Abstract
Speciation, the evolution of reproductive isolation between populations, serves as the driving force for generating biodiversity. Postzygotic barriers to gene flow, such as F1 hybrid sterility and inviability, play important roles in the establishment and maintenance of biological species. F1 hybrid incompatibilities in taxa that obey Haldane's rule, the observation that the heterogametic sex suffers greater hybrid fitness problems than the homogametic sex, are thought to often result from interactions between recessive-acting X-linked loci and dominant-acting autosomal loci. Because they play such prominent roles in producing hybrid incompatibilities, we examine the dominance and nature of epistasis between alleles derived from Drosophila persimilis that confer hybrid male sterility in the genetic background of its sister species, D. pseudoobscura bogotana. We show that epistasis elevates the apparent dominance of individually recessive-acting QTL such that they can contribute to F1 hybrid sterility. These results have important implications for assumptions underlying theoretical models of hybrid incompatibilities and may offer a possible explanation for why, to date identification of dominant-acting autosomal “speciation genes” has been challenging.
Keywords: postzygotic, gene flow, reproductive isolation, Haldane's rule, speciation
INTRODUCTION
The genetic changes underlying the evolution of reproductive isolation have long been an important research topic in the study of speciation. Gene flow between populations can be restricted either via prezygotic barriers that act before the fusion of the egg and the sperm or postzygotic barriers that act after gamete fusion. Postzygotic barriers, such as hybrid sterility or inviability, are thought to result from negative epistatic interactions between the genomes of two diverging populations (Dobzhansky 1936; Muller 1942) To date, many studies on postzygotic reproductive isolation have been devoted specifically to elucidating the causes of Haldane's (Haldane 1922) rule and more generally to the causes of F1 problems in hybrid males (Coyne and Orr 2004).
Postzygotic isolating barriers between disparate taxa largely conform to Haldane's (1922) rule, which observes that when hybrids of one sex are sterile or inviabile, it is more often the heterogametic (XY or ZW) sex. While several theories have been proposed to explain this asymmetry (Charlesworth et al. 1987; Wu and Davis 1993; Wu et al. 1996), the dominance theory (Muller 1940, 1942; Turelli and Orr 1995, 2000) appears to explain the majority of the observed cases, as it applies whether males or females are the heterogametic sex. The dominance theory simply posits that alleles underlying hybrid incompatibilities are, on average, partially recessive. Under this condition of partial recessivity, the expression of X-linked incompatible recessive alleles in the heterogametic sex (e.g., XY) outweighs the potentially greater number of incompatibilities suffered by the homogametic sex (e.g., XX, which could carry up to twice as many incompatibility alleles as the XY sex), resulting in Haldane's rule. (Note that this explanation assumes that the Y chromosome does not contain sufficient alleles, either because of its reduced size or gene content, to mask the effects of hemizygous X-linked alleles.)
The dominance theory predicts that recessive X-linked loci from one species interact with dominant autosomal loci from another species to produce incompatibilities in heterogametic-sex hybrids. This description implies that the evolution of hybrid incompatibilities necessarily involves epistasis between loci from different species (figure 1a) and could also involve epistasis between multiple loci from one or both species (figures 1b and 1c). Empirical studies provide strong evidence for both types of interactions (see references below). Additionally, genetic analyses of hybrid incompatibilities should be able to localize the recessive X-linked loci and the dominant-acting autosomal loci. However, while the former have been relatively accessible (Guenet et al. 1990; Cabot et al. 1994; Perez and Wu 1995; True et al. 1996; Macdonald and Goldstein 1999; Orr and Irving 2001; Oka et al. 2004; Storchova et al. 2004; Masly and Presgraves 2007), their dominant autosomal counterparts have remained far more elusive and are rarely mapped with precision (Slotman et al. 2004).
Figure 1. Epistasis between hybrid incompatibility QTL.
