Abstract
Interstimulus “jitter” involves randomization of intervals between successive stimulus events, and can facilitate performance on go/no-go tests among healthy adults, though its effect in clinical populations is unclear. Children with Attention-deficit/Hyperactivity Disorder (ADHD) commonly exhibit deficient response control, leading to increased intra-subject variability (ISV), which has been linked to anomalous functioning within frontal circuits, as well as their interaction with posterior “default mode” regions. We examined effects of interstimulus jitter on response variability in 39 children, ages 9–14 years (25 ADHD, 14 controls). Participants completed 2 computerized go/no-go tests: one with fixed interstimulus interval (ISI) and one with jittered ISI. Repeated measures analysis of variance (ANOVA) revealed a significant group-by test interaction, such that introduction of jitter produced a significant decrease in ISV among children with ADHD, but not among controls. Whereas children with ADHD were significantly more variable than controls on the go/no-go test with fixed ISI, their performance with jittered ISI was equivalent to that of controls. Jittering stimulus presentation provides a nonpharmacologic mechanism for improving response control in ADHD. This bottom-up approach may be mediated by increases in vigilance through noradrenergic circuits that facilitate maintenance of frontal circuits critical to response control.
Keywords: Executive function, Childhood, Variability, Attention, Continuous performance test, Noradrenergic, Locus ceruleus
INTRODUCTION
Several converging lines of research suggest that indices of performance from tasks assessing response control may be robust intermediate endophenotypes of Attention-deficit/Hyperactivity Disorder (ADHD). Response control is a basic characteristic of human behavior, reflecting an individual’s ability to efficiently and accurately choose a preferred response while inhibiting the choice of a less preferred or incorrect response (Mostofsky & Simmonds, 2008). Children with ADHD commonly exhibit deficiencies in response control, leading to disinhibited responding (Wodka et al., 2007), as well as slow (Harris et al., 1995) and variable (Di Martino et al., 2008; Vaurio, Simmonds, & Mostofsky, 2009) response times.
While impaired response inhibition has long been considered a core feature of ADHD (Barkley, 1997), there has been accumulating evidence in recent years that other aspects of response control are affected in ADHD. Prominent among these is intra-subject variability (ISV). which is assessed by measuring the variability within each individual’s reaction time (RT) series. ISV is thought to represent efficiency of response preparation and selection, with lower ISV (less variability) reflecting more efficient responding (Rommelse et al., 2008). Increased ISV in ADHD has also been extensively reported in a variety of paradigms including stop-signal (Klein, Wendling. Huettner, Ruder. & Peper, 2006), sustained attention (Bellgrove et al.. 2005), continuous performance (Epstein et al., 2006), flanker (Di Martino et al., 2008), oculomotor (Mahone, Mostofsky, Lasker, Zee, & Denckla, 2009), and working memory tasks (Karatekin, 2004). Furthermore, ISV has been reported to be “normalized” in children with ADHD on paradigms with fast event rates and incentives (Andreou et al., 2007; Kuntsi, Wood, van der Meere, & Asherson, 2009). Thus, ISV may in fact prove to be a more robust intermediate endophenotype than measures of inhibitory failure, as it is associated with diagnostic characteristics of ADHD and is seen in close family members of individuals with ADHD, suggesting a genetic mechanism for expression of the phenotype (Bidwell, Willcutt, DeFries, & Pennington, 2007).
Functional neuroimaging studies have been particularly relevant in identifying neural correlates of ISV (Kelly, Uddin. Biswall, Castellanos. & Milham, 2008). Studies have generally found lower ISV to be associated with increased activation in premotor and prefrontal cortex (Simmonds et al., 2007), as well as interconnected subcortical structures, the basal ganglia and thalamus (Rubia, Smith, & Taylor, 2007). In a study of adults performing a flanker task, decreased ISV was also found to be associated with anti-correlation of activity in a frontal “task positive” region (anterior cingulate) and that in a “default mode” region (precuneus).
In a pair of recent functional magnetic resonance imaging (fMRI) studies, the neural correlates of ISV in children were examined using a simplified go/no-go task with minimized cognitive demands (with green = go and red = no-go). For typically developing children, lower ISV was found to be associated with increased activity in the rostral supplementary motor area (pre-SMA) (Simmonds et al., 2007), a region known to be critical for motor response control and selection (Isoda & Hikosaka, 2007; Mostofsky & Simmonds, 2008). In contrast, for children with ADHD, increased pre-SMA activation was associated with greater ISV; furthermore, lower ISV in children with ADHD was instead associated with increased activation in a region of the midline prefrontal cortex, rostral to the pre-SMA (in BA8) (Suskauer et al., 2008). The findings suggest that children with ADHD may be able to compensate for impaired response control through recruitment of top-down mechanisms mediated through prefrontal circuits. While this mechanism appears to be effective for some children with ADHD, it may. in some respects, be disadvantageous. Reliance on prefrontal cortex for what is typically more automatic-response control may preclude the use of those prefrontal resources for higher order, more novel executive functions.
