Abstract
Transplantation of endothelial cells (EC) for therapeutic vascularization is a promising approach in tissue engineering but has yet to be proven effective in clinical trials. This cell-based therapy is hindered by significant apoptosis of EC upon transplantation as well as poor recruitment of host mural cells to stabilize nascent vessels. Here, we address these deficiencies by augmenting endothelial cell transplantation with dual delivery of vascular endothelial growth factor (VEGF) – to improve survival of transplanted EC – and monocyte chemotactic protein-1 (MCP-1) – to induce mural cell recruitment. We produced alginate microparticles that deliver VEGF and MCP-1 with distinct release kinetics and that can be integrated into a collagen/fibronectin (protein) gel construct for delivery of EC. Combined delivery of VEGF and MCP-1 increased functional vessel formation from transplanted EC and also led to a higher number of smooth muscle cell-invested vessels than did EC therapy alone. Despite the well-known role of MCP-1 in inflammation, these beneficial effects were accomplished without a long-term increase in monocyte/macrophage recruitment or a shift to a pro-inflammatory (M1) macrophage phenotype. Overall, these data suggest a potential benefit of combined delivery of MCP-1 and VEGF from EC-containing hydrogels as a strategy for therapeutic vascularization.
Keywords: growth factor, controlled drug release, tissue engineering, angiogenesis, collagen hydrogel, alginate
1. Introduction
The lack of control over microvasculature formation in therapeutic vascularization and tissue engineering remains a key roadblock to the realization of clinical benefits from these fields. Initial attempts to grow blood vessels for therapeutic purposes consisted of molecular delivery approaches [1] and progressed to vascular cell therapy methods [2, 3]. However, molecular delivery for therapeutic vascularization has yet to show efficacy in human clinical trials [4, 5], while cell therapy is plagued by grossly inefficient engraftment after transplantation that can lead to impaired functionality [6]. More recently, strategies combining molecular delivery and cell therapy for therapeutic vascularization in hindlimb ischemia models have been reported [7, 8]. While promising, these studies have examined the delivery of a single cytokine, vascular endothelial growth factor (VEGF), which effectively drives angiogenesis and improves survival of transplanted endothelial cells (EC), but does not by itself promote the formation of mature, stable vasculature [7, 9, 10].
VEGF-based therapies have consistently failed in clinical trials, which is not surprising considering that blood vessel formation requires not only EC -- which express the VEGF cognate receptor VEGF-R2 -- but also pericytes and smooth muscle cells (SMC) -- which may not express VEGF-R2 [11]. To stimulate recruitment and organization of the multiple cell types required for vascular development, delivery of multiple growth factors has been demonstrated in a number of systems [9, 10, 12-15], with the general goal of attempting to sequentially induce the formation and stabilization of new microvessels. However, the data from studies involving combinations of factors are in some cases contradictory, likely because doses and rates of delivery of factors differ between studies. For example, sequential delivery of VEGF and platelet-derived growth factor-BB (PDGF-BB) was successful at stimulating growth of mature vessels over several weeks [9]; however, simultaneous delivery of these same two factors did not lead to stable vessel formation [10]. A recent study suggests VEGF may actually inhibit the response of SMC or pericytes to PDGF [16]. This new finding suggests that an approach that mediates recruitment of host mural cells via a different mediator (other than PDGF) may yield more success at inducing rapid formation of mature vessels and necessitates placement of greater emphasis on both the careful selection of the therapeutic proteins for vascularization applications and the need to optimize the dosage, rate, and duration of their delivery.
In addition to growth factor delivery strategies for therapeutic vascularization, cell-based approaches have been employed. These include the delivery of endothelial progenitor cells [17], genetic modification of EC [18, 19], and co-transplantation of multiple cell types [20]. The efficacy of progenitor cell delivery may be limited by the lack of incorporation of these cells into healthy or developing vessels, as progenitor cell homing is vastly increased to vessels that are heavily damaged, including those that may be beyond repair [21, 22]. For this reason, progenitors and/or stem cells may accumulate in areas where vascularization is irrelevant. It is also unclear what constitutes a true vascular progenitor cell: it is now thought that many early studies claiming to use progenitor cells were in fact employing mononuclear phagocytes, which can promote angiogenesis but do not give rise to mature endothelial cells [23]. Pre-clinical studies involving injections of these cells have shown some promise, but it is unclear whether the chief benefit of the delivered cells is participation in angiogenesis via physical incorporation into neovessels [24] or secretion of angiogenic growth factors [22]. Additionally, genetic modification of EC to improve their survival via overexpression of anti-apoptotic genes has proven effective [19, 25], but, currently, this approach is not easily translatable to the clinical setting. Incorporation of multiple cell types into implants is another popular strategy. Co-transplantation of EC and SMC [20] or mesenchymal stem cells [26] – now acknowledged to likely be pericytes [27] – resulted in morphometric changes and increased persistence of vessels derived from transplanted cells. However, clinical use of such strategies would require cultivation of multiple cell types, and therapy would be limited to non-acute conditions. Further, the contractile nature of mural cells imposes mechanical constraints on cell delivery matrices that may convolute or reduce the efficacy of incorporation of other cell types for tissue engineering. Therefore, new strategies for delivery of multiple growth factors to support vascular cell transplantation are needed.