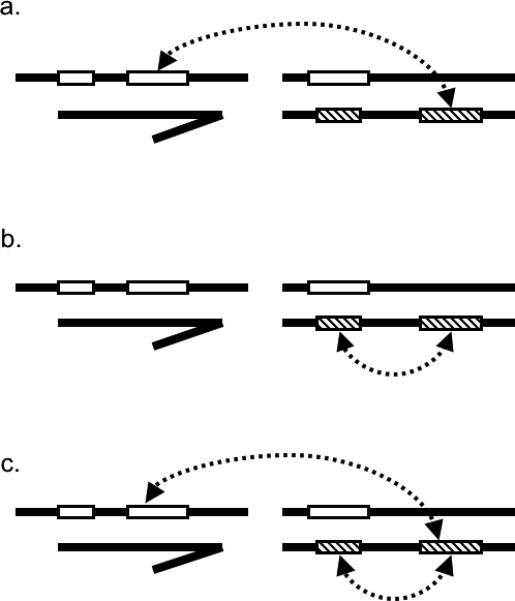
Epistasis necessarily occurs between incompatibility-conferring loci from different species (a). However, epistasis may also occur between multiple loci within a single species (b) and/or between multiple loci from both species (c). Open rectangles indicate incompatibility loci. Dashed arrows indicate epistatic interactions between loci. Note that not all possible interactions are depicted.
Several studies of Drosophila species pairs have highlighted the importance of epistatic interactions when considering the causes of hybrid male sterility. Hybrid males between D. simulans and its sister species D. mauritiana are rendered completely sterile only if the D. mauritiana allele of the X-linked gene Odysseus and an adjacent X-chromosome region interact with one or more D. simulans autosomal regions (Palopoli and Wu 1994; Perez and Wu 1995; Davis and Wu 1996). Similarly, sterility of hybrid males between the Bogota and USA subspecies of D. pseudoobscura is conditional upon interactions between Bogota alleles at two or more X-chromosome loci and USA alleles at two or more autosomal loci (Orr and Irving 2001; Phadnis and Orr 2009). Finally, Naveira and Fontdevila (1986, 1991) noted that hybrid male sterility between the species of the D. buzzatii species group results from epistasis between promiscuous (i.e., interchangeable) segments of the autosomes. Although these studies have acknowledged the significant contribution of epistasis to hybrid male sterility, none have explicitly examined the effects of epistasis among putatively dominant autosomal sterility-conferring loci that should contribute to Haldane's rule.
While epistasis must contribute to Haldane's rule, the pattern of interactions causing sterility in hybrids is less clear. Recent studies (reviewed in Coyne and Orr 2004) suggest that F1 hybrid male sterility likely results not from “major genes” of large effect but instead from multiple factors that individually have small effects and interact epistatically to cause sterility. More specifically, sterility appears to result from epistasis between non-interchangeable conspecific alleles in addition to epistasis between the alleles derived from the different species (Palopoli and Wu 1994; Perez and Wu 1995; Wu and Hollocher 1998).
Here, we examine the pattern of epistasis and its effect on the dominance of three autosomal QTL underlying hybrid male sterility between Drosophila persimilis and D. pseudoobscura bogotana. Results from our previous study (Chang and Noor 2007) suggest that three autosomal QTL (one on each major autosome) from D. persimilis interact with a D. p. bogotana background to produce sterile hybrid males. Specific epistatic interactions between these regions (i.e., between the three QTL) were not carefully examined in our previous study because of the variable genetic background of our backcross population.
The genetics underlying hybrid male sterility between D. persimilis and D. p. bogotana thus lends itself to addressing the two aforementioned implications of the dominance theory. In this study, we first introgressed QTL from D. persimilis into an otherwise completely D. p. bogotana genetic background to assess the dominance of each QTL individually. Second, we introgressed heterozygous and homozygous combinations of QTL to evaluate the effect of epistasis on the dominance of the sterility-conferring QTL. Results from this study show strong epistasis between these autosomal factors, and, unexpectedly, that epistasis modifies the apparent dominance of loci underlying hybrid male sterility. These results suggest that assessments of the dominance of individually examined QTL are not necessarily applicable when considering the causes of Haldane's rule.