It is therefore important to examine whether “bottom-up” mechanisms that are instead facilitated by external manipulations in task design to increase vigilance and resulting readiness to respond can also contribute to improved response control in ADHD. In a recently published study (Wodka, Simmonds. Mahone. & Mostofsky, 2009), we piloted such an approach. hypothesizing that the introduction of uneven intervals between successive stimuli (“jitter”) would enhance response preparatory state, effectively keeping people “on their toes,” and in doing so improve ability to efficiently control responding. Consistent with our hypothesis, we found that a moderate degree of jitter does, in fact, improve response control in healthy adults.
The aim of the present study was to examine the impact of moderate interstimulus jitter on response control in children with and without ADHD. We hypothesized that the introduction of interstimulus jitter would facilitate performance on go/no-go tasks in both groups, but with greater relative effect among those with ADHD.
METHODS
Participants
Participants were recruited as part of a larger study examining brain mechanisms in ADHD. All participants and their parents signed a consent form that met Institutional Review Board standards. Children were between 9 and 14 years old, and had Full Scale IQ (FSIQ) scores of 70 or higher on the Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV). Children were excluded if a history of speech/language disorder or word reading difficulties was identified, either through telephone screening before the initial visit, or based on prior school assessment (completed within one year). Further exclusion criteria included evidence of visual or hearing impairment, or history of other neurological disorder. Parents of participants were screened by telephone to obtain demographic information, school, and developmental history. Children with ADHD who were taking stimulant medication were removed from the medication on the day of and day prior to testing. Children with ADHD taking psychotropic medications other than stimulants were excluded. A total of 39 children (14 control, 25 ADHD) were included in the present investigation.
Following initial telephone screening, participants were screened for psychiatric diagnoses using a structured parent interview (Diagnostic Interview for Children and Adolescents–Fourth Edition, DICA-IV). Additionally, ADHD-specific and broad behavior rating scales (Conners’ Parent/Teacher Rating Scale–Revised, CPRS-R/CTRS-R; ADHD Rating Scale-IV) were used to confirm ADHD diagnosis using the following criteria: (1) positive Diagnostic and Statistical Manual–Fourth edition (DSM-IV) ADHD diagnosis on DICA-IV; and, (2) T-scores greater than 65 on the DSM-IV Hyperactive/Impulsive or Inattentive scales of the CPRS-R or CTRS-R; and, (3) 6 of 9 DSM-IV symptoms met (item rating of 2 or 3) on the Hyperactive/Impulsive or Inattention scales of the ADHD Rating Scale-IV, home or school version. Children with DSM-IV diagnoses other than Oppositional Defiant Disorder and Specific Phobias were excluded. Additional exclusionary criteria for the control group included any history of mental health services for behavior or emotional problems, history of academic problems requiring school-based intervention services, or history of defined primary reading or language-based learning disability. Parents of controls also completed the DICA-IV, CPRS-R, and ADHD Rating Scale-IV, and teachers completed the CTRS-R and teacher form of the ADHD Rating Scale-IV Controls with T-scores greater than 60 on either the DSM-IV Inattentive or Hyperactive/Impulsive scales of the CPRS-R or CTRS-R. or item ratings of 2 or greater for 4 or more symptoms of inattention or hyperactivity/impulsivity from the ADHD Rating Scale-IV (Home or School), were also excluded. All participants were screened for word reading difficulties, which were defined as a score less than the 25th percentile on the Basic Reading Composite of the Woodcock Johnson-III Tests of Achievement.
On the day of the assessment, children were administered the WISC-IV, reading measures, and go/no-go tests. Go/no-go tests were administered in a counterbalanced sequence, with both groups experiencing the task orders equally.