Here, we report a modular system capable of delivery of multiple therapeutic proteins to support transplantation of fully differentiated EC, close analogs of which would be readily obtainable from any patient in a clinical setting. Our group previously developed a hydrogel containing an alginate microparticulate system capable of tunable VEGF delivery that improved the engraftment and therapeutic efficacy of transplanted, genetically-modified EC [7]. In the present report, we transplanted unmodified EC and extended the delivery system to incorporate monocyte chemotactic protein-1 (MCP-1), also known as chemokine (C-C motif) ligand 2 (CCL2). MCP-1 is a well-characterized arteriogenic factor that has been shown to induce a significant host vascularization response in as little as 7 days [28]. Additionally, SMC have the receptor for MCP-1 (CCR2) and have been shown to proliferate in response to its presence [29]. Therefore, we hypothesized that controlled delivery of VEGF – by improving survival and engraftment of transplanted EC – and MCP-1 – by promoting SMC recruitment and vessel maturation – would improve the therapeutic effect of EC transplantation in an in vivo mouse model.
2. Materials and Methods
2.1. Materials, cells, and animals
Alginate (M. pyrifera, ~250cP, ~50kDa) was obtained from Sigma (St. Louis, MO, USA) and was purified of endotoxins as described previously [7]. Hydroxypropylmethylcellulose (HPMC), HEPES buffer (1M), sodium hydroxide solution (1N), and sodium bicarbonate solution (7.5%) were all from Sigma and used as received. Calcium chloride, Span 80, Tween 80, iso-octane, and iso-propanol were purchased and used as previously reported [7]. Collagen (rat tail, type I) was from BD (Franklin Lakes, NJ, USA) and fibronectin (human plasma) was obtained from Chemicon (Temecula, CA, USA). DuoSet ELISA kits for VEGF and MCP-1 were purchased from R&D Systems (Minneapolis, MN). MCP-1 (recombinant human) was from Peprotech (Rocky Hill, NJ, USA) and VEGF (165aa, recombinant human) was a generous gift of the Biological Resources Branch of the National Cancer Institute. HUVEC were isolated and cultured by routine methods. C.B-17 SCID/Bg mice were obtained from Taconic Farms (Germantown, NY, USA). All experiments were carried out by trained personnel under protocols approved by the Yale Human Investigations and Yale Animal Care and Use Committees.
2.2. Hydrogel construct preparation
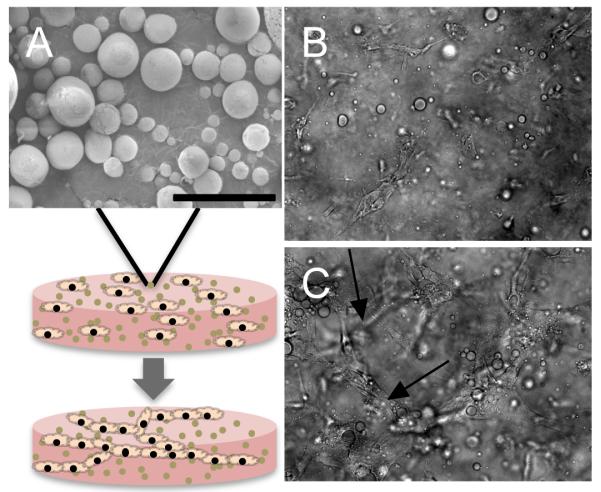
Alginate:HPMC microparticles were prepared essentially as previously described [30]. CaCl2 was the only agent used for crosslinking in this study. Microparticles were characterized by scanning electron microscopy (Fig. 1A) and their size was determined as previously described [30] (mean wet diameter = 9.0±0.3μm). The bioactivity of encapsulated VEGF was confirmed as previously reported [7, 30]. Bioactivity of encapsulated MCP-1 was assessed via a monocyte migration assay (J. Roh and C. Breuer, unpublished data). Total mass released was controlled by varying the initial loading with respect to alginate:HPMC mass of both MCP-1 (50ng/mg; “Lo”, 500ng/mg; “Med”, 1000ng/mg; “Hi”) and VEGF (200ng/mg; “Lo”, 600ng/mg; “Med”, 1000ng/mg; “Hi”). Particles were suspended in collagen/fibronectin (protein) solution as previously described [7] and gels were allowed to polymerize for 30 minutes at 370C (Fig. 1). Release profiles were obtained as previously noted [30] and all concentrations were measured via ELISA. Gels containing particles and unmodified HUVEC behaved similarly to those with particles and genetically modified HUVEC that overexpress Bcl-2 [7] (Fig. 1B,C), forming multicellular cords when incubated in vitro for 18h (arrows, Fig. 1C).