MATERIALS AND METHODS
F1 hybrid females between D. persimilis and D. p. bogotana were backcrossed to D. p. bogotana males. The females were then genotyped at three microsatellite markers linked to the three chromosomal inversions (two on the X-chromosome and one on the 2nd-chromosome) distinguishing these species. Those females heterozygous or homozygous for the D. p. bogotana arrangement were subsequently genotyped for microsatellite markers flanking each of three QTL, one on each autosome, conferring hybrid male sterility. Selecting for the D. p. bogotana arrangement ensured that the X-chromosome in the subsequent backcross hybrid males derived only from D. p. bogotana, as the two inversions and their recombination-suppression effects on the X-chromosome span the majority of the chromosome. Furthermore, this process also guaranteed that the sterility-conferring loci mapped in this study reside outside the inversions and are comparable to those fine-mapped in Chang and Noor (2007)
Females heterozygous for the sterility loci markers were selected and used for successive backcrosses to D. p. bogotana males: three individual “single introgression” lines (Q2, Q3, and Q4, with the numbers designating the autosome on which the QTL resides) were created such that each introgression line contained a QTL from D. persimilis that conferred hybrid male sterility against a D. p. bogotana genetic background. Ten generations of backcrossing were performed to ensure that the genetic background was purged of D. persimilis alleles other than those at the three sterility-conferring QTL (see figure 2a). Males from the 11th generation backcross were assayed for fertility following the methods of Coyne (1984): testes were dissected in Ringer's solution and sperm motility was determined. This assay of sterility is conservative, as we have shown previously that some hybrid males bearing motile sperm fail to produce offspring after mating with females in the laboratory (Campbell and Noor 2001). The males were then genotyped at the relevant microsatellite markers (see DNA preparation and genotyping methods in Chang and Noor (2007). Primer sequences for all markers used in this study are available in Table S1.
Figure 2.
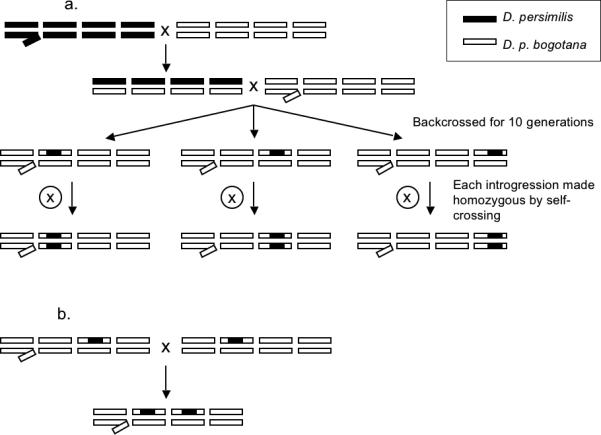
A. Crossing scheme for single introgression. D. persimilis males were crossed to D. p. bogotana females. F1 females were backcrossed to D. p. bogotana males for 10 generations until the genetic background was devoid of D. persimilis alleles except for the QTL of interest. Three introgression lines were created for each autosomal QTL (Q2, Q3, and Q4). Males homozygous for each QTL introgression were generated by self-crossing within each line. B. Example of cross for multiple introgressions. Example of a cross to generate male flies heterozygous for both the 2nd - and the 3rd-chromosome QTL.
To create homozygous introgression lines for each of the three autosomal QTL, males and females from within each single introgression line were self-crossed. This was possible because none of the introgression lines bearing one copy of the D. persimilis allele at one locus produced sterile hybrid males (see Results). Male offspring from these crosses were then assayed for fertility and genotyped at the relevant markers.
To examine the effect of epistasis on the dominance of sterility-conferring loci, males from one introgression line were crossed to females of another introgression line to produce “combination introgression” lines. For example, to assay the heterozygous effect (if any) of both the 2nd- and 3rd-chromosome QTL (i.e., Q2 + Q3) on hybrid male fertility, heterozygous Q3 males were crossed to heterozygous Q2 females and vice versa (figure 2b). These Q2 + Q3 males were assayed for fertility and genotyped at the relevant markers. Though 26 total heterozygous/homozygous combinations of QTL were possible, not all were produced and assayed. We did not produce some genotypic combinations because males bearing more than one D. persimilis allele were often incapable of producing progeny (see Results), in some cases even when individuals produced small numbers (e.g., less than 5) of motile sperm were observed (and hence classed as “fertile” by our conservative assay).
RESULTS
Dominance of Individual QTL Introgressions
To assess the dominance of individual autosomal QTL, each sterility-conferring QTL from D. persimilis was introgressed via 11 generations of backcrossing into an otherwise D. p. bogotana genetic background. In contrast to the results from our original backcross analysis (Chang and Noor 2007), our data here suggest that two of the three QTL (Q2 and Q3, the 2nd- and 3rd-chromosome QTL, respectively) behaved recessively when examined alone. Virtually all flies bearing the D. persimilis allele at either Q2 or Q3 were fully fertile (figure 3, genotypes 2 and 4), while virtually all flies homozygous for the D. persimilis allele at either Q2 or Q3 produced no visible motile sperm (figure 3, genotypes 3 and 5). Strikingly, the D. persimilis allele at the 4th-chromosome QTL (Q4) showed no effect on hybrid male sterility (figure 3, genotypes 6 and 7). Indeed, a vigorous self-perpetuating strain was constructed that is homozygous for the Q4 introgression.