Study Measures
Go/no-go tests
Participants were seated in front of a computer that flashed red and green spaceships. They were instructed to push a button with their right index finger as quickly as possible in response to green spaceships only. Use of familiar color elements (green for “go”; red for ‘“no-go”) minimized the working memory load of the test. Two versions of the go/no-go test were administered as part of the present study. In the fixed interstimulus interval (ISI) condition, cues appeared on the screen for 300 ms and were presented once every 1500 ms (1500 ms interstimulus interval). Cues were weighted towards green spaceships at a ratio of 3:1 (162 go cues; 54 no-go cues), intensifying the need to inhibit a rapid, habitual skeleto-motor response. In the jittered ISI condition, stimuli were presented with a variable ISI, using a moderate (33.3%) level of jitter in which five ISIs were presented randomly, ranging from 1000 to 2000 ms (i.e., 1000, 1250, 1500, 1750, 2000). The total time of each task was 6 mins 30 s. For both measures, variables of interest included omission rate, commission rate, mean RT (for correct hits), and intra-subject variability (ISV) – which was calculated as (standard deviation of response time)/(mean response time).
Data Analysis
Distributions of all variables were examined and square root transformations were used for those variables showing excessive skewness. Group comparisons of demographic. IQ. and go/no-go test scores were analyzed using analyses of variance (ANOVAs) for continuous variables and chi-square tests for categorical variables. Repeated measures ANOVAs were used to examine the moderating effect of jitter on go/no-go test performance for each of the four variables of interest. Effect sizes for each were calculated using partial eta-squared ( ).
RESULTS
Demographics
The study included 39 participants: 25 ADHD (80% male), 14 control (50% male), of which 84% were Caucasian, 12% were African-American, 2% Asian, 1%> Hispanic, and 1% mixed race. Within the ADHD group, there were 10 with Inattentive, 1 with Hyperactive-Impulsive, and 14 with Combined subtypes. Participants ranged in age from 9 to 14 years, with an average age of 11.1 years (ADHD mean = 10.9 ± 1.5; control mean = 11.3 ± 1.6). There were no significant differences between groups in age, sex distribution, socioeconomic status (SES), or racial composition. The control group had significantly higher FSIQ than the ADHD group, F(1, 37) = 9.41, p= .004, , but not Verbal Comprehension Index (VCI), F(1, 37) = 2.60. p= .113, . Given the overlap between components of IQ and dependent measures in this study (especially those involving response preparation/processing speed), it was felt that covarying for FSIQ was not appropriate when measuring group differences on executive control (Dennis et al., 2009). Additionally, a recent metaanalysis of the effects of attention on IQ assessment noted that children with ADHD taking stimulant medications had a mean increase of 6 to 7 IQ points compared to stimulant-naïve children who had been tested, suggesting that reduced IQ scores relative to typically developing peers may be driven by attentional problems and suboptimal test-taking behavior (Jepsen, Fagerlund, & Mortensen, 2009).
Group Differences for Go/No-go Variables
Means and standard deviations for go/no-go variables are listed in Table 1. For the fixed ISI condition, children with ADHD had significantly greater omission rates. F(1, 37) = 4.43, p = .05, , and greater ISV, F(1, 37) = 9.80, p = .003, , than controls, with no significant differences in commission rate or mean RT. In contrast, for the jittered ISI condition, there were no significant differences between ADHD and control groups on any of the four variables of interest (omissions, commissions, mean RT, ISV).
Table 1.
Performance on go/no-go tests
| Control (n = 14) |
ADHD (n = 25) |
||||||
|---|---|---|---|---|---|---|---|
| Go/No-Go Condition | Mean | SD | Mean | SD | p | ||
| Fixed | |||||||
| Omission rate | 0.012 | 0.015 | 0.046 | 0.059 | .042 | .107 | |
| Omission rate* | 0.088 | 0.072 | 0.168 | 0.138 | .050 | .100 | |
| Commission rate | 0.235 | 0.194 | 0.294 | 0.215 | .404 | .019 | |
| Mean RT | 463.979 | 132.904 | 464.154 | 117.519 | .997 | .000 | |
| ISV | 0.229 | 0.067 | 0.348 | 0.134 | .003 | .209 | |
| Jittered | |||||||
| Omission rate | 0.025 | 0.032 | 0.038 | 0.044 | .370 | .022 | |
| Omission rate* | 0.113 | 0.118 | 0.162 | 0.111 | .209 | .042 | |
| Commission rate | 0.250 | 0.200 | 0.316 | 0.200 | .295 | .030 | |
| Mean RT | 461.173 | 98.666 | 445.282 | 92.133 | .617 | .007 | |
| ISV | 0.247 | 0.074 | 0.286 | 0.094 | .190 | .046 | |
Note. RT = response time in ms; ISV = Intra-subject Variability (SD/Mean RT in ms).
