Abstract
Five human RecQ helicases (WRN, BLM, RECQ4, RECQ5, RECQ1) exist in humans. Of these, three are genetically linked to diseases of premature aging and/or cancer. Neither RECQ1 nor RECQ5 has yet been implicated in a human disease. However, cellular studies and genetic analyses of model organisms indicate that RECQ1 (and RECQ5) play an important role in the maintenance of genomic stability. Biochemical studies of purified RECQ1 protein demonstrate that the enzyme catalyzes DNA unwinding and strand annealing, and these activities are likely to be important for its role in DNA repair. RECQ1 also physically and functionally interacts with proteins involved in genetic recombination. In this review, we will summarize our current knowledge of RECQ1 roles in cellular nucleic acid metabolism and propose avenues of investigation for future studies.
Keywords: RECQ1 (RECQL), RecQ, helicase, genomic stability, DNA repair
Introduction
RECQL or RECQL1 (hereafter designated RECQ1) belongs to a class of DExH-containing DNA helicases that have been implicated in diseases of premature aging, cancer, and chromosomal instability (for review, see [1, 2]). These include Werner syndrome, Bloom's syndrome, and Rothmund-Thomson syndrome that have mutations in the WRN, BLM, and RECQ4 genes, respectively. Although the clinical importance of RECQ1 is yet to be fully appreciated, it is becoming increasingly apparent that RECQ1, the first of the human RecQ helicases to be discovered, has unique and important roles in cellular DNA metabolism. RECQ1 is the most abundant of the five human RecQ helicases, and RECQ1 depletion studies indicate its importance in human cells for chromosomal stability. Genetic studies of model organisms with mutations in genes encoding proteins with sequence homology to RECQ1 also demonstrate its importance in genome homeostasis. Advances in understanding the structural and biochemical properties of RECQ1 have led to further insights to its proposed molecular functions. These topics will be discussed to provide a conceptual basis for how RECQ1 might help cells to cope with DNA damage. We also suggest important avenues for RECQ1 research that may help in understanding one of the less characterized human RecQ helicases.
RECQ1-like Helicases and Their Genetic Functions in Model Organisms
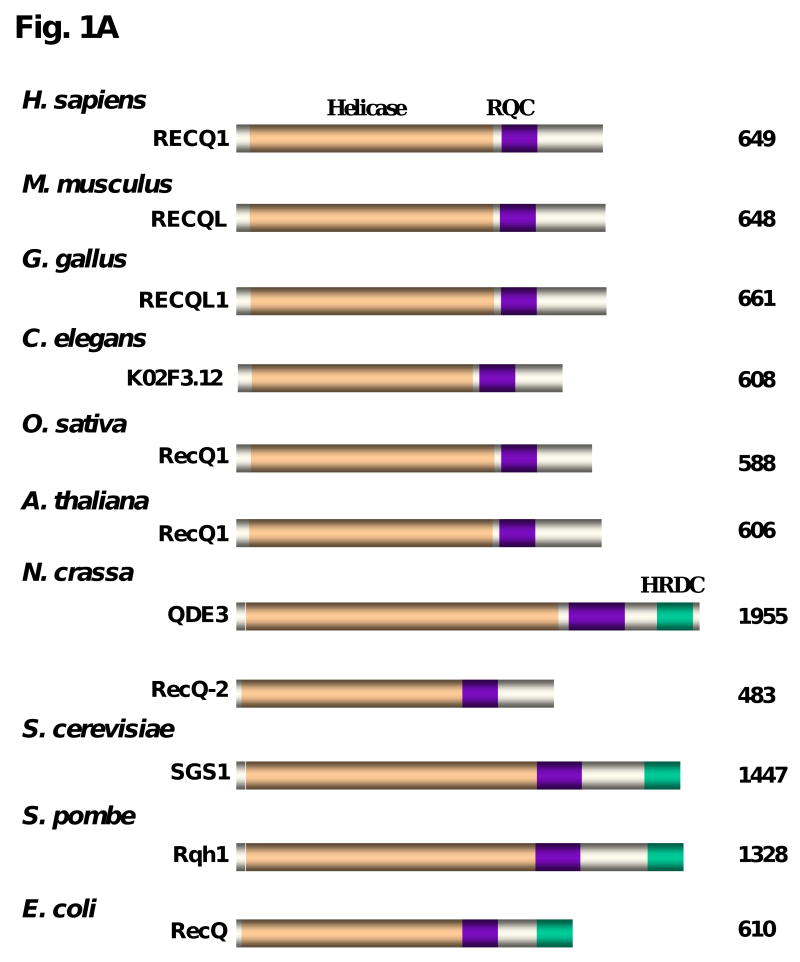
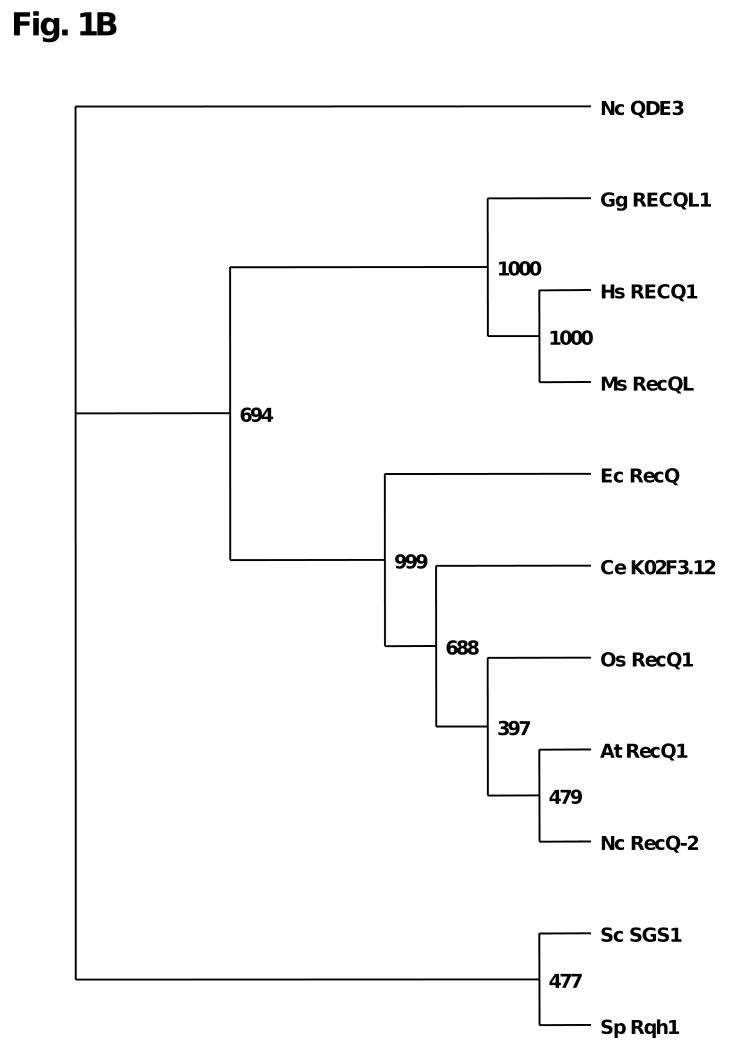
Close inspection of the RECQ1 amino acid sequence reveals similarity in the helicase core domain to a number of RECQ1-like proteins from different species, as shown in Fig. 1A. In addition to the helicase core domain containing the seven conserved amino acid motifs, two additional regions can be found in a number of the RecQ helicases [3], including the RECQ1-like helicases. The RecQ C-terminal region (RQC) that has been implicated in protein interactions and DNA binding is present in all the RECQ1-like proteins shown. However, the Helicase and RNase D C-terminal Domain (HRDC), necessary for the function of BLM in Holliday Junction (HJ) dissolution and important for DNA structure binding [4], is absent in human and mouse RECQ1, but can be found in RECQ1-like helicases for certain species. The human RECQ1 gene was identified by two groups [5, 6] a decade after the discovery of the E. coli RecQ gene [7]. Phylogenetic analysis of the RECQ1-like helicases reveals that human RECQ1 is most closely related by sequence to mouse and chicken (Fig. 1B).
Fig. 1. Alignment and phylogenetic tree of RECQ1-like helicases across species.
Panel A, The conserved helicase core domain is indicated by yellow, the RecQ C-terminal domain (RQC) in purple, and the Helicase and RNase D C-terminal domain (HRDC) in green. Panel B, The amino acid sequences of the full length proteins in Panel A were aligned and a phylogenetic tree was constructed by ClustalX2 with number of bootstrap trials at 1000. The image was generated in Treeview. Branch numbers refer to bootstrap values.
Cellular studies demonstrate that RECQ1 plays an important role in human and mouse for chromosomal stability and the DNA damage response ([8], and discussed below). The use of model genetic systems have enable researchers to study the functions of RECQ1-like helicases in whole organisms, and in some cases suggests diverse physiological roles. Because chicken B-lymphocyte DT40 cells are genetically easy to manipulate, they have been widely used to study DNA repair genes, including those encoding the RecQ helicases [9]. Although recq1 single knockout DT40 cells were not significantly different from the wild-type cells in cell growth, sensitivity to methyl methanesulfonate (MMS), or sister chromatid exchange (SCE) frequency, recq1 blm double knockout DT40 cells grew slower than blm single knockout cells due to an increased population of dead cells [10]. A higher incidence of SCE was observed in mitomycin C-treated recq1 blm mutant cells compared to blm cells (Wang et al., 2003), suggesting that recq1 can compensate for a blm deficiency. However, Otsuki et al. reported that disruption of the recq1 gene in a req5 blm mutant background did not affect UV or MMS survival, UV-induced SCE, or the frequency of damage-induced mitotic chiasma, leading the authors to conclude that RECQ1 might not function in DNA replication or repair in chicken cells [11].
