Abstract
Magnetic nanoparticles (MNPs) represent a class of non-invasive imaging agents that have been developed for magnetic resonance (MR) imaging. These MNPs have traditionally been used for disease imaging via passive targeting, but recent advances have opened the door to cellular-specific targeting, drug delivery, and multi-modal imaging by these nanoparticles. As more elaborate MNPs are envisioned, adherence to proper design criteria (e.g. size, coating, molecular functionalization) becomes even more essential. This review summarizes the design parameters that affect MNP performance in vivo, including the physicochemical properties and nanoparticle surface modifications, such as MNP coating and targeting ligand functionalizations that can enhance MNP management of biological barriers. A careful review of the chemistries used to modify the surfaces of MNPs is also given, with attention paid to optimizing the activity of bound ligands while maintaining favorable physicochemical properties.
Keywords: Magnetic nanoparticle, Molecular targeting, MRI, Contrast agents, Gene therapy, Drug release, Bioconjugation, Biological barriers, Blood Brain Barrier, Surface modification, Physicochemical properties
1. Introduction
Advances in nanotechnology and molecular biology are rapidly enabling the development of nanoparticles (NPs) with specific functional properties that address the shortcomings of traditional disease diagnostic and therapeutic agents [1, 2, 3]. Brighter, tissue-specific imaging probes are being developed with NP technology to visualize and help diagnose disease at its earliest stages, in some cases, even prior to disease manifestation [4, 5]. Concurrently, NPs are being developed as drug carriers thanks to careful nanostructure construction (tailored drug release characteristics, low immunogenicity, etc.) yielding improved treatment efficacy and reduction of unwanted side effects [6, 7]. Significantly, these imaging and delivery facilities have been combined into unique NP formulations through clever combinations of nanoscaled materials, enabling simultaneous in vivo diagnostic imaging and drug delivery for real-time treatment tracking [7, 8].
Among the broad spectrum of nanoscale materials being investigated for biomedical use, magnetic nanoparticles (MNPs) have gained significant attention due to their intrinsic magnetic properties, which enable tracking through the radiology cornerstone, magnetic resonance (MR) imaging [8]. This class of NPs include metallic, bimetallic, and superparamagnetic iron oxide nanoparticles (SPIONs) [8, 9]. The latter of which has been widely favored because of its inoffensive toxicity profile [10, 11, 12] and reactive surface that can be readily modified with biocompatible coatings [13, 14, 15, 16] as well as targeting, imaging, and therapeutic molecules [15, 16, 17, 18]. This flexibility has led to SPION use in magnetic separation [19], biosensor [20, 21], in vivo medical imaging [8, 22, 23], drug delivery [18, 24], tissue repair [25], and hyperthermia [26] applications.
Currently, a number of SPIONs are in early clinical trials or experimental study stages [8, 9, 15], and several formulations have been approved for clinical use for medical imaging and therapeutic applications. Notable examples include: Lumiren® for bowel imaging [11], Feridex IV® for liver and spleen imaging [27], Combidex® for lymph node metastases imaging [28], and most recently, Ferumoxytol® for iron replacement therapy [29]. The physicochemical profiles of these SPIONs provide passive targeting, but not the higher level targeting offered by bioligands. Addition of bioactive molecules to the SPION surface can increase the targeting specificity of NPs [8, 9, 17, 30, 31], producing contrast agents that specifically illuminate targeted tissue and drug carriers that don’t interact with healthy tissue [8, 18, 19, 31, 32, 33, 34]. Development in this area represents a majority of SPION research today.
The creation of next generation SPIONs that can specifically target and eliminate or illuminate damaged tissue requires careful engineering of the size, shape, coating, and surface modifications. Thorough consideration of each design parameter must be evaluated to produce a NP that can overcome biological barriers and carry out its function. In doing so, targeting molecules must be chosen based on their physical properties in addition to their binding characteristics, and integrated into the NP system in such a way that they remain functionally active. In vivo use of SPION imaging preparations require attention to each of these design parameters, while SPION drug delivery systems must additionally anticipate the routes of NP uptake by target cells and the controlled release of their payloads. Herein, we will review these design considerations and fabrication strategies for the development of NPs for in vivo imaging and targeted drug delivery.
2. Nanoparticle design considerations
Before synthesis, MNP design requires fundamental understandings of the nature of the nanostructure as (1) a pharmaceutical construct that must navigate the body in search of its target, (2) a biocompatible entity that will not harm the patient, and (3) a contrast agent used in an external, biomedical imaging system. Here, we will consider the first of these areas, specifically looking at the physiological barriers that a MNP must overcome to gain access to its cellular target, and the NP’s physical characteristics that can promote this functionality in vivo.
2.1 In vivo barriers
Intrinsic to the body’s defense system are a series of “biological barriers” that serve to protect the body against foreign entities, including injected therapeutics and contrast agents, keeping them from reaching their intended destinations [1]. These barriers can restrict NP function by blocking their movement, causing physical changes to them, or by inducing a negative host response using biochemical signaling [35].
Upon intravascular administration, NPs immediately encounter blood, a high ionic strength, heterogenous solution, that can induce NP agglomeration, altering their magnetic properties and inducing particle sequestration. Additionally, NPs can nonspecifically interact with plasma proteins (which can trigger the adaptive immune system), extracellular matrices, and non-targeted cell surfaces while in the blood stream [36]. In each case, the NP is in danger of prematurely binding to or being taken up by cells before reaching its target tissue.
In addition to coping with the vascular environment, NPs must overcome various anatomical size restrictions which limit NP access to target tissue (e.g. extravasation of lymph-targeting NPs from the blood vessels) [1]. These size limitations are especially stringent when targeting certain organs like the brain and kidney [37]. For instance, in the brain, endothelial cells and reinforcing astrocyte cells limit levels of pinocytosis and form tight junctions between cells at the blood-brain interface, yielding a structural and metabolic barrier referred to as the blood brain barrier (BBB) [38]. Here, only NPs of sufficient small sizes and appropriate physiochemical properties may pass the BBB.
Biological barriers are not unique to extracellular spaces; in fact intracellular barriers are a critical reason many drugs and drug delivery systems fail. NP systems are no exception. Once a cell-specific NP has bound to the membrane of its target, it is typically taken up by the cell through receptor-mediated endocytosis, where it is trafficked intracellularly via endosomal compartments for processing and destructions through acidification of the endosomes [39]. Most of these endosomes are then translocated into lysosomes where hydrolytic and enzymatic reactions completely metabolize macromolecules. Many therapeutics, such as DNA and siRNA, are susceptible to lysosomal degradation, rendering them ineffective upon cellular processing. However, carriers can be engineered to avoid this fate by facilitating endosomal escape prior to lysosomal trafficking [35]. NPs that are able to demonstrate endosomal escape may still be required to breach additional biological barriers, such as the nuclear membrane, as is required for effective gene therapy. Each of these obstacles illustrates a demand placed on the engineers of a given system, and must be addressed in the preparation of the core and surface properties of the NP.
2.2 Physicochemical considerations
NP pharmacokinetics and cellular uptake in vivo, including their ability to manage biological barriers, are largely related to NP physicochemical properties, including morphology, hydrodynamic size, charge, and other surface properties [40, 41]. These properties are dictated by the types, structures, and orientations of the materials that comprise the NP. Typically, an MNP consists of a magnetically active core coated with a stabilizing shell to which targeting ligands and additional imaging modalities are anchored. Therapeutic agents can then be embedded in the shell structure or chemically bonded to its surface. At each stage of its design, the size, charge, hydrophobicity, shape, and orientation of the NP’s constituent materials must be considered with regards to overall NP physiochemical properties.
2.2.1 Hydrodynamic Size
NP biodistribution appears to be significantly influenced by its physicochemical properties [37, 42]. Hydrodynamic size, for instance, (1) helps govern the NP concentration profile in the blood vessel [43, 44, 45], (2) affects the mechanism of NP clearance, and (3) dictates the permeability of NPs out of the vasculature [46]. In the case of the former, Decuzzi et al produced models suggesting that smaller sized, spherical NPs observed higher diffusion rates, increasing the NP concentration at the center of a blood vessel, thus limiting interactions with endothelial cells and prolonging the NP blood circulation time [45].
Hydrodynamic size also affects NP clearance from circulation [37, 47, 48, 49, 50, 51]. For instance, it has been reported that small NPs (< 20 nm) are excreted renally [47, 52], while medium sized NPs (30–150 nm) have accumulated in the bone marrow [53], heart, kidney and stomach [52], and large NPs (150–300 nm) have been found in the liver and spleen [54]. While these size ranges provide general clearance mechanisms, other physical parameters simultaneously affect NP mobility.
As previously discussed, nanoparticle size affects the ability of NPs to extravasate from the vasculature. While most endothelial barriers allow NPs < 150 nm in diameter to pass, more stringent barriers, such as the BBB are far more restrictive. The BBB allows passive diffusion of only small (< 500 Da MW), neutrally charged lipid soluble molecules, prohibiting > 98% of all potential neurotherapeutics and contrast agents from passing through the BBB [55, 56]. In addition, a vast majority of developed NPs have been unable to breach the BBB [38]. Consequently, this has become an area of intense research [38, 56, 57, 58, 59], with broad ramifications in the development of treatment strategies for brain tumors, Parkinson’s, Alzheimer’s, and Huntington’s diseases [1, 57, 60, 61, 62, 63]. In the quest towards determining the influence of NP size on BBB permeability Sonavane et al recently reported that gold NPs of 15 to 50 nm in hydrodynamic size could permeate across the BBB, while larger NPs, specifically 100 and 200 nm sized could not [64]. However, it should be noted that reviews of the literature have suggested that BBB permeability is likely influenced by all physiochemical properties discussed here and NP size may not alone dictate NP permeability across the BBB [56].
2.2.2 Shape
In investigating the effects of NP shape on biodistribution, a limited number of comparative studies have been performed evaluating the biodistribution of non-spherical and rod shaped NPs [65, 66, 67, 68, 69, 70]. It has been suggested that anisotropically shaped NPs can avoid bioelimination better than spherical NPs [67]. In one notable study by Geng et al, the authors demonstrated a relationship by which an increase in the length-to-width aspect ratio of the nanotubes correlated with increased in vivo blood circulation time of nanotubes [70]. Nanotube shaped MNPs have also been evaluated in vivo and found to have similarly enhanced blood circulation times over the spherical counterparts [71, 72]. Although these findings are promising more studies are needed to identify exactly what aspect ratios yield most dramatic influence on NP pharmacokinetics.
2.2.3 Surface properties
NP charge and hydrophobicity can affect NP biodistribution by limiting or enhancing interactions of NPs with the adaptive immune system, plasma proteins, extracellular matrices and non-targeted cells [36]. Specifically, hydrophobic and charged NPs have short circulation times due to adsorption of plasma proteins (opsonization) which can lead to recognition by the reticuloendothelial system (RES), followed by removal from circulation [41]. Positively charged NPs can also bind with non-targeted cells (typically negatively charged) leading to non-specific internalization. In addition, hydrophobic groups on the surface of NPs induce the agglomeration of the NPs upon injection, leading to rapid removal by the RES.
To limit NP-host interactions, surface engineering has led to the development of stealth NPs. Surface modification with molecules like the hydrophilic polyethylene glycol (PEG) have been shown to reduce the potential for opsonization through steric repulsion, prolonging NP circulation times [73]. The utility of organic coatings will be properly addressed in later sections.
2.3 Directing nanoparticles in vivo
The specificity of NPs for select tissues is critical in both diagnostic imaging and drug-based therapies [16, 74, 75]. In both cases, nonspecific cell binding can place healthy tissue at risk. To limit non-specific binding, NPs have been engineered to have an affinity for target tissues through passive, active, and magnetic targeting approaches.
Passive targeting uses the predetermined physicochemical properties of a given NP to specifically migrate to a given tissue region. For example, targeting of solid tumor tissue can be achieved through passive mechanism termed enhanced permeation and retention (EPR) [76]. This phenomenon is based on the principle that tumor cells, in an effort to grow rapidly, stimulate production of new blood vessels (the neovasculature) that are poorly organized and have leaky fenestrations. This enables extravasation of small macromolecules and NPs out of the vasculature, into the tumor tissue [77, 78]. Due to inefficient lymphatic drainage, there is poor clearance of these agents, leading to selective accumulation of these agents [79, 80]. However, EPR is limited to specific metastatic solid tumors, and successful implementation is dependent upon a number of factors including degree of capillary disorder, blood flow, and lymphatic drainage rate, making effective management difficult.
Because passive targeting is available for only certain in vivo applications and does not necessarily guarantee internalization of NPs by targeted cells, NPs can be additionally modified with molecular targeting ligands to employ active cell targeting [81, 82]. NP assemblies are now decorated with targeting molecules, complementary to unique receptors on target cells, to actively target only diseased tissue. A number of SPION systems have implemented targeting ligands into their design with varying success, including: small organic molecules [81, 83, 84], peptides [71, 85, 86, 87, 88], proteins [89], antibodies [90, 91, 92], and aptamers [93, 94, 95]. In addition to the type of ligand used, active targeting is affected by targeting molecule density and by the size and shape of the NP.
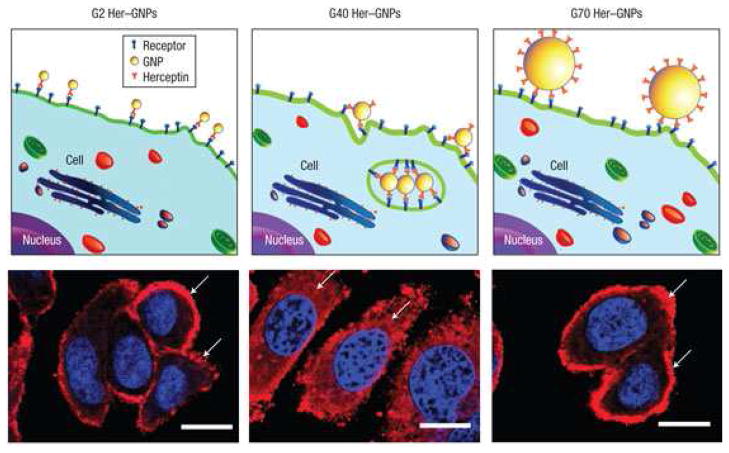
Recent studies indicated that the density and molecular organization of bound ligands significantly influence NP binding to target cells due to the multivalency phenomenon [86, 96]. Multivalency is the enhanced binding avidity phenomenon observed when multiple ligands simultaneously bind with multiple receptors between two surfaces [97, 98, 99]. Several NP systems have been engineered to achieve higher affinities to their cellular targets utilizing this principle [86, 100, 101]. Notably, in a study of cross-linked iron oxide (CLIO) NPs decorated with varying densities of the RGD peptide (4.1, 20, and 52 peptides per NP), it was shown that simultaneous ligand binding could be increased with higher RGD presentation, but beyond a given ligand density, multivalent interactions were sterically hindered [100]. In addition, multivalency is also affected by NP size [102]. In a notable study by Jiang et al NPs of sizes ranging from 2–100 nm were decorated with targeting herceptin antibodies and evaluated for ability to bind and be internalized by targeted cells [102]. Through a series of experiments it was revealed that NPs smaller than 25 nm lack the ability to present multiple ligands to a cell, unlike larger variants, limiting any potential multivalency binding effects. At the same time, larger NPs are not as readily endocytosed by cells, limiting their functionality for certain applications. Notably NPs of 25–50 nm were revealed to be most suitable for multivalent binding and endocytosis. This is graphically represented in Figure 1 where the larger NP, decorated with targeting antibodies, is able to form more multivalent interactions with the cell receptors of the targeted cell. NPs of varying size (2, 40, and 70 nm) coated with antibodies showed variable degrees of internalization by the target cell, as observed by fluorescence imaging.
Figure 1.
Illustrations with corresponding fluorescence images of ErbB2 receptor localization after treatment with different-sized heeceptin bound to gold NPs (Her–GNPs). In the fluorescence images of cells arrows indicate ErbB2 receptors, and the nucleus is counterstained with DAPI (blue) (scale bars=10 microm). Reprinted by permission from Macmillan Publishers Ltd: Nature Nanotechnology [102] Copyright 2008.
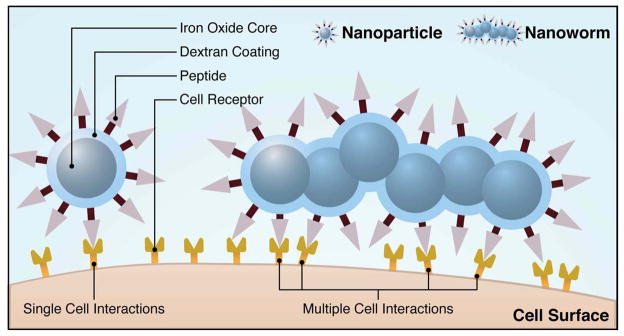
NP shape has also been shown to influence NP targeting abilities. A secondary study by Decuzzi et al hypothesized that oblique-shaped particles, that have been decorated with targeting molecules, show greater cell binding affinity compared with spherical NPs [43]. This theory has been supported by several recent studies [65, 66, 71, 103]. Most notably, in a comparative study between F3 peptide-modified spherical (5 nm) and rod shaped (5 nm × 5 nm joined cores) “nanoworm” MNP assemblies, the elongated “nanoworm” construct showed enhanced cell binding [71]. The enhanced multivalent interactions of the nanoworm are conceptually illustrated in Figure 2. As shown the elongated shape of the MNP can bind more targeting molecules with the target cells compared with a spherical NP.
