data expressed as means ± SD or median (25th–75th percentile interquartile range);
p<0.05 by T-test. HLHS, hypoplastic left heart syndrome; TGA, transposition great arteries; DILV, double inlet left ventricle; IAA, interrupted aortic arch; VSD, ventricular septal defect; SpO2; pulse oximetry saturation; rSO2, regional brain oxygen saturation.
Table 2.
Operative and Postoperative Patient Data
| Parameter | Single Ventricle, n=36 | Two Ventricle, n=32 | P value |
|---|---|---|---|
| Age (days) | 7 (5–10.5) | 8 (5.5–13.5) | 0.33 |
| Weight (kg) | 3.02 ± 0.36 | 3.30 ± 0.52 | 0.011* |
| Operation: | |||
| Norwood Stage I Palliation | 35 | NA | |
| ASO, AAA, PAB | 1 | NA | |
| ASO | 0 | 21 | NA |
| Truncus arteriosus repair | 0 | 5 | NA |
| AAA, VSD repair | 0 | 6 | NA |
| Preoperative mechanical ventilation (no., %) | 7 (19) | 6(19) | 0.81 |
| CPB time (min) | 216 ± 58 | 220 ± 68 | 0.77 |
| AoXcl time (min) | 103 ± 36 | 128 ± 44 | 0.01* |
| Lowest temperature (°C) | 17.5 ± 0.7 | 23.5 ± 3.6 | <0.001* |
| Hematocrit during hypothermia (%) | 30.2 ± 2.2 | 31.2 ± 1.7 | 0.07 |
| DHCA time (min) | 13 ± 8 (30 pts) | 11 ± 5 (4 pts) | 0.60 |
| ACP time (min) | 76 ± 20 (36 pts) | 27 ± 17 (7 pts) | <0.001* |
| ACP flow rates (ml/kg/min) | 56 ± 10 (36 pts) | 63 ± 13 (7 pts) | 0.08 |
| Intraoperative rSO2 minutes <45% | 45 ± 49 | 15 ± 31 | 0.004 |
| Intraoperative rSO2 minutes ≥45% | 410 ± 105 | 396 ± 117 | 0.60 |
| Postoperative rSO2 minutes <45% | 287 ± 408 | 1 ± 3 | <0.001 |
| Postoperative rSO2 minutes ≥45% | 3779 ± 582 | 4136 ± 326 | 0.003 |
Data expressed as means ± SD or median (25th–75th percentile interquartile range);
p<0.05 by T-test. SV, single ventricle; 2V, two ventricle; ASO, arterial switch operation; AAA, aortic arch advancement; PAB, pulmonary artery banding; CPB, cardiopulmonary bypass; AoXcl, aortic crossclamp time; DHCA, deep hypothermic circulatory arrest; ACP, antegrade cerebral perfusion; rSO2, regional cerebral oxygen saturation.
MRI data from the pre- and 7-day postoperative scans are presented in Table 3. Sixty eight patients had a first MRI, and 67 patients had the second MRI, 56 (84%) at 7–10 days postoperatively. For the primary outcome variable, there was a 36% incidence of new WMI, infarction, or hemorrhage, with 45% of SV patients having these new findings, vs. 25% of 2V patients (p=0.13). For other important outcome variables, there was a 15% incidence of new postoperative WMI (23% SV vs. 6% 2V, p=0.09). 6 patients had resolution of mild WMI between the pre- and postoperative scans. The severity of postoperative brain injury, estimated by our severity of injury score, was 3.5 ± 3.9, and was not different between SV and 2V groups; however, this represented a significant increase from a preoperative injury score of 2.0± 2.7 for the entire cohort. (p=0.012). Figures 1 and 2 display representative patients’ MRI scans.