In Neurospora crassa, two helicases (QDE3, RecQ-2) sharing sequence similarity to RECQ1 exist, and both are important for the DNA damage response [12, 13]. Mutation in the gene encoding the RECQ1-like helicase QDE3, an RNA-dependent RNA polymerase (QDE1), or an Argonaute protein containing a PIWI domain (QDE2) result in a quelling defect in RNA silencing [14-16]. QDE3 alone is involved in a key step of activation and maintenance of RNA silencing [17]. Recently, a novel small RNA (qiRNA) was identified on the basis of its interaction with QDE2 [18]. The newly found qiRNA is 20-21 nucleotides long with a strong preference for uridine at the 5′ end, and mostly from the ribosomal DNA locus. It is likely that this qiRNA is the counterpart of mammalian piRNA (for review, see [19, 20]), which was first observed in fruit fly to be associated with PIWI protein, QDE2 in Neurospora crassa. Interestingly, the qde3 mutation abolished qiRNA production, indicating that QDE3 is required for qiRNA biogenesis [18]. Consistent with the Neurospora genetic results, RECQ1 was found in a piRNA complex isolated from rat testis [21]. The piRNA protein complex contained ATPase and DNA helicase activities as well as RNA cleavage activity that would be predicted to be catalyzed respectively by RECQ1 and a conserved Argonaute protein responsible for RNA-guided cleavage of target RNAs.
Proteins sharing sequence similarity with RECQ1 have been identified in the plants O. sativa and A. thaliani (Fig. 1A). Expression of rice RecQ1 increased with exposure to various DNA damaging agents, suggesting that RecQ1 may be involved in DNA repair [22]. In addition to its role in the DNA damage response, rice RECQ1 is required for RNA silencing induced by particle bombardment for inverted-repeat DNA, which is likely formed by transposon elements [23]. It has been proposed that mammalian RecQ helicases might also have a function in gene silencing, but studies using mouse models for Wrn, Blm, and RecQ1 suggest that they are not essential for sequence-specific mRNA degradation in response to dsRNA [24]. It is possible that certain mammalian RecQ helicases are involved in the production of small RNA molecules. With novel small RNAs and gene silencing pathways being discovered, the importance of mammalian RECQ1-like helicases in defending genome integrity through gene silencing remains to be determined.
Prospective Importance of RECQ1 in Mammalian Cells
Although a human disease has not yet been genetically liked to mutations in the RECQ1 gene, cellular phenotypes associated with RECQ1 deficiency in mouse and human indicate that RECQ1 has a uniquely important role in genomic integrity. We will summarize what is known about the cellular importance of RECQ1, pointing out some hypotheses for the functions of RECQ1 that will be considered more extensively at the molecular level in the section entitled Structural Features and Biochemical Functions of RECQ1.
Chromosomal Instability in RECQ1-Deficient Cells
Primary embryonic fibroblasts from Recql-null mice display aneuploidy, spontaneous chromosomal breakage, frequent translocation events, and elevated SCE [25]. In RECQ1-deficient human [26] and mouse [25] cells there is an increased load of DNA damage as exemplified by the accumulation of γH2AX foci, a marker of double strand breaks. Transient knockdown of RECQ1 in human cells resulted in significantly elevated spontaneous SCE [26]. It is plausible that a role of RECQ1 in homologous recombinational repair helps cells to cope with strand breaks that arise directly from DNA damage or are a consequence of broken replication forks at sites of replication blockage [8]. An alternative hypothesis is that RECQ1 helps cells to circumvent the consequences of DNA damage that may occur at replication forks. However, given the genetic evidence that RECQ1-deficient cells are sensitive to ionizing radiation that introduces strand breaks [25, 26], we favor the hypothesis that RECQ1 plays a direct role in the repair of strand breaks and hence the maintenance of chromosomal stability through a mechanism that is yet to be fully understood.
A platform for understanding the role of RECQ1 in SCE suppression begins with understanding how the sequence-related BLM helicase suppresses SCE. In human cells, BLM physically interacts with Top3α, and the two proteins together have the ability to catalyze double HJ dissolution on model DNA substrates in a reaction that requires BLM-mediated ATP hydrolysis and the active-site tyrosine residue of Top3α [27]. This reaction gave rise exclusively to non-cross-over products, as predicted from the hemicatenane model, and supports a proposed role of BLM with Top3α as a suppressor of SCEs. RMI1 (BLAP75) promotes this BLM-dependent dissolution of the homologous recombination (HR) intermediate by recruiting Top3α to the double HJ [28, 29]. Interestingly, BLM appears to be unique in the double HJ dissolution reaction since WRN, RECQ1 and RECQ5 all failed to substitute for BLM [4, 28]. Moreover, association of Top3α and BLAP75 with BLM stimulates its HJ unwinding activity; however, neither WRN nor E. coli RecQ HJ unwinding was stimulated by Top3α/BLAP75 [30]. Very recently, a new component of the BLM-Top3α complex, designated RMI2, was identified that is important for the stability of the BLM protein complex [31, 32]. RMI2 deficiency in vertebrate cells results in chromosomal instability [31, 32], suggesting its function as a tumor suppressor. RMI2 enhanced the double HJ dissolvase activity of the BLM-Top3α complex [31], indicating that additional proteins are likely to be involved. In fact, other proteins were isolated with the RMI2 complex, including the mismatch repair complex MSH2/6, RPA, and the Fanconi Anemia proteins FANCM and FAAP24 [31].
The suppression of recombinant cross-over products that are detected as sister chromatid exchanges is thought to be specific to the coordinate functions of yeast Sgs1 and Top3, and its human counterparts, BLM and Top3α. However, RECQ5 and RECQ1 also interact with Top3α [33, 34], and elevated SCE is also found in fibroblasts from RECQ5 [35] or RECQ1 [25] knockout mice as well as human cells depleted of RECQ1 by RNA interference [26]. These studies suggest that RecQ helicases participate in non-redundant pathways to suppress cross-overs during mitosis [8].
Although RECQ1 was not observed in vitro to substitute for BLM in the BLM-Top3α complex double HJ dissolution reaction [4], RECQ1 may interact with Top3α in a related protein complex with additional factors and perform a function important for genomic stability. Conceivably, in a BLM-impaired condition, RECQ1 or another RecQ helicase may partially substitute for BLM through its protein partnership with a topoisomerase.
A thorough investigation of RECQ1 protein interactions and their biological significance would likely provide insight to the potentially multiple roles of RECQ1 in DNA repair, one of which may be double strand break repair by a putative function in DNA end-processing (see RECQ1 Protein Interactions). Although RECQ1 may play a role in modulating homologous recombinational repair, a function of RECQ1 in nonhomologous end-joining, a major pathway for repair of double strand breaks throughout the cell cycle in mammalian cells [36], is also a possibility.
RECQ1 is Required for Normal Proliferation in Human Cells
HeLa cells transfected with control- or RECQ1-shRNA plasmid were found to be impaired for cell growth by a colony forming assay [26]. RECQ1 deficiency in transformed HeLa cells led to perturbation of normal cell cycle progression and compromised cellular ability to maintain G2/M checkpoint [26]. In an independent study, silencing RECQ1 expression through RNA interference was observed to induce mitotic catastrophe specifically in growing cancer cells since normal human fibroblasts were refractory to the anti-proliferative effects of siRNA-RECQ1 [37]. Local and systemic administration of RECQ1-siRNA mixed with polyethyleneimine polymer or cationic liposomes prevented cancer cell proliferation in mouse models of cancer without detectable side effects [38]. Based on their findings, the authors suggested that RECQ1-siRNA formulated with a cationic liposome and introduced through intravenous injection may be a useful approach to target specific cancers.
The high copy number of RECQ1 in cancer cells may provide a growth advantage in certain human tumors [37, 39]. Mitotic death of cancer cells induced by RECQ1 silencing or RECQ1-specific small molecule inhibitors is a potential strategy for anti-cancer therapy, as suggested for other helicases [40, 41].
Structural Features and Biochemical Functions of RECQ1
Recently, the crystal structure of a truncated form of RECQ1 was solved, representing an important advance in the field. Biochemical studies characterized the DNA branch migration activity of RECQ1. Analyses of RECQ1 helicase and strand annealing activities revealed a better understanding of its interactions with DNA substrates and some differences in substrate specificity of RECQ1 compared to the other RecQ helicases. Finally, studies of RECQ1 protein interactions and advances in understanding the functions of RECQ1 protein partners have led to new hypotheses for the cellular role of RECQ1.
Structure of Truncated RECQ1
A crystal structure of a catalytically active truncated RECQ1 (RECQ1T1, residues 49–616 (of 649) of RECQ1, followed by a C-terminal tag of 22 amino acids) with ADP-Mg2+ complex was reported [42]. The overall structure of RECQ1T1 was similar to that of E. coli RecQ [43] for both the core helicase domain and the Zn2+ binding domain residing just after the helicase core. However, the Winged Helix (WH) domain in the C-terminal region of RECQ1T1 is positioned differently from that of the corresponding region in E. coli RecQ. The bacterial and human RecQ structures also display a difference in the β-hairpin structure that forms part of the WH fold, which is significantly longer in RECQ1T1. Biochemical characterization of a RECQ1T1 β-hairpin Y564A mutant suggests that the β-hairpin in RECQ1 may play a critical role in DNA strand separation [42]. Overall, the structure of RECQ1T1 shares major structural features with E. coli RecQ; however, the subtle differences may be highly important for certain unique aspects of the catalytic activities of RECQ1. The DNA substrate specificity and ability of RECQ1 to efficiently catalyze branch migration and assume alternative conformational states for DNA unwinding or strand annealing are discussed below.