Figure 2.
Conceptual scheme illustrating the varying multivalent affinity interactions between receptors on a cell surface and targeting ligands on a nanospheres versus a nanoworm. Conceptual adaptation from the figure previously published [71].
In addition to engineering NPs for tissue targeting, some researchers have used external magnetic systems to help direct MNPs localization in a strategy called magnetic targeting [18, 104]. This involves focusing high field, high gradient, or rare earth magnets on the target site, inducing accumulation of the highly magnetically susceptible MNPs. Recently this technique was successfully implemented in a clinical trial to deliver the chemotherapeutic, doxorubicin, to hepatocarcinoma cells [105]. While successful, the effectivity of magnetic targeting is limited to target tissue close to the body’s surface, due to loss of magnetic field strength further away from the magnetic source.
2.4 Drug loading and Release
When loaded with a therapeutic payload, NPs that are appropriately designed, can act as efficient drug delivery systems, offering limited non-specific cell interactions, controlled therapeutic release, flexible drug loading (a variety of drugs can be loaded), and delivery tracking using an NP imaging modality. Like NPs developed for diagnostic imaging, drug-carrying NPs require careful physicochemical and targeting design, but also require additional considerations paid to drug loading, transport, and release [24, 106, 107]. First, the NP must be able to carry and protect a significant drug payload, typically determined by the type of coating and method of loading (e.g. covalent bonding). Second, multiple drugs can be loaded to overcome cellular drug resistance, and improve overall cell kill efficiencies, but requires careful NP planning to accommodate the different therapeutics. Third, the release mechanism and rate of the therapeutic cargo unloading should be modulated for optimal therapeutic efficiency. For instance, the concentration and duration of release of drug after intracellular uptake can be predetermined, or in the case of gene therapy, the release of cargo can be adjusted to respond to the cell cycle, triggering release at an optimal time.
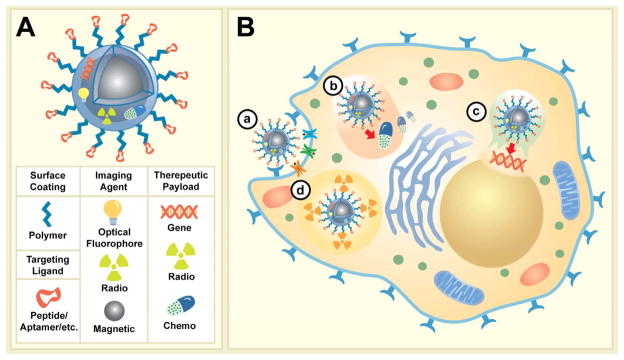
Additional application driven considerations are required based on the chosen therapeutics [8]. Figure 3 describes the blueprint of multifunctional imaging/therapeutic MNPs and the local activity of several categories of therapeutic agents designed for cancer therapy. As shown MNPs developed for these dual applications may carry targeted moieties extended from NP surface via polymeric tethers (e.g. PEG), and may carry multiple imaging reporters (optical, radio, magnetic), and therapies in the forms of biotherapeutics (i.e. gene), chemotherapeutics (i.e. chemical drug formulations), and/or radiotherapeutics (i.e. radionucleotides) (Figure 3A). At the cellular level different therapeutic mechanism can then be activated depending on the choice of therapy integrated onto MNPs (Figure 3B). For instance as shown in Figure 3B, therapeutic peptides/antibodies function by binding to and inactivating cell surface receptors, possibly requiring receptor-mediated internalization (a); chemotherapeutic agents require internalization and slow intracellular drug release (b); DNA and siRNA gene therapy requires action in the perinuclear region or nucleus, necessitating cellular uptake followed by escape from endosomal compartments (c); lastly, radiotherapeutics require cellular internalization (d).
Figure 3.
Illustration of multifunctional imaging/therapeutic MNPs anatomy and potential mechanisms of action at the cellular level. (A) A multifunctional MNP modified with targeting ligands extended from MNP surface with polymeric extenders, imaging reporters (optical, radio, magnetic), and potential therapeutic payloads (gene, radio, chemo). (B) Four possible modes of action for various therapeutic agents; a) Specific MNP binding to cell surface receptors (i.e. enzymes/proteins) facilitate their internalization and/or inactivation, b) controlled intercellular release of chemotherapeutics; c) release of gene therapeutic materials post endosomal escape and subsequent targeting of nucleus; and d) intracellular decay of radioactive materials.
2.5 Toxicity
To ensure a developed MNP system poses no threat to the patient after administration, toxicity of the individual components and NP as a whole must be evaluated. When evaluating NP toxicity it is necessary to both consider how the assembled NP system will interact with the body during its functional lifetime, and how the independent components will affect the body during biodegradation and liver processing [10]. Nanotoxicology is an emerging area of research, but additional studies are needed to better understand the body’s response to nanoparticulates. For an in-depth discussion of nanotoxicology concerns please refer to recent articles on this area published by Longmire et al. and Vega-Villa et al. [37, 108], or for a more comprehensive review please refer to the book published by Zhao et al. [109]. Typically, MNPs are not excreted from the body as a construct, necessitating the use of components that can individually be biodegraded by the body.
3. Fabrication of target-specific magnetic nanoparticles (MNPs)
In order to take advantage of our knowledge of the NP bioresponses and targeting techniques detailed above, to control the physicochemical properties of NPs, we need to understand and implement controlled synthesis and coating processes. In the following sections we will discuss some of the techniques used in the MNP field and detail the significant design parameters that can assist in synthesizing target-specific NPs.
3.1 MNP core fabrication
Typically comprised of an organic coating and multiple functional molecules at its surface, the magnetic functionality of MNPs for MR imaging is dictated by the composition, size, and shape of its magnetic core. These NP cores have been made from different materials and with varying sizes, shapes, uniformities, and magnetic properties [8, 13, 14, 110, 111]. Specifically, MNPs have been formed from pure iron and cobalt metals [69, 112], alloys such as CoPt3 [113], FePt [114], FeZn [115], and from iron oxides [15], including magnetite (Fe3O4) and maghemite (γ-Fe2O3) [15, 116, 117]. The iron oxides have also been doped to enhance their magnetic properties to form MFe2O4 structures where M is a +2 cation such as Mn, Fe, Co or Ni [30, 118]. While these MNPs make excellent MR contrast agents, those containing cobalt, nickel, and manganese are potentially toxic, making them poor candidates for clinical use until advanced coatings and chelating agents are developed [119]. Alternatively, the non-doped iron oxides degrade to their non-toxic iron and oxygen components, making them particularly attractive as NP cores [120]. Of the two, magnetite is typically preferred due to its superior magnetic properties and will be the focus of the remainder of this section [15].
SPIONs can be fabricated by either top-down (mechanical attrition) or bottom-up (chemical synthesis) approaches. However, chemical routes are better suited to produce nanoparticles with uniform composition and size (typical deviations of > 10% in less than 10% of the nanoparticle batch) [121]. The solution chemical methods include standard iron chloride co-precipitation, co-precipitation in constrained environments, thermal decomposition and/or reduction, hydrothermal synthesis, and polyol synthesis [8, 15, 111]. An excellent review of these methods has been presented by Lauret et al [15].
While each method has its own specific advantages, the most common preparation method is that of co-precipitation of Fe2+/Fe3+ salt solutions with the addition of base under an inert atmosphere. Here, NPs are formed by a nucleation and growth mechanism that typically allows for good monodispersity of the end product by optimization of the conditions that yield a short nucleation event followed by a slower growth phase. Type of salts employed (e.g. chlorides, sulfates, nitrates), ratio of Fe2+/Fe3+, temperature, pH and ionic strength all affect the properties of the synthesized SPIONs [111]. While adequate, standard co-precipitation methods have difficulty yielding consistent SPION size, shape, and polydispersity, and can introduce impurities and surface defects into the particles, compromising their magnetic properties [13].
To help improve the uniformity and stability of SPIONs, modifications of the standard co-precipitation approach have been investigated. Specifically, several studies added polymers or polyelectrolytes to the iron chloride solution during co-precipitation to specifically tune the size, shape, and crystallinity of the SPIONs [13, 122, 123, 124, 125]. In one study, the SPION core was tuned between 7–14 nm by changing the concentration of poly (acrylic acid) in solution [123], while in another, graft PEG-g-poly(glycerol monoacrylate) polymers were used to modulate SPION size [125]. Importantly, these polymers may act as surface coatings after the nucleation and growth processes are complete. These coatings are sometimes referred to as being “in situ” because they are present during nanoparitcle synthesis. Unfortunately, this approach can limit the crystallinity of the formed SPIONs, which may negatively affect their magnetic susceptibility.
Recently, new synthesis techniques have been developed using high-temperature decomposition methods and organic iron precursors [30, 116]. In one notable study by Sun et al. high-temperature reaction of iron (III) acetylacetonate, Fe(acac)3, in phenyl ether in the presence of alcohol, oleic acid, and oleylamine, yielded monodisperse, hydrophobic magnetite NPs with tunable sizes of 4–20nm [116]. The limitation of these synthesis approaches is that additional steps are required to remove the hydrophobic coating, or to modify the surfaces with an amphiphilic surfactant to render the NPs usable for biomedical applications. Though these new techniques yield more uniform NPs with superior magnetic properties, the co-precipitation method continues to be most widely used for biomedical applications because of ease of implementation and need for less hazardous materials and procedures.
3.2 Coating of SPIONs
After synthesis, unmodified SPIONs are stable in high and low pH solutions, but use in vivo requires that SPIONs be coated. These surface coatings, typically comprised of small organic molecules and polymers, function to (1) protect against iron oxide core agglomeration, (2) provide chemical handles for the conjugation of drug molecules, targeting ligands, and reporter moieties, and (3) limit non-specific cell interactions. Additionally, polymeric coatings have been engineered to enhance SPION pharmacokinetics, endosomal release, and tailored drug loading and release behaviors. To serve these coating functions, a diverse group of polymers have been investigated including PEG [81, 126, 127, 128, 129, 130, 131, 132], dextran [133, 134, 135, 136], chitosan [137, 138, 139, 140], PEI [104, 141, 142, 143, 144, 145], and phospholipids [145, 146, 147].
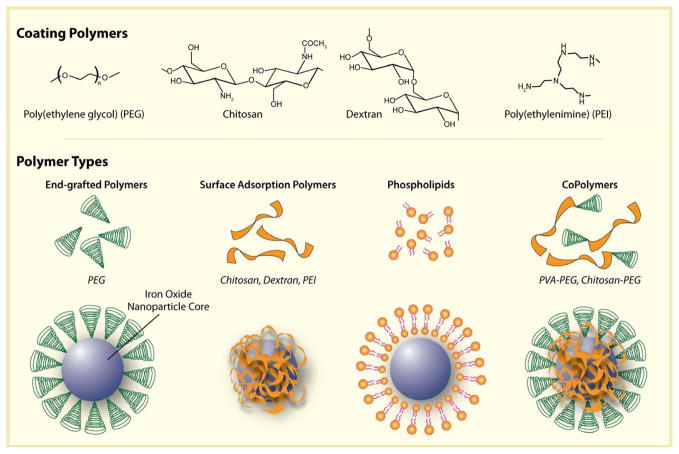
SPION coating can be achieved via a number of approaches, including in situ coating, post-synthesis adsorption and post-synthesis end grafting [15] (Figure 4). In situ and post synthesis modification with polysaccharides and copolymers lead to coatings that uniformly encapsulate cores. Alternatively, end grafted polymers (e.g. PEG) are anchored to the NP surface by the polymer end groups, forming brush like extensions. Liposome and micelle-forming molecules create a shell around the SPION core. These structures retain hydrophobic regions that can be used for drug encapsulation. Each technique retains specific advantages and disadvantages depending upon the polymer employed (e.g. ease of coating, number of functional groups, etc.)
Figure 4.
Illustration depicting the assembly of polymers onto the surface of magnetic nanoparticle cores.
Coating materials and immobilization strategies each influence the magnetic properties of MNPs in different ways. Several studies have revealed that the coating thickness, and hydrophobicity can drastically affect the magnetic properties of MNPs [145, 148]. In particular, LaConte et al showed that thicker coatings lowered R2 relaxivities [148], while Duan et al determined that polymer coatings of decreasing hydrophobicity (e.g. PEI versus octadecene coating) caused higher R2 relaxivities [145]. This illustrates the combinatorial effect that these different parameters play on the final properties of a given nanoparticle system. In the proceeding sections, we will detail some of the most common organic coatings, including their methods of attachment, functionality, and specific examples of use.
3.3 Organic surface coatings
3.3.1 Poly(ethylene glycol) PEG
PEG is a biocompatible linear synthetic polyether that can be prepared with a wide range of sizes and terminal functional groups [149]. For decades variations of this polymer have been used clinically as excipients in FDA approved pharmaceutical formulations [150]. They are neutral, hydrophilic molecules in biological fluids, which helps to improve the dispersity and blood circulation time of the SPIONs they are bound to [51, 81, 130, 131, 151, 152, 153]. PEG-coated (or PEGylated) SPIONs are commonly regarded to as “stealth” nanoparticles because they are not readily recognized by the RES [73]. This limits their use in imaging macrophages or other RES-related cells [154], but the same characteristic makes them ideally suited for use in target-specific cell labeling after modification with targeting ligands [155, 156, 157].
At sizes below 100,000 Da, PEG polymers are considered amphiphilic and are soluble in water as well as in many organic solvents, including methylene chloride, ethanol, toluene, acetone, and chloroform. This allows PEG assembly at the SPION surface using a variety of chemistries that require use of either aqueous or organic solvents. For example, Lutz et al demonstrated in situ coating of PEG onto SPIONs precipitated under aqueous conditions [158], while Kohler et al grafted PEG to SPIONs via a silane group in the organic solvent, toluene [131]. Significantly, in the latter study a hetrobifunctional PEG was prepared that could covalently attached to the SPION surface by one end and then functionalized with targeting ligands, imaging reporter molecules, or therapeutic agents by the other end [84, 85, 89, 155, 156, 159]. This represents a polymeric design strategy that is conscious of both efficient surface coating and functionalization after PEG attachment.
3.3.2 Dextran
Dextran is a branched polysaccharide comprised of glucose subunits that can be prepared with sizes ranging from 10 to 150 kDa. The polymer’s wide use in SPION coatings has been attributed to its biocompatibility and its polar interactions (chelation and hydrogen bonding) that give dextran a high affinity for iron oxide surfaces [160]. As such many of the clinically approved SPION preparations are dextran coated [120, 161, 162, 163, 164, 165]. Typically, these coatings are prepared by in situ techniques, as was first described in 1982 by Molday and Mackenzie [134]. Since then, various forms of dextran polymers, including carboxydextran and carboxymethyl dextran, have been used to coat SPIONs with varying hydrodynamic sizes [15].
Conventional dextran coatings are based on hydrogen bonding, making the polymer susceptible to detachment, but the polymers have been crosslinked after SPION attachment using epichlohydrin and ammonia, forming a CLIO [166]. CLIOs, have become a versatile platform that has demonstrated a high circulation half life in blood with no acute toxicity [167]. However, due to the use of epichlohydrin and an inability to degrade and clear from the body, their use in a clinical setting is unlikely [9]. To replace the need for cross-linking, Duguet et al created a multistep process to covalently bind dextran to the SPION surface using silane chemistry, and is expected to yield increased interest [136, 168].
3.3.3 Chitosan
Chitosan is a cationic, hydrophlilic polymer that is also nontoxic, biocompatible, and bioabsorbable, which has made it a popular material for drug delivery applications in recent years [169, 170]. This is primarily due to its large abundance in nature, biocompatibility, and ease of functionalization. For decades chitosan and its derivatives have been used to form polymeric nanoparticles through electrostatic complexation with nucleic acids and various pharmaceutical formulations [169], only recently being used in combination with magnetic nanoparticles [140, 171, 172, 173, 174, 175, 176].
To coat SPIONs with chitosan, it has been found that direct, in situ coating is problematic because of its poor solubility at pH values necessary to precipitate SPIONs [170]. Chitosan-coated SPIONs have been produced, though, by physically adsorbing chitosan onto oleic acid-coated nanoparticles yielding spherically shaped SPIONs (15 nm diameter) [175]. The cationic nature of the polymer allows complexation with genetic material making it suitable for use as a gene delivery carrier, even when used as a SPION coating. For example, Bhattarai et al. loaded chitosan-coated iron oxide nanoparticles with anionic adenovirus vectors through electrostatic interactions [174]. These SPIONs were used to enhance gene transfection. In addition to its bioadsorbtive properties, chitosan possesses both amino and hydroxyl functional groups, which can be used for SPION functionalization with targeting, imaging, and therapeutic agents.
3.3.4 Polyethyleneimine (PEI)
PEI is another water soluble cationic polymer that can take both linear and branched forms [177]. For decades PEI-based polymers has been used for gene delivery thanks to their ability to complex with DNA, facilitate endosomal release via the “proton sponge effect”, and guide intracellular trafficking of their cargo into the nucleus [177, 178]. To capitalize on these properties, PEI has been integrated into SPION coatings in recent years [141, 142, 143, 144, 179]. Naturally, the most common application of these constructs has been for in vitro cell transfection with either DNA or siRNA nucleotides [141, 180].