Figure 3. Effect on male sterility of single QTL introgressions.
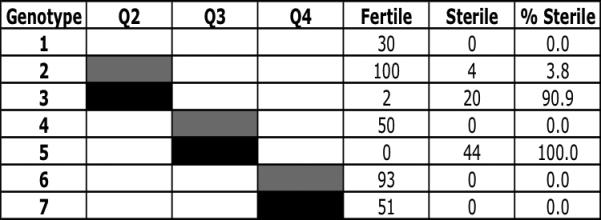
Percent of males sterile for each genotype at the three QTL is given. White rectangles represent homozygous D. p. bogotana genotype. Grey rectangles represent heterozygous D. persimilis / D. p. bogotana genotype. Black rectangles represent homozygous D. persimilis genotype.
Epistasis and Dominance of Multiple Inter-Chromosomal QTL Introgressions
To detect epistatic effects among the D. persimilis alleles at the hybrid male sterility QTL, co-introgressions of either one (heterozygous) or two (homozygous) copies of the D. persimilis allele for multiple QTL were performed. While 26 combinations of heterozygous and/or homozygous introgressions are possible, not all genotypes were assayed for fertility. The results from single-locus introgressions (above) show that male sterility is nearly complete when Q2 and Q3 are homozygous for the D. persimilis allele, which, because of the sterility observed in some genotypic combinations (see below), rendered many genotypes extremely difficult to produce.
Figure 4 shows the effects of heterozygous and homozygous combinations of D. persimilis QTL introgressions on hybrid male sterility. In contrast to an individual heterozygous introgressions of Q2 or Q3, which both yielded fertile males, cointrogression of heterozygous Q2 with heterozygous Q3 rendered 43.0% of the males sterile, suggesting strong epistasis between the D. persimilis alleles at these two QTL. However, heterozygous Q4, which had no effect when introgressed alone, failed to significantly increase male sterility when co-introgressed with heterozygous Q2. Similarly, no effect on sterility was apparent in co-introgressions of heterozygous Q4 with heterozygous Q3. Thus, heterozygous introgressions of two of the three QTL suggest that epistasis between Q2 and Q3 produced an elevated “composite dominance” of these two QTL while two-locus epistasis (i.e., Q2 + Q4 and Q3 + Q4) appeared to have no effect on Q4.
Figure 4. Effect on male sterility of multiple QTL introgressions.

Percent of males sterile for each genotype at the three QTL is given. White rectangles represent homozygous D. p. bogotana genotype. Grey rectangles represent heterozygous D. persimilis / D. p. bogotana genotype. Black rectangles represent homozygous D. persimilis genotype.
To further investigate the role of Q4 in causing hybrid male sterility, we produced introgression genotypes containing two copies of the D. persimilis allele in a D. p. bogotana background in combination with heterozygous introgressions of Q2 or Q3. 15% of males with heterozygous Q2 and homozygous Q4 introgressions were sterile, in comparison to 4% of sterile males with heterozygous Q2 and Q4 introgressions; this difference is not statistically significant (figure 4, genotype 5 vs. genotype 3; p = 0.16, Fisher's Exact test). In addition, 0% of males with heterozygous Q3 and homozygous Q4 introgressions were sterile, which is not significantly different from the 5% of sterile males with heterozygous Q3 and Q4 introgressions previously observed (figure 4, genotype 6 vs. genotype 4; p = 0.25, Fisher's Exact test). These data suggest that Q4 had little or no effect on hybrid male sterility between D. persimilis and D. p. bogotana when in combination with only one of the two other QTL conferring hybrid male sterility between D. persimilis and D. p. bogotana.