DNA Substrate Specificity of RECQ1 Helicase
RECQ1 is a 3′ to 5′ DNA helicase as determined by a conventional strand displacement assay with a DNA substrate that contains an intervening ssDNA between two duplexes within the same DNA substrate [44] or oligonucleotide-based substrates with 3′ ssDNA overhangs [45]. For a detailed discussion of the DNA substrate specificity of RECQ1 helicase activity, please see [8, 46]. For standard duplex DNA substrates, RECQ1 was shown to unwind (in order or preference) forked duplex, 3′ overhang or 3′-flap, 5′-flap, and synthetic replication fork structures. Thus, RECQ1 unwinds conventional duplex DNA substrates representing key replication and repair intermediates that lack single-strand character in the 3′, 5′, or both arms adjacent to the duplex. Some more recent developments in this area will be discussed here.
RECQ1, like a number of other RecQ helicases (e.g., WRN, BLM, and Sgs1) unwinds a synthetic HJ structure to generate a splayed arm product in a reaction dependent on ATP hydrolysis [47]. The bacterial HJ recognition protein RuvA inhibits RECQ1 branch migration, suggesting that RECQ1 initiates unwinding at the junction crossover. The N-terminal region of RECQ1 is required for HJ unwinding and also protein oligomerization, suggesting that the N-terminal domain or higher order oligomer formation promoted by this region is necessary for RECQ1 to disrupt HJs [46]. If oligomerization is required for RECQ1 to unwind HJs, this scenario would be similar to that of WRN which was recently shown to unwind the HJ structure as a tetramer [48].
RECQ1 also unwinds the three-stranded D-loop structure [47]. D-loops can form during an early step in homologous recombination when a recombinase protein binds ssDNA and mediates invasion of the ssDNA molecule into a homologous duplex. RECQ1 unwinds D-loops with either a protruding single-stranded 3′- or 5′-tail by releasing the invading third strand from D-loop structures in an ATP- and protein concentration-dependent manner; however, preferential unwinding of the D-loop with a protruding single-stranded 3′-tail was observed over the entire range of RECQ1 protein concentrations.
Recently, two groups reported that RECQ1 fails to unwind G-quadruplex substrates under conditions that the helicase efficiently unwinds standard duplex DNA substrates [46, 49]. The inability to resolve this particular form of alternate DNA structure distinguishes RECQ1 from WRN, BLM, Sgs1, or E. coli RecQ helicases which have the ability to efficiently unwind a variety of G-quadruplex DNA substrates [3]. A comparison of RECQ1 and BLM helicase substrate specificity revealed several other differences including the inability of RECQ1 to unwind a DNA-RNA hybrid, catalyze fork regression, or displace plasmid D-loops lacking a 3′-tail [46]. Curiously, RECQ1 but not BLM, was able to unwind four-armed synthetic HJ structures that lacked an homologous core [46].
Aside from helicase-catalyzed unwinding of DNA structures, certain RecQ helicases (BLM [50], RECQL5 [51]) can use their motor ATPase to inhibit Rad51-mediated strand exchange by stripping RAD51 from DNA, a function that is thought to be important for regulating homologous recombinational repair; however, RECQ1 is unable to do so [50]. RECQ1, WRN and BLM all fail to displace streptavidin from a biotinylated oligonucleotide under conditions that each helicase is active as a DNA unwinding enzyme [52]. In contrast, the SF2 helicase FANCJ efficiently disrupts the high affinity biotin-streptavidin interaction, destabilizes the Rad51-ssDNA interaction, and also inhibits Rad51 strand exchange [52]. Presumably the differences in substrate specificity between RECQ1 and other SF2 helicases are important for their respective roles in cellular DNA metabolism.
RECQ1 Helicase Activity on Damaged DNA Substrates
The importance of RECQ1 for cell proliferation and an appropriate DNA damage response is apparent from cellular studies in mouse and human. Since the RecQ helicases have important roles in DNA repair and act as DNA translocases, it has been informative to characterize their ability to unwind damaged DNA molecules.
Effect of Benzopyrene Adduct on RECQ1 Helicase Activity
Benzo[a]pyrene (BaP) in the diet and air from combustion of fuel and tobacco form bulky DNA adducts that impede replication and induce mutations by causing replication errors. BaP DE-dG trans adducts occupy the minor groove, but do not significantly distort the double helix of B-form DNA [53-55]. RECQ1 helicase activity on the BaP DE-dG adducted substrate was inhibited in a strand-specific manner, i.e., when the adduct was positioned in the strand that RECQ1 is presumed to translocate based on its demonstrated 3′ to 5′ directionality of unwinding [56]. A possible mechanism for the profound inhibition of RECQ1 helicase activity by the minor groove BaP DE-dG adduct is suggested by a recently identified conserved helix-turn-helix motif found in RecQ helicases that was shown to mediate minor groove binding in the human DNA repair protein O6-alkylguanine-DNA alkyltransferase [57]. Further studies of minor groove modifications on RECQ1 helicase activity are warranted since this form of damage is a consequence of many different agents that induce genotoxic stress, including both noncovalent and covalent damage. For example, the WRN and BLM helicases were profoundly sensitive to helicase inhibition by the minor groove binders distamycin and netropsin [58].
Effect of Backbone Discontinuity on RECQ1 Helicase Activity
Structural data for Superfamily (SF) 2 helicases to which RECQ1 belongs suggest that this class of DNA unwinding enzymes interacts with nucleic acids by contacts with the phosphodiester backbone. In contrast, SF1 helicases bind nucleic acids through hydrophobic interactions with the bases [59]. For example, the ability of SF1 Pcr helicase to interact directly with bases is proposed to be important for the base flipping mechanism it uses to unwind duplex DNA [60]. RECQ1 helicase activity was tested on forked duplex substrates that harbored a synthetic polyglycol modification to the sugar phosphate backbone within the duplex region in either the non-translocating or translocating strand that the enzyme is presumed to translocate. The polyglycol modification spans 3 base pairs within duplex DNA or 6 nucleotides within unwound ssDNA. RECQ1 helicase was inhibited in a strand-specific manner such that the polyglycol modification in only the translocating strand blocked RECQ1 unwinding [61]. Endogenous alkyl phosphotriester lesions or other sugar phosphate backbone modifications that disrupt strand continuity are predicted to inhibit RECQ1 in a strand-specific manner. This may be distinct from those SF1 helicases that intimately interact with the bases during unwinding.
Effect of Thymine Glycol on RECQ1 Helicase Activity
Thymine glycol, a base lesion that results from ionizing radiation and other forms of oxidative stress [62], induces a significant localized structural change to DNA with the thymine glycol largely extrahelical [63]. Thymine glycol is considered a lethal lesion as a consequence of its blocking effect on cellular DNA replication and transcription. RECQ1 was tested on a forked duplex substrate with a single thymine glycol in either the translocating or nontranslocating strand within the duplex region. Inhibition of RECQ1 helicase activity was observed only when the thymine glycol resided in the translocating strand [64]. The single-stranded DNA binding protein Replication Protein A (RPA), which physically interacts with RECQ1 and stimulates its unwinding activity on long undamaged DNA substrates [65], was tested for its ability to stimulate RECQ1 unwinding of the DNA substrate with the thymine glycol in the helicase translocating or non-translocating strand. RPA was unable to stimulate RECQ1 helicase activity on the DNA substrate with thymine glycol in the translocating strand; however, RPA stimulated RECQ1 helicase activity on the substrate with thymine glycol in the non-translocating strand [64]. Thus, RPA stimulates RECQ1 helicase activity in a strand-specific manner, i.e., RPA stimulates DNA unwinding by RECQ1 when the thymine glycol is positioned in the non-translocating strand for the 3′ to 5′ RECQ1 helicase. Interestingly, RPA stimulated DNA unwinding of the FANCJ 5′ to 3′ helicase in a strand-specific manner as well, only when the thymine glycol was positioned in the opposite strand that FANCJ translocates. The high affinity binding of RPA to ssDNA containing thymine glycol is likely to be a mechanistic component of the functional interaction between RPA and the SF2 helicases RECQ1 and FANCJ [64]. A model was proposed in which RPA binds with high affinity to the exposed thymine glycol in the unwound ssDNA opposite to the strand the helicase is tracking along. The physical interaction of RPA with the helicase is also likely to be important since a heterologous ssDNA binding protein from E. coli failed to stimulate helicase-catalyzed DNA unwinding of DNA substrates harboring the thymine glycol base damage.