SPION attachment has been performed by in situ coating [179], post-synthesis adsorption [145], and post-synthesis grafting [143, 144]. While successful binding has been shown, several problems remain, including PEI’s intrinsic toxicity [177] and low colloidal stability of SPION constructs in biological solutions [143, 181].
3.3.5 Liposomes and Micelles
Liposomes and micelles, spherical aggregates of amphiphilic molecules, can be used to coat SPIONs in two ways: post-synthesis incorporation or by synthesizing SPIONs directly within their open core. In the first case, water-soluble SPIONs have been confined to the aqueous center of the liposome [135, 182], or alternatively, hydrophobic SPIONs can be coated with micelles around the structure [183, 184, 185]. In the second case, SPIONs can be precipitated in the liposomal core, which yield highly uniform NPs with sizes as small as 15 nm in diameter [184].
SPION coating with either liposomal and micellular structures provide SPIONs with important advantages, especially when using the construct in drug delivery applications, including: (1) simple and easy surface modification, (2) convenient encapsulation of pharmaceuticals inside the amphiphilic substructures, and (3) sequestration and protection of pharmaceuticals from the body until degraded in target cells [186]. However, when applying these coatings, there is a danger in coating agglomerates rather than discrete SPION cores in micellular or phospholipid structures, leading to poor physiochemical and magnetic properties [187].
3.3.6 Copolymers
Copolymers have been developed to take advantage of the distinct functionalities derived from its constituents. For example, Veiseh et al demonstrated that by joining PEI and PEG polymers, a new polymer structure is created that can both complex DNA to facilitate cell transfection (PEI functionality), and enable a stealth profile necessary for molecular targeting of cancer cells (PEG functionality) [188].
The advantages these copolymers provide can be applied to SPION coatings. For instance, Kievit et al recently developed a SPION coated with a copolymer of PEG-g-chitosan-g-PEI and demonstrated its use as a DNA delivering nanovector [189]. This study demonstrated that a coating comprised of three polymers grafted together was ideally suitable for DNA complexation, stabilization of NP for in vivo use, and gene transfection. Guo et al developed a triblock PEG-poly(methacrylic acid)-poly(glycerol monomethacrylate) copolymer that was used to coat SPIONs via in situ coating (23 nm diameter) [190]. This yielded a unique pH sensitive coating with a hydrophobic center layer that could encapsulate drug molecules, preferentially releasing the therapeutics in the acidic environment of the cellular endosome. Similar copolymers are being investigated that can attach to the SPION surface by layer-by-layer deposition directed by matching of electrostatic interactions [191], hydrophilic/hydrophobic interactions [183, 184], and covalently grafting polymer layers to base coatings [192].
3.4 Surface modification chemistry
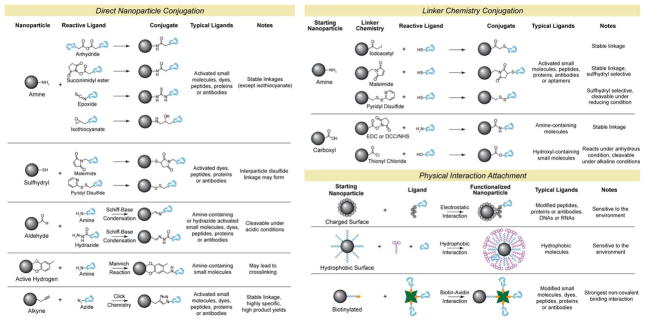
A number of chemical approaches have been used for the conjugation of targeting, therapeutic, and imaging reporter molecules with NP surfaces. These can be categorized into covalent linkage strategies (direct nanoparticle conjugation, click chemistry, covalent linker chemistry) and physical interactions (electrostatic, hydrophilic/hydrophobic, affinity interactions). The choice of chemistry is dictated, in part, by the physicochemical properties and functional groups found on the SPION coating and ligand to be linked. The primary goal is to bind the targeting, imaging, or therapeutic moiety without compromising its functionality once attached. Functionality in such assemblies is dictated by the nature of the ligand (e.g. conformation of biomolecules) and the manner in which it is attached. For example, if an antibody is bonded to the NP such that its recognition site is shielded, it may lose its ability to bind a target. Furthermore, engineering the attachment of therapeutics carries the additional burden of integrating a release mechanism into the NP. Table 1 lists several examples of chemistries that can be used at the SPION surface, reflecting a broad spectrum of approaches that have been evaluated in the literature. The reactions have been categorized by conjugation strategy, NP functional group, and the reactive functional group on the ligand to be attached. Also listed are unique features of each linkage formed. For detailed information on each of these agents and conjugation strategies, readers are directed to refer to G. Hermanson’s handbook [193]. In the following sections we will review unique advantages and drawbacks of each strategy described in Table 1, and highlights example application of the strategy in bioconjugation.
Table 1.
Examples of various SPION surface modification chemistry.
 |
3.4.1 Covalent linkages
Covalent linkages are strong and stable bonds, which can be specifically formed between functional groups, typically amino, carboxylic acid, and thiol groups found on the NP surface and conjugated ligands. Usually, these functional groups are added to the NP surface via its polymer coating, which can dictate both the type and number of functional groups on each NP. These chemical handles are found either on the body of the polymer (chitosan, PEI, dextran) or at their terminal ends (PEG). More binding sites can be added per polymer chain, on its body, thus affecting the total number of reactive groups available. For example dextran-coated SPIONs (38 nm) have been reported with 62 reactive amino groups per NP [194], while a larger PEG-coated SPION (64 nm) was reported to have 26 reactive amino groups per NP [89]. These same chemical groups are also found on the targeting, optical, or therapeutic agent to be covalently attached. To link the functional groups a host of chemistries are available (Table 1), which are subdivided into direct reaction, click chemistry, and linker strategies.
3.4.1.1 Direct nanoparticle conjugation
In direct reaction strategies functional groups at the NP surfaces are either directly bonded to reactive ligands, or a linkage reaction is facilitated with the aid of catalysts. As listed in Table 1, NP surfaces functionalized with amine, sulfhydryl, aldehyde, and active hydrogen functional groups can be targeted. These strategies are particularly suitable for small molecule conjugation. In one notable study, 46 different NP systems were prepared from the same dextran-coated base NP, each functionalized with a different small molecule [194]. However, the efficiency of these chemistries is variable, and with the exception of amine-functionalized NPs, direct conjugation methods are susceptible to intercalating or cross-linking. Specifically, NPs may crosslink due to disulfide linkage formation between NPs, or when multiple NPs bind a single ligand bearing multiple amino functional groups. Moreover, biomolecules are not natively reactive with NPs, requiring initial modification prior to conjugation. This can be a challenging task because many biomolecules can loose bioactivity through modification, and often as demonstrated in a study by Shellenberger et al, only precise, limited modifications can be tolerated [195]. Therefore, chemical modification must be controlled to limit loss of biofunctionality. However, it is typically difficult to perform with direct conjugation agents such as glutaraldehyde. This amine group crosslinking reagent can denature proteins and peptides (abundant with amine groups), and thus has limited applicability for biomolecule-NP attachment.
3.4.1.2 Click Chemistry
“Click” chemistry is a relatively new approach of direct conjugation, developed by Sharpless et al almost a decade ago [196]. Representing a set of Cu-catalyzed azide–alkyne chemistries (Table 1), click chemistry was developed to make conjugations between bioactive surfaces easier and less harsh to biomolecule ligands [197, 198]. Specifically, click reactions are fast, efficient, require mild reaction conditions (aqueous environment, relatively neutral pH), and create water-soluble and biocompatible linkages (electron configuration similar to amide bonds) [198]. Compared to other direct conjugation strategies this method of attachment offers several unique features. First, azide and alkyne reactive groups are highly specific for one another, and unreactive with most functional groups, ensuring specific conjugation at the desired location(s) on the reactive moiety. Second, the formed bonds are highly stable. This is in contrast to amide bonds, which can be cleaved by hydrolysis reactions, and disulfide linkages that are susceptible to cleavage under reducing environments. Third, the formed linkages are extremely rigid, which helps to maintain conformation of reacted moieties at the MNP surface and prevents their cross interactions. Combined, these features enable the production of highly oriented linkages engineered to ensure optimal reaction activity and efficiency. This technique appears to be specially suited for attachment of targeting moieties where orientation and stability of linkages are particularly important. As such this chemistry has been implemented with SPIONs, effective at binding targeting biomolecules to the NP surface with > 90% efficiency under mild reaction conditions and reaction times of 5–8 hours [199, 200].
Though this technique has many unique advantages there are limitations to its implementation. First, even though the formed linkages are biocompatible, the Cu catalyst needed to drive the reaction can lead to problems in vivo if not properly purified before use. Excess Cu consumption has been linked to a number of disorders such hepatitis, neurological disorders, kidney diseases, and Alzheimer’s disease [198]. Therefore, extensive measures are required to remove all of the catalyst from MNP solutions post-conjugation. Second, the highly stable linkages formed may inhibit MNP biodegradation. Further investigation is needed towards the long-term toxicity of MNPs developed using click chemistries.
3.4.1.3 Linker Chemistry
These linker molecules offer control over the molecular orientation of bound ligands, critical when protecting targeting ligand functionality. Additionally linkers can be selectively cleaved for use in ligand quantification, controlled release and other applications. SPION surfaces with amine or carboxylic acid functional groups have been modified with heterobifunctional molecules and further reacted with ligands as shown in Table 1. The most common approach here is the use of a linker molecule that binds the amine group of a SPION surface with the sulfhydryl of a biomolecule. In the case of proteins and peptides cystine amino acid residues can be targeted for reaction. If no reactive cystine amino acids are present, lysine, or terminal primary amine groups can be thiolated with trauts reagent (2-Mercaptoethylamine·HCl reagents) [156], or SATA (N-Succinimidyl S-Acetylthioacetate) [85]. In the latter case the introduced sulfhydryl group is initially protected to prevent undesired crosslinking prior to reaction with NPs. Oligonucleotide based molecules such siRNA biomolecules have been chemically synthesized to contain sulfhydryls which then could be conjugated to SPIONs using linker chemistry [201]. This chemistry method is especially suitable for reactions with complex biological molecules where multiple reactive sites and sensitivity to over-labeling are a concern.
The superiority of linker chemistry over direct conjugation strategies was highlighted in a notable study by Hogman et al where SPIONs decorated with the transferring (Tf) protein were prepared using pyridyl disulfide (PD) heterobifunctional linker chemistry or direct conjugation through Shiff base catalyzed reaction [202]. The comparison revealed that conjugation using PD linker chemistry allowed approximately a 4-fold increase in the number of Tf molecules attached per SPION, and a 10-fold improvement of binding and uptake by cells, resulting in a 16× more sensitive SPION for imaging. Linker chemistry offers better control over the binding sites used in ligand conjugations, increasing the number of active Tf proteins at the NP surface. In addition, a milder reactive condition of this chemistry limits the oxidative conditions that may harm the bioactivity of the protein during conjugation.
Pyridyl disulfide (PD) heterobifunctional linkers are also interesting because they produce cleavable disulfide linkages and a quantifiable reaction byproduct, which can be used to evaluate efficiency of reaction. Schellenberger et al demonstrated this utility in the preparation of SPIONs decorated with annexin V targeting ligands [203]. Though the bonds formed via PD linker chemistry are sensitive to reducing environments, more stable linkers, like iodoacetyls or maleimides, can be used if the application demands it. Linker chemistry does have its drawbacks, including covalent complexation between NPs or ligands, requiring stepwise NP modification prior to ligand attachment, and some linker chemistries require long reaction times and purifications between each step. This also may contribute to the low product yield, as loss of desired product can occur at each purification stage.
SPIONs decorated with carboxylic acid groups can be covalently bonded to biomolecules bearing primary amines through EDC/NHS linkers, which form amide linkages. This approach has been used in the attachment of aptamer [204] and folic acid [131] to SPIONs. While effective for the attachment of molecules that have only one amino group, it is difficult to control the binding orientation of ligands with multiple amines, often leading to inactivation of the ligands. Therefore, amino-decorated NPs conjugated to ligands using sulfhydryl-based linker chemistry, as described above, is the preferred conjugation approach for the attachment of peptides, proteins, antibodies, and enzymes to SPIONs.
3.4.2 Physical interactions
Physical interactions include electrostatic, hydrophilic/hydrophobic, and affinity interactions, as highlighted in Table 1. There are several unique advantages of this chemistry, including: rapid speed of binding, high efficiencies, and no need for intermediate modification steps. Electrostatic interactions have particularly proved useful in the assembly of plasmid DNA onto SPIONs. Several research groups have demonstrated this utility by creating SPIONs coated with cationic polymers of PEI, which are then complexed with negatively-charged plasmid DNA molecules [141, 142, 143, 144, 180]. A recent study also demonstrated the feasibility of using electrostatic interactions for binding cationic proteins to an anionic SPION surface [205].
Hydrophobic/hydrophilic interactions have proved highly useful when adsorbing hydrophobic drugs onto SPIONs. For this application, SPIONs are engineered with hydrophobic layers that can adsorb hydrophobic drugs that are then triggered for release intracellularly when the coating degrades [32, 206, 207]. This strategy has drawbacks, which include NP sensitivity to environmental conditions and low control over molecular orientation of bound ligands. Thus while suitable for drug delivery applications where the attached molecule is released for functionality, attachment of targeting ligands through these strategies are unattractive.
Affinity interactions on the other hand have shown to be very effective for bioconjugation of targeting ligands to SPIONs [89, 208]. As shown in Table 1 SPION surfaces can be modified with streptavidin, which specifically binds biotinylated molecules. The linkage formed is highly stable and the strongest of all non-covalent linkages chemistries. Unlike hydrophobic and electrostatic interactions, affinity binding is not sensitive to environmental conditions such as changes in pH, salinity, or hydrophilicity. Using this strategy Gunn et al produced high affinity multivalent display of targeted SPIONs for immunotherapy applications [89].
3.5 SPION targeting strategies
Targeting agents, including antibodies, proteins, peptides, aptamers and small organic molecules, have been used in SPION systems as targeting agents against specific surface markers on target cells.
Table 2 describes several examples for each targeting agent type. These agents have been subcategorized into small organic molecules, peptide, aptamers, and antibodies. Listed is the name of targeting ligand used, its target, and application. It should be noted that some of these agents serve dual purposes, such as chlorotoxin (CTX), which can act as both targeting agent and brain tumor therapeutic. In addition, some of these agents can help initiate endocytosis of the NPs to which they are bound, making them particularly attractive for drug delivery applications.
Table 2.
Example molecular targeting strategies combined with SPIOS, their cellular targets, applications, and functionality for therapeutic applications.
| Name | Target | Application | Internalized ? | Therapeutic ? | Published Reports | |
|---|---|---|---|---|---|---|
| Small Organic Molecules | Folic acid | Folate receptor | Breast cancer imaging | Yes | No | [84] |
| Methotrexate | Folate receptor | Brain tumor imaging and therapy | Yes | Yes | [83, 209] | |
| Non-peptidic RGD mimetic | avβ3 integrin | Integrin positive cell imaging | No | No | [210] | |
| Mimetic of the sialyl Lewisx | E-selectin | Inflammatory disease imaging | Unclear | No | [88] | |
| Peptides | RGD | avβ3 integrin | Breast cancer imaging | No | No | [86, 100] |
| Chlorotoxin | MMP-2 | Brain tumor imaging and therapy | Yes | Yes | [85, 155, 156, 159] | |
| Synaptotagm in I, C2 domain | Phospholipids | Apoptosis imaging | No | No | [211] | |
| VHSPNKK | Endothelial vascular adhesion molecule-1 | Cardiovascular disease imaging | Yes | No | [212] | |
| EPPT1 (YCAREPPT RTFAYWG) | Underglycosylated mucin-1 antigen | Multiple tumor type imaging | Yes | No | [213] | |
| Aptamers | A10 RNA aptamer | Prostate-specific membrane antigen | Prostate cancer imaging | Yes | No | [204] |
| Thrm-A and Thrm-B DNA aptamers | Human alpha-thrombin protein | Serum protein detection | N/A | No | [95] | |
| Proteins | Annexin V | Phosphatidylserine | Apoptosis imaging | No | No | [203, 214] |
| Luteinizing hormone releasing hormone (LHRH) | LHRH receptor | Breast cancer imaging | Yes | No | [74] | |
| Transferrin | Transferrin receptor | Breast cancer imaging | Yes | No | [75] | |
| Antibodies | Monoclonal antibody A7 | Colorectal carcinoma | Colon cancer imaging | No | No | [215] |
| Herceptin (Trastuzumab) | Her2/neu (Breast cancer) | Breast cancer imaging and therapy | No | Yes | [216, 217] | |
| Rituxan (Rituximab) | CD20 antigen (B-cell non-Hodgkin lymphoma) | Lymphoma imaging therapy | No | Yes | [218] |
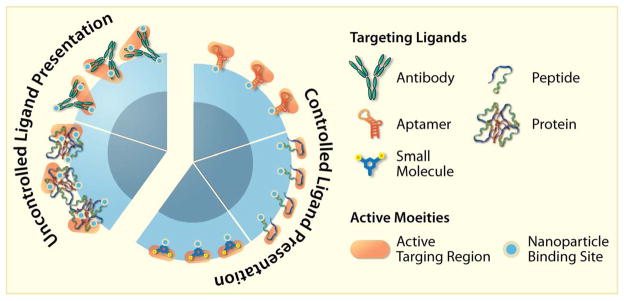
While each targeting agent enables SPION binding specificity, the type of ligand and method of NP attachment can significantly affect its targeting capabilities. Figure 5 gives a graphical representation of how these molecules are typically organized onto the NP surface, and depict some of the inherent advantages and disadvantages of each ligand. For instance, relatively bulky proteins and antibodies are difficult to assemble onto a surface. This can be due to the lack of consistent covalent binding orientation of the protein (there are often many active functional groups per molecule, and it’s difficult to regulate which gets bonded), or due to non-specific physical interactions by a protein’s charged, hydrophobic or hydrophilic regions with the NP surface before being covalently attached. Importantly, this loss of control can limit the presentation of protein binding sites outward, lowering its binding activity.
