Abstract
Multiple sclerosis (MS) is an inflammatory demyelinating disorder of the central nervous system (CNS). Recent studies suggest diverse mechanisms underlying demyelination, including a subset of lesions involving interplay between metabolic insult to oligodendrocytes and inflammatory mediators. For mice of susceptible strains, cuprizone feeding results in oligodendrocyte cell loss and demyelination of the corpus callosum. Remyelination ensues, and has been extensively studied. Cuprizone-induced demyelination remains incompletely characterized. Here we show that mice lacking type 2 CXC chemokine receptor (CXCR2) are relatively resistant to cuprizone-induced demyelination, and CXCR2+ neutrophils from the circulation play an essential role in cuprizone-induced demyelination. Findings support a novel two-hit process of cuprizone-induced demyelination, mirroring proposals about pathogenesis of MS lesions featuring extensive oligodendrocyte cell loss. These data indicate that cuprizone-induced demyelination will provide a useful model for certain aspects of MS pathogenesis.
INTRODUCTION
Multiple sclerosis (MS) is characterized pathologically by inflammation, demyelination, gliosis and axonal injury. Importantly, lesions of MS vary widely in the fate of oligodendrocytes, exhibiting a spectrum from widespread oligodendrocyte cell death to preservation of most oligodendrocytes 1. Based in part on these findings, actively-demyelinating MS lesions have been tentatively separated into three patterns, comprising the vast majority of analyzed biopsy material 1, 2. Patterns I and II have been proposed to be driven mainly by inflammatory processes with relatively low levels of oligodendrocyte cell loss, and can be effectively modeled by experimental autoimmune encephalomyelitis (EAE) 3. By contrast, pattern III lesions are suggested to occur when oligodendrocytes are subjected to environmental factors (viral or chemical) which produce metabolic stress. Oligodendrocyte cell loss is proposed to occur after additional pathological processes involving inflammation 1, 2. Although there is ongoing discussion whether these lesion patterns represent discrete subtypes of MS or temporal evolution of the disease, investigators agree that neuropathologically distinct actively-demyelinating MS lesions can be observed, 4. Animal models of pattern III lesions have not been validated 5.
Pattern III lesions of MS might involve ‘dying back gliopathy’, as the distal elements of oligodendrocyte processes show the earliest signs of degeneration 6. Feeding mice the copper chelator cuprizone (bis(cyclohexylidenehydrazide)) inhibits mitochondrial function 7 and causes CNS demyelination 8. More than a quarter-century ago, Ludwin 9 described dying-back gliopathy in cuprizone-induced demyelination. Lesions of cuprizone-induced demyelination show additional similarities with pattern III lesions of MS, including indistinct lesion borders and abundant accumulation of lesional microglia, but only a sparse hematogenous leukocyte response 2, 10, 11.
Early findings using the cuprizone model suggested a direct cell-autonomous toxicity to oligodendrocytes 7. This hypothesis is convincingly refuted by three lines of experimentation: First, cuprizone exposure to primary oligodendrocytes in vitro causes metabolic stress but not cell death, unless cultures are supplemented with inflammatory cytokines 12, 13. Second, MBP-IFN-γ-tg mice are relatively resistant to cuprizone-induced demyelination, although oligodendrocytes demonstrate metabolic stress in the form of reduced myelin protein mRNAs 14. Third, B6 mice lacking the neuronal nitric oxide synthase (nNOS; NOS-I) are relatively resistant to cuprizone-induced demyelination 15. Together these findings suggest that oxidative/nitrative stress causes mitochondrial impairment and nNOS plays a role in cuprizone-induced demyelination. Experimental studies using gene-targeted mice also demonstrate roles for inflammatory mediators in the kinetics of cuprizone-induced demyelination or myelin repair (16, 17 and references therein).
CXCR2 has been implicated in inflammation, oligodendroglial biology and myelin disorders. 18. In the developing spinal cord, CXCR2 is required for accurate positioning and timely proliferation of oligodendrocyte progenitor cells (OPCs) 19, 20. Mice lacking CXCR2 are relatively resistant to EAE, although T cells from Cxcr2−/− mice show vigorous myelin-specific recall responses and EAE susceptibility is recovered by transfer of CXCR2+ neutrophils 21. BALB/c strain Cxcr2−/− and Cxcr2+/+ mice are equally susceptible to cuprizone, but mice in this study show only very modest demyelination, reflecting the known cuprizone-resistance of BALB/c mice 22. This report also attempted immunolocalization of CXCR2, but the antibodies were found subsequently to be non-specific 23.