In contrast to those male flies carrying only two heterozygous introgressions of QTL from D. persimilis, 94% of males bearing heterozygous introgressions of Q2, Q3, and Q4 (figure 4, genotype 7) were sterile. This strongly suggests that Q4 interacted epistatically with Q2 and Q3 to produce nearly complete sterility and confirms the results from our original backcross analysis (Chang and Noor 2007) in which Q4 contributed significantly to hybrid male sterility. (Because nearly all the male flies heterozygous for the D. persimilis allele at all three QTL were sterile, the genotype “heterozygous Q2, heterozygous Q3, and homozygous D. persimilis Q4” was not assayed.) The dominant effect of Q4 was manifest only in combination with a single copy of the D. persimilis allele of both Q2 and Q3.
Effect of Introgression Size on Hybrid Male Sterility
As a crude test of whether the sterility we observed in hybrid males between D. persimilis and D. p. bogotana was caused by the size of the introgression itself (the “additive threshold” model; Naveira and Maside 1998) or by interactions of specific loci (the “weak allele-strong interaction” model; Hollocher and Wu 1998), we introgressed a 3.23 Mb region from the D. persimilis 2nd-chromosome into an otherwise D. p. bogotana background. This introgressed region is greater in size than the introgression Q2 by 1.47 Mb. Introgression of this segment of chromosome 2 in combination with Q3 and Q4 failed to produce any sterile hybrid males, showing that the hybrid male sterility we observed in this study was not a direct result of the sizes of the individual introgressed regions.
DISCUSSION
While epistasis is widely considered to play a role in hybrid incompatibilities, few studies have formally identified specific epistatic interactions between foreign autosomal loci that contribute to hybrid unfitness. Here, using an introgression approach, we explicitly test the nature of epistasis between the three autosomal QTL (one on each major autosome) from D. persimilis that confer hybrid male sterility in an otherwise completely D. p. bogotana genetic background. We show that none of the three QTL is capable of producing substantial hybrid sterility when a single (i.e., heterozygous) D. persimilis allele is introgressed. However, almost 50% of males carrying both the 2nd- and 3rd-chromosome QTL introgressions are sterile. Introgressions of the 2nd- and 4th-chromsome QTL or the 3rd- and 4th-chromosome QTL have almost no effect on male sterility, but almost 100% of males carrying one copy of all three QTL are sterile.
These results demonstrate that manifestation of hybrid male sterility between D. persimilis and D. p. bogotana depends critically on epistasis that is highly specific in nature. That we failed to observe sterility when a large region not associated with a QTL was introgressed along with two of our focal QTL further underscores the importance of QTL identity. Our results thus do not support an “additive threshold” model (Naveira and Maside 1998) for hybrid incompatibility, as the sterility-conferring genetic factors do not appear to be interchangeable. Instead, hybrid male sterility between these species appears to result from interactions between specific loci: the individually weak QTL have a formidable effect on male fertility when all three are co-introgressed. This result is consistent with several studies (Tao et al. 2001; Presgraves et al. 2003; Tao et al. 2003b; Phadnis and Orr 2009) that have mapped individual genes underlying hybrid incompatibilities.
Our results further suggest that epistasis between the hybrid sterility QTL modifies the apparent dominance of these QTL. Both the chromosome-2 and chromosome-3 QTL from D. persimilis behave completely recessively when introgressed individually into a D. p. bogotana background. Epistasis appears to elevate the “composite dominance” of these two QTL, as nearly half the males carrying cointrogressions of these QTL are sterile. (We favor the expression “composite dominance” as a simple descriptor of the overall dominance of 2 QTL over traditional quantitative genetics terms such as “additive-by-additive,” “additive-by-dominance,” and “dominance-by-dominance,” which more specifically describe the effects of epistasis on additivity and dominance between conspecific loci.) The addition of one copy of the 4th-chromsome QTL, which alone had no effect on hybrid male sterility even when homozygous, to the other two QTL produced nearly complete male sterility. Thus, the 4th-chromosome QTL, via epistasis, plays an indispensible role in the establishment of a postzygotic barrier to gene flow.
The modification of dominance by epistasis represents a theoretical void in our understanding of genetic interactions. While several theories (Fisher 1928; Kacser and Burns 1981; Keightley 1996; Phadnis and Fry 2005) exist to explain the evolution of dominance itself, these theories are not necessarily suitable for explaining the patterns observed in this study. Existing theories address only how new recessive mutations arising in populations (within species) exhibit or achieve dominance. Here, we examine the dominance of QTL from one species in another species’ genetic background. The heterospecific nature of the interactions we address diminish the applicability of within-species models. Furthermore, it is possible that our metric of fertility affected the dominance relationships observed. If an underlying additive basis to the effects of these QTL in a foreign background exists, but fertility is scored as a threshold trait, one could observe similar patterns. Nonetheless, the interactions we document exist and may have biased the conclusions of previous studies using similar metrics (see below).