Figure 5.
Illustration of the supermolecular assembly and presentation of targeting antibodies, proteins, peptides, aptamers and small molecules on the surfaces of SPIONs. Note that protein and antibody assembly is difficult to control. Small organic molecules do assemble well but their small size may cause their active targeting regions to be sterically blocked by polymeric coatings. Peptides and aptamers assembly can be controlled through their engineering, and can be modified to assemble in a manner that ensures their active sites are available for interaction with targets on cell surfaces.
Alternatively, smaller peptides and nucleic acid-based aptamers can be engineered to have only one active molecular handle per molecule, to ensure consistent linking to the NP surface, and no loss of binding activity. This has been shown to provide significant multivalent binding activity [9, 96, 100]. In addition, small organic molecules can also be engineered to assemble in high densities, but may require long linker molecules to ensure that the NP coating does not obscure the active region.
In addition to ligand bioactivity, these molecules also affect NP stability and immunogenicity. For instance, antibodies and proteins are often derived from non-human animal sources, which create the possibility of unwanted immune responses. Alternatively, peptides and aptamers can be chemically synthesized and have been shown to be non immunogenic [219].
4. MNP drug delivery vehicles
4.1 Chemotherapeutic agents
Chemotherapeutics encompass a broad category of small organic drug formulations, which have been developed to initiate a therapeutic response via cytotoxic, cytostatic, or antineoplastic effects. Most chemotherapeutics do not have cell-targeting capabilities (notable exceptions are highlighted in Table 2) and can elicit unwanted side effects when internalized by healthy cells. However, their integration into target-specific NP formulations can limit unwanted side effects, while increasing the dosage at the diseased tissue.
Successful NP drug delivery devices have a prolonged circulation time (small particles < 200 nm hydrodynamic size), are internalized by targeted cells, can carry a chemotherapeutic payload, and can be engineered to release its drugs after cell internalization. Currently, several chemical drug formulations have been combined with MNPs, including paclitaxel, doxorubicin, and methotrexate (MTX), all specifically developed for cancer therapy [206, 209, 220, 221].
To successfully integrate a drug into a NP system, several design strategies can be explored, including physical complexation with hydrophobic drugs [190, 206, 207, 220, 222], or covalent bonding with cleavable linkages for intracellular release [209]. Drugs loaded through hydrophobic interactions are typically encapsulated within the NP coating, limiting non-specific cell interactions. This approach is advantageous in applications where a drug being delivered could seriously harm non-targeted tissue.
Alternatively, in applications where the drug, such as MTX has an affinity for the target cell, it can be advantageous to graft the drug to the surface of the NP [83, 156, 209]. Kohler et al. first demonstrated this utility in a study where MTX was covalently attached to the surface of a PEG-coated SPION via a cleavable amide linkage [209]. Recently, Sun et al further modified the same SPION system with CTX to enhance the NP’s targeting abilities against brain tumor cells [156].
4.2 Radiotherapeutics
Radionuclides (particularly β-emitters) can function as therapeutic agents, because their localized decay in target cells generates DNA damaging free radicals, which can induce apoptosis. Over the past few decades various targeting strategies including conjugation with antibodies and peptides, have been investigated to help direct radionuclide away from healthy tissue, paralleling chemotherapeutic development [223].
SPIONs and other nanocarriers have recently been evaluated as radionuclides [224]. Compared to the integration of chemotherapeutics into a NP system, radionuclides pose a unique engineering challenge, because they are continuously decaying. In addition, while biotherapeutics and, to some extent, chemotherapeutics can be engineered to illicit therapeutic effects only on to their target cells, radiotherapeutics can damage practically any cell of the body. To limit the possibility of non-specific cellular damage, the SPION-radiocleotide complex must remain intact, even after cell uptake, during the radiation decay. This can limit the potential for radionucleotide interaction with non-targeted cells.
Most of the radionuclide-containing SPIONs developed so far have been prepared using 188Re radioactive isotopes (17 hour half-life) [225, 226, 227, 228]. Histidine decorated SPIONs are known to chelate these radionucleotides enabling attachment. These studies include 188Re-enabled SPIONs that were functionalized with albumin [225], or with specific antibodies for liver cancer targeting. The latter study successfully demonstrated the ability to specifically induce cell death in the targeted liver cancer cell line in vitro [227].
4.3 Biotherapeutics
4.3.1 Therapeutic peptides/antibodies
Peptides and antibodies function in a cell-specific manner, eliciting therapeutic effects by inhibiting or stimulating various cellular pathways making them attractive therapeutic agents. These biotherapeutics can be used against a number of cell mechanisms, including: activation of apoptotic/necrotic pathways, function blocking (e.g. interfering with cell adhesion, cell surface receptors, angiogenesis, or inhibiting protease and kinase action), and immune response stimulation [229].
SPIONs can be used as biotherapeutic carriers for peptides, and more importantly, these NPs can leverage the multivalent display of the biotherapeutics to improve its potency as a therapeutic agent, itself. At the same time, some biological pathways may require that the SPIONs be internalized to be effective. Here, SPIONs must have an appropriate size (~ 25–50 nm hydrodynamic size [102]) and coating (e.g. PEG, which can facilitate internalization [81]) to induce uptake. In a recent report, Veiseh et al demonstrated the enhanced potency of the CTX peptide at the SPION surface [159]. The CTX peptide has a high affinity for a set of lipid raft-anchored complexes that contains matrix metalloproteinase-2 (MMP-2) and chloride ion channels which are necessary to sustain the glioma cancer cell’s invasive nature. These NPs showed improved internalization via receptor-mediated endocytosis, subsequently impeding the target cell’s ability to invade neighboring tissue.
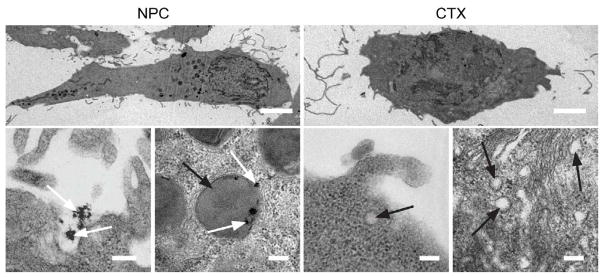
In this study, CTX-decorated SPIONs (NP-CTX) exhibited substantially enhanced cellular uptake, and showed an increased invasion inhibition rate compared to free CTX (98% versus 45%). As shown in Figure 6, TEM studies of glioma cells treated with NP-CTX (Figure 6a) or free CTX (Figure 6b) revealed that NP-CTX treatment facilitated internalization of larger volumes of MMP2 containing lipid rafts compared to free CTX. Additional assays were performed, illustrating that NP-CTX, owing to multivalency, was more efficient at limiting glioma cell invasion by promoting the internalization of cell surface-bound peptidases and volume regulating channels.
Figure 6.
TEM images showing increased membrane uptake subsequent to NP-CTX (NPC in figure) binding. Scale bars represent 5 mm for whole cell images (first row) and 200 nm for high magnification images (second row). White and black arrows identify NP-CTX and endosomes, respectively. Reproduced with permission from Wiley-VCH Verlag GmbH & Co. KGa: Small [159] Copyright 2009.
SPIONs decorated with antibodies have also been evaluated for therapeutic applications. A notable example includes the use of the antibody Herceptin (commercially marketed as Trastuzumab), which targets the Her2/neu receptor [230]. Her2/neu is characterized to be a growth factor, upregulated on cell surfaces of 20–30% of early stage breast cancer tumors, and essential for cell proliferation. Clinical evidence has revealed that the interaction of Herceptin with this receptor leads to its inactivation, and subsequent inhibition of cell proliferation. As such SPIONs have been decorated with Herceptin, and evaluated as targeting/biotherapeutic NPs [216, 217].
4.3.2 Gene therapy
Gene therapy describes the use of DNA and antisense RNA (siRNA) technologies for therapeutic gene expression, and expression silencing of defective genes, respectively. These therapeutics have been integrated into MNP preparations, which have helped to protect the nucleic acids against enzymatic degradation and facilitate cellular internalization and endosomal release [104, 231]. To accomplish this, most MNP systems have been engineered using cationic polymers such as PEI [143], polyamidoamine [232], or chitosan [174]. These coatings can complex negatively charged nucleic acids, and assist endosomal release by inducing acidification of endosomal vesicles (“the proton sponge effect”) [180]. While these approaches have shown great success in vitro, their applicability in vivo has been limited because of toxicity and stability concerns. Highly cationic MNPs, specifically PEI coated ones, have shown poor stability in biological solutions, and potential for in vivo toxicity [181]. New preparations that can address these problems are still needed.
One alternative to cationic coatings was offered in a study by Medarova et al [201]. Here, antisense RNA was bonded to CLIO NPs by covalent linkages, while a cell penetrating peptide was used to facilitate transfection. This strategy proved to be highly successful for the delivery of therapeutic siRNAs to human colorectal carcinoma tumors in vivo, and represents the first targeted/siRNA MNP used for therapeutic application.
5. Applications of SPIONs for in vivo imaging
5.1 Imaging modalities
SPIONs were developed specifically as MR imaging contrast agents, but new preparations are being developed that incorporate multiple imaging moieties onto the MNP for use in integrated imaging systems [233]. These multimodal agents can assist investigators to visualize the MNP across different platforms, including MR, optical, or nuclear imaging systems [234]. This offers clinicians the ability to obtain a variety of pathologic information using the unique imaging capabilities of each system, with a common contrast agent [233].
5.1.1 MR Imaging
MR imaging, one of the most effective tools in medicine, offers clinicians the ability to non-invasively obtain anatomic, and metabolic/functional information with high spatial and temporal resolution [28]. This imaging technique uses high magnetic fields to align the nuclear magnetization of the body’s hydrogen atoms to which a radio frequency (RF) pulse is applied that changes the alignment of these nuclei. When the RF pulse is removed, the nuclei “relax” back to their original state. This process can be measured by either its longitudinal relaxation (T1) or transverse relaxation (T2), each of which can be used to generate an MR image. Variation in relaxation rates corresponds to image contrast, allowing for discrimination between tissue types. MR imaging has the benefit of a high three dimensional spatial resolution and high contrast differentiation between soft tissues, which enables simultaneous extraction of physiological, molecular and anatomical information.
Contrast agents developed for MR imaging include MNPs and paramagnetic chelates (e.g. Gd atoms chelated by DTPA, DTPA-BMA, and DTPA-BMEA). The localized interaction of these agents with protons of water molecules creates contrast by reducing T1 or T2 decay relaxation times. SPIONs are typically more effective at shortening T2 rather than T1 relaxation times [22].
Currently, with more advanced MR imaging systems and contrast agents this technique can offer a spatial resolution of 10–100 μm, with no imaging depth limitations (unlike other imaging systems) [233]. At the same time, MR imaging requires relatively long acquisition times (minutes to hours), that patients be placed in the confines of the MR imaging machine, and shows high sensitivity to motion artifacts.
5.1.2 Optical imaging
Optical imaging in vivo typically relies on the monitoring of photons emitted from fluorescent agents in the near infrared range (NIR; ~650–900 nm wavelengths), because of their ability to efficiently pass through biological tissue [22, 235]. These fluorescent molecules absorb light of a particular wavelength, and emit light back at a longer wavelength [233].
Fluorescent contrast agents are most often used in fluorescence reflectance imaging and fluorescence-mediated topography systems. These techniques are highly useful in molecular screening of surface-based diseases or facilitation of disease resection. State of the art systems provide resolutions on the order of millimeters, and imaging depths up to 10 cm from the surface [233]. Significantly, these techniques offer continuous imaging capabilities (imaging is on the order of seconds). Conversely, optical imaging suffers from poor resolution and limited penetration of light.
5.1.3 Positron Emission Tomography (PET) Imaging
Positron emission tomography (PET) is a nuclear medicine imaging technique that relies on using pairs of high-energy gamma rays produced indirectly by decay of positron emitting radio nucleotides (tracers) introduced into the body. Through this technique, quantitative, tracer concentration dependent, three-dimensional images can be constructed illuminating functional biological processes. Independently this technique does not provide anatomical information. Currently available PET systems offer a relatively poor effective spatial resolution of approximately 1 mm [236]. However, in comparison to other imaging modalities PET offers extremely high sensitivity allowing for detection of as few as 10−11 moles of radionuclides deposited in the living subjects.
Radionuclides used for PET imaging include isotopes with short half-lives. Some examples include carbon−11 (~20 min), nitrogen−13 (~10 min), oxygen−15 (~2 min), and fluorine−18 (~110 min). For imaging applications these agents can either be incorporated into metabolites (e.g. sugar, water, oxygen), or linked to targeting ligands or nanoparticles including MNPs [236].
5.1.4. Conventional imaging compounds vs. MNPs
The purpose of a contrast-imaging agent is to report its location, thus providing physiological or biochemical information about the tissue around it. The success of the agent is dependent on both the concentration at which it accumulates at target site and the strength of the signal it emits. For example, radionuclides can produce highly energetic signals where as few as 10−11 moles of radonucleitides can be detected. At this signal to noise ratio a single targeting ligand carrying a radionucleotide can be detected in a living organism [236]. A fluorescent contrast agent has a detection sensitivity of ~10−8 moles, one thousand times weaker. This means that to achieve the same sensitivity as PET, an optical imaging agent would have to either accommodate 1000 fluorophores or the target site would have to bind 1000 targeting ligand-fluorophore conjugates. Neither scenario could be practically reached. Gadolinium chelates in water emit signals detectable ~10−4 mole concentrations roughly 10,000 times even weaker than fluorophores making it much more difficult to image using this agent.
An NP platform can be used to overcome these limitations. The number of imaging reporter and targeting ligands can be tuned to achieve desired signal to noise sensitivity. MNPs are ideally suited for use in these imaging platforms because of their intrinsic magnetic properties. Their detectability is dependent on the size, crystallinity, and coating of the material. However formulations can be created with a detection sensitivity of 10−12 M [237]. In practice the magnetic sensitivity depends on parameters such as magnetic field strength, gradient characteristics, and acquisition time [236].
MNPs can be additionally modified with other reporters to create multimodal imaging agents. For example NIR fluorophores have been attached to MNPs to create multimodal contrast agents that offer both the high spatial and temporal resolution and deep tissue penetration of MR imaging and rapid response and sensitivity of optical imaging [22]. Applications of these constructs include cell death monitoring, intra-operative imaging, and epithelial lesion detection [233]. Several studies highlighted the use of these constructs for preoperative diagnosis, and intra-operative resection of brain tumors [85, 238, 239]. SPIONs have similarly been labeled with 64Cu radionuclides and arginine-glycine-aspartic (RGD) peptides to create a multifunctional PET/MRI contrast agent for imaging of integrin expressing tumors [240]. The multimodal imaging approach can facilitate verification of the accuracy in tumor detection and provide additional information regarding the pathology of the tumor.
5.2 Dual imaging and drug delivery applications
MNPs engineered as drug delivery devices retain the ability to track their movement through the body. This is significant because it allow clinicians to monitor the effectivity of injected therapeutics to reach their target sites. There remains significant flexibility in the contrast agents implemented in these constructs and the manner in which drugs are delivered. Here we profile two notable examples of what this technology can offer.
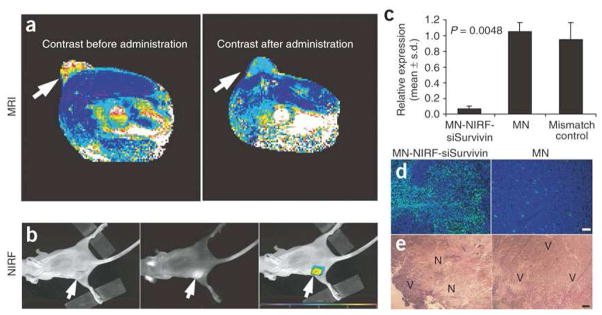
Medarova et al recently developed a CLIO modified with a NIR fluorophore, therapeutic siRNA sequences, and a cell penetrating peptide [201]. The MNP used passive targeting by the EPR effect to direct tumor localization. In vivo, these MNPs demonstrated therapeutic efficacy against target tissue, as determined by real time PCR and histological evaluation, while simultaneously demonstrating image contrast in both MR and optical imaging (Figure 7).
Figure 7.