We placed the Cxcr2−/− targeted allele on the cuprizone-susceptible B6 background, to evaluate the role of CXCR2 in toxin-induced inflammatory demyelination, caused either by cuprizone feeding or by LPC injection. Unexpectedly, we found that demyelination in the cuprizone model was contingent upon CXCR2+ neutrophils, suggesting a two-step mechanism for oligodendrocyte cell loss: (1) cuprizone-induced inhibition of mitochondrial function in oligodendrocytes, along with nitrative stress associated with nNOS activity in CNS-resident cells, induce metabolic stress and vulnerability to apoptotic cell death; followed by (2) a ‘second hit’ which requires functions delivered by CXCR2+ blood-derived neutrophils. Our findings extend the utility of cuprizone-induced demyelination as a productive model for pattern III lesions of MS.
RESULTS
Resistance of Cxcr2−/−→Cxcr2+/+ mice to cuprizone-induced demyelination
We compared the effects of cuprizone feeding in Cxcr2+/+ and Cxcr2−/− mice. On the B6 background, Cxcr2+/+ mice showed robust demyelination following cuprizone feeding, enabling us readily to discern differences between Cxcr2+/+ and Cxcr2−/− mice. Cxcr2−/− mice were resistant to cuprizone-induced demyelination as compared with Cxcr2+/+ mice after 6 weeks (Fig. 1a; 1c). Toluidine blue staining (Supplementary Fig. 1) and EM studies were done after 4 weeks (Fig. 1b; 1d) to evaluate early active demyelination and confirmed striking differences between Cxcr2+/+ and Cxcr2−/− mice.
Figure 1.
Cxcr2−/− mice are relatively resistant to cuprizone-induced demyelination. Cxcr2−/− or Cxcr2+/+ mice received cuprizone for 1, 2, 3, 4 or 6 weeks before evaluation of demyelination in matched serial sections. (a) Black-gold staining showed robust demyelination at Cxcr2+/+ mice (a, upper panels), but not Cxcr2−/− (a, lower panels) following 3 weeks and 6 weeks of cuprizone feeding. (b) EM studies showed demyelination in corpus callosum of Cxcr2+/+ mice, but not Cxcr2−/− at 4 weeks of cuprizone feeding. (c) Quantification of percent demyelinated area in corpus callosum at 6 weeks of cuprizone feeding for (a). (d) Quantification of myelinated axons in corpus callosum at 4 weeks of cuprizone feeding for (b). **, P<0.01, comparing Cxcr2−/− and Cxcr2+/+ mice. Scale bar: (a), 250μm; (b), 2μm
Reduced myelin protein mRNA but limited apoptosis in Cxcr2−/− mice
Cuprizone-induced demyelination follows a stereotyped time course in B6 mice: initial changes are observed after 1-2 weeks of cuprizone feeding, when myelin protein mRNAs are reduced by 75% 24. Oligodendrocyte cell loss is observed by 3 weeks, accompanied by first signs of demyelination. Peak accumulation of inflammatory cells comprised of microglia (~90%) and hematogenous cells (~10%) is seen at 4 weeks 11. By 6 weeks, the corpus callosum shows maximal demyelination. Remyelination is observed during 2 to 4 weeks after resuming a cuprizone-free diet 8. We evaluated whether cuprizone caused acute toxic effects and cell death for oligodendrocytes in Cxcr2+/+ and Cxcr2−/− mice. After 1 or 2 weeks of cuprizone feeding, analysis of corpus callosum for mRNA encoding the myelin proteins MBP and CNPase showed equivalent and severe reduction in both Cxcr2−/− and Cxcr2+/+ mice (Fig. 2a), demonstrating cuprizone-induced metabolic toxicity for oligodendrocytes equally in both groups of mice. However, at 3 and 4 weeks, myelin protein mRNAs showed a substantial and significant rebound in the corpus callosum of Cxcr2−/−, but not Cxcr2+/+ mice despite continuous cuprizone feeding of both groups (Fig. 2a). These results suggested that damage to oligodendrocytes in Cxcr2+/+ mice was severe and irreversible, while being transient in Cxcr2−/− mice. We tested this hypothesis with TUNEL assays, finding that apoptotic cells were significantly elevated at 3 weeks over controls only in Cxcr2+/+ mice (Fig. 2b-c). Furthermore, EM images readily demonstrated nuclei morphologically consistent with apoptotic cells in the corpus callosum of Cxcr2+/+ mice after 4 weeks of cuprizone feeding (Supplementary Fig. 2). These results correlated well with the relative resistance to demyelination in cuprizone-fed Cxcr2−/− mice.