Table 3.
Standard MRI Data, Pre- and 7-Day Postoperative MRI Scans
| Parameter | Single Ventricle, n=36 | Two Ventricle, n=32 | P value, SV vs. 2V | Total |
|---|---|---|---|---|
| MRI postoperative day | 7 (7–9) | 7 (7–9) | 1 | 7 (7–9) |
| Preoperative WMI, infarct, or IP hemorrhage, no., (%) | 7 (19) | 4 (13) | 0.83 | 11 (28) |
| Preoperative WMI, no., (%) | 5(13) | 6 (19) | 0.83 | 11 (16) |
| Preoperative infarct, no., (%) | 5 (14) | 7 (22) | 0.59 | 12 (18) |
| Preoperative IP hemorrhage, no., (%) | 0 | 0 | 1 | 0 |
| Preoperative punctate lesions, no., (%) | 3 (8) | 1 (3) | 0.62 | 4 (6) |
| Preoperative MRS elevated lactate, no., (%) | 2 (6) | 2 (6) | 1 | 4 (6) |
| Preoperative SDH, no., (%) | 11 (31) | 3 (9) | 0.05* | 14 (21) |
| Preoperative DVST no., (%) | 1 (3) | 1 (3) | 1 | 2 (3) |
| Preoperative IVH no., (%) | 0 | 2 (6) | 0.22 | 2 (3) |
| Any preoperative finding, no., (%) | 19 (53) | 19 (59) | 0.76 | 38 (57) |
| 7-day postoperative WMI, infarction, or IP hemorrhage, no., (%) | 22 (63) | 14 (44) | 0.19 | 36 (54) |
| 7-day postoperative WMI, no., (%) | 13(37)† | 2 (6) | 0.006* | 15 (22) |
| 7-day postoperative infarct, no., (%) | 11 (31) | 11 (34) | 1 | 22 (33) |
| 7-day postoperative IP hemorrhage, no., (%) | 3 (9) | 2 (6) | 1 | 5 (8) |
| 7-day postoperative punctate lesions, no., (%) | 6 (17) | 10 (31)† | 0.29 | 16 (24)‡ |
| 7-day postoperative MRS elevated lactate, no., (%) | 0 | 0 | 1 | 0 |
| 7-day postoperative SDH, no., (%) | 3 (8)† | 7 (22) | 0.18 | 10 (15) |
| 7-day postoperative DVST, no., (%) | 3 (9) | 4 (13) | 0.70 | 7 (11) |
| 7-day postoperative IVH, no., (%) | 1 (3) | 3 (9) | 0.34 | 4 (6) |
| Any 7-day postoperative finding, no., (%) | 23 (66) | 20(63) | 0.99 | 43 (65) |
| New 7-day postoperative WMI, infarct, or IP hemorrhage, no., (%) | 16 (45) | 8 (25) | 0.13 | 24 (36) |
| New 7-day postoperative WMI | 8 (23) | 2 (6) | 0.09 | 10 (15) |
| Any new 7-day postoperative finding, no., (%) | 18 (51) | 20 (63) | 0.51 | 38 (58) |
| Any pre- or 7-day postoperative finding, no., (%) | 26 (72) | 25 (78) | 0.78 | 51 (76) |
| Congenital malformations, no., (%) | 0 | 2 (6) | 0.22 | 2 (3) |
| Preoperative MRI Total Maturity Score (TMS) | 11.8±1.5 | 11.8±1.0 | 0.93 | 11.8±1.3 |
| Patients with TMS ≤ 10, no., (%) | 8 (22) | 6(19) | 0.96 | 14 (21) |
| Preoperative total MRI abnormality score | 1.8±2.6 | 2.3±2.8 | 0.47 | 2.0± 2.7 |
| 7-day postoperative total MRI abnormality score | 3.8±4.2† | 3.2±3.7 | 0.57 | 3.5±3.9‡ |
| Pre/postop change in total MRI abnormality score | 1.9 ±3.2 | 0.9±3.2 | 0.22 | 1.5± 3.2 |