(a) In vivo MRI of mice bearing subcutaneous LS174T human colorectal adenocarcinoma (arrows). There was a significant drop in T2 relaxivity in images acquired after administration of the contrast agent (P = 0.003), indicating probe delivery. (b) A high-intensity NIRF signal on in vivo optical images associated with the tumor following injection of MNP-NIRF-siSurvivin confirmed the delivery of the probe to this tissue (left, white light; middle, NIRF; right, color-coded overlay). (c) Quantitative RT-PCR analysis of survivin expression in LS174T tumors after injection with either MNP-NIRF-siSurvivin, a mismatch control or the parental magnetic nanoparticle (MNP). Data are representative of three separate experiments. (d) Note distinct areas with a high density of apoptotic nuclei (green) in tumors treated with MNP-NIRF-siSurvivin (left). Such areas were not identified in tumors treated with the control MNPs (right). Sections were counter-stained with 6-diamidino-2-phenylindole (DAPI, blue). (e) H&E staining of frozen tumor sections revealed considerable eosinophilic areas of tumor necrosis (N) in tumors treated with MNP-NIRF-siSurvivin (left). Tumors treated with MNPs were devoid of necrotic tissue (right). Purple hematoxiphilic regions (V) indicate viable tumor tissues. Scale bar, 50 μm. Reprinted by permission from Macmillan Publishers Ltd: Nature Medicine [201], copyright 2007.
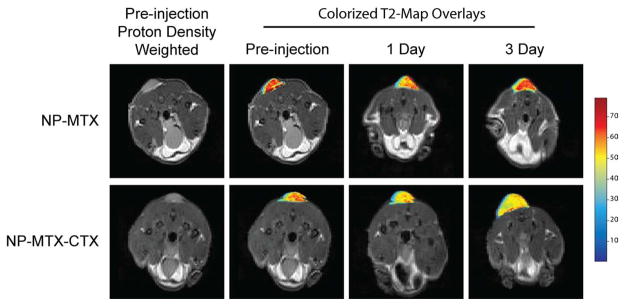
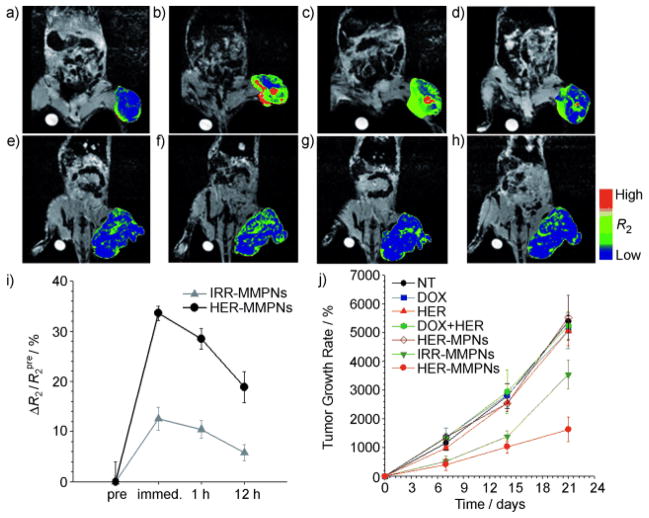
In a study by Sun et al, active cell targeting was shown by PEG-coated SPIONs to which the chemotherapeutic, MTX, and targeting molecule, CTX, were attached [155]. Shown in Figure 8 are MR images obtained from mice with flank 9L brain tumors injected with NP-MTX or NP-MTX-CTX. The selective contrast enhancement of the 9L brain tumor by these SPIONs indicates preferential accumulation compared with the same SPION construct without the CTX peptide in a 3-day study.
Figure 8.
Axial cross sections displaying 9L tumors of mice before injection of nanoparticle conjugates and 1 and 3 days post-injection. T2 map overlays of the tumor region show decreased T2 for both NP-MTX and NP-MTX-CTX nanoprobe conjugates 1 day after administration. However, the reduction is more significant and uniform in tumor of mouse receiving NP-MTX-CTX. A total of 3 days post-injection, the tumor T2 values of the mouse receiving NP-MTX-CTX remained at the decreased level, while those of mouse receiving NP-MTX returned to the post-injection level suggesting clearance of NP-MTX from tumor tissue. (CTX = Chlorotoxin, MTX = Methotrexate, NP = Nanoparticle). Reproduced with permission from Future Medicine Ltd: Nanomedicine [156] Copyright 2008.
In another recent study by Yang et al. simultaneous targeted drug delivery and MR imaging of breast cancer tumors were demonstrated through the use magneto-polymeric nanohybrids (MMPNs) composed of magnetic nanocrystals and doxorubicin (chemotherapeutic agent) which were simultaneously encapsulated within an amphiphilic block copolymer shell [241]. The surfaces of these micelles were additionally functionalized with the breast cancer targeting/therapeutic ligand, anti-Herceptin antibody. In vivo evaluations of this nanoparticle system were performed in nude mice bearing NIH3T6.7 breast cancer tumors. Shown in Figure 9 are MR images acquired from tumor bearing mice at various time points prior to and post injection with either HER-MMPNs (a–d), or a control irrelevant IgG human antibody (IRR) IRR-MMNPs (e–h). The quantitative evaluation of MR images revealed preferential accumulation of the targeted MNPs compared to the control MNPs (i). The therapeutic functionality of the MNPs developed in this study were additionally evaluated and it was determined that the HER-MNNP’s which were decorated with targeting ligands and loaded with doxorubicin were most effective in inhibiting tumor growth (j). Combined, these findings illustrate the functionality and efficacy of targeted multifunctional MNPs for simultaneous MR imaging and drug delivery.
Figure 9.
a) MR images and their color maps (tumor region) of cancer-targeting events of HER-MMPNs (a-d) and IRR-MMPNs (e-h) in NIH3T6.7 cells implanted in mice at various time intervals: a,e) preinjection; b,f) immediately; c,g) 1 h; d,h) 12 h after injection of the MMPNs. i) ΔR2/R2pre graph versus time before and after injection of MMPNs. j) Comparative therapeutic-efficacy study in an in vivo model. Reproduced with permission from Wiley-VCH Verlag GmbH & Co. KGa: Angew. Chem. Int. Ed. [241]
6. Conclusions
Advancements in our ability to fabricate MNPs with greater control over physicochemical and bioactive properties have led to new NP candidates for imaging and therapeutic use. These formulations offer (1) disease diagnosis at their earliest stages and improved pre-operative staging, (2) delivery of therapeutics specifically to diseased tissue, limiting unwanted side effects, and (3) non-invasive monitoring capabilities of new therapeutics. To take advantage of these imaging and therapeutic opportunities, it is critical to be aware of the design parameters discussed, especially when more elaborate constructs are prepared.
Importantly, MNP success ultimately depends upon its ability to bypass in vivo barriers. This is highly influenced by the physicochemical properties of MNPs. Size, shape, and surface chemistry dictate in vivo behavior, including biodistribution, biocompatibility, and pharmacokinetics. As such, these parameters can be tuned to achieve enhanced targeting via passive, active, and magnetic targeting mechanisms. Active targeting, in particular, offers high sensitivity due to the ability to direct MNP localization, but requires added attention be paid to the targeting agent used, and the method of MNP attachment employed. A number of bioconjugation strategies including physical methods, covalent strategies, and click chemistries are available, each having distinct advantages. In addition to assisting in disease imaging, cell targeting by MNPs can also assist in disease treatment if a therapeutic payload is integrated into the MNP. This requires additional design considerations, though, including type of therapeutic, method of release, and intracellular activity.
As we move forward, better characterization tools are needed to both evaluate new MNPs and better understand their behavior in the body. Controlled studies of individual physicochemical parameters will offer NP engineers the basic understanding to successfully build more elaborate and functional MNPs. At the same time, questions about MNP elimination and long term toxicity remain barriers to clinical entry. Once these concerns are addressed, MNPs will move closer to clinical application, improving the diagnosis, treatment, and monitoring of our most unmanageable diseases.
Acknowledgments
This work is supported in part by NIH grants (R01CA119408, R01EB006043, and R01CA134213). O.V. would like to acknowledge support through an NCI/NSF IGERT fellowship. J.G. would like to acknowledge NIH training grant (T32GM065098). We would like assistance from Mr. Chen Fang and Mr. Forrest Kievit in preparing tables 1 and 2 respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferrari M. Cancer nanotechnology: Opportunities and challenges. Nature Reviews Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 2.Sanvicens N, Marco MP. Multifunctional nanoparticles - properties and prospects for their use in human medicine. Trends in Biotechnology. 2008;26:425–433. doi: 10.1016/j.tibtech.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Farokhzad OC, Langer R. Nanomedicine: Developing smarter therapeutic and diagnostic modalities. Advanced Drug Delivery Reviews. 2006;58:1456–1459. doi: 10.1016/j.addr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 4.He J, VanBrocklin HF, Franc BL, Seo Y, Jones EF. Nanoprobes for medical diagnosis: Current status of nanotechnology in molecular imaging. Current Nanoscience. 2008;4:17–29. [Google Scholar]
- 5.Matsuura N, Rowlands JA. Towards new functional nanostructures for medical imaging. Medical Physics. 2008;35:4474–4487. doi: 10.1118/1.2966595. [DOI] [PubMed] [Google Scholar]
- 6.Boyd BJ. Past and future evolution in colloidal drug delivery systems. Expert Opinion on Drug Delivery. 2008;5:69–85. doi: 10.1517/17425247.5.1.69. [DOI] [PubMed] [Google Scholar]
- 7.Torchilin VP. Multifunctional nanocarriers. Advanced Drug Delivery Reviews. 2006;58:1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Sun C, Lee JSH, Zhang MQ. Magnetic nanoparticles in MR imaging and drug delivery. Advanced Drug Delivery Reviews. 2008;60:1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy JR, Weissleder R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Advanced Drug Delivery Reviews. 2008;60:1241–1251. doi: 10.1016/j.addr.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 11.Wang YXJ, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. European Radiology. 2001;11:2319–2331. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence R. Development and comparison of iron dextran products. Pda Journal of Pharmaceutical Science and Technology. 1998;52:190–197. [PubMed] [Google Scholar]
- 13.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Gupta AK, Naregalkar RR, Vaidya VD, Gupta M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine. 2007;2:23–39. doi: 10.2217/17435889.2.1.23. [DOI] [PubMed] [Google Scholar]
- 15.Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, Muller RN. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chemical Reviews. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 16.Misra RDK. Magnetic nanoparticle carrier for targeted drug delivery: perspective, outlook and design. Materials Science and Technology. 2008;24:1011–1019. [Google Scholar]
- 17.McCarthy JR, Kelly KA, Sun EY, Weissleder R. Targeted delivery of multifunctional magnetic nanoparticles. Nanomedicine. 2007;2:153–167. doi: 10.2217/17435889.2.2.153. [DOI] [PubMed] [Google Scholar]
- 18.Dobson J. Magnetic nanoparticles for drug delivery. Drug Development Research. 2006;67:55–60. [Google Scholar]
- 19.Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. Journal of Physics D-Applied Physics. 2003;36:R167–R181. [Google Scholar]
- 20.Zhao ZL, Bian ZY, Chen LX, He XW, Wang YF. Synthesis and surface-modifications of iron oxide magnetic nanoparticles and applications on separation and analysis. Progress in Chemistry. 2006;18:1288–1297. [Google Scholar]
- 21.Perez JM, Josephson L, O’Loughlin T, Hogemann D, Weissleder R. Magnetic relaxation switches capable of sensing molecular interactions. Nature Biotechnology. 2002;20:816–820. doi: 10.1038/nbt720. [DOI] [PubMed] [Google Scholar]
- 22.Frullano L, Meade TJ. Multimodal MRI contrast agents. Journal of Biological Inorganic Chemistry. 2007;12:939–949. doi: 10.1007/s00775-007-0265-3. [DOI] [PubMed] [Google Scholar]
- 23.Corot C, Robert P, Idee JM, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Advanced Drug Delivery Reviews. 2006;58:1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Duran JDG, Arias JL, Gallardo V, Delgado AV. Magnetic colloids as drug vehicles. Journal of Pharmaceutical Sciences. 2008;97:2948–2983. doi: 10.1002/jps.21249. [DOI] [PubMed] [Google Scholar]
- 25.Solanki A, Kim JD, Lee KB. Nanotechnology for regenerative medicine: nanomaterials for stem cell imaging. Nanomedicine. 2008;3:567–578. doi: 10.2217/17435889.3.4.567. [DOI] [PubMed] [Google Scholar]
- 26.Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. International Journal of Hyperthermia. 2008;24:467–474. doi: 10.1080/02656730802104757. [DOI] [PubMed] [Google Scholar]
- 27.Bonnemain B. Superparamagnetic agents in magnetic resonance imaging: Physicochemical characteristics and clinical applications - A review. Journal of Drug Targeting. 1998;6:167–174. doi: 10.3109/10611869808997890. [DOI] [PubMed] [Google Scholar]
- 28.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. New England Journal of Medicine. 2003;348:2491–U5. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 29.Singh A, Patel T, Hertel J, Bernardo M, Kausz A, Brenner L. Safety of Ferumoxytol in Patients With Anemia and CKD. American Journal of Kidney Diseases. 2008;52:907–915. doi: 10.1053/j.ajkd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Huh YM, Jun Y, Seo J, Jang J, Song HT, Kim S, Cho EJ, Yoon HG, Suh JS, Cheon J. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nature Medicine. 2007;13:95–99. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 31.Goya GF, Grazu V, Ibarra MR. Magnetic nanoparticles for cancer therapy. Current Nanoscience. 2008;4:1–16. [Google Scholar]
- 32.Jurgons R, Seliger C, Hilpert A, Trahms L, Odenbach S, Alexiou C. Drug loaded magnetic nanoparticles for cancer therapy. Journal of Physics-Condensed Matter. 2006;18:S2893–S2902. [Google Scholar]
- 33.Chertok B, Moffat BA, David AE, Yu FQ, Bergemann C, Ross BD, Yang VC. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29:487–496. doi: 10.1016/j.biomaterials.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tartaj P, Morales MD, Veintemillas-Verdaguer S, Gonzalez-Carreno T, Serna CJ. The preparation of magnetic nanoparticles for applications in biomedicine. Journal of Physics D-Applied Physics. 2003;36:R182–R197. [Google Scholar]
- 35.Belting M, Sandgren S, Wittrup A. Nuclear delivery of macromolecules: barriers and carriers. Advanced Drug Delivery Reviews. 2005;57:505–527. doi: 10.1016/j.addr.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Davis ME. Non-viral gene delivery systems. Current Opinion In Biotechnology. 2002;13:128–131. doi: 10.1016/s0958-1669(02)00294-x. [DOI] [PubMed] [Google Scholar]
- 37.Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine. 2008;3:703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Begley DJ. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacology & Therapeutics. 2004;104:29–45. doi: 10.1016/j.pharmthera.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Bareford LA, Swaan PW. Endocytic mechanisms for targeted drug delivery. Advanced Drug Delivery Reviews. 2007;59:748–758. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Molecular Pharmaceutics. 2008;5:487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chouly C, Pouliquen D, Lucet I, Jeune JJ, Jallet P. Development of superparamagnetic nanoparticles for MRI: Effect of particle size, charge and surface nature on biodistribution. Journal of Microencapsulation. 1996;13:245–255. doi: 10.3109/02652049609026013. [DOI] [PubMed] [Google Scholar]
- 42.Reschel T, Konak C, Oupicky D, Seymour LW, Ulbrich K. Physical properties and in vitro transfection efficiency of gene delivery vectors based on complexes of DNA with synthetic polycations. Journal Of Controlled Release. 2002;81:201–217. doi: 10.1016/s0168-3659(02)00045-7. [DOI] [PubMed] [Google Scholar]
- 43.Decuzzi P, Ferrari M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials. 2006;27:5307–5314. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 44.Decuzzi P, Lee S, Bhushan B, Ferrari M. A theoretical model for the margination of particles within blood vessels. Annals Of Biomedical Engineering. 2005;33:179–190. doi: 10.1007/s10439-005-8976-5. [DOI] [PubMed] [Google Scholar]
- 45.Decuzzi P, Causa F, Ferrari M, Netti PA. The effective dispersion of nanovectors within the tumor microvasculature. Annals Of Biomedical Engineering. 2006;34:633–641. doi: 10.1007/s10439-005-9072-6. [DOI] [PubMed] [Google Scholar]