Figure 1.
Composite hydrogel/microparticle construct for EC transplantation. (A) Scanning electron micrograph of alginate microparticle. Microparticles are mixed with collagen solution and EC, which spontaneously organize into cords (arrows) within the gel. Images of EC- and particle-containing gels (B) 1h and (C) 18h after casting. All pictures are magnified 200x, scale bars = 50μm.
2.3. In vivo studies
For all studies, mice were anesthetized with isoflurane via the open-drop method following a protocol established by the Yale Institutional Animal Care and Use Committee. Following polymerization, gels were immediately implanted into mice in the subcutaneous position on the abdominal wall, as previously described [20]. Constructs were harvested at 2 weeks post-implantation. Initial studies to evaluate different doses of MCP-1 and VEGF separately were conducted in 4 mice/group (n=4). Studies employing the optimized doses of each growth factor as well as the combination of those doses were conducted with 6 mice/group (n=6), and data are representative of 2 independent experiments. For all studies, HUVEC were from the same donors.
2.4. Implant characterization
Upon retrieval at 2 weeks, gel grafts were fixed in formalin and embedded in paraffin for immunohistochemical analysis. Vessels were characterized via sections stained with primary antibodies reactive to smooth muscle cell alpha-actin (αSMA) or human-specific CD31 (both from Abcam, Cambridge, MA, USA; 1:100). Hematoxylin and eosin (H&E) staining was also used for this purpose. A primary antibody to F4/80 (AbD Serotec, Raleigh, NC, USA; 1:100) was employed to determine macrophage content of gels, and antibodies to arginase I (arginase) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and inducible nitric oxide synthase (iNOS) (Abcam) were utilized for macrophage phenotyping. Species-appropriate biotinylated secondary antibodies (JacksonImmuno, West Grove, PA, USA; 1:100) were used to detect the primary antibody, followed by addition of an avidin-binding complex and AEC detection kit used for color development (Vector Laboratories, Burlingame, CA, USA).
Vascular density was determined by manual counting of mid-graft sections by an observer (S.M.J.) blinded to the sample identity. Functional vessels derived from transplanted EC were identified as structures having distinctive lumens that contained visible erythrocytes and stained positive for human CD31. All vessels in 2-3 high-powered fields (enough to account for ~100% of the gel area) were counted for each sample. Positively stained area for various antibodies was determined through a common procedure, in which representative sections consisting of >50% of the total sample area were photographed and analyzed with ImageJ software. For each picture, background was uniformly subtracted with a rolling ball radius of 50 pixels. Pictures were then subjected to color deconvolution based on standard pixel intensities established for AEC-developed sections. Images were then thresholded to a common value and the stained area was measured. Total section area per image was measured separately using the freehand trace tool, and the % stained area was derived from these two values for each image. Vessel diameters were also determined via ImageJ, using the straight line tracing tool.
Macrophage content and phenotype was determined following similar analyses of F4/80-, iNOS-, and arginase-stained sections. To compare macrophage phenotype between groups, a ratio of the fold change of arginase- and iNOS-staining relative to the no particles control group was calculated (Fig. 6G).
Figure 6.
Delivery of MCP-1 does not result in a sustained increase of macrophages infiltrating implanted gels or a switch to a pro-inflammatory phenotype. Grafts were retrieved at 2 weeks and stained with antibodies against (A) pan-macrophage marker F4/80, (B) M2-macrophage effector molecule arginase I, and (C) M1-macrophage effector molecule iNOS, quantitation in (D-F). Representative sections from grafts with MCP-1 and VEGF delivery (M/V) shown. (G) Proportion of arginase to iNOS positive staining was used to generate a relative comparison of M2/M1 macrophage ratios for each group. Arrows indicate examples of positive staining. *P<0.05, scale bars = 50μm, n=6.
2.5. Statistical analysis
Unless indicated, data are represented as mean ± standard error of the mean. Statistical significance was determined using a student’s T-test or a one-way ANOVA with a Bonferroni post hoc correction. P values of less than 0.05 were considered significant. All statistical calculations were performed using GraphPad Prism software.