Data expressed as numbers of patients and percentages for MRI findings, and median days (25th–75th percentile interquartile range) for MRI days postoperatively; or means ± SD for numerical scores. P values are between groups.
p≤0.05 for between group differences.
p≤0.05 for pre- to postoperative difference within groups.
p≤0.05 for pre- to postoperative difference for both groups combined. WMI, white matter injury; IP, intraparenchymal; MRS, magnetic resonance spectroscopy; SDH, subdural hemorrhage; DVST, dural sinovenous thrombosis; IVH, intraventricular hemorrhage.
Figure 1.
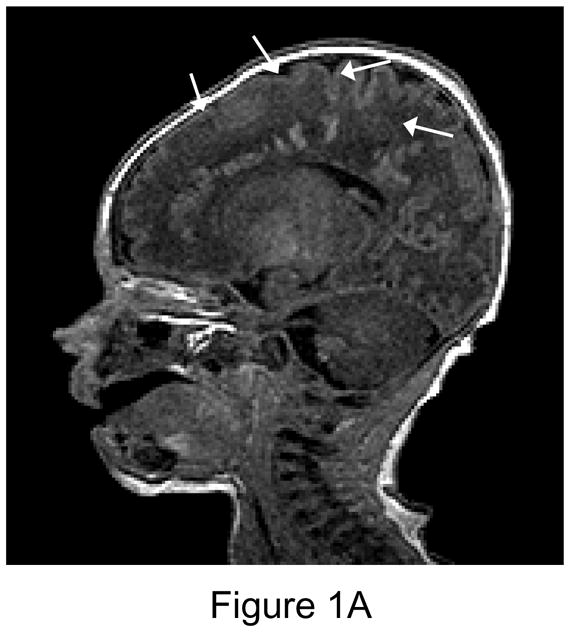
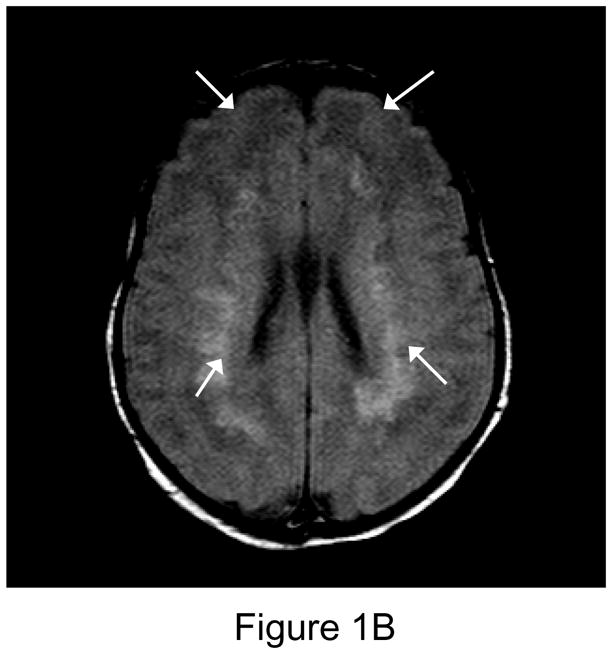
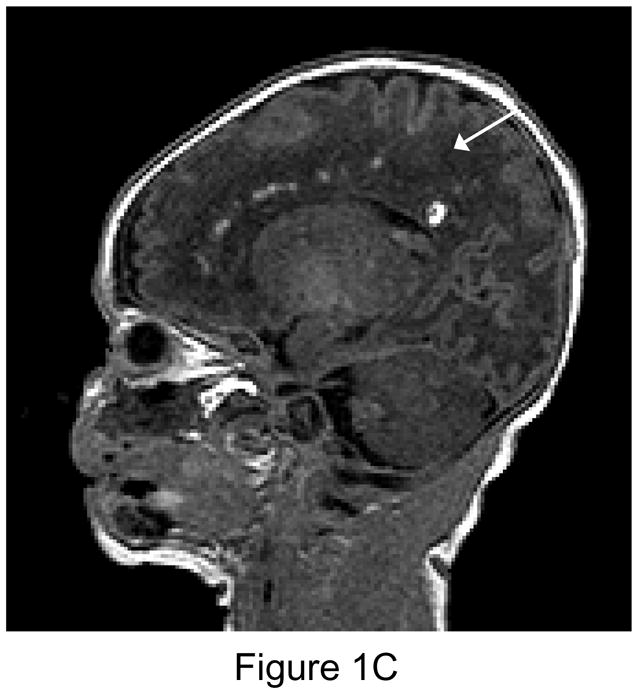
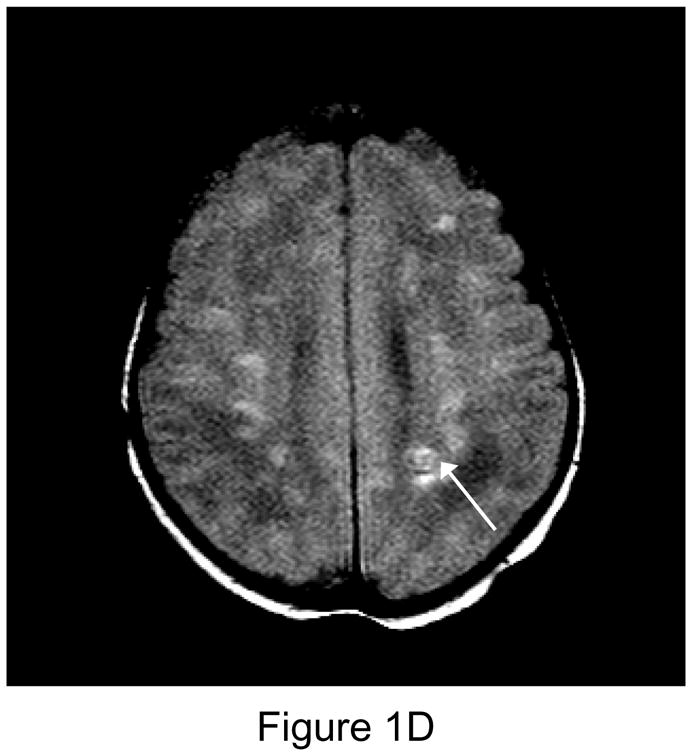
1A. Preoperative sagittal T1-weighted MR image of a 35 week gestational age infant with hypoplastic left heart syndrome. Extensive white matter injury (WMI) is present in the periventricular areas. (arrows). 1B. Preoperative axial proton-density T2 weighted image. Again note extensive WMI (arrows). 1C. 7-day postoperative T1 sagittal MRI after Norwood Stage I palliation. Note new intraparenchymal/intraventricular hemorrhage and infarction in the left paritrigonal region (arrow). 1D. Proton density T2-weighted image. Again note WMI and new hemorrhage (arrow). This patient had the single highest injury score on both preoperative, and postoperative MRI injury scale, at 11 points preoperatively, and 21 points postoperatively. (Refer to MRI Scoring Table in Appendix).
Figure 2.
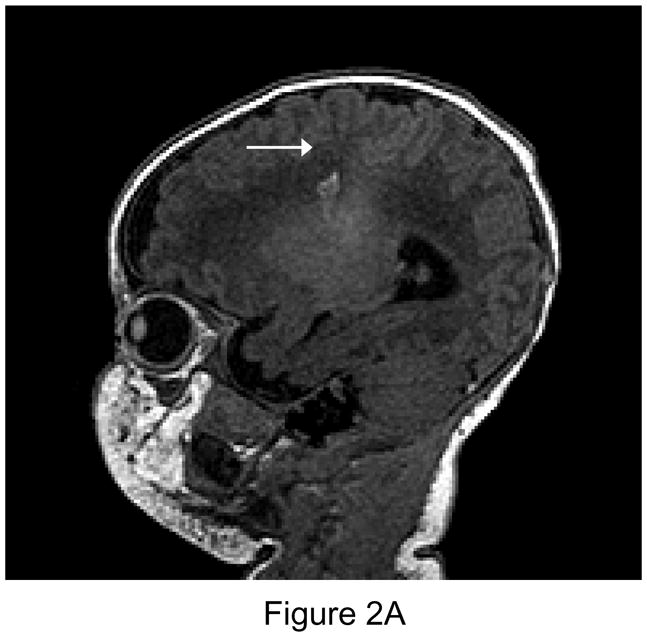
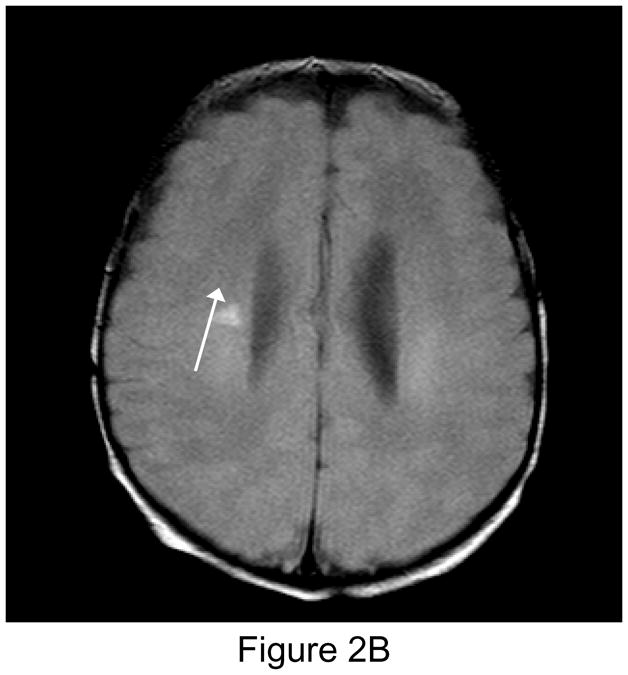
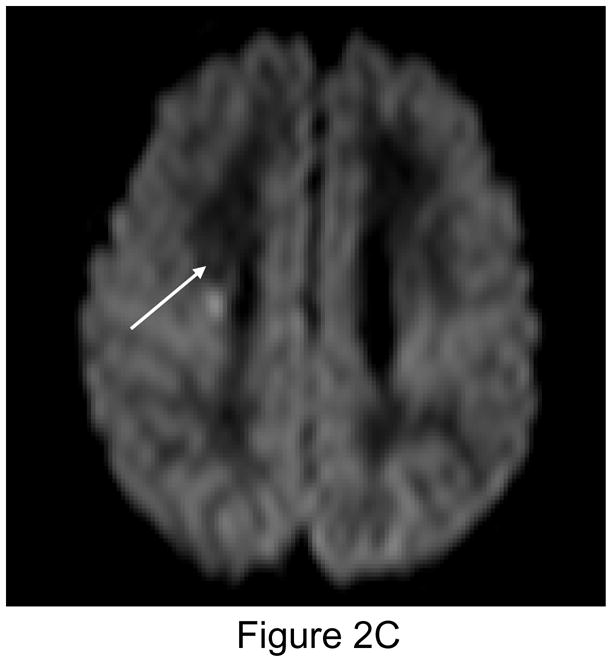
Figure 2A (left): New postoperative infarction (white arrow) in a patient after Norwood Stage I palliation for hypoplastic left heart syndrome, on T1 weighted sagittal imaging in the right posterior fronto-parietal deep white matter, and extending to the lentiform nucleus, measuring 5 × 7 mm. Figure 2B (center): T2 weighted image. Figure 2C (right): Matching area of restricted diffusion (white arrow).
Secondary outcome variables included preoperative WMI, infarction, or hemorrhage, seen in 28% of patients (28% in both SV and 2V groups). Preoperative WMI was observed in 16% (13% SV vs. 19% 2V, p=0.83). Late death was observed in 7/35 SV patients, vs. none of 32 2V patients, (p=0.01).