- 46.Chavanpatil MD, Khdair A, Panyam J. Nanoparticles for cellular drug delivery: Mechanisms and factors influencing delivery. Journal Of Nanoscience And Nanotechnology. 2006;6:2651–2663. doi: 10.1166/jnn.2006.443. [DOI] [PubMed] [Google Scholar]
- 47.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nature Biotechnology. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moghimi SM. Mechanism of splenic clearance of blood-cells and particles - Towards development of new splenotropic agents. Advanced Drug Delivery Reviews. 1995;17:103–115. [Google Scholar]
- 49.Moghimi SM, Hunter AC. Capture of stealth nanoparticles by the body’s defences. Critical Reviews in Therapeutic Drug Carrier Systems. 2001;18:527–550. [PubMed] [Google Scholar]
- 50.Zamboni WC. Concept and clinical evaluation of carrier-mediated anticancer agents. Oncologist. 2008;13:248–260. doi: 10.1634/theoncologist.2007-0180. [DOI] [PubMed] [Google Scholar]
- 51.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacological Reviews. 2001;53:283–318. [PubMed] [Google Scholar]
- 52.Banerjee T, Mitra S, Singh AK, Sharma RK, Maitra A. Preparation, characterization and biodistribution of ultrafine chitosan nanoparticles. International Journal of Pharmaceutics. 2002;243:93–105. doi: 10.1016/s0378-5173(02)00267-3. [DOI] [PubMed] [Google Scholar]
- 53.Moghimi SM. EXPLOITING BONE-MARROW MICROVASCULAR STRUCTURE FOR DRUG-DELIVERY AND FUTURE THERAPIES. Advanced Drug Delivery Reviews. 1995;17:61–73. [Google Scholar]
- 54.Moghimi SM. MECHANISMS OF SPLENIC CLEARANCE OF BLOOD-CELLS AND PARTICLES - TOWARDS DEVELOPMENT OF NEW SPLENOTROPIC AGENTS. Advanced Drug Delivery Reviews. 1995;17:103–115. [Google Scholar]
- 55.Pardridge WM. Drug and gene targeting to the brain with molecular Trojan horses. Nature Reviews Drug Discovery. 2002;1:131–139. doi: 10.1038/nrd725. [DOI] [PubMed] [Google Scholar]
- 56.Koo YEL, Reddy GR, Bhojani M, Schneider R, Philbert MA, Rehemtulla A, Ross BD, Kopelman R. Brain cancer diagnosis and therapy with nanoplatforms. Advanced Drug Delivery Reviews. 2006;58:1556–1577. doi: 10.1016/j.addr.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 57.Linazasoro G, Nanotechnologies N. Potential applications of nanotechnologies to Parkinson’s disease therapy. Parkinsonism & Related Disorders. 2008;14:383–392. doi: 10.1016/j.parkreldis.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 58.Lockman PR, Mumper RJ, Khan MA, Allen DD. Nanoparticle technology for drug delivery across the blood-brain barrier. Drug Dev Ind Pharm. 2002;28:1–13. doi: 10.1081/ddc-120001481. [DOI] [PubMed] [Google Scholar]
- 59.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Advanced Drug Delivery Reviews. 2001;47:65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Yang LL, Chen Z, Shin DM. Application of nanotechnology in cancer therapy and imaging. Ca-a Cancer Journal for Clinicians. 2008;58:97–110. doi: 10.3322/CA.2007.0003. [DOI] [PubMed] [Google Scholar]
- 61.Elder JB, Liu CY, Apuzzo MLJ. Neurosurgery in the realm of 10(-9), part 2: applications of nanotechnology to neurosurgery - Present and future. Neurosurgery. 2008;62:269–284. doi: 10.1227/01.neu.0000315995.73269.c3. [DOI] [PubMed] [Google Scholar]
- 62.Silva GA. Neuroscience nanotechnology: Progress, opportunities and challenges. Nature Reviews Neuroscience. 2006;7:65–74. doi: 10.1038/nrn1827. [DOI] [PubMed] [Google Scholar]
- 63.Emerich DF, Thanos CG. Targeted nanoparticle-based drug delivery and diagnosis. Journal of Drug Targeting. 2007;15:163–183. doi: 10.1080/10611860701231810. [DOI] [PubMed] [Google Scholar]
- 64.Sonavane G, Tomoda K, Makino K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids and Surfaces B-Biointerfaces. 2008;66:274–280. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 65.Gratton SEA, PohhauS PD, Lee J, Guo I, Cho MJ, DeSimone JM. Nanofabricated particles for engineered drug therapies: A preliminary Biodistribution study of PRINT (TM) nanoparticles. Journal Of Controlled Release. 2007;121:10–18. doi: 10.1016/j.jconrel.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Son SJ, Bai X, Nan A, Ghandehari H, Lee SB. Template synthesis of multifunctional nanotubes for controlled release. Journal Of Controlled Release. 2006;114:143–152. doi: 10.1016/j.jconrel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 67.Liu Z, Cai WB, He LN, Nakayama N, Chen K, Sun XM, Chen XY, Dai HJ. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nature Nanotechnology. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 68.Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, Bianco A, Kostarelos K. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3357–3362. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park SJ, Kim S, Lee S, Khim ZG, Char K, Hyeon T. Synthesis and magnetic studies of uniform iron nanorods and nanospheres. Journal of the American Chemical Society. 2000;122:8581–8582. [Google Scholar]
- 70.Geng Y, Dalhaimer P, Cai SS, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nature Nanotechnology. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park JH, von Maltzahn G, Zhang LL, Schwartz MP, Ruoslahti E, Bhatia SN, Sailor MJ. Magnetic iron oxide nanoworms for tumor targeting and imaging. Advanced Materials. 2008;20:1630–1635. doi: 10.1002/adma.200800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park JH, von Maltzahn G, Zhang L, Derfus AM, Simberg D, Harris TJ, Ruoslahti E, Bhatia SN, Sailor MJ. Systematic surface engineering of magnetic nanoworms for in vivo tumor targeting. Small. 2009;5:694–700. doi: 10.1002/smll.200801789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nature Reviews Drug Discovery. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 74.Leuschner C, Kumar C, Hansel W, Soboyejo W, Zhou JK, Hormes J. LHRH-conjugated magnetic iron oxide nanoparticles for detection of breast cancer metastases. Breast Cancer Research and Treatment. 2006;99:163–176. doi: 10.1007/s10549-006-9199-7. [DOI] [PubMed] [Google Scholar]
- 75.Kresse M, Wagner S, Pfefferer D, Lawaczeck R, Elste V, Semmler W. Targeting of ultrasmall superparamagnetic iron oxide (USPIO) particles to tumor cells in vivo by using transferrin receptor pathways. Magnetic Resonance in Medicine. 1998;40:236–242. doi: 10.1002/mrm.1910400209. [DOI] [PubMed] [Google Scholar]
- 76.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. Journal Of Controlled Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 77.Jain RK. Transport of molecules, particles, and cells in solid tumors. Annual Review of Biomedical Engineering. 1999;1:241–263. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 78.Jain RK. Delivery of molecular medicine to solid tumors: lessons from in vivo imaging of gene expression and function. Journal Of Controlled Release. 2001;74:7–25. doi: 10.1016/s0168-3659(01)00306-6. [DOI] [PubMed] [Google Scholar]
- 79.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mnneil SE. Nanotechnology for the biologist. Journal of Leukocyte Biology. 2005;78:585–594. doi: 10.1189/jlb.0205074. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Y, Kohler N, Zhang MQ. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 2002;23:1553–1561. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 82.Sinha R, Kim GJ, Nie SM, Shin DM. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Molecular Cancer Therapeutics. 2006;5:1909–1917. doi: 10.1158/1535-7163.MCT-06-0141. [DOI] [PubMed] [Google Scholar]
- 83.Kohler N, Sun C, Wang J, Zhang MQ. Methotrexate-modified superparamagnetic nanoparticles and their intracellular uptake into human cancer cells. Langmuir. 2005;21:8858–8864. doi: 10.1021/la0503451. [DOI] [PubMed] [Google Scholar]
- 84.Sun C, Sze R, Zhang MQ. Folic acid-PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRI. Journal of Biomedical Materials Research Part A. 2006;78A:550–557. doi: 10.1002/jbm.a.30781. [DOI] [PubMed] [Google Scholar]
- 85.Veiseh O, Sun C, Gunn J, Kohler N, Gabikian P, Lee D, Bhattarai N, Ellenbogen R, Sze R, Hallahan A, Olson J, Zhang MQ. Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Letters. 2005;5:1003–1008. doi: 10.1021/nl0502569. [DOI] [PubMed] [Google Scholar]
- 86.Montet X, Montet-Abou K, Reynolds F, Weissleder R, Josephson L. Nanoparticle imaging of integrins on tumor cells. Neoplasia. 2006;8:214–222. doi: 10.1593/neo.05769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Montet X, Weissleder R, Josephson L. Imaging pancreatic cancer with a peptide-nanoparticle conjugate targeted to normal pancreas. Bioconjugate Chemistry. 2006;17:905–911. doi: 10.1021/bc060035+. [DOI] [PubMed] [Google Scholar]
- 88.Boutry S, Laurent S, Vander Elst L, Muller RN. Specific E-selectin targeting with a superparamagnetic MRI contrast agent. Contrast Media & Molecular Imaging. 2006;1:15–22. doi: 10.1002/cmmi.87. [DOI] [PubMed] [Google Scholar]
- 89.Gunn J, Wallen H, Veiseh O, Sun C, Fang C, Cao JH, Yee C, Zhang MQ. A multimodal targeting nanoparticle for selectively labeling T cells. Small. 2008;4:712–715. doi: 10.1002/smll.200701103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Artemov D, Mori N, Okollie B, Bhujwalla ZM. MR molecular imaging of the Her-2/neu receptor in breast cancer cells using targeted iron oxide nanoparticles. Magnetic Resonance in Medicine. 2003;49:403–408. doi: 10.1002/mrm.10406. [DOI] [PubMed] [Google Scholar]
- 91.Hu FQ, Wei L, Zhou Z, Ran YL, Li Z, Gao MY. Preparation of biocompatible magnetite nanocrystals for in vivo magnetic resonance detection of cancer. Advanced Materials. 2006;18:2553–2556. [Google Scholar]
- 92.Huh YM, Jun YW, Song HT, Kim S, Choi JS, Lee JH, Yoon S, Kim KS, Shin JS, Suh JS, Cheon J. In vivo magnetic resonance detection of cancer by using multifunctional magnetic nanocrystals. Journal of the American Chemical Society. 2005;127:12387–12391. doi: 10.1021/ja052337c. [DOI] [PubMed] [Google Scholar]
- 93.Schafer R, Wiskirchen J, Guo K, Neumann B, Kehlbach R, Pintaske J, Voth V, Walker T, Scheule AM, Greiner TO, Hermanutz-Klein U, Claussen CD, Northoff H, Ziemer G, Wendel HP. Aptamer-based isolation and subsequent imaging of mesenchymal stem cells in ischemic myocard by magnetic resonance imaging. Rofo-Fortschritte Auf Dem Gebiet Der Rontgenstrahlen Und Der Bildgebenden Verfahren. 2007;179:1009–1015. doi: 10.1055/s-2007-963409. [DOI] [PubMed] [Google Scholar]
- 94.Yigit MV, Mazumdar D, Kim HK, Lee JH, Dintsov B, Lu Y. Smart “Turn-on” magnetic resonance contrast agents based on aptamer-functionalized superparamagnetic iron oxide nanoparticles. Chembiochem. 2007;8:1675–1678. doi: 10.1002/cbic.200700323. [DOI] [PubMed] [Google Scholar]
- 95.Yigit MV, Mazumdar D, Lu Y. MRI detection of thrombin with aptamer functionalized superparamagnetic iron oxide nanoparticles. Bioconjugate Chemistry. 2008;19:412–417. doi: 10.1021/bc7003928. [DOI] [PubMed] [Google Scholar]
- 96.Hong S, Leroueil PR, Majoros IJ, Orr BG, Baker JR, Holl MMB. The binding avidity of a nanoparticle-based multivalent targeted drug delivery platform. Chemistry & Biology. 2007;14:105–113. doi: 10.1016/j.chembiol.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 97.Wright D, Usher L. Multivalent binding in the design of bioactive compounds. Current Organic Chemistry. 2001;5:1107–1131. [Google Scholar]
- 98.Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angewandte Chemie-International Edition. 1998;37:2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 99.Munson PJ, Rodbard D. COMPUTER MODELING OF SEVERAL LIGANDS BINDING TO MULTIPLE RECEPTORS. Endocrinology. 1979;105:1377–1381. [PubMed] [Google Scholar]
- 100.Montet X, Funovics M, Montet-Abou K, Weissleder R, Josephson L. Multivalent effects of RGD peptides obtained by nanoparticle display. Journal of Medicinal Chemistry. 2006;49:6087–6093. doi: 10.1021/jm060515m. [DOI] [PubMed] [Google Scholar]
- 101.Natarajan A, Xiong CY, Gruettner C, DeNardo GL, DeNardo SJ. Development of multivalent radioimmunonanoparticles for cancer imaging and therapy. Cancer Biotherapy and Radiopharmaceuticals. 2008;23:82–91. doi: 10.1089/cbr.2007.0410. [DOI] [PubMed] [Google Scholar]
- 102.Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size-dependent. Nature Nanotechnology. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 103.Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Letters. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 104.McBain SC, Griesenbach U, Xenariou S, Keramane A, Batich CD, Alton E, Dobson J. Magnetic nanoparticles as gene delivery agents: enhanced transfection in the presence of oscillating magnet arrays. Nanotechnology. 2008;19 doi: 10.1088/0957-4484/19/40/405102. [DOI] [PubMed] [Google Scholar]
- 105.Wilson MW, Kerlan RK, Fidelman NA, Venook AP, LaBerge JM, Koda J, Gordon RL. Hepatocellular carcinoma: Regional therapy with a magnetic targeted carrier bound to doxorubicin in a dual MR imaging/conventional angiography suite - Initial experience with four patients. Radiology. 2004;230:287–293. doi: 10.1148/radiol.2301021493. [DOI] [PubMed] [Google Scholar]
- 106.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature Reviews Drug Discovery. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 107.Namdeo M, Saxena S, Tankhiwale R, Bajpai M, Mohan YM, Bajpai SK. Magnetic Nanoparticles for Drug Delivery Applications. Journal Of Nanoscience And Nanotechnology. 2008;8:3247–3271. doi: 10.1166/jnn.2008.399. [DOI] [PubMed] [Google Scholar]
- 108.Vega-Villa KR, Takemoto JK, Yanez JA, Remsberg CM, Forrest ML, Davies NM. Clinical toxicities of nanocarrier systems. Advanced Drug Delivery Reviews. 2008;60:929–938. doi: 10.1016/j.addr.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 109.Zhao Y, Nalwa HS, editors. Nanotoxicology - Interactions of Nanomaterials with Biological Systems American Scientific Publishers. Stevenson Ranch; CA: 2006. [Google Scholar]
- 110.Gupta AK, Gupta M. Synthesis and surface engineering of superparamagnetic iron oxide nanoparticles for drug delivery and cellular targeting. In: Kumar R, editor. Handbook of particulate drug delivery. American Scientific Publishers; USA: 2007. [Google Scholar]
- 111.Lu AH, Salabas EL, Schuth F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angewandte Chemie-International Edition. 2007;46:1222–1244. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- 112.Puntes VF, Krishnan KM, Alivisatos AP. Colloidal nanocrystal shape and size control: The case of cobalt. Science. 2001;291:2115–2117. doi: 10.1126/science.1057553. [DOI] [PubMed] [Google Scholar]
- 113.Shevchenko EV, Talapin DV, Rogach AL, Kornowski A, Haase M, Weller H. Colloidal synthesis and self-assembly of COPt3 nanocrystals. Journal of the American Chemical Society. 2002;124:11480–11485. doi: 10.1021/ja025976l. [DOI] [PubMed] [Google Scholar]
- 114.Sun SH, Murray CB, Weller D, Folks L, Moser A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science. 2000;287:1989–1992. doi: 10.1126/science.287.5460.1989. [DOI] [PubMed] [Google Scholar]
- 115.Grasset F, Labhsetwar N, Li D, Park DC, Saito N, Haneda H, Cador O, Roisnel T, Mornet S, Duguet E, Portier J, Etourneau J. Synthesis and magnetic characterization of zinc ferrite nanoparticles with different environments: Powder, colloidal solution, and zinc ferrite-silica core-shell nanoparticles. Langmuir. 2002;18:8209–8216. [Google Scholar]
- 116.Sun SH, Zeng H. Size-controlled synthesis of magnetite nanoparticies. Journal of the American Chemical Society. 2002;124:8204–8205. doi: 10.1021/ja026501x. [DOI] [PubMed] [Google Scholar]