Square Root transformation.
Effects of Jitter Condition
Repeated measures ANOVAs, using group as the between groups variable, and jitter condition (fixed vs. jittered) as the repeated measure, revealed no significant effects for jitter condition or group–by condition interaction for omissions, commissions, or mean RT. In contrast, there was a significant group–by condition interaction effect for ISV, Pillai’s V = 0.125, p = .027. η2 = 0.125 (Figure 1). In order to examine the nature of the interaction, repeated measures ANOVAs were completed separately for each group. Within the ADHD group, children had significantly greater ISV on the fixed condition than on the jittered condition, F(1, 24) = 6.33, p = .019, . In contrast, the difference in ISV between the fixed and jittered conditions among controls was not significant, F(1, 13) = 1.54, p = .237, .
Fig. 1.
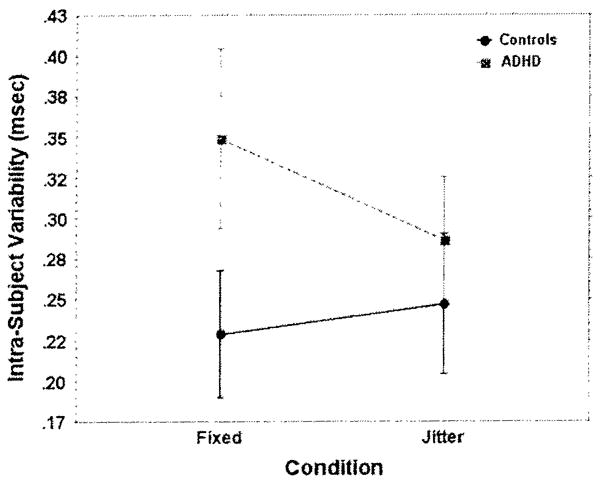
Intra-subject Variability (ISV) by Test. Children with ADHD had significantly greater ISV than controls (p = .003) on the go/no-go test with fixed interstimulus interval (ISI), but not on the test with jittered ISI (p = .190). Additionally, children with ADHD had significantly greater ISV on the go/no-go test with fixed ISI than they did on the test with jittered ISI (p = .019), whereas controls did not differ in ISV on the two tests (p = .237).
DISCUSSION
The current study sought to examine the impact of moderate jitter on response control in children with and without ADHD. On the go/no-go task with fixed ISI, the ADHD and control groups did not differ in commissions or mean response time; however, the ADHD group had significantly more omissions and increased ISV compared to controls. Of note, the effect size for differences in ISV was large ( ), and approximately ten times the magnitude of the effect size for commission errors ( ). This finding is consistent with the growing literature that suggests that ISV may be a stronger behavioral phenotype in ADHD than inhibitory control.
In contrast to the robust group differences on the fixed ISI go/no-go task, there were no group differences on any variable for the jittered ISI task, and all effect sizes were small ( for all). In other words, introduction of moderate jitter essentially “normalized” the performance of children with ADHD, with respect to lapses in attention (omissions) and sustained response control (ISV). Furthermore, children in the ADHD group performed significantly better on the jittered versus the fixed ISI task. Because the order of administration was counterbalanced, this difference does not appear to be a function of test order.
The introduction of jitter, and its seeming “normalization” of the ADHD population, requires us to examine the process utilized in preparing a response to a stimulus, and the dysfunction found in that process in children with ADHD. Between stimulus perception and choice to respond lie several critical executive function skills, including sustaining attention, inhibition of off-task behavior, and preparedness to respond (Denckla, 1996). Increased intra-subject variability in responding may depend in part on vulnerabilities related to response preparation (Pashler & Johnston, 1989); however, the frequent intrusion of large reaction times may also be an indication of loss of vigilance or factors independent of stimulus familiarity or long-term memory processes (Gilden & Hancock, 2007). Current research suggests that response selection and inhibition are closely related processes dependent on mechanisms important to motor response preparation (Mostofsky & Simmonds. 2008). Electrophysiological research findings suggest that pre-SMA circuits are crucial for accurate response selection and inhibition (Isoda & Hikosaka, 2007). Given the importance of the role of pre-SMA circuitry, an association may exist between optimal response preparation and optimal response efficiency and accuracy, as seen in individuals with lower ISV (Wodka et al., 2009).