The most important independent variable tested was the preoperative TMS, according to our study hypothesis. The preoperative TMS was 11.8 ± 1.5 in the SV group, and 11.8 ± 1.0 in the 2V group. After multivariable analysis, low TMS (increasing brain immaturity) was associated with preoperative WMI (p=0.002), and also postoperative late death (p=0.008). Low brain maturity rated using the TMS score was associated with increased severity of postoperative brain injury, with 7 of 13 patients with TMS ≤ 10 (equal to brain maturity of 35 weeks gestational age or less21) having a postoperative brain injury score of 6 or above (moderate injury), vs. only 10 of 54 with TMS >10. (p=0.01, χ-square). In addition, preoperative WMI was more likely with TMS <10 (5/13 vs. 6/55, p=0.03, χ-square), as was postoperative WMI (6/13 vs. 9/54, p=0.05, χ-square).
We considered sustained cerebral desaturation (total minutes of rSO2 <45% in the intra- and postoperative periods) an important independent variable, but did not find an association between this parameter and any measure of postoperative MRI brain injury. Eighteen of 35 SV patients had greater than 240 minutes of rSO2 <45% in the intra- and postoperative periods; 11 of these 18 had new postoperative MRI lesions, vs. 8 of 17 who did not have prolonged low rSO2. (p=0.62, χ-square). No 2V patient had more than 120 minutes of rSO2 <45% in the intra-and postoperative periods; 17 of 32 had no rSO2 minutes <45%.
SV diagnosis was another important independent variable, and was associated with the presence of postoperative WMI (p=0.02), and also with late death (p=0.04). Of all other independent variables tested, there was an association of new postoperative WMI/infarction/hemorrhage with low sustained diastolic blood pressure (p=0.039). The initial Spearman bivariate analyses, and complete final linear regression models for preoperative MRI injury, presence of postoperative MRI injury, and new postoperative MRI injury, are reported in Tables 4–6.
Table 4.
Non-Parametric Spearman Bivariate and Multivariate Linear Regression Correlations With Brain Injury: Preoperative Brain Injury
| Independent Variable | Bivariate Dependent Variable R Value: WMI | Multivariate B value (95% C.I.), p value | Bivariate Dependent Variable R Value: WMI/hemorrhage/infarction | Multivariate B value (95% C.I.), p value |
|---|---|---|---|---|
| SV/2V | 0.055 | -- | −0.005 | -- |
| GA | −0.165 | -- | −0.164 | -- |
| BW | −0.055 | -- | −0.075 | -- |
| 1″ apgar | −0.024 | -- | 0.042 | -- |
| 5″ apgar | −0.061 | -- | 0.104 | -- |
| BAS Y/N | 0.070 | -- | 0.045 | -- |
| Lowest SpO2 sustained 10″ | −0.209* | −0.011 (−0.028 to 0.007), p=0.224 | −0.082 | -- |
| Min rSO2<45% | −0.112 | -- | −0.197 | -- |
| TMS | −0.232* | −0.176 (−0.284 to − 0.068), - p=0.002 | −0.147 | -- |
| Percentage of variance explained by the final block entry linear regression model | -- | 12.6% | -- | NA |
Spearman Correlation test;
= entered into linear regression analysis by block entry method.
Table 6.