The results together demonstrate that the assessments of gene action at a single locus can be misleading when considering how hybrid incompatibilities can arise between species (see also Fitzpatrick 2008). The dominance theory explaining Haldane's rule (Muller 1940, 1942; Turelli and Orr 1995, 2000) predicts that F1 genetic incompatibilities, such as hybrid male sterility, are often caused by interactions between recessive X-linked loci from one species and dominant autosomal loci from another. Based on this theory, genetic studies should be able to localize dominant autosomal loci precisely, as well as to map their recessive X-linked counterparts. Contrary to these expectations, however, the dominant-acting autosomal loci have been comparatively difficult to detect, though several recessive-acting autosomal loci (True et al. 1996; Tao et al. 2003a; Tao and Hartl 2003; Tao et al. 2003b; Masly and Presgraves 2007) underlying hybrid incompatibilities have been isolated from exhaustive introgression studies.
We offer a possible explanation for this discrepancy: strong epistasis among autosomal loci may moderate their detectable effects in interspecific backcross or introgression analyses. As we observe in this study, individual QTL may behave recessively in isolation but dominantly in combination. Only by taking into account epistatic interactions between the QTL were we able to detect “dominant” effects of these loci that would operate in an F1 hybrid. Introgression studies in particular may often entirely miss QTL underlying hybrid incompatibilities. Loci that behave similarly to the chromosome-4 QTL in this study may appear to have no effect though, through epistasis, they may dramatically reduce the fitness of F1 hybrids.
Furthermore, autosomal recessive “speciation genes” that have previously been isolated (e.g., Nup96, Presgraves et al. 2003; Tmy, Tao et al. 2001, 2003b) may also contribute directly to reduced F1 male fitness. Our data suggest that, in the case of speciation genes such as Nup96 and Tmy, should their autosomal interactors be cloned, the composite effect of these genes and their interactors could potentially cause lethality or sterility in F1 hybrids. Introgression studies by Tao and colleagues (Tao et al. 2003a; Tao and Hartl 2003; Tao et al. 2003b) offer another intriguing opportunity to assess the applicability of these results. These researchers mapped many autosomal loci causing hybrid male sterility between D. simulans and D. mauritiana. While all these loci behaved recessively, the F1 males between these species were completely sterile. Consistent with our hypothesis, “trans-heterozygotes” of some pairs of alleles showed reduced fertility (Tao and Hartl 2003). It would be enlightening to examine whether epistasis between more of their sterility QTL can elevate this partial sterility to increased or complete sterility via composite dominance.
In this study, we only address epistasis between alleles from D. persimilis in a D. p. bogotana genetic background. Certainly, these D. persimilis alleles must interact with one or more D. p. bogotana alleles to produce sterile hybrid males between these two species. Furthermore, D. p. bogotana alleles could interact with each other as well. However, neither of these latter two classes of interactions is within the scope of this study. As a result, we can only say that interactions between a minimum of 3 genes from D. persimilis and at least one from D. p. bogotana are required to cause hybrid male sterility in this species pair, but likely many more loci are involved.
We also note that our current study examines the effect of hybrid male sterility QTL on a group and not on individuals. In other words, our results rely on observations of the number of males that are sterile vs. the number of males that are fertile rather than number of nonfunctional vs. functional sperm within an individual. This latter approach was impractical for our studies: hybrid male sterility between D. persimilis and D. p. bogotana could originally only be detected in our introgressions when large sample sizes were utilized, thus rendering sperm counts of individual males unfeasible. Yet, the effects of the QTL and of epistasis between them were present when using the group approach. Nevertheless, we acknowledge that we may have missed other contributing loci.
In summary, epistasis between hybrid male sterility QTL appears indispensable for the manifestation of this incompatibility between D. persimilis and D. p. bogotana. In the absence of epistatic interactions, individually recessive-acting loci cannot contribute to F1 hybrid sterility. Whether this observation applies more broadly to hybrid incompatibilities between other species pairs can only be resolved by additional future studies. The paucity of individually dominant-acting “speciation loci” to date suggests that perhaps their necessary interactors have not yet been detected. The elucidation of the genetic and cellular mechanisms causing hybrid incompatibilities will depend on identification of all members of the interacting genetic complex.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank S. Bennett, M. Nowak, D. Presgraves, J. True, and two anonymous reviewers for comments on the manuscript. This work was funded by National Science Foundation grant 0808029 to A.S.C. and grants 0509780 and 0715484 to M.A.F.N. as well as National Institutes of Health grant GM076051 to M.A.F.N.