Jittering stimulus onset likely enhances response preparatory state by increasing vigilance, effectively keeping people “on their toes,” and in doing so improve ability to efficiently control responding. It follows that this effect of increased vigilance on readiness to respond may be mediated by bottom-up noradrenergic projections from the locus ceruleus. Dysfunction within these and other brainstem catecholaminergic systems (in particular, dopmainergic projections from the substantia nigra) have been hypothesized to play a role in the pathogenesis of ADHD (Sergeant, Geurts, Huijbregts, Scheres, & Oosterlaan, 2003). This is in large part due to observations of response to stimulant medications that enhance catecholaminergic transmission, as well as the more recently observed effect of atomoxetine, which selectively inhibits reuptake of norepinephrine (Pliszka, 2005). As such, the use of jitter may represent an effective nonpharmacologic approach for improving response control in ADHD.
Future investigations of the influence of jitter on response control should take into account methods by which activation of response control may be more closely examined (i.e., fMRI and event-related potentials) in conjunction with go/no-go tasks. Additionally, it will be important to examine the impact of jitter on motivational (energetic) factors, as well as impaired delay aversion, which have been also described as fundamental deficits in ADHD (Sonuga-Barke. Wiersema, van der Meere, & Roeyers, 2009). Strategies emphasizing moderate unpredictability in classroom settings may be effective in ameliorating some inattention symptoms in ADHD by improving overall response preparation; continued research is warranted to examine this hypothesis.
Several limitations to the current findings should be considered. The relatively small sample size precluded further examination of the contribution of age, sex, ADHD subtype, or the differential impact of jitter on “raw” response time standard deviation (compared with ISV; Klein et al., 2006). The sample had wide age range (9–14 years), and developmental neurobiological changes related to response control occurring during this period may have contributed to observed deficits in the later-maturing children with ADHD. Reductions in overall gray matter volume and prefrontal volume occur during this age range. Considering the relative “delay” in brain maturation associated with ADHD, group differences may be driven by the younger age of the sample (Shaw et al., 2007). Future research should continue to examine in detail the elements of response variability that are facilitated by introduction of moderate jitter (i.e., examination of ex-Gaussian distributions) to determine whether jitter facilitates reduction of “lapses” in attention or better response control throughout the task (Vaurio et al., 2009).
Acknowledgments
The authors report no conflicts of interest. Portions of this manuscript were presented as a poster at the Annual Meeting of the American Academy of Clinical Neuropsychology in San Diego. California on June 18, 2009. This work was supported by HD-24061 (Intellectual and Developmental Disabilities Research Center), R01 NS043480, R01 NS047781. R0I MH085328, K02 NS044850, P50 HD 52121, and the Johns Hopkins University School of Medicine Institute for Clinical and Translational Research, an NUT/NCRR CTSA Program, UL1-RR025005. Drs. Mostofsky and Mahone contributed equally as mentors in this work.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A, et al. Reaction time performance in ADHD: Improvement under fast-incentive condition and familial effects. Psychological Medicine. 2007;37:1703–1715. doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Hawi Z, Kirley A, Fitzgerald M, Gill M, Robertson IH. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: Sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005;43:1847–1857. doi: 10.1016/j.neuropsychologia.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Bidwell LC, Willcutt EG, DeFries JC, Pennington BF. Testing for neuropsychological endophenotypes in siblings discordant for attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;62:991–998. doi: 10.1016/j.biopsych.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. Connors’ Ratings Scale-Revised (CRS-R) Austin, TX: Pro-Ed; 1997. [Google Scholar]
- Denckla MB. Biological correlates of learning and attention: What is relevant to learning disability and attention-deficit/hyperactivity disorder? Developmental and Behavioral Pediatrics. 1996;17:1–6. [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ghaffari M, Curchak J, Reiss P, Hyde C, Vannucci M, et al. Decomposing intrasubject variability in children with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2008;64:607–614. doi: 10.1016/j.biopsych.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulis AD, Reid R. ADHD rating scale-IV. New York: Guilford Press; 1998. [Google Scholar]
- Epstein JN, Conners CK, Hervey AS, Tonev ST, Arnold LE, Abikoff HB, et al. Assessing medication effects in the MTA study using neuropsychological outcomes. Journal of Child Psychology and Psychiatry. 2006;47:446–456. doi: 10.1111/j.1469-7610.2005.01469.x. [DOI] [PubMed] [Google Scholar]