RECQ1 Strand Annealing and Assembly State Controlled by Nucleotide-induced Conformational Switch
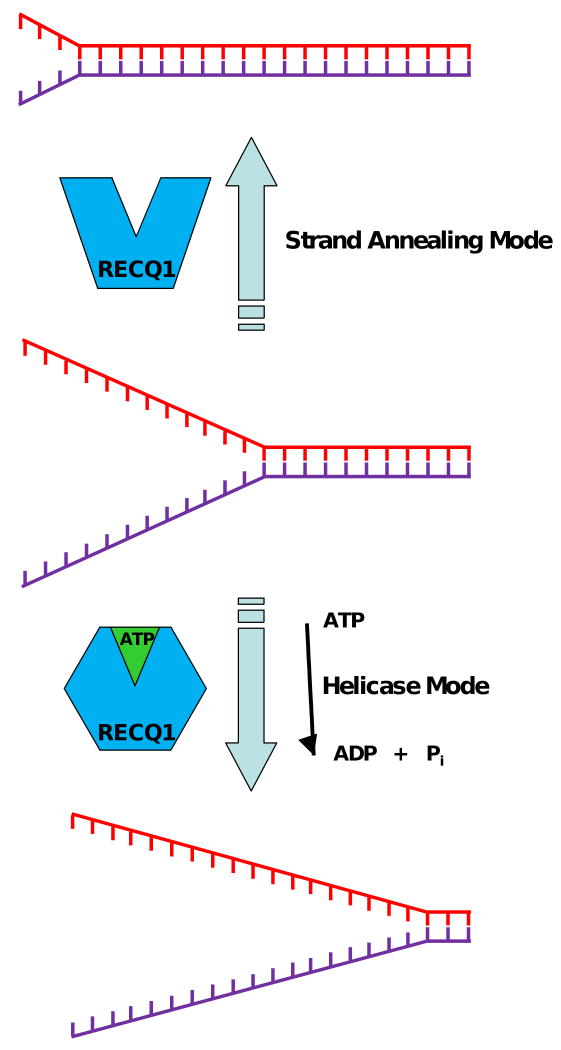
RECQ1, like other DNA helicases and motor ATPases (Table 1), was found to catalyze strand annealing of complementary single-strand DNA molecules [47]. ATP binding to RECQ1 induces a conformational change in the protein that serves as a molecular switch from a strand-annealing to a DNA-unwinding mode [47] (Fig. 2). Different quaternary states of RECQ1, modulated by ssDNA and ATP binding, are associated with its strand annealing or DNA unwinding activities [66]. RECQ1 efficiently promotes strand annealing as a higher order oligomer (pentamer or hexamer) whereas smaller oligomeric states (dimer or monomer) act to unwind duplex DNA. Electron microscopy reconstructions of the higher order oligomeric form revealed a cage-like structure that forms a hollow channel (see [66] for details). It would be of interest to determine if the physical interaction of RECQ1 with other proteins confers a preference for one assembly state or another, thereby regulating its dual enzymatic activities. For example, RPA which physically interacts with RECQ1 and stimulates its helicase activity [45, 65], may help RECQ1 to favor its smaller oligomeric state that efficiently unwinds DNA.
Table 1. DNA Helicases and Motor ATPases that Catalyze Strand Annealing.
| Proteins | References | |
|---|---|---|
| Human | RECQ1 | [47, 66] |
| BLM | [103, 104] | |
| RECQ4 | [104, 105] | |
| WRN | [104, 106] | |
| RECQ5β | [107] | |
| DNA2 | [108] | |
| CSB | [109] | |
| PIF1 | [110] | |
| HARP | [111] | |
| RAD54 | Personal communication, A. Mazin | |
| Yeast | DNA2 | [108] |
| Drosophila | BLM | [104, 112] |
| Bacteriophage | UVsW (T4) | [113] |
| Archea | Hjm/Hel308A (S. tokodaii) | [114] |
| Hel112 (S. solfataricus) | [115] |
Fig. 2. Nucleotide-induced conformational switch controls RECQ1 helicase and strand annealing activities.
A model is presented depicting the importance of ATP-induced conformational change in RECQ1 for regulation of its catalytic activity as a DNA helicase or strand annealing protein. Assembly state of RECQ1 in terms of oligomerization is not depicted in the model.
From a biological standpoint, it is very important to determine the significance of RECQ1 strand annealing and DNA unwinding activities. As discussed more extensively in [8], the coordinate action of DNA unwinding and annealing may play a role in fork regression or synthesis dependent strand annealing, a pathway of double strand break repair. The elevated SCEs and spontaneous γ-H2AX foci in RECQ1-deficient cells would be consistent with a role of RECQ1 in a pathway of homologous recombinational repair. Coordination between strand annealing and DNA unwinding would likely influence directional migration of a D-loop or HJ structure.
RECQ1 DNA Branch Migration Activity
RECQ1 was reported to promote ATP-dependent three-stranded and four-stranded DNA branch migration, showing greater efficiency in the three-stranded reaction [67]. RECQ1 strongly preferred to promote branch migration in the 3′ to 5′ direction for both three-stranded and four-stranded reactions. The unidirectional 3′ to 5′ polarity for branch migration distinguishes RECQ1 from BLM helicase and RAD54 which do not display significant preference in branch migration directionality [67]. Given that the N-terminal region of RECQ1 is required for Holliday junction unwinding and also protein oligomerization [46], it will be of interest to determine the assembly state of the RECQ1 species that promotes branch migration, and the functions of RECQ1 protein domains in DNA branch migration activity.
The ability of RECQ1 to promote branch migration with 3′ to 5′ polarity enables RECQ1 to disrupt three-stranded D-loop recombination intermediates arising from invasion of the protruding 5′ ssDNA tail into the recipient duplex [67]. This particular form of the D-loop is inert to extension by a DNA polymerase which requires a base paired nucleotide with a free 3′ hydroxyl. Therefore, the D-loop substrate efficiently branch-migrated by RECQ1 in vitro may represent a dead-end substrate intermediate of homologous recombination that arises during double strand break repair. It is proposed that through its branch migration activity, RECQ1 may prevent the accumulation of potentially toxic homologous recombination intermediates and facilitate the restart of collapsed replication forks.
RECQ1 Protein Interactions
From nuclear extract co-immunoprecipitation experiments and biochemical assays with purified recombinant proteins, RECQ1 was found to interact with several DNA repair factors that regulate genetic recombination. These include MSH2/6, MLH1-PMS2, EXO1 [68], and RAD51 [26]. RECQ1 stimulated the endonucleolytic and exonucleolytic incision activities of EXO1 through an ATP-independent protein interaction [68], suggesting that RECQ1 and EXO1 may function together in the resolution of DNA structural intermediates formed during DNA repair or replication; however, the biological significance of the protein interaction is not yet known.
It is conceivable that the increased sensitivity of mouse and human RECQ1-deficient cells to ionizing radiation [25, 26] reflects a role of RECQ1 with structure-specific nucleases in end processing of strand breaks [69]. Recently, a number of studies from several laboratories have provided evidence that EXO1 plays an important role in end resection during double strand break repair. Exo1 and Sgs1, the sole RecQ homolog in S. cerevisiae, were shown to have a role in long range 5′ strand resection at double strand breaks at a step after initial trimming by the MRX/Sae2 complex ([70-72]. Furthermore, Sgs1 is required in order for Dna2 to compensate for the loss of Mre11 nuclease during repair of IR-induced damage [73]. Using fission yeast as a model system, it was shown that in the absence of MRN, EXO1 becomes the major nuclease required for efficient meiotic recombination [74]. Recently, the Kowalczykowski lab demonstrated that the BLM helicase stimulates the nucleolytic activity of EXO1 on a linearized plasmid DNA molecule in an ATP-independent manner [75]. The earlier finding that WRN interacts physically and functionally with EXO1 [76] suggests that EXO1 may be a conserved protein partner of the RecQ helicases. Conceivably, the interaction of EXO1 with the various human RecQ helicases (RECQ1, WRN, BLM) is tailored for specific situations such as repair of strand breaks, restoration of blocked replication forks, or at specialized DNA structures such as telomeres.
Avenues for Investigation of RECQ1 Biological Function
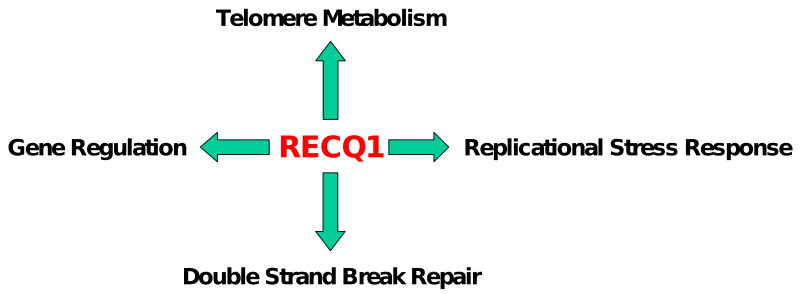
RECQ1 polymorphism studies have suggested that RECQ1 may play a role in cancer [77, 78]. However, mutations in RECQ1 (or RECQ5β) have not yet been directly linked to a genetic disease and the RECQ1 knockout mouse lacks organismal or tissue-specific phenotypes [25]. Therefore, understanding the role of RECQ1 in nucleic acid metabolism and suppression of pathological symptoms may be more elusive than other DNA helicases. We will focus this portion of the review on some recent discoveries that lead us to propose research directions for understanding how RECQ1 is important (Fig. 3).
Fig. 3. Potential cellular functions of RECQ1 in DNA metabolism.
See text for details.
Does RECQ1 Have a Role at Telomeres?
Certain members of the RecQ helicase family have been implicated in telomere metabolism (for review, see [79]). S. cerevisiae Sgs1 plays a role in telomere metabolism [80-82]. In mammalian cells, both WRN and BLM helicases are also believed to be involved in telomere maintenance (for review, see [79]). However, FISH and flow cytometry analyses of mouse Recql+/+, Recql+/−, and Recql−/− splenocytes and thymocytes did not reveal any differences in telomere length [25]. Expression levels of the known family members WRN, BLM, RECQL4, and RECQL5 were not altered in Recql knockout mice, suggesting that if one or more of these helicases compensates for the absence of RECQL, the normal level of expression is sufficient for this compensation. The genetic background and/or tissue specificity of RECQL function may protect the mouse from severe abnormalities that might be expected from the magnitude of chromosomal aberrations detected at the cellular level. For example, WRN-deficient mice lack a disease phenotype [83, 84]; however, late-generation telomerase- and WRN-deficient mice (mTerc−/− Wrn−/−) displayed clinical symptoms of premature aging and the types of tumors typically observed in Werner syndrome patients [85, 86]. It is plausible that the manifestation of disease phenotypes of RECQL deficiency in mice is related to telomere maintenance or some other event critical for genomic stability.
Genetic data from yeast implicate the sole RecQ homolog Sgs1 in telomere metabolism through a recombination mediated pathway known as Alternative Lengthening of Telomeres (ALT)[81, 87]. A recent study using a de novo telomere addition assay showed that Sgs1 is likely required for Exo1 to act at telomeres in C strand degradation [88]. The authors provide further evidence that Sgs1 controls telomere length maintenance by regulating telomere processing.