3. Results
3.1. Optimization of growth factor dosage
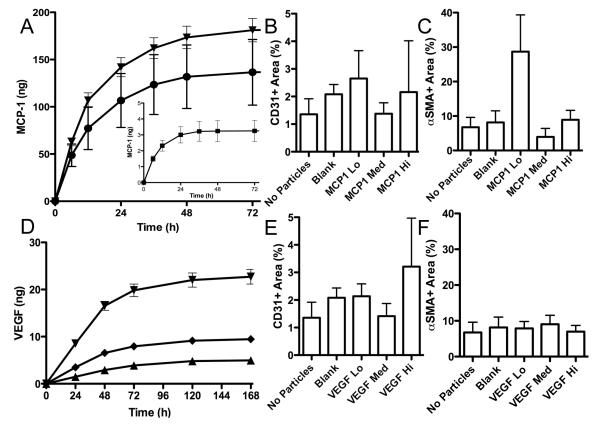
To determine the efficacy of combined delivery of VEGF and MCP-1, we first attempted to optimize the dosage of each protein independently. MCP-1 is a known mitogen for SMC [29]; therefore, we evaluated the presence and abundance of SMC in implanted protein hydrogels that contained HUVEC and microparticles delivering different doses of MCP-1 (Fig. 2A). In implants retrieved at 2 weeks, there was a trend towards increased SMC in gels with the lowest dose of MCP-1 delivered in this study (MCP-1 Lo; 50ng/mg initial loading) (Fig. 2C), while no effect on the survival of EC was observed (Fig. 2B). A similar experiment was conducted to optimize VEGF dosage, this time in an effort to enhance EC survival as measured by human-specific CD31+ staining. Distinct doses of VEGF were delivered from the same mass of particles (Fig. 2D) revealing a trend towards increased EC survival in the highest dosage of VEGF examined (Fig. 2E). No effect of VEGF dose on SMC migration into gels was observed (Fig. 2F), which is consistent with previous studies [7].
Figure 2.
MCP-1 and VEGF dosing. (A) MCP-1 and (D) VEGF were released at 3 different total doses from gels based on varied initial loading into microparticles. (B,E) CD31+ area and (C,F) αSMA+ area were measured using imageJ software in gels retrieved after 2 weeks implantation (n=4).
3.2. Vessel formation in implanted hydrogels
Based on data collected in the dosing studies, an implant capable of concurrent EC transplantation and dual delivery of VEGF and MCP-1 at optimal doses was constructed (Fig. 3). To evaluate the effectiveness of this composite gel (M/V), HUVEC-containing gels of the following compositions were also assessed: without particles (no particles), with particles loaded with no growth factors (blank), with particles containing only MCP-1 (MCP1), and with particles containing only VEGF (VEGF). Vascular density was assessed via evaluation of hCD31-stained slides in conjunction with H&E stained sections (Fig. 4). Though there was a trend towards an increase in vessels associated with delivery of MCP-1 and VEGF alone, only the combination therapy yielded a statistically significant increase in vessel density compared to EC-laden gels unsupported by protein delivery (P=0.043) (Fig. 4F). It should be noted that these vessels stained positive for human-specific CD31 (inset, Fig. 4E), indicating that they are constituted primarily by transplanted HUVEC as opposed to infiltrating host EC; a negligible number of vessels containing visible erythrocytes on H&E-stained sections stained positive for mouse-specific CD31 in this study. Analysis of vessel morphometry revealed that more large vessels were formed in M/V grafts than in blank or MCP1 gels (Fig. 4G), as measured by an increase in average vessel diameter between the conditions (P<0.001) (Fig. 4H).
Figure 3.
Optimized dosing for combined delivery. (A) Percentage and (B) total mass released of MCP-1 and VEGF in gels releasing both factors simultaneously (n=3).
Figure 4.
Vessel formation within gels is increased by combined delivery of MCP-1 and VEGF. H&E stained sections of implants, retrieved after 2 weeks, with: (A) no particles, (B) blank particles (Blank), (C) MCP-1 releasing-particles (MCP1), (D) VEGF releasing-particles (VEGF), and (E) particles releasing both MCP-1 and VEGF (M/V), quantitation in (F). Vessels (arrows) stained positive for human-specific CD31 (inset, E). (G,H) Measurement of vessel diameter showed an increase in the average diameter of vessels formed in M/V grafts compared to blank and MCP1 grafts and also in VEGF grafts compared to MCP1 grafts. *P<0.05, ***P<0.001, scale bars = 50μm, n=6.
3.3. Maturation of vessels in implanted hydrogels
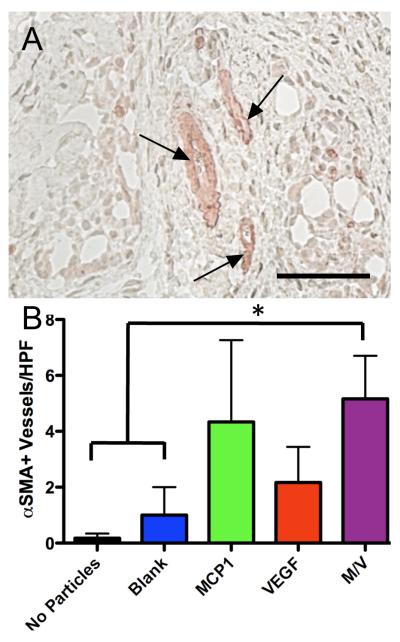
Association of mural cells with nascent vessels is necessary to promote their maturation. We assessed the number of vessels invested with SMC (Fig. 5) and found that combined delivery resulted in a significant increase in αSMA+ vessels relative to gels without particles (P=0.009) or with particles containing no growth factors (P=0.046) (Fig. 5B). There was also a trend towards an increase in αSMA+ vessels associated with delivery of MCP-1 alone, but these data were not significant.