LITERATURE CITED
- Cabot EL, Davis AW, Johnson NA, Wu C-I. Genetics of reproductive isolation in the Drosophila simulans clade: complex epistasis underlying hybrid male sterility. Genetics. 1994;137:175–189. doi: 10.1093/genetics/137.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RV, Noor MAF. Assessing hybrid male fertility in Drosophila species: Correlation between sperm motility and production of offspring. Drosoph. Inf. Serv. 2001;84:6–9. [Google Scholar]
- Chang AS, Noor MA. The genetics of hybrid male sterility between the allopatric species pair Drosophila persimilis and D. pseudoobscura bogotana: dominant sterility alleles in collinear autosomal regions. Genetics. 2007;176:343–349. doi: 10.1534/genetics.106.067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B, Coyne JA, Barton NH. The relative rates of evolution of sex chromosomes and autosomes. American Naturalist. 1987;130:113–146. [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sinauer Associates, Inc.; Sunderland, MA: 2004. [Google Scholar]
- Davis AW, Wu C-I. The broom of the sorcerer's apprentice: the fine structure of a chromosomal region causing reproductive isolation between two sibling species of Drosophila. Genetics. 1996;143:1287–1298. doi: 10.1093/genetics/143.3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. Studies of hybrid sterility. II. Localization of sterility factors in Drosophila pseudoobscura hybrids. Genetics. 1936;21:113–135. doi: 10.1093/genetics/21.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. The possible modification of the response of the wild type to recurrent mutations. American Naturalist. 1928;62:115–126. [Google Scholar]
- Guenet J, Nagamine C, Simon-Chazottes D, Montagutelli X, Bonhomme F. Hst-3: an X-linked hybrid sterility gene. Genet. Res. 1990;53:163–165. doi: 10.1017/s0016672300035254. [DOI] [PubMed] [Google Scholar]
- Haldane JBS. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 1922;12:101–109. [Google Scholar]
- Kacser H, Burns JA. The molecular basis of dominance. Genetics. 1981;97:639–666. doi: 10.1093/genetics/97.3-4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley PD. A metabolic basis for dominance and recessivity. Genetics. 1996;143:621–625. doi: 10.1093/genetics/143.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald SJ, Goldstein DB. A quantitative genetic analysis of male sexual traits distinguishing the sibling species Drosophila simulans and D. sechellia. Genetics. 1999;153:1683–1699. doi: 10.1093/genetics/153.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masly JP, Presgraves DC. High-resolution genome-wide dissection of the two rules of speciation in Drosophila. PLoS Biology. 2007;5:e243. doi: 10.1371/journal.pbio.0050243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HJ. Bearings of the Drosophila work on systematics. In: Huxley J, editor. New Systematics. Clarendon Press; Oxford: 1940. pp. 185–268. [Google Scholar]
- Muller HJ. Isolating mechanisms, evolution and temperature. Biol. Symp. 1942;6:71–125. [Google Scholar]
- Naveira H, Fontdevila A. The evolutionary history of Drosophila buzzatii. XII. The genetic basis of sterility in hybrids between D. buzzatii and its sibling D. serido from Argentina. Genetics. 1986;114:841–857. doi: 10.1093/genetics/114.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveira H, Fontdevila A. The evolutionary history of D. buzzatii. XXI. Cumulative action of multiple sterility factors on spermatogenesis in hybrids of D. buzzatii and D. koepferae. Heredity. 1991;67:57–72. doi: 10.1038/hdy.1991.65. [DOI] [PubMed] [Google Scholar]
- Naveira HF, Maside XR. The genetics of hybrid male sterility in Drosophila. Oxford University Press; Oxford: 1998. [Google Scholar]