The interaction of human RECQ1 with EXO1 [76] may also be important in telomere metabolism. EXO1 nuclease activity was shown to have a role in stability of chromosome ends (for review, see [89]). In a proposed model, EXO1 removes the lagging strand template strand to yield a 3′ ssDNA overhang structure that can be properly processed to maintain telomere stability. As an auxiliary factor for EXO1, RECQ1 may facilitate the 5′ end trimming function of EXO1. Using a procedure called Proteomics of Isolated Chromatin Segments (PICh) that isolates genomic DNA with its associated proteins, it was found that RECQ1 and other proteins were purified with human telomeric chromatin specifically in cells that use the ALT pathway [90]. It will be of interest to determine whether RECQ1 interacts with telomere factors such as the single-stranded DNA binding protein POT1 and the telomeric sequence specific binding protein TRF2 to which WRN interacts [91-93]. RECQ1 and EXO1 may collaborate in a complex to accurately process chromosome ends. This may be in addition to an interactive role of RECQ1 and EXO1 in double strand break repair.
Involvement of RECQ1 in Herpes Virus Lytic DNA Replication
RECQ1 depletion resulted in a significantly reduced DNA content compared to control HeLa cells [26]. Mitogenic efficiency was also reduced as evaluated by bromodeoxyuridine incorporation as a measurement of DNA synthesis. Therefore, RECQ1-depleted cells are impaired for their ability to synthesize DNA as compared to control siRNA treated cells. The impaired cell proliferation of RECQ1-deficient cells may reflect a direct role of RECQ1 in cellular DNA replication that is required during a specialized circumstance such as replicational stress that occurs from endogenous DNA damage.
Although RECQ1 has not been directly implicated in replication, evidence suggests that some of the other RecQ helicases have roles in replication, either during initiation or elongation. Certain RecQ helicases (e.g., WRN [94]) are associated with a DNA replication complex isolated from mammalian cells. Although the mammalian RecQ helicases are not essential for replication since the corresponding mutant cell lines are viable, this does not exclude a more specialized role. Viral DNA replication can provide a window to the identification of nuclear protein factors in replication. A helicase deficiency can negatively affect viral DNA replication which relies heavily on the host cell machinery. For example, herpes simplex virus yield is reduced in WRN-deficient cells compared to control human fibroblast cells, and WRN is associated with the viral single-stranded DNA binding protein ICP8 [95].
Herpesvirus lytic DNA replication requires the cis-acting element, origin, and trans-acting factors, including virally encoded origin-binding protein, DNA replication enzymes, and auxiliary factors. Two lytic DNA replication origins (ori-Lyt) of Kaposi's sarcoma-associated herpesvirus (KSHV) have been identified, and two virally encoded proteins (RTA and K8) have been shown to bind to the origins [96]. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) contains two functional lytic origins of DNA replication [97]. Using a nuclear extract derived from the primary effusion lymphoma cell line BCBL-1 that carries latent KSHV and a biotin-labeled DNA fragment of KSHV ori-Lyt core domain as bait, Wang et al. (2008) employed a DNA affinity purification and mass spectrometry procedure to show that RECQ1 is physically associated with KSHV ori-Lyt through K8 and RTA [98]. RECQ1 was able to be co-immunoprecipitated by both K8 and RTA viral proteins, suggesting that RECQ1 is an integral component of the pre-replication complex. Interestingly, RECQ1 is the only cellular protein that was detected in the pre-replication complex, suggesting a unique function of RECQ1 in initiation of viral DNA replication.
More recently, it was found that RECQ1 is associated with the OriLyt and Zta of another virus, Epstein-Barr virus (EBV)[99]. Depletion of RECQ1 by shRNA resulted in reduced lytic DNA replication. Identification of RECQ1 in the KSHV and EBV replication complexes suggests that the cellular RECQ1 helicase may be involved in unwinding origin DNA during the initiation of KSHV and EBV lytic DNA replication. Future studies such as CHIP experiments to examine the association of RECQ1 with DNA replication factories at origins should be encouraged. Replication of certain regions of the genome may require a RecQ helicase for replication initiation (RECQ4, [100]) or normal fork progression during replicational stress (WRN, [101]).
RECQ1 and Gene Regulation
Genotoxic stress in the form of DNA damage can lead to mutations which predispose individuals to cancer. Cellular senescence might also be enhanced by DNA damage which can suppress tumor formation [102]. A growing field of interest is how persistent DNA damage triggers senescence-associated phenotypes. Cellular studies demonstrating increased DNA damage load in RECQ1-deficient cells suggests that RECQ1, the most abundant of the RecQ helicases, plays an important role in the DNA damage response. One hypothesis is that RECQ1 plays a role in the regulation of gene expression as a component of the DNA damage response. Currently, we have undertaken a microarray analyses of gene expression patterns in primary mouse embryonic fibroblasts (MEFs) from RECQ1 knockout and wild-type mice. MEFs were exposed to a variety of DNA damaging agents and allowed to recover for short or long time periods. RNA was then collected and examined by microarray analyses to determine if differences between RECQ1 wild-type and knockout MEFs exist, and if there are stress-specific gene expression changes. Our unpublished results indicate that RECQ1 knockout MEFs show gene expression changes as a function of the DNA damage treatment that are clearly different from those of MEFs from the wild-type littermates. Further studies in this area are underway, leading us to believe that RECQ1 status influences the gene expression response after various forms of DNA damage (Wu et al., manuscript in preparation).
Summary
In this review, we have attempted to provide an up to date summary of the research pertaining to RECQ1. In many areas, this research lags behind that of the other RecQ helicases. However, certain recent advances including the genetic characterization of RECQ1 in model systems and the demonstration of cellular phenotypes in RECQ1-deficient mammalian cells suggest a unique role of RECQ1 in cellular DNA metabolism. A linkage to disease or cancer may await the future of RECQ1 research, in which case ongoing studies in basic research to provide a molecular understanding of RECQ1 functions and pathways will likely aid in understanding the basis for phenotypes associated with RECQ1 deficiency.
Acknowledgments
This work was supported by the Intramural Research program of the NIH, National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brosh RM, Jr, Bohr VA. Human premature aging, DNA repair and RecQ helicases. Nucleic Acids Res. 2007;35:7527–7544. doi: 10.1093/nar/gkm1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanada K, Hickson ID. Molecular genetics of RecQ helicase disorders. Cell Mol Life Sci. 2007;64:2306–2322. doi: 10.1007/s00018-007-7121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma S, Doherty KM, Brosh RM., Jr Mechanisms of RecQ helicases in pathways of DNA metabolism and maintenance of genomic stability. Biochem J. 2006;398:319–337. doi: 10.1042/BJ20060450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu L, Chan KL, Ralf C, Bernstein DA, Garcia PL, Bohr VA, Vindigni A, Janscak P, Keck JL, Hickson ID. The HRDC domain of BLM is required for the dissolution of double Holliday junctions. EMBO J. 2005;24:2679–2687. doi: 10.1038/sj.emboj.7600740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puranam KL, Blackshear PJ. Cloning and characterization of RECQL, a potential human homologue of the Escherichia coli DNA helicase RecQ. J Biol Chem. 1994;269:29838–29845. [PubMed] [Google Scholar]