Figure 5.
Combined delivery results in increased number of SMC-invested vessels. (A) αSMA+ vessels (arrows) in a gel releasing MCP-1 and VEGF at 2 weeks, quantified in (B). *P<0.05, scale bar = 50μm, n=6.
3.4. Macrophage infiltration into implants
Though MCP-1 was selected for delivery in this study because of its ability to stimulate SMC, it is also a potent chemoattractant for monocytes/macrophages [31], as its name implies. Therefore, we sought to determine if MCP-1 delivery led to potentially deleterious accumulation of pro-inflammatory M1 macrophages in implanted grafts. Since retrieval and isolation of cells from implanted grafts was limited, we chose to analyze macrophage phenotype via immunohistochemical analysis. Staining with an antibody reactive to the mouse macrophage-specific antigen F4/80 revealed no differences in overall macrophage infiltration associated with the addition of particles to gels or with the delivery or MCP-1, VEGF, or both (Fig. 6A,D). The same grafts were also stained for arginase (Fig. 6B,E) – which is preferentially upregulated in the M2 population of macrophages that are known to promote angiogenesis and tissue repair [32]– and iNOS (Fig. 6C,F) – which is upregulated in M1 (pro-inflammatory) but not M2 macrophages [32]. A trend towards an increased M2 phenotype associated with protein delivery was suggested by these data (Fig. 6G).
4. Discussion
The rapid formation of a stable microvasculature is critical to the function of engineered tissues. Establishment of a blood vessel network through EC transplantation is a promising strategy, but poor engraftment efficiency and a limited ability of such cells to recruit stabilizing mural cells hinders this approach. Here, we demonstrate that dual delivery of VEGF and MCP-1 enhances the vessel forming potential of transplanted EC, both in number and in size. We further show that this combined delivery strategy yields a higher number of mature, SMC-invested vessels than are formed via EC transplantation in the absence of supporting growth factor delivery. Finally, the dose of MCP-1 was optimized to impart a therapeutic effect without inducing increases in macrophage recruitment to implants or stimulating a transition to a pro-inflammatory macrophage phenotype.
We previously explored the effect of EC transplanted within protein gels containing alginate microparticles encapsulating VEGF on survival and vessel formation [7]. In this study, we expanded this system to also deliver MCP-1. Encapsulation of MCP-1 in alginate microspheres was less efficient than encapsulation of VEGF [7, 30]. This result is not surprising considering the lower MW of MCP-1 (~9kDa) compared to VEGF (~45kDa). In addition, VEGF encapsulation in alginate is mediated through VEGF’s heparin-binding domain [33], which MCP-1 lacks. For these same reasons, the duration of release of MCP-1 was significantly less than that previously reported for VEGF in the same particle system [7, 30]. Interestingly, short-term expression of MCP-1 has been shown to lead to improved reperfusion in a hindlimb ischemia model [34] while a longer duration of expression yielded no therapeutic benefit in conjunction with FGF-2 delivery [35] despite the established relationship between these two factors [36]. Therefore, it is reasonable to conclude that the duration of release of MCP-1 exhibited by these scaffolds could lead to the therapeutic effects observed in this study. Furthermore, prolonged release of MCP-1 may lead to a more deleterious biological response, such as chronic inflammation. Importantly, we did not observe an increase in accumulation of total or M1 macrophages accompanying delivery of MCP-1 (Fig. 6), despite the fact that CCR2 expression is recognized as an indicator of pro-inflammatory phenotype in macrophages [32]. We believe that the low dose and duration of MCP-1 delivery in this study prevents systemic activation of macrophages leading to inflammation, while allowing for activation of endothelium and local recruitment of SMC. Also, as mentioned previously, recent data show that VEGF expression can serve as a negative regulator of pericyte-EC interactions [16]. Therefore, extended delivery of either of these factors may not be optimal for inducing rapid microvascular patency.
In addition to delivery duration, optimization of the dose of therapeutic proteins is critical to achieving a desired outcome. Previous work has shown that high levels of VEGF delivery can lead to vascular regression [37] and formation of pathologic, as opposed to physiologic, vasculature [38]. To determine the best dose in our experimental setting, we evaluated three different doses of VEGF (Fig. 2D) and found that the highest one led to the greatest benefit (Fig. 2E). We note that the dose delivered in this case is lower than that used in other studies in similar models [7, 39]. Furthermore, VEGF delivery from the multifunctional gels used in these experiments has previously been shown to be localized to the area of the implant, with little of the VEGF entering the systemic circulation [7]. Therefore, off-target effects from VEGF delivery in this therapy are unlikely.