Non-Parametric Spearman Bivariate and Multivariate Linear Regression Correlations With Brain Injury: NEW Postoperative Brain Injury
| Independent Variable | Bivariate Dependent Variable R Value: NEW WMI | Multivariate B value (95% C.I.), p value For NEW WMI | Bivariate Dependent Variable R Value: NEW WMI/hemorrhage/infarction | Multivariate B value (95% C.I.), p value For NEW WMI/hemorrhage/infarction |
|---|---|---|---|---|
| SV/2V | −0.182 | -- | 0.112 | -- |
| GA | −0.105 | -- | −0.110 | -- |
| TMS | 0.034 | -- | 0.029 | -- |
| Preop MRI WMI | 0.094 | -- | 0.297* | 0.256 (0.066 to 0.447), p=0.009 |
| Preop MRI WMI/hem/inf | −0.100 | -- | 0.082 | -- |
| CPB time | 0.042 | -- | 0.194 | -- |
| Aoxcl time | −0.162 | -- | 0.016 | -- |
| ACP time | 0.085 | -- | −0.170 | -- |
| DHCA time | 0.120 | -- | 0.012 | -- |
| Postop low SPB sustained 30″ | −0.097 | -- | −0.083 | -- |
| Postop low DBP sustained 30″ | 0.109 | -- | 0.226* | 0.034 (0.002 to 0.067), p=0.039 |
| Intra/postop rSO2<45% total minutes | 0.052 | -- | 0.086 | -- |
| Postop Low SpO2 sustained 30″ | −0.119 | -- | 0.189 | -- |
| Percentage of variance explained by the final block entry linear regression model | -- | -- | -- | 14.7% |
Spearman Correlation test;
= entered into linear regression analysis by block entry method.
The third MRI was completed on 45 patients; Appendix IV reports the MRI findings. New findings were seen in 27% of patients, and a total of 29% had any abnormality detected. 58% of patients had partial or complete resolution of findings from the first or second MRI.
Appendix IV.
MRI Data, 3rd MRI Scan at Age 3–6 Months
| Parameter | Single Ventricle, n=24 | Two Ventricle, n=21 | P value | Total |
|---|---|---|---|---|
| Age at 3rd MRI, days | 150 ± 50 | 189 ± 71 | 0.04* | 169 ± 63 |
| MRI sedation complications, no., (%) | 0 | 0 | 1 | 0 |
| WMI, no., (%) | 4 (17) | 1 (5) | 0.35 | 5 (11) |
| Infarct, no., (%) | 3 (13) | 1 (5) | 0.61 | 4 (9) |
| IP hemorrhage, no., (%) | 1 (4) | 1(5) | 1 | 2 (4) |
| Punctate lesions, no., (%) | 3(13) | 4 (19) | 0.69 | 7 (16) |
| MRS lactate, no., (%) | 1 (4) | 0 | 1 | 1(2) |
| SDH, no., (%) | 2 (8) | 0 | 0.49 | 2 (5) |
| DVST no., (%) | 1 (4) | 0 | 1 | 1 (2) |
| IVH, no., (%) | 1 (4) | 0 | 1 | 1 (2) |
| Any finding, no., (%) | 8 (33) | 5 (24) | 0.71 | 13 (29) |
| New finding, no., (%) | 8 (33) | 4(19) | 0.46 | 12 (27) |
| Complete resolution of findings, no., (%) | 5(21) | 12 (57) | 0.03* | 17 (38) |
| Partial resolution of findings, no., (%) | 6 (25) | 3 (14) | 0.47 | 9 (20) |
| Never abnormal findings | 5 (21) | 2(10) | 0.42 | 7 (16) |
| MRI Total Maturity Score (TMS) | 17.5 ± 2.0 | 19.7 ± 1.3 | <0.001* | 18.5 ± 2.0 |
| Total MRI abnormality score | 2.3 ± 3.1 | 0.8 ± 1.7 | 0.05* | 1.6± 2.7 |
Data expressed as numbers of patients and percentages for MRI findings, and median days (25th–75th percentile interquartile range) for MRI days postoperatively.
p < 0.05 for between group differences.
p<0.05 for pre- to postoperative difference within groups. WMI, white matter injury; IP, intraparenchymal; MRS, magnetic resonance spectroscopy; SDH, subdural hemorrhage; DVST, dural sinovenous thrombosis; IVH, intraventricular hemorrhage.
Discussion
The major new finding in this study is that brain immaturity in neonates with CHD is associated with both pre- and postoperative brain injury, particularly WMI. Our patients exhibited an increased incidence of brain immaturity by TMS score. The mean TMS for both groups was 11.8 (equal to 38 weeks’ gestational age for brain maturation), and the number of patients with TMS ≤ 10 (equal to brain maturity of 35 weeks gestational age or less21) was not different between groups. However, 14 of 68 patients (21%) had TMS ≤ 10, but only 4 of 68 (6%) had gestational age less than 36 weeks. In a normal population these proportions should be similar, but are significantly different in this CHD population (p=0.023, χ-square).
Brain injury on MRI from brain immaturity is also found among premature infants, in whom non-cystic WMI on MRI is the primary brain injury in the perinatal and early neonatal period.17 The WMI in prematures is manifest by focal lesions, white matter volume loss, thinning corpus callosum, and diffuse, excessive signal intensity.16 Immature oligodendrocyte white matter precursors in the rapidly developing premature neonatal brain are vulnerable to injury from hypoxia-ischemia.18,19 The peak numbers of these immature oligodendrocytes is at 23 to 32 weeks, which corresponds to the peak period of WMI in premature infants.22 Patients with CHD and immature brains could thus have their period of peak vulnerability extended to later than 32 weeks, into the period when neonatal cardiac surgery with bypass is commonly performed. Inflammation associated with pre- or postnatal infections also damages white matter in premature infants;18 it is possible that the inflammatory response from cardiopulmonary bypass contributes to the WMI in CHD patients.23
The neurodevelopmental delays in both CHD and in premature infants are qualitatively similar, and include attention deficit disorder, problems with language and fine motor function and visual-motor integration.2 These problems may be explained by a common mechanism of brain injury, namely WMI. Galli et al also found that WMI is frequent in surgical neonates with CHD, observing a 54% incidence of WMI on postoperative brain MRI, vs. only 6% in infants aged 1–6 months at the time of surgery.24 In another important finding from our study, brain immaturity was associated with late death in our single ventricle cohort, suggesting that this factor could also play a role in the pathophysiology of these patients’ demise.