- 6.Seki M, Miyazawa H, Tada S, Yanagisawa J, Yamaoka T, Hoshino S, Ozawa K, Eki T, Nogami M, Okumura K. Molecular cloning of cDNA encoding human DNA helicase Q1 which has homology to Escherichia coli Rec Q helicase and localization of the gene at chromosome 12p12. Nucleic Acids Res. 1994;22:4566–4573. doi: 10.1093/nar/22.22.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakayama H, Nakayama K, Nakayama R, Irino N, Nakayama Y, Hanawalt PC. Isolation and genetic characterization of a thymineless death-resistant mutant of Escherichia coli K12: identification of a new mutation (recQ1) that blocks the RecF recombination pathway. Mol Gen Genet. 1984;195:474–480. doi: 10.1007/BF00341449. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S, Brosh RM., Jr Unique and important consequences of RECQ1 deficiency in mammalian cells. Cell Cycle. 2008;7:989–1000. doi: 10.4161/cc.7.8.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seki M, Otsuki M, Ishii Y, Tada S, Enomoto T. RecQ family helicases in genome stability: lessons from gene disruption studies in DT40 cells. Cell Cycle. 2008;7:2472–2478. doi: 10.4161/cc.7.16.6462. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Seki M, Narita Y, Nakagawa T, Yoshimura A, Otsuki M, Kawabe Y, Tada S, Yagi H, Ishii Y, Enomoto T. Functional relation among RecQ family helicases RecQL1, RecQL5, and BLM in cell growth and sister chromatid exchange formation. Mol Cell Biol. 2003;23:3527–3535. doi: 10.1128/MCB.23.10.3527-3535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otsuki M, Seki M, Inoue E, Abe T, Narita Y, Yoshimura A, Tada S, Ishii Y, Enomoto T. Analyses of functional interaction between RECQL1, RECQL5, and BLM which physically interact with DNA topoisomerase IIIalpha. Biochim Biophys Acta. 2007;1782:75–81. doi: 10.1016/j.bbadis.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Kato A, Akamatsu Y, Sakuraba Y, Inoue H. The Neurospora crassa mus-19 gene is identical to the qde-3 gene, which encodes a RecQ homologue and is involved in recombination repair and postreplication repair. Curr Genet. 2004;45:37–44. doi: 10.1007/s00294-003-0459-3. [DOI] [PubMed] [Google Scholar]
- 13.Pickford A, Braccini L, Macino G, Cogoni C. The QDE-3 homologue RecQ-2 cooperates with QDE-3 in DNA repair in Neurospora crassa. Curr Genet. 2003;42:220–227. doi: 10.1007/s00294-002-0351-6. [DOI] [PubMed] [Google Scholar]
- 14.Catalanotto C, Nolan T, Cogoni C. Homology effects in Neurospora crassa. FEMS Microbiol Lett. 2006;254:182–189. doi: 10.1111/j.1574-6968.2005.00037.x. [DOI] [PubMed] [Google Scholar]
- 15.Cogoni C, Macino G. Isolation of quelling-defective (qde) mutants impaired in posttranscriptional transgene-induced gene silencing in Neurospora crassa. Proc Natl Acad Sci U S A. 1997;94:10233–10238. doi: 10.1073/pnas.94.19.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond SM, Caudy AA, Hannon GJ. Post-transcriptional gene silencing by double-stranded RNA. Nat Rev Genet. 2001;2:110–119. doi: 10.1038/35052556. [DOI] [PubMed] [Google Scholar]
- 17.Cogoni C, Macino G. Posttranscriptional gene silencing in Neurospora by a RecQ DNA helicase. Science. 1999;286:2342–2344. doi: 10.1126/science.286.5448.2342. [DOI] [PubMed] [Google Scholar]
- 18.Lee HC, Chang SS, Choudhary S, Aalto AP, Maiti M, Bamford DH, Liu Y. qiRNA is a new type of small interfering RNA induced by DNA damage. Nature. 2009;459:274–277. doi: 10.1038/nature08041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klattenhoff C, Theurkauf W. Biogenesis and germline functions of piRNAs. Development. 2008;135:3–9. doi: 10.1242/dev.006486. [DOI] [PubMed] [Google Scholar]
- 20.O'Donnell KA, Boeke JD. Mighty Piwis defend the germline against genome intruders. Cell. 2007;129:37–44. doi: 10.1016/j.cell.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa S, Nakano T, Bartel DP, Kingston RE. Characterization of the piRNA complex from rat testes. Science. 2006;313:363–367. doi: 10.1126/science.1130164. [DOI] [PubMed] [Google Scholar]
- 22.Saotome A, Kimura S, Mori Y, Uchiyama Y, Morohashi K, Sakaguchi K. Characterization of four RecQ homologues from rice (Oryza sativa L. cv. Nipponbare) Biochem Biophys Res Commun. 2006;345:1283–1291. doi: 10.1016/j.bbrc.2006.04.134. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Samadder PP, Tanaka Y, Ohira T, Okuizumi H, Yamaoka N, Miyao A, Hirochika H, Ohira T, Tsuchimoto S, Ohtsubo H, Nishiguchi M. OsRecQ1, a QDE-3 homologue in rice, is required for RNA silencing induced by particle bombardment for inverted repeat DNA, but not for double-stranded RNA. Plant J. 2008;56:274–286. doi: 10.1111/j.1365-313X.2008.03587.x. [DOI] [PubMed] [Google Scholar]
- 24.Stein P, Svoboda P, Stumpo DJ, Blackshear PJ, Lombard DB, Johnson B, Schultz RM. Analysis of the role of RecQ helicases in RNAi in mammals. Biochem Biophys Res Commun. 2002;291:1119–1122. doi: 10.1006/bbrc.2002.6578. [DOI] [PubMed] [Google Scholar]
- 25.Sharma S, Stumpo DJ, Balajee AS, Bock CB, Lansdorp PM, Brosh RM, Jr, Blackshear PJ. RECQL, a Member of the RecQ Family of DNA Helicases, Suppresses Chromosomal Instability. Mol Cell Biol. 2007;27:1784–1794. doi: 10.1128/MCB.01620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma S, Brosh RM. Human RECQ1 Is a DNA Damage Responsive Protein Required for Genotoxic Stress Resistance and Suppression of Sister Chromatid Exchanges. PLoS ONE. 2007;2:e1297. doi: 10.1371/journal.pone.0001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu L, Hickson ID. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- 28.Raynard S, Bussen W, Sung P. A Double Holliday Junction Dissolvasome Comprising BLM, Topoisomerase III{alpha}, and BLAP75. J Biol Chem. 2006;281:13861–13864. doi: 10.1074/jbc.C600051200. [DOI] [PubMed] [Google Scholar]
- 29.Wu L, Bachrati CZ, Ou J, Xu C, Yin J, Chang M, Wang W, Li L, Brown GW, Hickson ID. BLAP75/RMI1 promotes the BLM-dependent dissolution of homologous recombination intermediates. Proc Natl Acad Sci U S A. 2006;103:4068–4073. doi: 10.1073/pnas.0508295103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bussen W, Raynard S, Busygina V, Singh AK, Sung P. Holliday junction processing activity of the BLM-Topo IIIalpha-BLAP75 complex. J Biol Chem. 2007;282:31484–31492. doi: 10.1074/jbc.M706116200. [DOI] [PubMed] [Google Scholar]
- 31.Singh TR, Ali AM, Busygina V, Raynard S, Fan Q, Du CH, Andreassen PR, Sung P, Meetei AR. BLAP18/RMI2, a novel OB-fold-containing protein, is an essential component of the Bloom helicase-double Holliday junction dissolvasome. Genes Dev. 2008;22:2856–2868. doi: 10.1101/gad.1725108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu D, Guo R, Sobeck A, Bachrati CZ, Yang J, Enomoto T, Brown GW, Hoatlin ME, Hickson ID, Wang W. RMI, a new OB-fold complex essential for Bloom syndrome protein to maintain genome stability. Genes Dev. 2008;22:2843–2855. doi: 10.1101/gad.1708608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson FB, Lombard DB, Neff NF, Mastrangelo MA, Dewolf W, Ellis NA, Marciniak RA, Yin Y, Jaenisch R, Guarente L. Association of the Bloom syndrome protein with topoisomerase IIIalpha in somatic and meiotic cells. Cancer Res. 2000;60:1162–1167. [PubMed] [Google Scholar]
- 34.Shimamoto A, Nishikawa K, Kitao S, Furuichi Y. Human RecQ5beta, a large isomer of RecQ5 DNA helicase, localizes in the nucleoplasm and interacts with topoisomerases 3alpha and 3beta. Nucleic Acids Res. 2000;28:1647–1655. doi: 10.1093/nar/28.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Y, Lu X, Barnes E, Yan M, Lou H, Luo G. Recql5 and Blm RecQ DNA helicases have nonredundant roles in suppressing crossovers. Mol Cell Biol. 2005;25:3431–3442. doi: 10.1128/MCB.25.9.3431-3442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieber MR. The mechanism of human nonhomologous DNA end joining. J Biol Chem. 2008;283:1–5. doi: 10.1074/jbc.R700039200. [DOI] [PubMed] [Google Scholar]
- 37.Futami K, Kumagai E, Makino H, Goto H, Takagi M, Shimamoto A, Furuichi Y. Induction of mitotic cell death in cancer cells by small interference RNA suppressing the expression of RecQL1 helicase. Cancer Sci. 2008;99:71–80. doi: 10.1111/j.1349-7006.2007.00647.x. [DOI] [PubMed] [Google Scholar]
- 38.Futami K, Kumagai E, Makino H, Sato A, Takagi M, Shimamoto A, Furuichi Y. Anticancer activity of RecQL1 helicase siRNA in mouse xenograft models. Cancer Sci. 2008;99:1227–1236. doi: 10.1111/j.1349-7006.2008.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawabe T, Tsuyama N, Kitao S, Nishikawa K, Shimamoto A, Shiratori M, Matsumoto T, Anno K, Sato T, Mitsui Y, Seki M, Enomoto T, Goto M, Ellis NA, Ide T, Furuichi Y, Sugimoto M. Differential regulation of human RecQ family helicases in cell transformation and cell cycle. Oncogene. 2000;19:4764–4772. doi: 10.1038/sj.onc.1203841. [DOI] [PubMed] [Google Scholar]
- 40.Aggarwal M, Brosh RM., Jr Hitting the bull's eye: novel directed cancer therapy through helicase-targeted synthetic lethality. J Cell Biochem. 2009;106:758–763. doi: 10.1002/jcb.22048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta R, Brosh RM., Jr Helicases as prospective targets for anti-cancer therapy. Anticancer Agents Med Chem. 2008;8:390–401. doi: 10.2174/187152008784220339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pike AC, Shrestha B, Popuri V, Burgess-Brown N, Muzzolini L, Costantini S, Vindigni A, Gileadi O. Structure of the human RECQ1 helicase reveals a putative strand-separation pin. Proc Natl Acad Sci U S A. 2009;106:1039–1044. doi: 10.1073/pnas.0806908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernstein DA, Zittel MC, Keck JL. High-resolution structure of the E.coli RecQ helicase catalytic core. EMBO J. 2003;22:4910–4921. doi: 10.1093/emboj/cdg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seki M, Yanagisawa J, Kohda T, Sonoyama T, Ui M, Enomoto T. Purification of two DNA-dependent adenosinetriphosphatases having DNA helicase activity from HeLa cells and comparison of the properties of the two enzymes. J Biochem. 1994;115:523–531. doi: 10.1093/oxfordjournals.jbchem.a124369. [DOI] [PubMed] [Google Scholar]