Figure 2.
Cxcr2−/− mice exhibit transiently-reduced myelin protein mRNA after cuprizone feeding, and little apoptotic cell death. (a) Cxcr2−/− or Cxcr2+/+ mice received cuprizone for 1 to 4 weeks before analysis of mRNA encoding myelin basic protein (MBP) and 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase), normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Real time PCR data showed equivalent and severe reduction in both Cxcr2−/− and Cxcr2+/+ mice after 1 or 2 weeks of cuprizone feeding. However, at 3 and 4 weeks of cuprizone feeding, myelin protein mRNAs showed a substantial and significant rebound in the corpus callosum of Cxcr2−/− but not Cxcr2+/+ mice. (b) TUNEL staining showed apoptotic cells were significantly elevated in Cxcr2+/+ mice at 3weeks of cuprizone feeding. DAPI counterstaining showed the nuclei of TUNEL positive cells. (c) Quantification of TUNEL positive cells in corpus callosum through 1 to 3 weeks of cuprizone-fed mice. *, P<0.05; **, P<0.01, comparing Cxcr2−/− and Cxcr2+/+ mice. NS, no significant difference. Scale bar: (b), 25μm.
Responses of the oligodendrocyte lineage to cuprizone
We recently found that CXCR2 antagonists accelerate myelin repair 25. To address the possibility that cuprizone-fed Cxcr2−/− mice underwent rapid demyelination and myelin repair, animals were evaluated with black-gold myelin staining at 1, 2, 3 and 6 weeks of cuprizone feeding (Fig. 1a; 1c) without finding evidence of myelin damage or repair in Cxcr2−/− mice. Supporting this interpretation, the G-ratios of remaining myelinated fibers in the corpus callosum of Cxcr2+/+ and Cxcr2−/− mice after 4 weeks of cuprizone feeding were not different, and were equal to those of control animals (Supplementary Fig. 3), indicating that remyelination had not extensively occurred in either Cxcr2+/+ or Cxcr2−/− mice at the 4-week time point. These findings were extended by analysis of the oligodendrocyte lineage during the early phase of cuprizone feeding. As previously reported by others 8, we found that 3 weeks of cuprizone feeding caused severe (>75%) loss of GST-π+ cells in the affected corpus callosum of Cxcr2+/+ mice (Fig. 3a; 3d), while Cxcr2−/− mice showed only moderate (<50%) loss of GST-π cells at the same time point (Fig. 3a; 3d). By contrast at 2 weeks, both groups of mice showed modest and equivalent loss of GST-π+ cells. These findings correlated with the time course of appearance of apoptotic cells in the corpus callosum and suggested loss of GST-π+ oligodendrocytes through apoptosis (Fig. 2b; 2c) and possibly through diminished expression of the GST-π marker. We evaluated PDGFRα+ cells, which represented oligodendrocyte progenitor cells (OPCs), at weeks 1-3 and week 6, to determine whether cuprizone equally affected all cells of the lineage. Cxcr2+/+ and Cxcr2−/− mice showed equivalent initial numbers of PDGFRα+ cells, which were significantly and equally reduced at 1 week and 2 weeks of cuprizone feeding, but rose remarkably by more than 300% only in Cxcr2+/+ mice after 6 weeks of cuprizone feeding (Fig. 3c; 3f). Analysis of olig2+ cells (representing both mature oligodendrocytes and OPCs) reflected the summation of these changes in GST-π+ and PDGFRα+ cells (Fig.3b; 3e). We concluded that cuprizone caused widespread apoptotic death of mature oligodendrocytes, in the corpus callosum of Cxcr2+/+ but not Cxcr2−/− mice. The remarkable proliferative reaction of PDGFRα+ cells in the corpus callosum of Cxcr2+/+ mice at 3 weeks and 6 weeks of cuprizone feeding (Fig. 3c; 3f) is proposed to reflect response to demyelination, which was robust only in Cxcr2+/+ mice (Fig. 3c; 3f). Interestingly, there was no change in the number or appearance of PDGFRα+ OPCs in the adjacent cortex across the time course of cuprizone feeding in either Cxcr2+/+ or Cxcr2−/− mice (Supplementary Fig. 4). This observation provided an internal technical control for the immunostaining and suggested that OPCs involved in the reparative tissue reaction to cuprizone-induced demyelination in B6 mice might be derived from sources other than tissue adjacent to the corpus callosum.