- 117.Di Marco M, Sadun C, Port M, Guilbert I, Couvreur P, Dubernet C. Physicochemical characterization of ultrasmall superparamagnetic iron oxide particles (USPIO) for biomedical application as MRI contrast agents. International Journal of Nanomedicine. 2007;2:609–622. [PMC free article] [PubMed] [Google Scholar]
- 118.Park J, An KJ, Hwang YS, Park JG, Noh HJ, Kim JY, Park JH, Hwang NM, Hyeon T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nature Materials. 2004;3:891–895. doi: 10.1038/nmat1251. [DOI] [PubMed] [Google Scholar]
- 119.Dobson J. Magnetic properties of biological materials. In: Barnes S, Greenebaum B, editors. Handbook of biological effects of electromagnetic fields: bioengineering and biophysical aspects of electromagnetic fields. Taylor and Francis/CRC Press; Boca Ranton: 2007. pp. 101–113. [Google Scholar]
- 120.Weissleder R, Stark DD, Engelstad BL, Bacon BR, Compton CC, White DL, Jacobs P, Lewis J. SUPERPARAMAGNETIC IRON-OXIDE - PHARMACOKINETICS AND TOXICITY. American Journal of Roentgenology. 1989;152:167–173. doi: 10.2214/ajr.152.1.167. [DOI] [PubMed] [Google Scholar]
- 121.Willard MA, Kurihara LK, Carpenter EE, Calvin S, Harris VG. Chemically prepared magnetic nanoparticles. International Materials Reviews. 2004;49:125–170. [Google Scholar]
- 122.Wormuth K. Superparamagnetic latex via inverse emulsion polymerization. Journal of Colloid and Interface Science. 2001;241:366–377. [Google Scholar]
- 123.Si S, Kotal A, Mandal TK, Giri S, Nakamura H, Kohara T. Size-controlled synthesis of magnetite nanoparticles in the presence of polyelectrolytes. Chemistry of Materials. 2004;16:3489–3496. [Google Scholar]
- 124.Bee A, Massart R, Neveu S. SYNTHESIS OF VERY FINE MAGHEMITE PARTICLES. Journal of Magnetism and Magnetic Materials. 1995;149:6–9. [Google Scholar]
- 125.Wan SR, Huang JS, Yan HS, Liu KL. Size-controlled preparation of magnetite nanoparticles in the presence of graft copolymers. Journal of Materials Chemistry. 2006;16:298–303. [Google Scholar]
- 126.Chen TJ, Cheng TH, Hung YC, Lin KT, Liu GC, Wang YM. Targeted folic acid-PEG nanoparticles for noninvasive imaging of folate receptor by MRI. Journal of Biomedical Materials Research Part A. 2008;87A:165–175. doi: 10.1002/jbm.a.31752. [DOI] [PubMed] [Google Scholar]
- 127.Herve K, Douziech-Eyrolles L, Munnier E, Cohen-Jonathan S, Souce M, Marchais H, Limelette P, Warmont F, Saboungi ML, Dubois P, Chourpa I. The development of stable aqueous suspensions of PEGylated SPIONs for biomedical applications. Nanotechnology. 2008;19 doi: 10.1088/0957-4484/19/46/465608. [DOI] [PubMed] [Google Scholar]
- 128.Park JY, Daksha P, Lee GH, Woo S, Chang YM. Highly water-dispersible PEG surface modified ultra small superparamagnetic iron oxide nanoparticles useful for target-specific biomedical applications. Nanotechnology. 2008;19 doi: 10.1088/0957-4484/19/36/365603. [DOI] [PubMed] [Google Scholar]
- 129.Sun C, Veiseh O, Gunn J, Fang C, Hansen S, Lee D, Sze R, Ellenbogen RG, Olson J, Zhang M. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small. 2008;4:372–9. doi: 10.1002/smll.200700784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xie J, Xu C, Kohler N, Hou Y, Sun S. Controlled PEGylation of monodisperse Fe3O4 nanoparticles for reduced non-specific uptake by macrophage cells. Advanced Materials. 2007;19:3163–3166. [Google Scholar]
- 131.Kohler N, Fryxell GE, Zhang MQ. A bifunctional poly(ethylene glycol) silane immobilized on metallic oxide-based nanoparticles for conjugation with cell targeting agents. Journal of the American Chemical Society. 2004;126:7206–7211. doi: 10.1021/ja049195r. [DOI] [PubMed] [Google Scholar]
- 132.Butterworth MD, Illum L, Davis SS. Preparation of ultrafine silica- and PEG-coated magnetite particles. Colloids and Surfaces a-Physicochemical and Engineering Aspects. 2001;179:93–102. [Google Scholar]
- 133.Shen T, Weissleder R, Papisov M, Bogdanov A, Brady TJ. MONOCRYSTALLINE IRON-OXIDE NANOCOMPOUNDS (MION) - PHYSICOCHEMICAL PROPERTIES. Magnetic Resonance in Medicine. 1993;29:599–604. doi: 10.1002/mrm.1910290504. [DOI] [PubMed] [Google Scholar]
- 134.Molday RS, MacKenzie D. Immunospecific ferromagnetic iron-dextran reagents for the labeling and magnetic separation of cells. J Immunol Methods. 1982;52:353–67. doi: 10.1016/0022-1759(82)90007-2. [DOI] [PubMed] [Google Scholar]
- 135.Bulte JWM, Ma LD, Magin RL, Kamman RL, Hulstaert CE, Go KG, The TH, Deleij L. SELECTIVE MR IMAGING OF LABELED HUMAN PERIPHERAL-BLOOD MONONUCLEAR-CELLS BY LIPOSOME MEDIATED INCORPORATION OF DEXTRAN-MAGNETITE PARTICLES. Magnetic Resonance in Medicine. 1993;29:32–37. doi: 10.1002/mrm.1910290108. [DOI] [PubMed] [Google Scholar]
- 136.Mornet S, Portier J, Duguet E. A method for synthesis and functionalization of ultrasmall superparamagnetic covalent carriers based on maghemite and dextran. Journal of Magnetism and Magnetic Materials. 2005;293:127–134. [Google Scholar]
- 137.Donadel K, Felisberto MDV, Favere VT, Rigoni M, Batistela NJ, Laranjeira MCM. Synthesis and characterization of the iron oxide magnetic particles coated with chitosan biopolymer. Materials Science & Engineering C: Biomimetic and Supramolecular systems. 2008;28:509–514. [Google Scholar]
- 138.Sasaki T, Iwasaki N, Kohno K, Kishimoto M, Majima T, Nishimura SI, Minami A. Magnetic nanoparticles for improving cell invasion in tissue engineering. Journal of Biomedical Materials Research Part A. 2008;86A:969–978. doi: 10.1002/jbm.a.31724. [DOI] [PubMed] [Google Scholar]
- 139.Ho KM, Li P. Design and synthesis of novel magnetic core-shell polymeric particles. Langmuir. 2008;24:1801–1807. doi: 10.1021/la702887m. [DOI] [PubMed] [Google Scholar]
- 140.Kim EH, Ahn Y, Lee HS. Biomedical applications of superparamagnetic iron oxide nanoparticles encapsulated within chitosan. Journal of Alloys and Compunds. 2007;434:633–636. [Google Scholar]
- 141.Steitz B, Hofmann H, Kamau SW, Hassa PO, Hottiger MO, von Rechenberg B, Hofmann-Amtenbrink M, Petri-Fink A. Characterization of PEI-coated superparamagnetic iron oxide nanoparticles for transfection: Size distribution, colloidal properties and DNA interaction. Journal of Magnetism and Magnetic Materials. 2007;311:300–305. [Google Scholar]
- 142.Chorny M, Polyak B, Alferiev IS, Walsh K, Friedman G, Levy RJ. Magnetically driven plasmid DNA delivery with biodegradable polymeric nanoparticles. Faseb Journal. 2007;21:2510–2519. doi: 10.1096/fj.06-8070com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Park IK, Ng CP, Wang J, Chu B, Yuan C, Zhang S, Pun SH. Determination of nanoparticle vehicle unpackaging by MR imaging of a T-2 magnetic relaxation switch. Biomaterials. 2008;29:724–732. doi: 10.1016/j.biomaterials.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 144.McBain SC, Yiu HHP, El Haj A, Dobson J. Polyethyleneimine functionalized iron oxide nanoparticles as agents for DNA delivery and transfection. Journal of Materials Chemistry. 2007;17:2561–2565. [Google Scholar]
- 145.Duan HW, Kuang M, Wang XX, Wang YA, Mao H, Nie SM. Reexamining the effects of particle size and surface chemistry on the magnetic properties of iron oxide nanocrystals: New insights into spin disorder and proton relaxivity. Journal of Physical Chemistry C. 2008;112:8127–8131. [Google Scholar]
- 146.Plassat V, Martina MS, Barratt G, Menager C, Lesieur S. Sterically stabilized superparamagnetic liposomes for MR imaging and cancer therapy: Pharmacokinetics and biodistribution. International Journal of Pharmaceutics. 2007;34:118–127. doi: 10.1016/j.ijpharm.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 147.Shtykova EV, Huang XL, Remmes N, Baxter D, Stein B, Dragnea B, Svergun DI, Bronstein LM. Structure and properties of iron oxide nanoparticles encapsulated by phospholipids with poly(ethylene glycol) tails. Journal of Physical Chemistry C. 2007;111:18078–18086. [Google Scholar]
- 148.LaConte LEW, Nitin N, Zurkiya O, Caruntu D, O’Connor CJ, Hu XP, Bao G. Coating thickness of magnetic iron oxide nanoparticles affects R-2 relaxivity. Journal of Magnetic Resonance Imaging. 2007;26:1634–1641. doi: 10.1002/jmri.21194. [DOI] [PubMed] [Google Scholar]
- 149.Mahato RI. Biomaterials for delivery and targeting of proteins and nucleic acids. CRC Press; Boca Ranton, Florida: 2005. [Google Scholar]
- 150.Fuertges F, Abuchowski A. The Clinical efficacy of Poly(ethylene glycol)-Modified Proteins. Journal Of Controlled Release. 1990;11:139–148. [Google Scholar]
- 151.Kim DK, Zhang Y, Kehr J, Klason T, Bjelke B, Muhammed M. Characterization and MRI study of surfactant-coated superparamagnetic nanoparticles administered into the rat brain. Journal of Magnetism and Magnetic Materials. 2001;225:256–261. [Google Scholar]
- 152.Tiefenauer LX, Tschirky A, Kuhne G, Andres RY. In vivo evaluation of magnetite nanoparticles for use as a tumor contrast agent in MRI. Magnetic Resonance Imaging. 1996;14:391–402. doi: 10.1016/0730-725x(95)02106-4. [DOI] [PubMed] [Google Scholar]
- 153.Illum L, Church AE, Butterworth MD, Arien A, Whetstone J, Davis SS. Development of systems for targeting the regional lymph nodes for diagnostic imaging: In vivo behaviour of colloidal PEG-coated magnetite nanospheres in the rat following interstitial administration. Pharmaceutical Research. 2001;18:640–645. doi: 10.1023/a:1011081210142. [DOI] [PubMed] [Google Scholar]
- 154.Papisov MI, Bogdanov A, Schaffer B, Nossiff N, Shen T, Weissleder R, Brady TJ. COLLOIDAL MAGNETIC-RESONANCE CONTRAST AGENTS - EFFECT OF PARTICLE SURFACE ON BIODISTRIBUTION. Journal of Magnetism and Magnetic Materials. 1993;122:383–386. [Google Scholar]
- 155.Sun C, Fang C, Stephen Z, Veiseh O, Hansen S, Lee D, Ellenbogen RG, Olson J, Zhang MQ. Tumor-targeted drug delivery and MRI contrast enhancement by chlorotoxin-conjugated iron oxide nanoparticles. Nanomedicine. 2008;3:495–505. doi: 10.2217/17435889.3.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sun C, Veiseh O, Gunn J, Fang C, Hansen S, Lee D, Sze R, Ellenbogen RG, Olson J, Zhang M. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small. 2008;4:372–379. doi: 10.1002/smll.200700784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen X, Zhang W, Laird J, Hazen SL, Salomon RG. Polyunsaturated phospholipids promote the oxidation and fragmentation of gamma-hydroxyalkenals: formation and reactions of oxidatively truncated ether phospholipids. J Lipid Res. 2008;49:832–46. doi: 10.1194/jlr.M700598-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lutz JF, Stiller S, Hoth A, Kaufner L, Pison U, Cartier R. One-pot synthesis of PEGylated ultrasmall iron-oxide nanoparticles and their in vivo evaluation as magnetic resonance imaging contrast agents. Biomacromolecules. 2006;7:3132–3138. doi: 10.1021/bm0607527. [DOI] [PubMed] [Google Scholar]
- 159.Veiseh O, Gunn J, Kievit F, Sun C, Fang C, Lee j, Zhang M. Inhibition of tumor cell invasion with chlorotoxin-bound superparamagnetic nanoparticles. Small. doi: 10.1002/smll.200800646. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tartaj P, Morales MP, Veintemillas-Verdaguer S, Gonzalez-Carreno T, Serna CJ. Synthesis, properties and biomedical applications of magnetic nanoparticles. Elsevier; Amsterdam, Netherlands: 2006. [Google Scholar]
- 161.Weissleder R, Stark DD, Compton CC, Wittenberg J, Ferrucci JT. FERRITE-ENHANCED MR IMAGING OF HEPATIC LYMPHOMA - AN EXPERIMENTAL-STUDY IN RATS. American Journal of Roentgenology. 1987;149:1161–1165. doi: 10.2214/ajr.149.6.1161. [DOI] [PubMed] [Google Scholar]
- 162.Weissleder R, Hahn PF, Stark DD, Rummeny E, Saini S, Wittenberg J, Ferrucci JT. MR IMAGING OF SPLENIC METASTASES - FERRITE-ENHANCED DETECTION IN RATS. American Journal of Roentgenology. 1987;149:723–726. doi: 10.2214/ajr.149.4.723. [DOI] [PubMed] [Google Scholar]
- 163.Weissleder R, Elizondo G, Wittenberg J, Lee AS, Josephson L, Brady TJ. ULTRASMALL SUPERPARAMAGNETIC IRON-OXIDE - AN INTRAVENOUS CONTRAST AGENT FOR ASSESSING LYMPH-NODES WITH MR IMAGING. Radiology. 1990;175:494–498. doi: 10.1148/radiology.175.2.2326475. [DOI] [PubMed] [Google Scholar]
- 164.Stark DD, Weissleder R, Elizondo G, Hahn PF, Saini S, Todd LE, Wittenberg J, Ferrucci JT. SUPERPARAMAGNETIC IRON-OXIDE - CLINICAL-APPLICATION AS A CONTRAST AGENT FOR MR IMAGING OF THE LIVER. Radiology. 1988;168:297–301. doi: 10.1148/radiology.168.2.3393649. [DOI] [PubMed] [Google Scholar]
- 165.Weissleder R, Elizondo G, Stark DD, Hahn PF, Marfil J, Gonzalez JF, Saini S, Todd LE, Ferrucci JT. THE DIAGNOSIS OF SPLENIC LYMPHOMA BY MR IMAGING - VALUE OF SUPERPARAMAGNETIC IRON-OXIDE. American Journal of Roentgenology. 1989;152:175–180. doi: 10.2214/ajr.152.1.175. [DOI] [PubMed] [Google Scholar]
- 166.Josephson L, Tung CH, Moore A, Weissleder R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-tat peptide conjugates. Bioconjugate Chemistry. 1999;10:186–191. doi: 10.1021/bc980125h. [DOI] [PubMed] [Google Scholar]
- 167.Wunderbaldinger P, Josephson L, Weissleder R. Crosslinked iron oxides (CLIO): A new platform for the development of targeted MR contrast agents. 2002:S304–S306. doi: 10.1016/s1076-6332(03)80210-6. [DOI] [PubMed] [Google Scholar]
- 168.Mornet S, Vasseur S, Grasset F, Duguet E. Magnetic nanoparticle design for medical diagnosis and therapy. Journal of Materials Chemistry. 2004;14:2161–2175. [Google Scholar]
- 169.Janes KA, Calvo P, Alonso MJ. Polysaccharide colloidal particles as delivery systems for macromolecules. Advanced Drug Delivery Reviews. 2001;47:83–97. doi: 10.1016/s0169-409x(00)00123-x. [DOI] [PubMed] [Google Scholar]
- 170.Kumar M, Muzzarelli RAA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chemical Reviews. 2004;104:6017–6084. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- 171.Li BQ, Jia DC, Zhou Y, Hu QL, Cai W. In situ hybridization to chitosan/magnetite nanocomposite induced by the magnetic field. Journal of Magnetism and Magnetic Materials. 2006;306:223–227. [Google Scholar]
- 172.Sipos P, Berkesi O, Tombacz E, St Pierre TG, Webb J. Formation of spherical iron(III) oxyhydroxide nanoparticles sterically stabilized by chitosan in aqueous solutions. Journal of Inorganic Biochemistry. 2003;95:55–63. doi: 10.1016/s0162-0134(03)00068-0. [DOI] [PubMed] [Google Scholar]
- 173.Bhattarai SR, Badahur KCR, Aryal S, Khil MS, Kim HY. N-Acylated chitosan stabilized iron oxide nanoparticles as a novel nano-matrix and ceramic modification. Carbohydrate Polymers. 2007;69:467–477. [Google Scholar]
- 174.Bhattarai SR, Kim SY, Jang KY, Lee KC, Yi HK, Lee DY, Kim HY, Hwang PH. Laboratory formulated magnetic nanoparticles for enhancement of viral gene expression in suspension cell line. Journal of Virological Methods. 2008;147:213–218. doi: 10.1016/j.jviromet.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 175.Kim EH, Lee HS, Kwak BK, Kim BK. Synthesis of ferrofluid with magnetic nanoparticles by sonochemical method for MRI contrast agent. Journal of Magnetism and Magnetic Materials. 2005;289:328–330. [Google Scholar]
- 176.Lee HS, Kim EH, Shao HP, Kwak BK. Synthesis of SPIO-chitosan microspheres for MRI-detectable embolotherapy. Journal of Magnetism and Magnetic Materials. 2005;293:102–105. [Google Scholar]
- 177.Kircheis R, Wightman L, Wagner E. Design and gene delivery activity of modified polyethylenimines. Advanced Drug Delivery Reviews. 2001;53:341–358. doi: 10.1016/s0169-409x(01)00202-2. [DOI] [PubMed] [Google Scholar]