- 45.Cui S, Klima R, Ochem A, Arosio D, Falaschi A, Vindigni A. Characterization of the DNA-unwinding activity of human RECQ1, a helicase specifically stimulated by human replication protein A. J Biol Chem. 2003;278:1424–1432. doi: 10.1074/jbc.M209407200. [DOI] [PubMed] [Google Scholar]
- 46.Popuri V, Bachrati CZ, Muzzolini L, Mosedale G, Costantini S, Giacomini E, Hickson ID, Vindigni A. The Human RecQ helicases, BLM and RECQ1, display distinct DNA substrate specificities. J Biol Chem. 2008;283:17766–17776. doi: 10.1074/jbc.M709749200. [DOI] [PubMed] [Google Scholar]
- 47.Sharma S, Sommers JA, Choudhary S, Faulkner JK, Cui S, Andreoli L, Muzzolini L, Vindigni A, Brosh RM., Jr Biochemical analysis of the DNA unwinding and strand annealing activities catalyzed by human RECQ1. J Biol Chem. 2005;280:28072–28084. doi: 10.1074/jbc.M500264200. [DOI] [PubMed] [Google Scholar]
- 48.Compton SA, Tolun G, Kamath-Loeb AS, Loeb LA, Griffith JD. The Werner syndrome protein binds replication fork and Holliday junction DNAs as an oligomer. J Biol Chem. 2008;283:24478–24483. doi: 10.1074/jbc.M803370200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Y, Shin-Ya K, Brosh RM., Jr FANCJ helicase defective in Fanconia Anemia and breast cancer unwinds G-quadruplex DNA to defend genomic stability. Mol Cell Biol. 2008;28:4116–4128. doi: 10.1128/MCB.02210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bugreev DV, Yu X, Egelman EH, Mazin AV. Novel pro- and anti-recombination activities of the Bloom's syndrome helicase. Genes Dev. 2007;21:3085–3094. doi: 10.1101/gad.1609007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu Y, Raynard S, Sehorn MG, Lu X, Bussen W, Zheng L, Stark JM, Barnes EL, Chi P, Janscak P, Jasin M, Vogel H, Sung P, Luo G. RECQL5/Recql5 helicase regulates homologous recombination and suppresses tumor formation via disruption of Rad51 presynaptic filaments. Genes Dev. 2007;21:3073–3084. doi: 10.1101/gad.1609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sommers JA, Rawtani N, Gupta R, Bugreev DV, Mazin AV, Cantor SB, Brosh RM., Jr FANCJ uses its motor ATPase to disrupt protein-DNA complexes, unwind triplexes, and inhibit rad51 strand exchange. J Biol Chem. 2009;284:7505–7517. doi: 10.1074/jbc.M809019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cosman M, de los SC, Fiala R, Hingerty BE, Singh SB, Ibanez V, Margulis LA, Live D, Geacintov NE, Broyde S. Solution conformation of the major adduct between the carcinogen (+)-anti-benzo[a]pyrene diol epoxide and DNA. Proc Natl Acad Sci U S A. 1992;89:1914–1918. doi: 10.1073/pnas.89.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de los SC, Cosman M, Hingerty BE, Ibanez V, Margulis LA, Geacintov NE, Broyde S, Patel DJ. Influence of benzo[a]pyrene diol epoxide chirality on solution conformations of DNA covalent adducts: the (-)-trans-anti-[BP]G.C adduct structure and comparison with the (+)-trans-anti-[BP]G.C enantiomer. Biochemistry. 1992;31:5245–5252. doi: 10.1021/bi00138a002. [DOI] [PubMed] [Google Scholar]
- 55.Fountain MA, Krugh TR. Structural characterization of a (+)-trans-anti-benzo[a]pyrene-DNA adduct using NMR, restrained energy minimization, and molecular dynamics. Biochemistry. 1995;34:3152–3161. doi: 10.1021/bi00010a004. [DOI] [PubMed] [Google Scholar]
- 56.Choudhary S, Doherty KM, Handy CJ, Sayer JM, Yagi H, Jerina DM, Brosh RM., Jr Inhibition of Werner Syndrome helicase activity by benzo[a]pyrene diol epoxide adducts can be overcome by Replication Protein A. J Biol Chem. 2006;281:6000–6009. doi: 10.1074/jbc.M510122200. [DOI] [PubMed] [Google Scholar]
- 57.Daniels DS, Woo TT, Luu KX, Noll DM, Clarke ND, Pegg AE, Tainer JA. DNA binding and nucleotide flipping by the human DNA repair protein AGT. Nat Struct Mol Biol. 2004;11:714–720. doi: 10.1038/nsmb791. [DOI] [PubMed] [Google Scholar]
- 58.Brosh RM, Jr, Karow JK, White EJ, Shaw ND, Hickson ID, Bohr VA. Potent inhibition of Werner and Bloom helicases by DNA minor groove binding drugs. Nucleic Acids Res. 2000;28:2420–2430. doi: 10.1093/nar/28.12.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singleton MR, Wigley DB. Modularity and specialization in superfamily 1 and 2 helicases. J Bacteriol. 2002;184:1819–1826. doi: 10.1128/JB.184.7.1819-1826.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dillingham MS, Soultanas P, Wiley P, Webb MR, Wigley DB. Defining the roles of individual residues in the single-stranded DNA binding site of PcrA helicase. Proc Natl Acad Sci U S A. 2001;98:8381–8387. doi: 10.1073/pnas.131009598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta R, Sharma S, Doherty KM, Sommers JA, Cantor SB, Brosh RM., Jr Inhibition of BACH1 (FANCJ) helicase by backbone discontinuity is overcome by increased motor ATPase or length of loading strand. Nucleic Acids Res. 2006;34:6673–6683. doi: 10.1093/nar/gkl964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wallace SS. Biological consequences of free radical-damaged DNA bases. Free Radic Biol Med. 2002;33:1–14. doi: 10.1016/s0891-5849(02)00827-4. [DOI] [PubMed] [Google Scholar]
- 63.Kung HC, Bolton PH. Structure of a duplex DNA containing a thymine glycol residue in solution. J Biol Chem. 1997;272:9227–9236. doi: 10.1074/jbc.272.14.9227. [DOI] [PubMed] [Google Scholar]
- 64.Suhasini AN, Sommers JA, Mason AC, Voloshin ON, Camerini-Otero RD, Wold MS, Brosh RM., Jr FANCJ helicase uniquely senses oxidative base damage in either strand of duplex DNA and is stimulated by Replication Protein A to unwind the damaged DNA substrate in a strand-specific manner. J Biol Chem. 2009;284:18458–18470. doi: 10.1074/jbc.M109.012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cui S, Arosio D, Doherty KM, Brosh RM, Jr, Falaschi A, Vindigni A. Analysis of the unwinding activity of the dimeric RECQ1 helicase in the presence of human Replication Protein A. Nucleic Acids Res. 2004;32:2158–2170. doi: 10.1093/nar/gkh540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muzzolini L, Beuron F, Patwardhan A, Popuri V, Cui S, Niccolini B, Rappas M, Freemont PS, Vindigni A. Different quaternary structures of human RECQ1 are associated with its dual enzymatic activity. PLoS Biol. 2007;5:e20. doi: 10.1371/journal.pbio.0050020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bugreev DV, Brosh RM, Jr, Mazin AV. RECQ1 Possesses DNA Branch Migration Activity. J Biol Chem. 2008;283:20231–20242. doi: 10.1074/jbc.M801582200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doherty KM, Sharma S, Uzdilla L, Wilson TM, Cui S, Vindigni A, Brosh RM., Jr RECQ1 helicase interacts with human mismatch repair factors that regulate gentic recombination. J Bio Chem. 2005;280:28085–28094. doi: 10.1074/jbc.M500265200. [DOI] [PubMed] [Google Scholar]
- 69.Sharma S, Sommers JA, Brosh RM., Jr Processing of DNA replication and repair intermediates by the concerted action of RecQ helicases and Rad2 structure-specific nucleases. Protein Pept Lett. 2008;15:89–102. doi: 10.2174/092986608783330369. [DOI] [PubMed] [Google Scholar]
- 70.Gravel S, Chapman JR, Magill C, Jackson SP. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev. 2008;22:2767–2772. doi: 10.1101/gad.503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mimitou EP, Symington LS. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008;455:770–774. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134:981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Budd ME, Campbell JL. Interplay of Mre11 nuclease with Dna2 plus Sgs1 in Rad51-dependent recombinational repair. PLoS ONE. 2009;4:e4267. doi: 10.1371/journal.pone.0004267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farah JA, Cromie GA, Smith GR. Ctp1 and Exonuclease 1, alternative nucleases regulated by the MRN complex, are required for efficient meiotic recombination. Proc Natl Acad Sci U S A. 2009;106:9356–9361. doi: 10.1073/pnas.0902793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nimonkar AV, Ozsoy AZ, Genschel J, Modrich P, Kowalczykowski SC. Human exonuclease 1 and BLM helicase interact to resect DNA and initiate DNA repair. Proc Natl Acad Sci U S A. 2008;105:16906–16911. doi: 10.1073/pnas.0809380105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sharma S, Sommers JA, Driscoll HC, Uzdilla L, Wilson TM, Brosh RM., Jr The exonucleolytic and endonucleolytic cleavage activities of human Exonuclease 1 are stimulated by an interaction with the carboxyl-terminal region of the Werner syndrome protein. J Biol Chem. 2003;278:23487–23496. doi: 10.1074/jbc.M212798200. [DOI] [PubMed] [Google Scholar]
- 77.Li D, Frazier M, Evans DB, Hess KR, Crane CH, Jiao L, Abbruzzese JL. Single Nucleotide Polymorphisms of RecQ1, RAD54L and ATM Genes Are Associated With Reduced Survival of Pancreatic Cancer. J Clin Oncol. 2006;24:1720–1728. doi: 10.1200/JCO.2005.04.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li D, Liu H, Jiao L, Chang DZ, Beinart G, Wolff RA, Evans DB, Hassan MM, Abbruzzese JL. Significant effect of homologous recombination DNA repair gene polymorphisms on pancreatic cancer survival. Cancer Res. 2006;66:3323–3330. doi: 10.1158/0008-5472.CAN-05-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Opresko PL. Telomere ResQue and preservation--roles for the Werner syndrome protein and other RecQ helicases. Mech Ageing Dev. 2008;129:79–90. doi: 10.1016/j.mad.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 80.Cohen H, Sinclair DA. Recombination-mediated lengthening of terminal telomeric repeats requires the Sgs1 DNA helicase. Proc Natl Acad Sci U S A. 2001;98:3174–3179. doi: 10.1073/pnas.061579598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang P, Pryde FE, Lester D, Maddison RL, Borts RH, Hickson ID, Louis EJ. SGS1 is required for telomere elongation in the absence of telomerase. Curr Biol. 2001;11:125–129. doi: 10.1016/s0960-9822(01)00021-5. [DOI] [PubMed] [Google Scholar]