- Oka A, Mita A, Sakurai-Yamatani N, Yamamoto H, Takagi N, Takano-Shimizu T, Toshimori K, Moriwaki K, Shiroishi T. Hybrid breakdown caused by substitution of the X chromosome between two mouse subspecies. Genetics. 2004;166:913–924. doi: 10.1534/genetics.166.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr HA, Irving S. Complex epistasis and the genetic basis of hybrid sterility in the Drosophila pseudoobscura Bogota-USA hybridization. Genetics. 2001;158:1089–1100. doi: 10.1093/genetics/158.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palopoli MF, Wu C-I. Genetics of hybrid male sterility between Drosophila sibling species: a complex web of epistasis is revealed in interspecific studies. Genetics. 1994;138:329–341. doi: 10.1093/genetics/138.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez DE, Wu C-I. Further characterization of the Odysseus locus of hybrid sterility in Drosophila: One gene is not enough. Genetics. 1995;140:201–206. doi: 10.1093/genetics/140.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadnis N, Fry JD. Widespread correlation between dominance and homozygous effects of mutations: implications for theories of dominance. Genetics. 2005;171:385–392. doi: 10.1534/genetics.104.039016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadnis N, Orr HA. A single gene causes both male sterility and segregation distortion in Drosophila hybrids. Science. 2009;323:376–379. doi: 10.1126/science.1163934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presgraves DC, Balagopalan L, Abmayr SM, Orr HA. Adaptive evolution drives divergence of a hybrid inviability gene between two species of Drosophila. Nature. 2003;423:715–719. doi: 10.1038/nature01679. [DOI] [PubMed] [Google Scholar]
- Slotman M, dellaTorre A, Powell JR. The genetics of inviability and male sterility in hybrids between Anopheles gambiae and A. arabiensis. Genetics. 2004;167:275–287. doi: 10.1534/genetics.167.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storchova R, Gregorova S, Buckiova D, Kyselova V, Divina P, Forejt J. Genetic analysis of X-linked hybrid sterility in the house mouse. Mamm. Genome. 2004;15:515–524. doi: 10.1007/s00335-004-2386-0. [DOI] [PubMed] [Google Scholar]
- Tao Y, Chen S, Hartl DL, Laurie CC. Genetic dissection of hybrid incompatibilities between Drosophila simulans and D. mauritiana. I. Differential accumulation of hybrid male sterility effects on the X and autosomes. Genetics. 2003a;164:1383–1398. doi: 10.1093/genetics/164.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Hartl DL. Genetic dissection of hybrid incompatibilities between Drosophila simulans and D. mauritiana. III. Heterogeneous accumulation of hybrid incompatibilities, degree of dominance, and implications for Haldane's Rule. Evolution. 2003;57:2580–2598. doi: 10.1111/j.0014-3820.2003.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Tao Y, Hartl DL, Laurie CC. Sex-ratio segregation distortion associated with reproductive isolation in Drosophila. Proc. Natl. Acad. Sci. USA. 2001;98:13183–13188. doi: 10.1073/pnas.231478798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Zeng Z-B, Li J, Hartl DL, Laurie CC. Genetic dissection of hybrid incompatibilities between Drosophila simulans and D. mauritiana. II. Mapping hybrid male sterility loci on the third chromosome. Genetics. 2003b;164:1399–1418. doi: 10.1093/genetics/164.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True JR, Weir BS, Laurie CC. A genome-wide survey of hybrid incompatibility factors by the introgression of marked segments of Drosophila mauritiana chromosomes into Drosophila simulans. Genetics. 1996;142:819–837. doi: 10.1093/genetics/142.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M, Orr HA. The dominance theory of Haldane's Rule. Genetics. 1995;140:389–402. doi: 10.1093/genetics/140.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M, Orr HA. Dominance, epistasis and the genetics of postzygotic isolation. Genetics. 2000;154:1663–1679. doi: 10.1093/genetics/154.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-I, Davis AW. Evolution of postmating reproductive isolation: The composite nature of Haldane's Rule and its genetic bases. American Naturalist. 1993;142:187–212. doi: 10.1086/285534. [DOI] [PubMed] [Google Scholar]
- Wu C-I, Hollocher H. Subtle is nature: The genetics of species differentiation and speciation. In: Howard DJ, Berlocher SH, editors. Endless Forms: Species and Speciation. Oxford University Press; New York: 1998. pp. 339–351. [Google Scholar]
- Wu C-I, Johnson NA, Palopoli MF. Haldane's rule and its legacy: why are there so many sterile males? Trends in Ecology and Evolution. 1996;11:281–284. doi: 10.1016/0169-5347(96)10033-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