Figure 3.
Differential response of cells of the oligodendrocyte lineage cells to cuprizone feeding in Cxcr2+/+ and Cxcr2−/− mice. Matched serial sections mice after 1 to 3 weeks or 6 weeks of cuprizone feeding were analyzed with GST-π antibodies (a), olig2 antibodies (b), or PDGFRα antibodies (c). Quantification of GST-π+ (d), olig2+ (e), and PDGFRα+ (f) cells in corpus callosum. *, P<0.05; **, P<0.01, comparing Cxcr2−/− and Cxcr2+/+ mice. Scale bar: (a), 25μm; (b), 50μm; (c), 250μm. Arrowheads: representative quantified cells.
Equal cuprizone-induced demyelination in chimeric mice
Both Cxcr2−/− and Cxcr2+/+ mice demonstrated transient toxic effects of cuprizone feeding but only Cxcr2+/+ oligodendrocytes underwent sufficient cell death to cause robust demyelination (Fig. 1, 2), and we proposed that this result suggested a ‘two-hit’ model for cuprizone-induced demyelination. We addressed the corollary hypothesis that myeloid CXCR2+ cells might be required for demyelination in this model. Initially, we examined the possibility that CNS microglia (derived from the myeloid lineage 26) might express CXCR2, as recently suggested based on immunohistochemistry 22. Using flow cytometry, we confirmed that the commercial antibodies used in this prior study were non-specific for CXCR2 as noted by the authors 23. In particular, commercial antibodies failed to stain CXCR2+ Gr1+ myeloid cells in flow cytometry assays (Supplementary Fig. 5), but equally stained brain and splenic tissue sections from Cxcr2+/+ and Cxcr2−/− mice (data not shown). We used validated polyclonal monospecific antibodies 21 to show that CD45dim microglia do not express CXCR2.(Supplementary Fig. 6).
The responses of Cxcr2+/+ and Cxcr2−/− mice to cuprizone began to diverge markedly at 3 weeks of feeding (Figs. 1-3), corresponding to the onset of substantial cellular inflammation including microglial proliferation and infiltration of CD11b+ myeloid hematogenous cells 11. Given lack of CXCR2 expression by microglia, we focused attention on CXCR2+ myeloid circulating cells. Radiation bone marrow chimeras were generated to address whether the presence of CXCR2 on hematogenous myeloid cells played a role in cuprizone-induced demyelination. We used Cxcr2+/− mice as donors for Cxcr2+/+ or Cxcr2−/− mice, since our colony was maintained using heterozygous breeders. This approach was conservative of animals, and also enabled direct comparison of littermates in experiments. Chimerism was analyzed 6 weeks after bone marrow transfer, by demonstrating heterozygous genotype in blood cells (not shown). Cxcr2+/+ mice exhibited a population of circulating Gr1+/CXCR2+ cells in the myeloid cell gate (Supplementary Fig. 7). In Cxcr2−/− mice, the myeloid cell gate contained an expanded Gr1+/CXCR2− population (Supplementary Fig. 7) as previously shown by others 27. Both Cxcr2+/−→Cxcr2+/+ and Cxcr2+/−→Cxcr2−/− chimeric mice showed similar Gr1+/CXCR2+ myeloid cell populations, when evaluated 6 weeks after chimerization (Supplementary Fig. 7). These results demonstrated recovery of the myeloid compartment in Cxcr2+/−→Cxcr2+/+ and Cxcr2+/−→Cxcr2−/− mice after bone marrow transfer and also showed that CXCR2+ cells in the circulation were virtually all Gr1+ myeloid cells.
Given the complexity of radiation chimerism as an experimental model 28, several control groups were evaluated. Findings of equal demyelination in Cxcr2+/+ and Cxcr2+/+→Cxcr2+/+ mice indicated that radiation chimerism did not modulate cuprizone-induced demyelination (Fig. 4a-b), consistent with a prior 29. An effect of haploinsufficiency at CXCR2 was excluded by the observation that Cxcr2+/− and Cxcr2+/+ mice developed an equal extent of demyelination (Fig. 4a-b).
Figure 4.