- 82.Johnson FB, Marciniak RA, McVey M, Stewart SA, Hahn WC, Guarente L. The Saccharomyces cerevisiae WRN homolog Sgs1p participates in telomere maintenance in cells lacking telomerase. EMBO J. 2001;20:905–913. doi: 10.1093/emboj/20.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lebel M, Leder P. A deletion within the murine Werner syndrome helicase induces sensitivity to inhibitors of topoisomerase and loss of cellular proliferative capacity. Proc Natl Acad Sci U S A. 1998;95:13097–13102. doi: 10.1073/pnas.95.22.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lombard DB, Beard C, Johnson B, Marciniak RA, Dausman J, Bronson R, Buhlmann JE, Lipman R, Curry R, Sharpe A, Jaenisch R, Guarente L. Mutations in the WRN gene in mice accelerate mortality in a p53-null background. Mol Cell Biol. 2000;20:3286–3291. doi: 10.1128/mcb.20.9.3286-3291.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang S, Multani AS, Cabrera NG, Naylor ML, Laud P, Lombard D, Pathak S, Guarente L, DePinho RA. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat Genet. 2004;36:877–882. doi: 10.1038/ng1389. [DOI] [PubMed] [Google Scholar]
- 86.Du X, Shen J, Kugan N, Furth EE, Lombard DB, Cheung C, Pak S, Luo G, Pignolo RJ, DePinho RA, Guarente L, Johnson FB. Telomere shortening exposes functions for the mouse Werner and Bloom syndrome genes. Mol Cell Biol. 2004;24:8437–8446. doi: 10.1128/MCB.24.19.8437-8446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee JY, Kozak M, Martin JD, Pennock E, Johnson FB. Evidence that a RecQ helicase slows senescence by resolving recombining telomeres. PLoS Biol. 2007;5:e160. doi: 10.1371/journal.pbio.0050160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonetti D, Martina M, Clerici M, Lucchini G, Longhese MP. Multiple pathways regulate 3′ overhang generation at S. cerevisiae telomeres. Mol Cell. 2009;35:70–81. doi: 10.1016/j.molcel.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 89.Tran PT, Erdeniz N, Symington LS, Liskay RM. EXO1-A multi-tasking eukaryotic nuclease. DNA Repair (Amst) 2004;3:1549–1559. doi: 10.1016/j.dnarep.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 90.Dejardin J, Kingston RE. Purification of proteins associated with specific genomic Loci. Cell. 2009;136:175–186. doi: 10.1016/j.cell.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Machwe A, Xiao L, Orren DK. TRF2 recruits the Werner syndrome (WRN) exonuclease for processing of telomeric DNA. Oncogene. 2004;23:149–156. doi: 10.1038/sj.onc.1206906. [DOI] [PubMed] [Google Scholar]
- 92.Opresko PL, von Kobbe C, Laine JP, Harrigan J, Hickson ID, Bohr VA. Telomere-binding protein TRF2 binds to and stimulates the Werner and Bloom syndrome helicases. J Biol Chem. 2002;277:41110–41119. doi: 10.1074/jbc.M205396200. [DOI] [PubMed] [Google Scholar]
- 93.Opresko PL, Mason PA, Podell ER, Lei M, Hickson ID, Cech TR, Bohr VA. POT1 stimulates RecQ helicases WRN and BLM to unwind telomeric DNA substrates. J Biol Chem. 2005;280:32069–32080. doi: 10.1074/jbc.M505211200. [DOI] [PubMed] [Google Scholar]
- 94.Lebel M, Spillare EA, Harris CC, Leder P. The Werner syndrome gene product co-purifies with the DNA replication complex and interacts with PCNA and topoisomerase I. J Biol Chem. 1999;274:37795–37799. doi: 10.1074/jbc.274.53.37795. [DOI] [PubMed] [Google Scholar]
- 95.Taylor TJ, Knipe DM. Proteomics of herpes simplex virus replication compartments: association of cellular DNA replication, repair, recombination, and chromatin remodeling proteins with ICP8. J Virol. 2004;78:5856–5866. doi: 10.1128/JVI.78.11.5856-5866.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.AuCoin DP, Colletti KS, Xu Y, Cei SA, Pari GS. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) contains two functional lytic origins of DNA replication. J Virol. 2002;76:7890–7896. doi: 10.1128/JVI.76.15.7890-7896.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin CL, Li H, Wang Y, Zhu FX, Kudchodkar S, Yuan Y. Kaposi's sarcoma-associated herpesvirus lytic origin (ori-Lyt)-dependent DNA replication: identification of the ori-Lyt and association of K8 bZip protein with the origin. J Virol. 2003;77:5578–5588. doi: 10.1128/JVI.77.10.5578-5588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y, Li H, Tang Q, Maul GG, Yuan Y. Kaposi's sarcoma-associated herpesvirus ori-Lyt-dependent DNA replication: involvement of host cellular factors. J Virol. 2008;82:2867–2882. doi: 10.1128/JVI.01319-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang P, Rennekamp AJ, Yuan Y, Lieberman PM. Topoisomerase I and RECQL1 function in Epstein-Barr Virus lytic reactivation. J Virol. 2009;83:8090–8098. doi: 10.1128/JVI.02379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sangrithi MN, Bernal JA, Madine M, Philpott A, Lee J, Dunphy WG, Venkitaraman AR. Initiation of DNA replication requires the RECQL4 protein mutated in Rothmund-Thomson syndrome. Cell. 2005;121:887–898. doi: 10.1016/j.cell.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 101.Sidorova JM, Li N, Folch A, Monnat RJ., Jr The RecQ helicase WRN is required for normal replication fork progression after DNA damage or replication fork arrest. Cell Cycle. 2008;7:796–807. doi: 10.4161/cc.7.6.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Campisi J, dda di FF. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 103.Cheok CF, Wu L, Garcia PL, Janscak P, Hickson ID. The Bloom's syndrome helicase promotes the annealing of complementary single-stranded DNA. Nucleic Acids Res. 2005;33:3932–3941. doi: 10.1093/nar/gki712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Machwe A, Xiao L, Groden J, Matson SW, Orren DK. RecQ family members combine strand pairing and unwinding activities to catalyze strand exchange. J Biol Chem. 2005;280:23397–23407. doi: 10.1074/jbc.M414130200. [DOI] [PubMed] [Google Scholar]
- 105.Macris MA, Krejci L, Bussen W, Shimamoto A, Sung P. Biochemical characterization of the RECQ4 protein, mutated in Rothmund-Thomson syndrome. DNA Repair (Amst) 2006;5:172–180. doi: 10.1016/j.dnarep.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 106.Muftuoglu M, Kulikowicz T, Beck G, Lee JW, Piotrowski J, Bohr VA. Intrinsic ssDNA annealing activity in the C-terminal region of WRN. Biochemistry. 2008;47:10247–10254. doi: 10.1021/bi800807n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garcia PL, Liu Y, Jiricny J, West SC, Janscak P. Human RECQ5beta, a protein with DNA helicase and strand-annealing activities in a single polypeptide. EMBO J. 2004;23:2882–2891. doi: 10.1038/sj.emboj.7600301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Masuda-Sasa T, Polaczek P, Campbell JL. Single strand annealing and ATP-independent strand exchange activities of yeast and human DNA2: possible role in Okazaki fragment maturation. J Biol Chem. 2006;281:38555–38564. doi: 10.1074/jbc.M604925200. [DOI] [PubMed] [Google Scholar]
- 109.Muftuoglu M, Sharma S, Thorslund T, Stevnsner T, Soerensen MM, Brosh RM, Jr, Bohr VA. Cockayne syndrome group B protein has novel strand annealing and exchange activities. Nucleic Acids Res. 2006;34:295–304. doi: 10.1093/nar/gkj410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gu Y, Masuda Y, Kamiya K. Biochemical analysis of human PIF1 helicase and functions of its N-terminal domain. Nucleic Acids Res. 2008;36:6295–6308. doi: 10.1093/nar/gkn609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yusufzai T, Kadonaga JT. HARP is an ATP-driven annealing helicase. Science. 2008;322:748–750. doi: 10.1126/science.1161233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weinert BT, Rio DC. DNA strand displacement, strand annealing and strand swapping by the Drosophila Bloom's syndrome helicase. Nucleic Acids Res. 2007;35:1367–1376. doi: 10.1093/nar/gkl831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nelson SW, Benkovic SJ. The T4 phage UvsW protein contains both DNA unwinding and strand annealing activities. J Biol Chem. 2007;282:407–416. doi: 10.1074/jbc.M608153200. [DOI] [PubMed] [Google Scholar]
- 114.Li Z, Lu S, Hou G, Ma X, Sheng D, Ni J, Shen Y. Hjm/Hel308A DNA helicase from Sulfolobus tokodaii promotes replication fork regression and interacts with Hjc endonuclease in vitro. J Bacteriol. 2008;190:3006–3017. doi: 10.1128/JB.01662-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.De FM, Aria V, Esposito L, De FM, Pucci B, Rossi M, Pisani FM. A novel DNA helicase with strand-annealing activity from the crenarchaeon Sulfolobus solfataricus. Biochem J. 2007;408:87–95. doi: 10.1042/BJ20070134. [DOI] [PMC free article] [PubMed] [Google Scholar]