It was also important to control the dose of MCP-1 used in this study (Fig. 2A), as it is a potent chemotactic factor for monocytes/macrophages [31] and plays a key role in the inflammatory response to biomaterial implants [40, 41]. Interestingly, we found that the lowest dose of MCP-1 that we examined resulted in the greatest number of SMC present in gels (Fig. 2C). It has been shown that picomolar doses of MCP-1 are capable of direct promotion of SMC migration and proliferation in vitro through induction of autocrine VEGF production [42]. Alternatively, it is possible that, in this study, MCP-1 mediated SMC recruitment indirectly, via EC activation. EC are known to express CCR2, and neovascularization associated with MCP-1 has been attributed to engagement of EC [43, 44]. This supposition is also supported by the fact that Bcl-2-transduced HUVEC, which effectively recruit pericytes and SMC in vivo when implanted within protein gels, secrete MCP-1 at higher levels in culture than non-transduced HUVEC (1299.6 ± 71.3 pg/106 cells > 1028.7 ± 60.4 pg/106 cells, P=0.007, n=3), which do not recruit mural cells in the same in vivo setting. Moreover, staining for CCR2 on αSMA+ cells indicated a lack of co-expression (not shown) at 2 weeks post-implantation. It has also been noted that aortic smooth muscle cells from CCR2 -/- mice respond directly to MCP-1 [45], providing strong evidence for the existence of a second MCP-1 receptor that may have played a role in these studies. The lack of efficacy of the higher doses of MCP-1 employed here may be the result of the saturation of cell surface receptors, preventing EC from interrogating microenvironmental protein gradients that would typically induce appropriate activation and SMC recruitment. This outcome is similar to the deleterious effect of high VEGF doses [37] and overexpression of PDGF-BB [46] on pericyte investment. Overall, these data show that optimized doses of therapeutic proteins delivered over relatively short periods of time can lead to beneficial biological outcomes over longer time scales.
Combining MCP-1 and VEGF delivery (Fig. 3) resulted in improved outcomes of EC transplantation (Fig. 4), as measured by an increase in total vessels formed (Fig. 4F), an increase in average diameter of vessels (Fig 4H), and an increase in SMC-invested vessels (Fig 5) compared to other conditions. There were also trends toward increased vascular density in grafts with transplanted EC supported by VEGF delivery in the absence of MCP-1 (Fig. 4) or delivery of MCP-1 in the absence of VEGF (Fig. 5); however, these results fell short of statistical significance in two independent experiments. It is not surprising that VEGF delivery alone could promote vessel formation [7], or that MCP-1 delivery alone could lead to an increase in SMC-invested vessels [28, 29]. Yet, VEGF delivery alone does not promote mature vessel formation as well as in combination with other factors [9] whereas MCP-1, while not efficient in promoting vasculogenesis or angiogenesis on its own, can support the generation of vessels lined by mural cells. Further, in the case of MCP-1, such vessel formation can be accomplished without interfering with EC-pericyte or EC-SMC interactions, a negative regulation that has been associated with PDGF-BB [16], another molecule used for combined delivery in therapeutic vascularization [9, 10]. Therefore, these results provide a new strategy for dual molecular delivery combined with EC transplantation that merits further evaluation.
Finally, these studies were targeted towards establishing a general approach to inducing therapeutic vascularization. Therefore, a subcutaneous implant model was chosen. Based on our previous experience with this model [18, 47], constructs were evaluated 2 weeks after implantation. We were also aware that, in the absence of a true physiologic need for vessel formation, such as a chronic inflammatory stimulus or in ischemia, vascular regression will occur over time [48]. Based on the results gathered here, further evaluation of combined VEGF and MCP-1 delivery with EC transplantation is warranted, especially in the setting of ischemia. The efficacy of VEGF delivery for therapeutic angiogenesis is well known, and this approach has already been shown to enhance survival of transplanted EC in a hindlimb ischemia model [7]. Additionally, MCP-1 is well studied as a potential mediator of therapeutic arteriogenesis [28, 49, 50]. Thus, the method described here could be adapted for use as a combined angiogenic/arteriogenic therapy to treat ischemic disease.
5. Conclusions
Though both molecular and cellular therapy approaches for therapeutic vascularization have been studied for many years, the application of molecular delivery to support cell-based therapy has only recently been tested. Combination therapy may be necessary for therapeutic vascularization to reach its clinical potential. The present study demonstrates the delivery of multiple therapeutic proteins – each with independent release kinetics and doses that facilitate their distinct biological functions – to enhance the efficacy of cell-based therapeutic vascularization. This strategy of combined delivery of MCP-1 and VEGF with EC transplantation bears further pre-clinical investigation in models of vascular disease and as part of more complex engineered tissues.