Cxcr2+/−→Cxcr2−/− chimeric mice exhibit equal cuprizone-induced demyelination to Cxcr2+/−→Cxcr2+/+ mice. Radiation bone marrow chimeras, Cxcr2+/+, +/− and −/− mice were fed cuprizone and analyzed for demyelination of the corpus callosum. Panels in (a) are representative of corresponding groups shown in (b). Effects of chimerization were evaluated by comparing Cxcr2+/+ to Cxcr2+/+→Cxcr2+/+ mice. Effects of haploinsufficiency on the response to cuprizone were assessed by comparing Cxcr2+/+ and Cxcr2+/− mice (b), and by comparing Cxcr2+/+→Cxcr2+/+ and Cxcr2+/−→Cxcr2+/+ mice (b). Two separate cohorts of Cxcr2+/−→Cxcr2+/+ mice were prepared and analyzed (b). Cxcr2+/−→Cxcr2−/− mice are susceptible to cuprizone-induced demyelination: confirmation using EM. Cxcr2+/−→Cxcr2+/+ or Cxcr2+/−→Cxcr2−/− mice were fed cuprizone for 4 weeks, before analysis of the rostral corpus callosum by EM (c), and quantification of myelinated axons and G-rations in the corpus callosum (d, e). NS: not significant. Scale bar: (a), 250μM; (c), 2μm.
After 6 weeks of cuprizone feeding, Cxcr2+/−→Cxcr2−/− chimeric mice exhibited an equivalent extent of cuprizone-induced demyelination to Cxcr2+/−→Cxcr2+/+ mice (Fig. 4a-b), suggesting that replacement of the CXCR2+ myeloid cells compartment had reversed resistance to cuprizone-induced demyelination. Observations using black-gold staining were confirmed through EM analysis (Fig. 4c-d). Cxcr2+/−→Cxcr2+/+ chimeric mice were generated and studied both in comparison to Cxcr2+/−→Cxcr2−/− chimeric mice and in comparison to Cxcr2+/+ mice, with reproducible results (Fig. 4a-b). We found equal G-ratios in Cxcr2+/+ and Cxcr2−/− mice (Supplementary Fig. 3) as well as in residual myelinated axons of Cxcr2+/−→Cxcr2+/+ and Cxcr2+/− Cxcr2−/− mice after 4 weeks of cuprizone feeding (Fig. 4e). These observations indicated that extent of myelin loss at 4 weeks solely represented demyelination, as there was no evidence for substantial remyelination in the chimeras after 4 weeks of cuprizone feeding. The most straightforward interpretation of our data was that functions expressed by CXCR2+ myeloid cells were required for oligodendrocyte cell loss and demyelination, after cuprizone challenge.
Resistance of Cxcr2−/−→Cxcr2+/+ mice to cuprizone-induced demyelination
Our findings raised the question whether absence of CXCR2 on myeloid cells was sufficient to render mice resistant to cuprizone-induced demyelination. This possibility was tested by construction and analysis of Cxcr2−/−→Cxcr2+/+ mice in comparison with Cxcr2+/+→Cxcr2+/+ mice in the cuprizone-feeding model of demyelination. Cxcr2−/−→Cxcr2+/+ mice were resistant to cuprizone-induced demyelination (Fig. 5a; 5c, upper panel), by comparison with Cxcr2+/+→Cxcr2+/+ mice. Further, similar to Cxcr2−/− mice, Cxcr2−/−→Cxcr2+/+ mice demonstrated only a moderate loss of oligodendrocytes (Fig. 5b; 5c, middle panel). Quantitative analysis showed significantly more GST-π+ oligodendrocytes in the corpus callosum of Cxcr2−/−→Cxcr2+/+ mice than in that of Cxcr2+/+→Cxcr2+/+ mice after 4 weeks of cuprizone feeding (Fig. 5c, middle panel; P<0.001). Additionally, in Cxcr2−/−→Cxcr2+/+ mice, the secondary inflammatory reactions to cuprizone-induced oligodendrocyte cell loss were also limited (Fig. 5b; 5c, lower panel). In summary, Cxcr2−/−→Cxcr2+/+ mice recapitulated the phenotype of Cxcr2−/− mice in cuprizone-induced demyelination. Taken together, results from studying radiation chimeras showed that CXCR2+ peripheral blood cells were essential for cuprizone-induced demyelination.
Figure 5.
Cxcr2−/−→Cxcr2+/+ mice are resistant to cuprizone-induced demyelination, and there is minimal reduction of GST-π+ cells or inflammatory reaction in corpus callosum of cuprizone-fed Cxcr2−/−→Cxcr2+/+ mice. Cxcr2−/−→Cxcr2+/+ or Cxcr2+/+→Cxcr2+/+ mice (generated and studied separately from those shown in Fig. 4) were fed cuprizone for 4 weeks and analyzed for demyelination (a; c, upper panel) or mature oligodendrocytes (b, upper panels; c, middle panel) or infiltrating leukocytes and reactive microglia (b, lower panels; c, lower panel). Arrowheads: mature oligodendrocytes. Arrows: CD45+ leukocytes. **, P<0.01. Scale bar: (a), 250μm; (b), 25μm.