We found a 36% incidence of new acute postoperative MRI changes consisting of WMI, infarction, and intraparenchymal hemorrhage; 15% of patients had new WMI. In previous studies, Mahle et al6 reported 24 neonates receiving pre- and postoperative brain MRI after cardiac surgery; 67% of patients had new postoperative brain lesions, including 42% with new WMI. Eighty eight percent of these patients were exposed to DHCA, and low-flow CPB was used in the remainder, with a median DHCA time of 50 minutes. Dent et al5 studied 15 neonates undergoing Norwood stage I palliation with ACP, and 11 patients (73%) had new lesions, including 47% with WMI, on postoperative MRI. Compared to patients in the current study, whose mean ACP flow rate is 57 ml/kg/min, the ACP flow rate in Dent’s study was less than half. In 53 cardiac surgical neonates with pre- and postoperative MRI, McQuillen et al14 also found that 36% of their 53 patients had some form of new postoperative brain injury; their patients had a 26% incidence of new WMI on postoperative MRI. DHCA use was limited in this cohort, and they found low-flow ACP was associated with new postoperative WMI.
We did not find an association with prolonged low brain rSO2 <45%, and postoperative brain injury, or mean rSO2 in the intraoperative or postoperative periods in this patient cohort, differing from previous studies.5,14 This was unexpected, and the potential explanations include the possibility that the patients who remained at low rSO2 values may have had other protective factors, i.e. hypoxic preconditioning. In addition, the rSO2 threshold for brain injury may have been lower than 45% in our patients; we did not test for lower thresholds, nor did we assess rSO2 area under the curve; these more sophisticated analyses will be the subject of a future publication.
Using our full MRI injury scale, we found a high overall incidence of patients with any abnormality on MRI scans (56% preoperative, 63% postoperative, 75% overall). This high incidence is due to the fact that we devised a comprehensive scoring system categorizing all detected abnormalities, even minor findings not included by other investigators.5,6,14,24 Twenty-five of 43 (58%) patients with postoperative findings had a mild injury (brain injury score <6), 15 (35%) had a moderate injury (brain injury score 6–10), and only 3 (7%) a severe injury (brain injury score>10).
Studies of preterm and term infants without CHD have demonstrated that hypoxic-ischemic changes on MRI such as WMI are predictive of long term neurodevelopmental abnormalities,25 but to date no studies have established the association between early brain MRI changes and later abnormal neurodevelopmental outcomes in the neonatal cardiac population. There is an urgent need to determine if there is such an association in the CHD population, to determine whether MRI will be an acceptable surrogate, in order to quickly determine the effects of any interventions to improve neurodevelopmental status.26 Our own data, and one other study report that many changes resolve by 3–6 months,24 suggesting the possibility that neonatal brain plasticity may allow remodeling after early insults.
This study has several important limitations. Although the Childs Total Maturity Score is a validated scale to assess structural brain maturity in infants with a gestational age of 23 to 41 weeks, we did not have our own concurrent normal control population studied on the same MRI scanner, with TMS assigned by the same neuroradiologist. Although our patients’ incidence and severity of new postoperative brain injury may be lower than in previous reports, judgments about the superiority of our bypass and neuromonitoring techniques cannot be made from this study because we did not have a control group utilizing alternate methods, i.e. a primary DHCA strategy with no neurological monitoring. Additionally, we do not yet have sufficient long term outcome data, so that our proposed brain injury scoring system has not yet been validated. The association between brain immaturity and late death in the single ventricle cohort is interesting but the number of patients is too small to make any definite conclusions. Finally, our final linear regression models account for only 9–30% of the factors associated with brain injury, meaning that we may not be measuring a number of other possible associated factors. These could include factors such as genotypic variation, inflammatory response, and any effect from hypoxic-ischemic preconditioning in these patients.
Many neonates undergoing cardiac surgery have congenital and acquired conditions that contribute to neurological disability and cannot be altered. In contrast, management strategies can be modified to limit perioperative insults.27 The important new finding of an association between MRI evidence of brain immaturity, with vulnerability to pre- and postoperative injury as well as late postoperative death, could have implications for management of these patients with regard to timing of delivery, or surgery with bypass. Our finding that the immature brain in neonatal CHD is more vulnerable to MRI injury needs to be confirmed in other patient cohorts, and if validated could potentially be the basis for controlled trials of alternative approaches to improve early and late neurodevelopmental outcomes in neonates with CHD.
Table 5.