- Gilden DL, Hancock H. Response variability in attention-deficit disorders. Psychological Science. 2007;18:796–802. doi: 10.1111/j.1467-9280.2007.01982.x. [DOI] [PubMed] [Google Scholar]
- Harris EL, Schuerholz LJ, Singer HS, Reader MJ, Brown JE, Cox C, et al. Executive function in children with Tourette syndrome and/or attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society. 1995;1:511–516. doi: 10.1017/s1355617700000631. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medical frontal cortex. Nature Neuroscience. 2007;10:240–248. doi: 10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- Jepsen JRM, Fagerlund B, Mortensen EL. Do attention deficits influence IQ assessment in children and adolescents with ADHD? Journal of Attention Disorders. 2009;12:551–562. doi: 10.1177/1087054708322996. [DOI] [PubMed] [Google Scholar]
- Karatekin C. A test of the integrity of the components of Baddelely’s model of working memory in attention-deficit/hyperactivity disorder (ADHD) Journal of Child Psychology and Psychiatry. 2004;45:912–926. doi: 10.1111/j.1469-7610.2004.t01-1-00285.x. [DOI] [PubMed] [Google Scholar]
- Kelly CAM, Uddin LQ, Biswall BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Wood AC, van der Meere J, Asherson P. Why cognitive performance in ADHD may not reveal true potential: Findings from a large population-based sample. Journal of the International Neuropsychological Society. 2009;15:570–579. doi: 10.1017/S135561770909081X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahone EM, Mostofsky SH, Lasker AG, Zee D, Denckla MB. Oculomotor anomalies in attention-deficit/hyperactivity disorder: Evidence for deficits in response preparation and inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:749–756. doi: 10.1097/CHI.0b013e3181a565f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: Two sides of the same coin. Journal of Cognitive Neuroscience. 2008;20:1–11. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Pashler H, Johnston JC. Chronometric evidence for central postponement in temporally overlapping tasks. Quarterly Journal of Experimental Psychology. 1989;41A:19–45. [Google Scholar]
- Pliszka SR. The neuropsychopharmacology of attention-deficit hyperactivity disorder. Biological Psychiatry. 2005;11:1385–1390. doi: 10.1016/j.biopsych.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Reich W, Welner Z, Herjanic B. The Diagnostic Interview for Children and Adolescents. 4. North Tonawanda, NY: Multi-Health Systems; 1997. (DICA-IV) [Google Scholar]
- Rommelse NNJ, Altink ME, Oosterlaan J, Beem L, Buschgens CJM, Buitelaar J, Sergeant JA. Speed, variability, and timing of motor output in ADHD: Which measures are useful for endophenotypic research? Behavioral Genetics. 2008;38:121–132. doi: 10.1007/s10519-007-9186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith A, Taylor E. Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery of impulsiveness. Child Neuropsychology. 2007;13:276–304. doi: 10.1080/09297040600770761. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, Huijbregts S, Scheres A, Oosterlaan J. On the top and bottom of ADHD: A neuropsychological perspective. Neuroscience and Biobehavioral Reviews. 2003;27:583–592. doi: 10.1016/j.neubiorev.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar JJ, Denckla MB, Mostofsky SH. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;46:2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Wiersema JR, van der Meere JJ, Roeyers H. Context-dependent dynamic processes in attention deficit/hyperactivity disorder: Differentiating common and unique effects of state regulation deficits and delay aversion. Neuropsychological Review. 2009 doi: 10.1007/s11065-009-9115-0. [DOI] [PubMed] [Google Scholar]
- Suskauer SJ, Simmonds DJ, Fotedar S, Blankner JG, Pekar JJ, Denckla MB, Mostofsky SH. Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: Differences in activation associated with response inhibition but not habitual motor response. Journal of Cognitive Neuroscience. 2008;20:478–493. doi: 10.1162/jocn.2008.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaurio RG, Simmonds DJ, Mostofsky SH. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47:2389–2396. doi: 10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 4. Minneapolis, MN: Pearson, Inc; 2003. (WISC-IV) [Google Scholar]
- Wodka EL, Mahone EM, Blankner JG, Gidley Larson JC, Fotedar S, Denckla MB, Mostofsky SH. Evidence that response inhibition is a primary deficit in ADHD. Journal of Clinical and Experimental Neuropsychology. 2007;29:345–356. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock Johnson Psychoeducational Battery. 3. Chicago, IL: Riverside Publishing; 2001. [Google Scholar]
- Wodka EL, Simmonds DJ, Mahone EM, Mostofsky SH. Moderate variability in stimulus presentation improves motor response control. Journal of Clinical and Experimental Neuropsychology. 2009;31:483–488. doi: 10.1080/13803390802272036. [DOI] [PMC free article] [PubMed] [Google Scholar]