For experiments shown in Fig. 5, cuprizone feeding was terminated after 4 weeks, as Cxcr2−/−→Cxcr2+/+ mice showed relatively high mortality during the chimerization protocol, and exhibited reduced body weight during cuprizone feeding. Nevertheless, on a weight/weight basis, Cxcr2−/−→Cxcr2+/+ mice consumed an equivalent amount of cuprizone-supplemented chow as did Cxcr2+/+→Cxcr2+/+ mice. Successful Cxcr2−/−→Cxcr2+/+ chimerization was verified by flow cytometry (Supplementary Fig. 8). We propose that that weight losses in Cxcr2−/−→Cxcr2+/+ chimeric mice, which came to resemble Cxcr2−/− mice in size, were likely caused by poor host defence against gastrointestinal flora due to incompetent neutrophil migration or effector response 30.
Impaired neutrophil effector functions in Cxcr2−/− mice
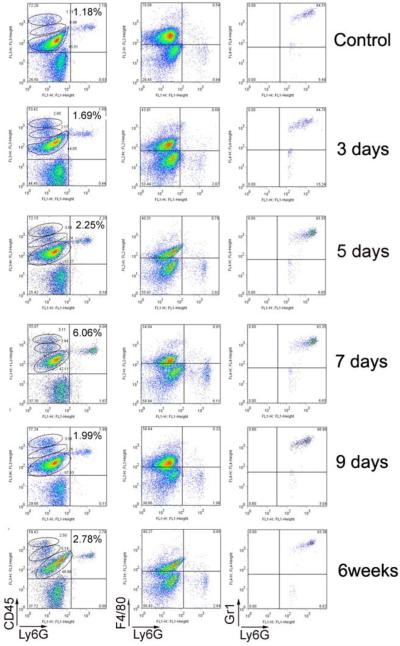
CXCR2 has been detected on various myeloid cells in vitro. We analyzed blood cells from Cxcr2+/+ mice, and showed that all Ly6G+ neutrophils were CXCR2+, and that all CXCR2+ cells were neutrophils (Fig. 6). Therefore, the biological effects of CXCR2 on myeloid cells of Cxcr2+/+ mice were restricted to neutrophils. We then performed kinetic analysis of myeloid cell entry into the corpus callosum of Cxcr2+/+ mice at days 3, 5, 7 and 9 of cuprizone feeding and at 6 weeks. CD45int/Ly6G+ neutrophils (lefthand column) increased significantly at day 7 from 1.2% (control) to 6.06% of the total population of cells isolated from the CNS. Analysis for F4/80 (macrophage marker) shown in the second column demonstrated that the Ly6G+ population was uniformly negative confirming that these cells were neutrophils, not macrophages. In the third column, the myeloid marker Gr1 (Ly6C/Ly6G) was shown to correlate with the neutrophil marker Ly6G, showing that most early infiltrating myeloid cells were neutrophils in this model (Fig. 7). We compared Cxcr2+/+ and Cxcr2−/− mice in a subsequent experiment at 5 days of cuprizone feeding, the earliest point at which Cxcr2+/+ mice showed significant entry of neutrophils into the CNS. Cxcr2−/− mice, which harbored an elevated number of neutrophils in the CNS at baseline, showed a large population of CD45int/Ly6G+/CXCR2− neutrophils in the CNS at this time point. Importantly, the fold increase of neutrophils over control (about 5-fold) and the percent of total CD45int/hi infiltrating cells represented by neutrophils at 5 days (27-30%) were equivalent in the Cxcr2+/+ and Cxcr2-/- mice (Fig. 8). We conclude that there is no recruitment deficit for neutrophils to the CNS of cuprizone-fed Cxcr2−/− mice, and that the demyelination-resistant phenotype is caused by deficient neutrophil effector responses.
Figure 6.
All Ly6G+ neutrophils are CXCR2+, and that all CXCR2+ cells are neutrophils. Gating on live cells (left), blood cells from Cxcr2+/+ mice were stained with Ly6GFITC and CXCR2PE, and analyzed by flow cytometry (right). These data represent three separate experiments, and four mice each experiment.
Figure 7.