- 178.Godbey WT, Wu KK, Mikos AG. Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5177–5181. doi: 10.1073/pnas.96.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Corti M, Lascialfari A, Marinone M, Masotti A, Micotti E, Orsini F, Ortaggi G, Poletti G, Innocenti C, Sangregorio C. Magnetic and relaxometric properties of polyethylenimine-coated superparamagnetic MRI contrast agents. Journal of Magnetism and Magnetic Materials. 2008;320:E316–E319. [Google Scholar]
- 180.Huth S, Lausier J, Gersting SW, Rudolph C, Plank C, Welsch U, Rosenecker J. Insights into the mechanism of magnetofection using PEI-based magnetofectins for gene transfer. Journal of Gene Medicine. 2004;6:923–936. doi: 10.1002/jgm.577. [DOI] [PubMed] [Google Scholar]
- 181.Petri-Fink A, Steitz B, Finka A, Salaklang J, Hofmann H. Effect of cell media on polymer coated superparamagnetic iron oxide nanoparticles (SPIONs): Colloidal stability, cytotoxicity, and cellular uptake studies. European Journal of Pharmaceutics and Biopharmaceutics. 2008;68:129–137. doi: 10.1016/j.ejpb.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 182.Martina MS, Fortin JP, Menager C, Clement O, Barratt G, Grabielle-Madelmont C, Gazeau F, Cabuil V, Lesieur S. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. Journal of the American Chemical Society. 2005;127:10676–10685. doi: 10.1021/ja0516460. [DOI] [PubMed] [Google Scholar]
- 183.Bulte JWM, de Cuyper M, Despres D, Frank JA. Short- vs. long-circulating magnetoliposomes as bone marrow-seeking MR contrast agents. Journal of Magnetic Resonance Imaging. 1999;9:329–335. doi: 10.1002/(sici)1522-2586(199902)9:2<329::aid-jmri27>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 184.Decuyper M, Joniau M. MAGNETOLIPOSOMES - FORMATION AND STRUCTURAL CHARACTERIZATION. European Biophysics Journal with Biophysics Letters. 1988;15:311–319. doi: 10.1007/BF00256482. [DOI] [PubMed] [Google Scholar]
- 185.Yang J, Lee TI, Lee J, Lim EK, Hyung W, Lee CH, Song YJ, Suh JS, Yoon HG, Huh YM, Haam S. Synthesis of ultrasensitive magnetic resonance contrast agents for cancer imaging using PEG-fatty acid. Chemistry of Materials. 2007;19:3870–3876. [Google Scholar]
- 186.Mulder WJM, Strijkers GJ, van Tilborg GAF, Griffioen AW, Nicolay K. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. Nmr in Biomedicine. 2006;19:142–164. doi: 10.1002/nbm.1011. [DOI] [PubMed] [Google Scholar]
- 187.Dagata JA, Farkas N, Dennis CL, Shull RD, Hackley VA, Yang C, Pirollo KF, Chang EH. Physical characterization methods for iron oxide contrast agents encapsulated within a targeted liposome-based delivery system. Nanotechnology. 2008;19 doi: 10.1088/0957-4484/19/30/305101. [DOI] [PubMed] [Google Scholar]
- 188.Veiseh O, Kievit FM, Gunn JW, Ratner BD, Zhang M. A ligand-mediated nanovector for targeted gene delivery and transfection in cancer cells. Biomaterials. 2009;30:649–657. doi: 10.1016/j.biomaterials.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kievit FM, Veiseh O, Bhattarai N, Fang C, Gunn JW, Lee D, Ellenbogen RG, Olson JM, Zhang M. PEI-PEG-Chitosan-Copolymer-Coated iron oxide nanoparticles for safe gene delivery: synthesis, complexation, and transfection Advanced Functional Materials. doi: 10.1002/adfm.200801844. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Guo M, Yan Y, Zhang HK, Yan HS, Cao YJ, Liu KL, Wan SR, Huang JS, Yue W. Magnetic and pH-responsive nanocarriers with multilayer core-shell architecture for anticancer drug delivery. Journal of Materials Chemistry. 2008;18:5104–5112. [Google Scholar]
- 191.Thunemann AF, Schutt D, Kaufner L, Pison U, Mohwald H. Maghemite nanoparticles protectively coated with poly(ethylene imine) and poly(ethylene oxide)-block-poly(glutamic acid) Langmuir. 2006;22:2351–2357. doi: 10.1021/la052990d. [DOI] [PubMed] [Google Scholar]
- 192.Xiang JJ, Tang JQ, Zhu SG, Nie XM, Lu HB, Shen SR, Li XL, Tang K, Zhou M, Li GY. IONP-PLL: a novel non-viral vector for efficient gene delivery. Journal of Gene Medicine. 2003;5:803–817. doi: 10.1002/jgm.419. [DOI] [PubMed] [Google Scholar]
- 193.Hermanson GT. Bioconjugate Techniques. Elsevier Inc; London, UK: 2008. [Google Scholar]
- 194.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotech. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 195.Schellenberger EA, Weissleder R, Josephson L. Optimal modification of annexin V with fluorescent dyes. Chembiochem. 2004;5:271–274. doi: 10.1002/cbic.200300741. [DOI] [PubMed] [Google Scholar]
- 196.Kolb HC, Finn MG, Sharpless KB. Click chemistry: Diverse chemical function from a few good reactions. Angewandte Chemie-International Edition. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 197.Lutz JF, Zarafshani Z. Efficient construction of therapeutics, bioconjugates, biomaterials and bioactive surfaces using azide-alkyne “click” chemistry. Advanced Drug Delivery Reviews. 2008;60:958–970. doi: 10.1016/j.addr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 198.Hein CD, Liu XM, Wang D. Click chemistry, a powerful tool for pharmaceutical sciences. Pharmaceutical Research. 2008;25:2216–2230. doi: 10.1007/s11095-008-9616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.von Maltzahn G, Ren Y, Park JH, Min DH, Kotamraju VR, Jayakumar J, Fogal V, Sailor MJ, Ruoslahti E, Bhatia SN. In vivo tumor cell targeting with “Click” nanoparticles. Bioconjugate Chemistry. 2008;19:1570–1578. doi: 10.1021/bc800077y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sun EY, Josephson L, Weissleder R. “Clickable” nanoparticles for targeted imaging. Molecular Imaging. 2006;5:122–128. [PubMed] [Google Scholar]
- 201.Medarova Z, Pham W, Farrar C, Petkova V, Moore A. In vivo imaging of siRNA delivery and silencing in tumors. Nature Medicine. 2007;13:372–377. doi: 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]
- 202.Hogemann D, Josephson L, Weissleder R, Basilion JP. Improvement of MRI probes to allow efficient detection of gene expression. Bioconjugate Chemistry. 2000;11:941–946. doi: 10.1021/bc000079x. [DOI] [PubMed] [Google Scholar]
- 203.Schellenberger EA, Sosnovik D, Weissleder R, Josephson L. Magneto/optical annexin V, a multimodal protein. Bioconjugate Chemistry. 2004;15:1062–1067. doi: 10.1021/bc049905i. [DOI] [PubMed] [Google Scholar]
- 204.Wang AZ, Bagalkot V, Vasilliou CC, Gu F, Alexis F, Zhang L, Shaikh M, Yuet K, Cima MJ, Langer R, Kantoff PW, Bander NH, Jon SY, Farokhzad OC. Superparamagnetic iron oxide nanoparticle-aptamer bioconjugates for combined prostate cancer imaging and therapy. Chemmedchem. 2008;3:1311–1315. doi: 10.1002/cmdc.200800091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schellenberger E, Schnorr J, Reutelingsperger C, Ungethum L, Meyer W, Taupitz M, Hamm B. Linking proteins with anionic nanoparticles via protamine: Ultrasmall protein-coupled probes for magnetic resonance imaging of apoptosis. Small. 2008;4:225–230. doi: 10.1002/smll.200700847. [DOI] [PubMed] [Google Scholar]
- 206.Jain TK, Richey J, Strand M, Leslie-Pelecky DL, Flask CA, Labhasetwar V. Magnetic nanoparticles with dual functional properties: Drug delivery and magnetic resonance imaging. Biomaterials. 2008;29:4012–4021. doi: 10.1016/j.biomaterials.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yu MK, Jeong YY, Park J, Park S, Kim JW, Min JJ, Kim K, Jon S. Drug-loaded superparamagnetic iron oxide nanoparticles for combined cancer imaging and therapy in vivo. Angewandte Chemie-International Edition. 2008;47:5362–5365. doi: 10.1002/anie.200800857. [DOI] [PubMed] [Google Scholar]
- 208.Pan D, Caruthers SD, Hu G, Senpan A, Scott MJ, Gaffney PJ, Wickline SA, Lanza GM. Ligand-directed nanobialys as theranostic agent for drug delivery and manganese-based magnetic resonance Imaging of vascular targets. Journal of the American Chemical Society. 2008;130:9186–9187. doi: 10.1021/ja801482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kohler N, Sun C, Fichtenholtz A, Gunn J, Fang C, Zhang MQ. Methotrexate-immobilized poly(ethylene glycol) magnetic nanoparticles for MR imaging and drug delivery. Small. 2006;2:785–792. doi: 10.1002/smll.200600009. [DOI] [PubMed] [Google Scholar]
- 210.Burtea C, Laurent S, Roch A, Vander Elst L, Muller RN. C-MALISA (cellular magnetic-linked immunosorbent assay), a new application of cellular ELISA for MRI. Journal of Inorganic Biochemistry. 2005;99:1135–1144. doi: 10.1016/j.jinorgbio.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 211.Zhao M, Beauregard DA, Loizou L, Davletov B, Brindle KM. Non-invasive detection of apoptosis using magnetic resonance imaging and a targeted contrast agent. Nature Medicine. 2001;7:1241–1244. doi: 10.1038/nm1101-1241. [DOI] [PubMed] [Google Scholar]
- 212.Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circulation Research. 2005;96:327–336. doi: 10.1161/01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- 213.Moore A, Medarova Z, Potthast A, Dai GP. In vivo targeting of underglycosylated MUC-1 tumor antigen using a multimodal imaging probe. Cancer Research. 2004;64:1821–1827. doi: 10.1158/0008-5472.can-03-3230. [DOI] [PubMed] [Google Scholar]
- 214.Schellenberger EA, Hogemann D, Josephson L, Weissleder R. Annexin V-CLIO: A nanoparticle for detecting apoptosis by MRI. Academic Radiology. 2002;9:S310–S311. doi: 10.1016/s1076-6332(03)80212-x. [DOI] [PubMed] [Google Scholar]
- 215.Toma A, Otsuji E, Kuriu Y, Okamoto K, Ichikawa D, Hagiwara A, Ito H, Nishimura T, Yamagishi H. Monoclonal antibody A7-superparamagnetic iron oxide as contrast agent of MR imaging of rectal carcinoma. British Journal of Cancer. 2005;93:131–136. doi: 10.1038/sj.bjc.6602668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Funovics MA, Kapeller B, Hoeller C, Su HS, Kunstfeld R, Puig S, Macfelda K. MR imaging of the her2/neu and 9.2.27 tumor antigens using immunospecific contrast agents. Magnetic Resonance Imaging. 2004;22:843–850. doi: 10.1016/j.mri.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 217.Sakamoto JH, Smith BR, Xie B, Rokhlin SI, Lee SC, Ferrari M. The molecular analysis of breast cancer utilizing targeted nanoparticle based ultrasound contrast agents. Technology in Cancer Research & Treatment. 2005;4:627–636. doi: 10.1177/153303460500400606. [DOI] [PubMed] [Google Scholar]
- 218.Cirstoiu-Hapca A, Bossy-Nobs L, Buchegger F, Gurny R, Delie F. Differential tumor cell targeting of anti-HER2 (Herceptin (R)) and anti-CD20 (Mabthera (R)) coupled nanoparticles. International Journal of Pharmaceutics. 2007;331:190–196. doi: 10.1016/j.ijpharm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 219.Ireson CR, Kelland LR. Discovery and development of anticancer aptamers. Molecular Cancer Therapeutics. 2006;5:2957–2962. doi: 10.1158/1535-7163.MCT-06-0172. [DOI] [PubMed] [Google Scholar]
- 220.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. Acs Nano. 2008;2:889–896. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hu SH, Tsai CH, Liao CF, Liu DM, Chen SY. Controlled Rupture of Magnetic Polyelectrolyte Microcapsules for Drug Delivery. Langmuir. 2008;24:11811–11818. doi: 10.1021/la801138e. [DOI] [PubMed] [Google Scholar]
- 222.Zhang J, Misra RDK. Magnetic drug-targeting carrier encapsulated with thermosensitive smart polymer: Core-shell nanoparticle carrier and drug release response. Acta Biomaterialia. 2007;3:838–850. doi: 10.1016/j.actbio.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 223.Brans B, Linden O, Giammarile F, Tennvall J, Punt C. Clinical applications of newer radionuclide therapies. European Journal of Cancer. 2006;42:994–1003. doi: 10.1016/j.ejca.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 224.Hamoudeh M, Kamleh MA, Diab R, Fessi H. Radionuclides delivery systems for nuclear imaging and radiotherapy of cancer. Advanced Drug Delivery Reviews. 2008;60:1329–1346. doi: 10.1016/j.addr.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 225.Zhang CF, Cao JQ, Yin DZ, Wang YX, Feng YL, Tan JJ. Preparation and radiolabeling of human serum albumin (HSA)-coated magnetite nanoparticles for magnetically targeted therapy. Applied Radiation and Isotopes. 2004;61:1255–1259. doi: 10.1016/j.apradiso.2004.03.114. [DOI] [PubMed] [Google Scholar]
- 226.Cao JQ, Wang YX, Yu JF, Xia JY, Zhang CF, Yin DZ, Hafeli UO. Preparation and radiolabeling of surface-modified magnetic nanoparticles with rhenium-188 for magnetic targeted radiotherapy. Journal of Magnetism and Magnetic Materials. 2004;277:165–174. [Google Scholar]
- 227.Liang S, Wang YX, Yu JF, Zhang CF, Xia JY, Yin DZ. Surface modified superparamagnetic iron oxide nanoparticles: as a new carrier for bio-magnetically targeted therapy. Journal of Materials Science-Materials in Medicine. 2007;18:2297–2302. doi: 10.1007/s10856-007-3130-6. [DOI] [PubMed] [Google Scholar]
- 228.Zhang CF, Sun HW, Xia JY, Yu JF, Yao S, Yin DZ, Wang YX. Synthesis of polyacrylamide modified magnetic nanoparticles and radiolabeling with Re-188 for magnetically targeted radiotherapy. Journal of Magnetism and Magnetic Materials. 2005;293:193–198. [Google Scholar]
- 229.Bhutia SK, Maiti TK. Targeting tumors with peptides from natural sources. Trends in Biotechnology. 2008;26:210–217. doi: 10.1016/j.tibtech.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 230.Ross JS, Fletcher JA, Bloom KJ, Linette GP, Stec J, Symmans WF, Pusztai L, Hortobagyi GN. Targeted therapy in breast cancer - The HER-2/neu gene and protein. Molecular & Cellular Proteomics. 2004;3:379–398. doi: 10.1074/mcp.R400001-MCP200. [DOI] [PubMed] [Google Scholar]
- 231.Mykhaylyk O, Zelphati O, Rosenecker J, Plank C. siRNA delivery by magnetofection. Current Opinion in Molecular Therapeutics. 2008;10:493–505. [PubMed] [Google Scholar]
- 232.Pan BF, Cui DX, Sheng Y, Ozkan CG, Gao F, He R, Li Q, Xu P, Huang T. Dendrimer-modified magnetic nanoparticles enhance efficiency of gene delivery system. Cancer Research. 2007;67:8156–8163. doi: 10.1158/0008-5472.CAN-06-4762. [DOI] [PubMed] [Google Scholar]
- 233.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219:316–333. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 235.Jobsis FF. NONINVASIVE, INFRARED MONITORING OF CEREBRAL AND MYOCARDIAL OXYGEN SUFFICIENCY AND CIRCULATORY PARAMETERS. Science. 1977;198:1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 236.Debbage P, Jaschke W. Molecular imaging with nanoparticles: giant roles for dwarf actors. Histochem Cell Biol. 2008;130:845–75. doi: 10.1007/s00418-008-0511-y. [DOI] [PubMed] [Google Scholar]
- 237.Gao J, Hillebrenner HL USPTO. US Patent Application. USA: 2008. Nanotubular probes as ultrasensitive MR contrast agent. [Google Scholar]
- 238.Trehin R, Figueiredo JL, Pittet MJ, Weissleder R, Josephson L, Mahmood U. Fluorescent nanoparticle uptake for brain tumor visualization. Neoplasia. 2006;8:302–311. doi: 10.1593/neo.05751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kircher MF, Mahmood U, King RS, Weissleder R, Josephson L. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Research. 2003;63:8122–8125. [PubMed] [Google Scholar]
- 240.Lee HY, Li Z, Chen K, Hsu AR, Xu C, Xie J, Sun S, Chen X. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J Nucl Med. 2008;49:1371–9. doi: 10.2967/jnumed.108.051243. [DOI] [PubMed] [Google Scholar]
- 241.Yang J, Lee CH, Ko HJ, Suh JS, Yoon HG, Lee K, Huh YM, Haam S. Multifunctional magneto-polymeric nanohybrids for targeted detection and synergistic therapeutic effects on breast cancer. Angew Chem Int Ed Engl. 2007;46:8836–8839. doi: 10.1002/anie.200703554. [DOI] [PubMed] [Google Scholar]