Non-Parametric Spearman Bivariate and Multivariate Linear Regression Correlations With Brain Injury: Presence of Postoperative Brain Injury
| Independent Variable | Bivariate Dependent Variable R Value: presence of postoperative WMI | Multivariate B value (95% C.I.), p value: presence of postoperative WMI | Bivariate Dependent Variable R Value: presence of postoperative WMI/hemorrhage/infarction | Multivariate B value (95% C.I.), p value: presence of postoperative WMI/hemorrhage/infarction |
|---|---|---|---|---|
| SV/2V | −0.328* | −0.458 (−0.749 to −0.168), p=0.002 | −0.191 | -- |
| GA | −0.095 | -- | −0.102 | -- |
| TMS | −0.097 | -- | −0.095 | -- |
| Preop MRI WMI | 0.412* | 0.556 (0.314 to 0.797), p<0.001 | NA | NA |
| Preop MRI WMI/hem/inf | NA | NA | 0.252* | 0.275 (0.015 to 0.535), p=0.039 |
| CPB time | 0.085 | -- | 0.065 | -- |
| Aoxcl time | −0.297† | -- | −0.190 | -- |
| ACP time | 0.230† | -- | 0.126 | -- |
| DHCA time | 0.215† | -- | 0.082 | -- |
| Postop low SPB sustained 30″ | −0.120 | -- | 0.005 | -- |
| Postop low DBP sustained 30″ | −0.019 | -- | 0.190 | -- |
| Intra/postop rSO2<45% total minutes | 0.133 | -- | 0.191 | -- |
| Postop Low SpO2 sustained 30″ | −0.160 | -- | −0.140 | -- |
| Percentage of variance explained by the final block entry linear regression model | -- | 30.2% | -- | 8.6% |
Spearman Correlation test;
= entered into linear regression analysis by block entry method.;
=failed mulitcollinearity screening.
Acknowledgments
Funding Sources: Dr. Andropoulos is supported in part by NIH National Institute of Child Health and Development grant 1R21 HD055501-01, Baylor College of Medicine General Clinical Research Center Grant #0942, funded by NIH M01 RR00188, and Charles A. Dana Foundation Brain Imaging Grant. The remainder of the funding support was from Texas Children’s Hospital Anesthesiology Research Fund, and the Texas Children’s Hospital Center for Cardiac Outcomes Research.
The authors thank Jhiyue Wang, PhD, and Zili Chu, PhD, for assistance with designing the MRI sequences and producing the post-processing data for MR spectroscopy. They also thank Tracie Jeffers, R.R.T., and Ivone Rodriguez, C.R.R.T., for support with MRI studies; Marcie Garcia R.N., M.S.., Debora East, R.N., B.S.N., Karol Arrington, R.N., and Kathleen Carberry R.N,., for assistance with patient enrollment, data collection and database design and maintenance.
Appendix I: Details of MRI Sequences
MR imaging was performed using a 1.5T scanner (Intera: Philips Medical Systems, Best, the Netherlands). Images obtained included standard T1 and T2 weighted images, as well as diffusion weighted imaging, diffusion tensor imaging, and MR spectroscopy. Technical details of the sequences are listed in the following table:
| Image type | TR/TE (ms) | Flip angle(°) | FOV (mm) | Section Thickness/gap (mm) | Number of slices | Acquisition/Reconstruction matrix size | Other |
|---|---|---|---|---|---|---|---|
| 3D FFE T1-weighted sagittal | 20/4.1 | 30 | 200 | 1.0/0.0 | 100 | 224/256 | |
| 3D TSE T2-weighted sagittal | 3200/130 | 90 | 200 | 1.0/0/0 | 140 | 240/256 | |
| SE PD/T2 weighted axial | 2500/18, 120 | 90 (refocus angle 140) | 200 | 5.0/1.0 | 22 | 352/512 | |
| FFE axial | 610/23 | 15 | 200 | 5.0/1.0 | 20 | 256/256 | |
| TSE T2-weighted coronal FLAIR | 11000/140 (TI 2600) | 90 | 200 | 3.0/−1.0 | 53 | 256/256 | |
| 15 direction axial DTI | 10071/90 | 90 | 256 | 2.7/0.0 | 55 | 96/128 | b = 0, 860 (sec/mm2) |
| Axial DWI | 10000/81 (IR delay 2100) | 90 | 200 | 6.0/1.0 | 20 | 112/256 | b = 0, 1000 (sec/mm2) |
| 3D SWI axial | 56/40 | 20 | 256 | 2.0/0.0- | 32 | 512/512 |
Abbreviations: TR, relaxation time; TE, echo time; FFE, fast field echo; FLAIR, fluid attenuated inversion recovery; SWI, susceptibility-weighted imaging; FOV, field of view; DWI, diffusion weighted imaging; ADC, average diffusivity coefficient; 3D, three-dimensional; PD, proton density;
MR Spectroscopic Methods
Proton spectra were be collected using 2D chemical shift imaging (CSI) technique with the region of interest preselected by means of point-resolved spectroscopy (PRESS) through the plane of the basal ganglia. 4 regions of interest included sample volumes in the left and right basal ganglia, and left and right thalami. Short echo time (TR/TE 1500/31 ms) were be used. The FOV for the 2D CSI is 160 × 160 mm, with 16 × 16 phase encoding steps, yielding 10-mm in-plane resolution.
In each subject, a single-voxel water reference spectrum was also obtained in the gray matter at the center of the 2D CSI plane for signal intensity calibration (TR/TE/NSA=5000/31/4). This is based on the assumption that the water content of the gray matter is relatively constant (approximately 78%).