Neutrophils infiltrate into the corpus callosum at early stages of cuprizone-induced demyelination. Cells from corpus callosum of Cxcr2+/+ mice fed with cuprizone for 3 days, 5 days, 7 days and 9 days and 6 weeks were stained with Ly6GFITC, F4/80PE, CD45PerCP and Gr1APC antibodies. In comparison with control, the CD45int/Ly6G+ neutrophils increased significantly at day 7 (left column). Analysis for F4/80 (macrophage marker) demonstrated that the Ly6G+ population is uniformly negative (middle column). The myeloid marker Gr1 (Ly6C) is correlated with the neutrophil marker Ly6G (right column). Each group has two mice. These data represent three separate experiments.
Figure 8.

Neutrophils infiltrate in Cxcr2+/+ and Cxcr2−/− mice at 5 days of cuprizone feeding. Although Cxcr2−/− mice showed elevated numbers of neutrophils in the CNS at baseline (left), the fold increase of neutrophils over control (about 5-fold) and the percent of total CD45int/hi infiltrating cells represented by neutrophils at 5 days (27-30%) were equivalent in the Cxcr2+/+ and Cxcr2−/− mice (right). Each group has two mice. These data represent two separate experiments.
To establish the role for early infiltrated neutrophils in cuprizone-induced demyelination in the Cxcr2+/+ context, we depleted neutrophils using Gr1 antibodies (RB6-8C5) starting two days before cuprizone feeding. Neutrophils depletion was verified by flow cytometry and persisted for one week (Supplementary Fig. 9). At 3weeks of cuprizone feeding, neutrophil-depleted mice showed fewer apoptotic cells in corpus callosum than mice injected with control antibodies (Supplementary Fig. 9). These data demonstrated that the presence of in the CNS as well as effector functions regulated by CXCR2 were ensential for cuprizone-induced demyelination.
Similar demyelination in LPC injected spinal cord
Cxcr2−/− mice are resistant both to EAE21 and cuprizone-induced demyelination (Fig. 1) raising the question whether deficiency of CXCR2 precluded detection or development of demyelinating CNS lesions. Injection of LPC into the dorsal columns of the thoracic spinal cord causes demyelination, with fully developed lesions at 2 days post-injection 31. LPC injections to Cxcr2+/+ or Cxcr2−/− mice caused an equivalent demyelinating lesion after 1, 2 and 3 days (Supplementary Fig. 10). This result showed that LPS-induced demyelination could be readily observed without CXCR2 signaling.
DISCUSSION
Here we report that Cxcr2−/− mice are relatively resistant to cuprizone-induced demyelination. This requirement for CXCR2 for toxin-induced demyelination was selective for the cuprizone model, as Cxcr2−/− mice were susceptible to LPC-mediated demyelination. Results from studying bone marrow chimeras showed that CXCR2+ neutrophils from the blood stream were necessary for cuprizone-induced demyelination, as Cxcr2−/−→Cxcr2+/+ mice were equally as refractory to demyelination as were Cxcr2−/− mice. Further, CXCR2+ neutrophils were sufficient to confer full susceptibility to demyelination in Cxcr2+/−→Cxcr2−/− chimeras. Unexpectedly we found that a brief burst of peripheral leukocyte infiltration of the corpus callosum at 5-7 days of cuprizone feeding which is characterized by CD45int/Ly6G+/Gr1+/CXCR2+ neutrophils whose effector functions are required for cuprizone-induced demyelination. These results indicated that CXCR2 played non-redundant functions, not compensated by CXCR1, for cuprizone-induced demyelination. As one corollary, it seems likely that CXCR2 ligands (rather than dual CXCR1/CXCR2 ligands) will be associated with this pathology.
It was important to exclude the possibility that Cxcr2−/− mice underwent accelerated demyelination/remyelination in response to cuprizone feeding. We provided several lines of evidence to address this prospect: first, histochemical staining across the time course of cuprizone feeding failed to disclose evidence of early demyelination in Cxcr2−/− mice; second, EM analysis at 4 weeks showed no evidence of extensive remyelination in Cxcr2−/− mice, as G-ratios of myelinated fibers were equal to those seen in control animals; third, evaluation by TUNEL at 1-3 weeks documented a virtual absence of apoptotic cells in the corpus callosum of Cxcr2−/− mice; fourth, analysis of the oligodendrocyte lineage demonstrated significantly greater loss of GST-π+ cells (at 3 weeks) and much more proliferation of PDGFRα+ cells (at 3 and 6 weeks) in Cxcr2+/+ mice during cuprizone feeding.