Acknowledgements
We thank Eleni Skokos for technical advice and helpful discussions. The authors acknowledge the National Institutes of Health for funding this research through grants HL 085416-01 (to W.M.S. and J.S.P.) and GM 072194-01 (to T.R.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
References
- [1].Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, et al. Therapeutic angiogenesis - a single intraarterial bolus of vascular endothelial growth-factor augments revascularization in a rabbit ischemic hind-limb model. Journal of Clinical Investigation. 1994;93:662–70. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–7. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bhattacharya V, McSweeney PA, Shi Q, Bruno B, Ishida A, Nash R, et al. Enhanced endothelialization and microvessel formation in polyester grafts seeded with CD34(+) bone marrow cells. Blood. 2000;95:581–5. [PubMed] [Google Scholar]
- [4].Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359:2053–8. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- [5].Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–65. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- [6].Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–21. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- [7].Jay SM, Shepherd B, Bertram JP, Pober J, Saltzman WM. Engineering of multifunctional gels integrating highly efficient growth factor delivery with endothelial cell transplantation. The FASEB Journal. 2008;22:2949–56. doi: 10.1096/fj.08-108803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Silva E, Kim ES, Kong H, Mooney D. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc Natl Acad Sci USA. 2008;105:14347–52. doi: 10.1073/pnas.0803873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- [10].Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–13. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- [11].Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- [12].Peirce SM, Price RJ, Skalak TC. Spatial and temporal control of angiogenesis and arterialization using focal applications of VEGF164 and Ang-1. Am J Physiol Heart Circ Physiol. 2004;286:H918–25. doi: 10.1152/ajpheart.00833.2003. [DOI] [PubMed] [Google Scholar]
- [13].Pike DB, Cai S, Pomraning KR, Firpo MA, Fisher RJ, Shu XZ, et al. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials. 2006;27:5242–51. doi: 10.1016/j.biomaterials.2006.05.018. [DOI] [PubMed] [Google Scholar]
- [14].Freeman I, Cohen S. The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials. 2009:1–10. doi: 10.1016/j.biomaterials.2008.12.057. [DOI] [PubMed] [Google Scholar]
- [15].Rophael JA, Craft RO, Palmer JA, Hussey AJ, Thomas GP, Morrison WA, et al. Angiogenic growth factor synergism in a murine tissue engineering model of angiogenesis and adipogenesis. Am J Pathol. 2007;171:2048–57. doi: 10.2353/ajpath.2007.070066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–13. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–12. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- [18].Enis DR, Shepherd BR, Wang Y, Qasim A, Shanahan CM, Weissberg PL, et al. Induction, differentiation, and remodeling of blood vessels after transplantation of Bcl-2-transduced endothelial cells. Proc Natl Acad Sci U S A. 2005;102:425–30. doi: 10.1073/pnas.0408357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schechner JS, Nath AK, Zheng L, Kluger MS, Hughes CC, Sierra-Honigmann MR, et al. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci U S A. 2000;97:9191–6. doi: 10.1073/pnas.150242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shepherd B, Jay SM, Saltzman WM, Tellides G, Pober J. Human aortic smooth muscle cells promote arteriole formation by coengrafted endothelial cells. Tissue Engineering Part A. 2009;15:165–73. doi: 10.1089/ten.tea.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pober JS. Is host endothelium a silver lining for allografts? Lancet. 2001;357:2–3. doi: 10.1016/S0140-6736(00)03558-3. [DOI] [PubMed] [Google Scholar]
- [22].Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–83. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–8. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- [25].Nor JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–84. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428:138–9. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- [27].Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- [28].Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res. 1997;80:829–37. doi: 10.1161/01.res.80.6.829. [DOI] [PubMed] [Google Scholar]
- [29].Selzman CH, Miller SA, Zimmerman MA, Gamboni-Robertson F, Harken AH, Banerjee A. Monocyte chemotactic protein-1 directly induces human vascular smooth muscle proliferation. Am J Physiol Heart Circ Physiol. 2002;283:H1455–61. doi: 10.1152/ajpheart.00188.2002. [DOI] [PubMed] [Google Scholar]