The first step in the data reduction for off line MR spectroscopy analysis is to import the localizer and 2D CSI data for each subject into a software package (Spectro Tool; Philips Medical Systems). A separate program written Interactive Data Language (IDL, Research Systems, Denver, CO) is used to convert the data in each region into a format for use in LCModel. Other corrections entered into the LCModel analysis included the relaxation effects of metabolites in the phantom and in normal brain tissue. Water scaling was done in the LCModel analysis by using the data from the water reference spectrum. The criterion for elevated lactate is the presence of elevated lactate doublet peak at 1.33 PPM, defined as lactate:creatine ratio >0.15, when signal to noise ratio is acceptable.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Welke KF, Shen I, Ungerleider RM. Current assessment of mortality rates in congenital cardiac surgery. Ann Thorac Surg. 2006;82:164–71. doi: 10.1016/j.athoracsur.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Wernovsky G, Shillingford AJ, Gaynor JW. Central nervous system outcomes in children with complex congenital heart disease. Curr Opin Cardiol. 2005;20:94–99. doi: 10.1097/01.hco.0000153451.68212.68. [DOI] [PubMed] [Google Scholar]
- 3.Creighton DE, Robertson CM, Sauve RS, Moddemann DM, Alton GY, Nettel-Aguirre A, et al. Neurocognitive, functional, and health outcomes at 5 years of age for children after complex cardiac surgery at 6 weeks of age or younger. Pediatrics. 2007;120:e478–86. doi: 10.1542/peds.2006-3250. [DOI] [PubMed] [Google Scholar]
- 4.Majnemer A, Limperopoulos C, Shevell M, Rosenblatt B, Rohlicek C, Tchervenkov C. Long-term neuromotor outcome at school entry of infants with congenital heart defects requiring open-heart surgery. J Pediatr. 2006;148:72–7. doi: 10.1016/j.jpeds.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 5.Dent CL, Spaeth JP, Jones BV, Schwartz SM, Glauser TA, Hallinan B, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2006;131:190–97. doi: 10.1016/j.jtcvs.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106(suppl I):I-109–I-114. [PubMed] [Google Scholar]
- 7.Atallah J, Joffe AR, Robertson CM, Leonard N, Blakley PM, Nettel-Aguirre A, et al. Two-year general and neurodevelopmental outcome after neonatal complex cardiac surgery in patients with deletion 22q11.2: A comparative study. J Thorac Cardiovasc Surg. 2007;134:772–779. doi: 10.1016/j.jtcvs.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Wypij D, Newburger JW, Rappaport LA, duPlessis AJ, Jonas RA, Wernovsky G, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397–1403. doi: 10.1016/s0022-5223(03)00940-1. [DOI] [PubMed] [Google Scholar]
- 9.du Plessis AJ. Mechanisms of brain injury during infant cardiac surgery. Semin Pediatr Neurol. 1999;6:32–47. doi: 10.1016/s1071-9091(99)80045-x. [DOI] [PubMed] [Google Scholar]
- 10.Fraser CD, Jr, Andropoulos DB. Principles of antegrade cerebral perfusion during arch reconstruction in newborns/infants. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2008:61–68. doi: 10.1053/j.pcsu.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andropoulos DB, Stayer SA, Diaz LK, Ramamoorthy C. Neurologic monitoring for congenital heart surgery. Anesth Analg. 2004;99:1365–75. doi: 10.1213/01.ANE.0000134808.52676.4D. [DOI] [PubMed] [Google Scholar]
- 12.Jonas RA, Bellinger DC, Rappaport LA, Wernovsky G, Hickey PR, Farrell DM, et al. Relation of pH strategy and developmental outcome after hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 1993;106:362–68. [PubMed] [Google Scholar]
- 13.Wypij D, Jonas RA, Bellinger DC, Del Nido PJ, Mayer JE, Jr, Bacha EA, et al. The effect of hematocrit during hypothermic cardiopulmonary bypass in infant heart surgery: results from the combined Boston hematocrit trials. J Thorac Cardiovasc Surg. 2008;135:355–60. doi: 10.1016/j.jtcvs.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 14.McQuillen PS, Barkovich AJ, Hamrick SE, Perez M, Ward P, Glidden DV, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–41. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 15.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–36. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, et al. Abnormal brain development in neonates with congenital heart disease. N Eng J Med. 2007;357:1928–38. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 17.Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003;143:171–79. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- 18.Glass HC, Bonifacio SL, Chau V, Glidden D, Poskitt K, et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics. 2008;122:299–305. doi: 10.1542/peds.2007-2184. [DOI] [PubMed] [Google Scholar]
- 19.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–95. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 20.Partridge SC, Vigneron DB, Charlton NN, Berman JI, Henry RG, Mukherjee P, et al. Pyramidal tract maturation after brain injury in newborns with heart disease. Ann Neurol. 2006;59:640–51. doi: 10.1002/ana.20772. [DOI] [PubMed] [Google Scholar]
- 21.Childs AM, Ramenghi LA, Cornette L, Tanner SF, Arthur RJ, Martinez D, et al. Cerebral maturation in premature infants: quantitative assessment using MR imaging. AJNR Am J Neuroradiol. 2001;22:1577–82. [PMC free article] [PubMed] [Google Scholar]
- 22.Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–12. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appachi E, Mossad E, Mee RB, Bokesch P. Perioperative serum interleukins in neonates with hypoplastic left heart syndrome and transposition of the great arteries. J Cardiothorac Vasc Anesth. 2007;21:184–190. doi: 10.1053/j.jvca.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 24.Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 25.Ment LR, Bada HS, Barnes P, Grant PE, Hirtz D, Papile LA, et al. Practice parameter: Neuroimaging of the neonate: Report of the Quality Standards Subcommittee of the America Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58:1726–38. doi: 10.1212/wnl.58.12.1726. [DOI] [PubMed] [Google Scholar]
- 26.McQuillen PS. Magnetic resonance imaging in congenital heart disease: what to do with what we see and don’t see? Circulation. 2009 Feb 10;119(5):660–2. doi: 10.1161/CIRCULATIONAHA.108.835744. [DOI] [PubMed] [Google Scholar]
- 27.Hsia TY, Gruber PJ. Factors influencing neurologic outcome after neonatal cardiopulmonary bypass: What we can and cannot control. Ann Thorac Surg. 2006;81:S2381–8. doi: 10.1016/j.athoracsur.2006.02.074. [DOI] [PubMed] [Google Scholar]