- [30].Jay SM, Saltzman WM. Controlled delivery of VEGF via modulation of alginate microparticle ionic crosslinking. Journal of controlled release : official journal of the Controlled Release Society. 2009;134:26–34. doi: 10.1016/j.jconrel.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rollins BJ. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol Med Today. 1996;2:198–204. doi: 10.1016/1357-4310(96)88772-7. [DOI] [PubMed] [Google Scholar]
- [32].Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- [33].Peters MC, Isenberg BC, Rowley JA, Mooney DJ. Release from alginate enhances the biological activity of vascular endothelial growth factor. J Biomater Sci Polym Ed. 1998;9:1267–78. doi: 10.1163/156856298x00389. [DOI] [PubMed] [Google Scholar]
- [34].Capoccia BJ, Gregory AD, Link DC. Recruitment of the inflammatory subset of monocytes to sites of ischemia induces angiogenesis in a monocyte chemoattractant protein-1-dependent fashion. J Leukoc Biol. 2008;84:760–8. doi: 10.1189/jlb.1107756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Heilmann C, Kostic C, Giannone B, Grawitz AB, Armbruster W, Lutter G, et al. Improvement of contractility accompanies angiogenesis rather than arteriogenesis in chronic myocardial ischemia. Vascular Pharmacology. 2006;44:326–32. doi: 10.1016/j.vph.2006.01.002. [DOI] [PubMed] [Google Scholar]
- [36].Fujii T, Yonemitsu Y, Onimaru M, Tanii M, Nakano T, Egashira K, et al. Nonendothelial Mesenchymal Cell-Derived MCP-1 Is Required for FGF-2-Mediated Therapeutic Neovascularization: Critical Role of the Inflammatory/Arteriogenic Pathway. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:2483–9. doi: 10.1161/01.ATV.0000244684.23499.bf. [DOI] [PubMed] [Google Scholar]
- [37].Davies N, Dobner S, Bezuidenhout D, Schmidt C, Beck M, Zisch AH, et al. The dosage dependence of VEGF stimulation on scaffold neovascularisation. Biomaterials. 2008;29:3531–8. doi: 10.1016/j.biomaterials.2008.05.007. [DOI] [PubMed] [Google Scholar]
- [38].Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113:516–27. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5:590–8. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- [40].Jay SM, Skokos E, Laiwalla F, Krady MM, Kyriakides TR. Foreign body giant cell formation is preceded by lamellipodia formation and can be attenuated by inhibition of Rac1 activation. Am J Pathol. 2007;171:632–40. doi: 10.2353/ajpath.2007.061213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kyriakides TR, Foster MJ, Keeney GE, Tsai A, Giachelli CM, Clark-Lewis I, et al. The CC chemokine ligand, CCL2/MCP1, participates in macrophage fusion and foreign body giant cell formation. Am J Pathol. 2004;165:2157–66. doi: 10.1016/S0002-9440(10)63265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Parenti A, Bellik L, Brogelli L, Filippi S, Ledda F. Endogenous VEGF-A is responsible for mitogenic effects of MCP-1 on vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;286:H1978–84. doi: 10.1152/ajpheart.00414.2003. [DOI] [PubMed] [Google Scholar]
- [43].Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- [44].Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy P. Monocyte Chemotactic Protein (MCP)-1 Promotes Angiogenesis via a Novel Transcription Factor, MCP-1-induced Protein (MCPIP) Journal of Biological Chemistry. 2008;283:14542–51. doi: 10.1074/jbc.M802139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schecter AD, Berman AB, Yi L, Ma H, Daly CM, Soejima K, et al. MCP-1-dependent signaling in CCR2(-/-) aortic smooth muscle cells. J Leukoc Biol. 2004;75:1079–85. doi: 10.1189/jlb.0903421. [DOI] [PubMed] [Google Scholar]
- [46].Au P, Tam J, Duda DG, Lin PC, Munn LL, Fukumura D, et al. Paradoxical effects of PDGF-BB overexpression in endothelial cells on engineered blood vessels in vivo. Am J Pathol. 2009;175:294–302. doi: 10.2353/ajpath.2009.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Suarez Y, Shepherd BR, Rao DA, Pober JS. Alloimmunity to human endothelial cells derived from cord blood progenitors. J Immunol. 2007;179:7488–96. doi: 10.4049/jimmunol.179.11.7488. [DOI] [PubMed] [Google Scholar]
- [48].Ehrbar M, Zeisberger SM, Raeber GP, Hubbell JA, Schnell C, Zisch AH. The role of actively released fibrin-conjugated VEGF for VEGF receptor 2 gene activation and the enhancement of angiogenesis. Biomaterials. 2008;29:1720–9. doi: 10.1016/j.biomaterials.2007.12.002. [DOI] [PubMed] [Google Scholar]
- [49].van Royen N, Hoefer I, Buschmann I, Kostin S, Voskuil M, Bode C, et al. Effects of local MCP-1 protein therapy on the development of the collateral circulation and atherosclerosis in Watanabe hyperlipidemic rabbits. Cardiovasc Res. 2003;57:178–85. doi: 10.1016/s0008-6363(02)00615-6. [DOI] [PubMed] [Google Scholar]
- [50].Voskuil M, van Royen N, Hoefer IE, Seidler R, Guth BD, Bode C, et al. Modulation of collateral artery growth in a porcine hindlimb ligation model using MCP-1. Am J Physiol Heart Circ Physiol. 2003;284:H1422–8. doi: 10.1152/ajpheart.00506.2002. [DOI] [PubMed] [Google Scholar]