These studies delineated the complexity of the response of the oligodendroglial lineage to cuprizone, showing that GST-π+ mature oligodendrocytes were extensively lost at 3 weeks through apoptosis, and that in situ proliferation of PDGFRα+ OPCs was the likely source of oligodendrocyte renewal. These findings can be extended by identifying the source of callosal OPCs which proliferate after demyelination. Our preliminary results argue that OPCs are derived from the progeny of neuronal progenitor cells in the subependymal zone (LL, LD, RMR, unpublished observations).
As noted above, we used bone marrow chimeras to demonstrate that CXCR2+ neutrophils were both necessary and sufficient for cuprizone-induced demyelination. Antibody-mediated depletion studies complemented these observations (Supplementary Fig. 9). Given these findings and the lack of CXCR2 on microglial cells (Supplementary Fig. 6), we concluded that it was unlikely that action of CXCR2 towards resident CNS cells caused cuprizone resistance in Cxcr2−/− mice. Our studies represent the first demonstration that peripheral circulating cells are essential for cuprizone-induced demyelination.
Mice lacking nNOS show a resistance to cuprizone-induced demyelination, accompanied by preservation of oligodendrocyte numbers, reduced oligodendrocyte apoptosis and minimal tissue reaction 15. The requirement for nNOS activity for cuprizone-induced demyelination is caused by its function within the CNS, as murine nNOS is expressed selectively in the nervous system (http://www.ncbi.nlm.nih.gov/UniGene/ESTProfileViewer.cgi?uglist=Mm.44249), and nNOS−/− mice do not exhibit abnormalities in immunity or inflammation. Further, in contrast to Cxcr2−/− mice, cuprizone-exposed nNOS−/− mice show only very modest reduction in abundance of myelin protein mRNAs in the corpus callosum. We interpret these results as indicating that CNS nNOS activity is required for inducing metabolic stress of oligodendrocytes during the early phases of cuprizone-induced demyelination.
The cuprizone-resistant phenotype of Cxcr2−/− mice was most reminiscent to that exhibited by MBP-IFN-γ-tg mice which over-express modest amounts of IFN-γ in the CNS, under control of the MBP promoter 14. Both Cxcr2−/− and MBP-IFN-γ–tg mice demonstrate preservation of oligodendrocytes and lack of tissue reaction after cuprizone-feeding. Early reduction of myelin protein mRNAs occurred equally in wildtype mice destined to develop demyelination and in MBP-IFN-γ-tg mice or Cxcr2−/− mice which were resistant. Myelin protein mRNAs returned to normal levels at 3weeks and 4weeks of cuprizone feeding in Cxcr2−/− but not MBP-IFN-γ-tg mice, possibly because IFN-γ also delays remyelination by inducing endoplasmic reticulum stress 32. These authors. 14 suggested that elevated levels of insulin-like growth factor(IGF)-1 might account for the resistance of MBP-IFN-γ-tg mice to cuprizone-induced demyelination. However, over-expression of IGF-1 in transgenic mice does not preclude demyelination, making this explanation less plausible 33.
Another IFN-γ-mediated effect may help to explain resistance to cuprizone in transgenic mice 34. Specifically, in both viral and autoimmune models, IFN-γ plays a critical role in neuroinflammation by restraining the production of CXCR2 ligands such as CXCL1 and CXCL2 thereby limiting invasion of the CNS by neutrophils 35-38. It is tempting to speculate that MBP-IFN-γ-tg mice resist cuprizone-induced demyelination, and resemble Cxcr2−/− mice in this regard, at least in part because production of CXCR2 ligands is blocked by IFN-γ. We don’t address here which CXCR2 ligands are implicated in cuprizone-induced demyelination, nor their cellular source. By analogy with pathologies of other models such as EAE and viral encephalitis, it seems most likely that local reactive astrocytes produce CXCL1 and CXCL2 during cuprizone-induced demyelination. Previous microarray studies provided support for this hypothesis24.
Our findings suggest a reconsideration of the interaction of neurotoxic and inflammatory mechanisms in the cuprizone-feeding model of demyelination. As a mitochondrial toxin, cuprizone provides a model for mitochondrial impairment in MS pathogenesis 39 and for compromised mitochondrial function in pattern III lesions in particular 40. We propose that, in both cuprizone-induced demyelination and pattern III MS lesions, mitochondrial impairment increases reactive oxygen species (ROS) generation and renders oligodendrocytes vulnerable to apoptosis. We hypothesize that neuronal nNOS activity leads to additional oxidative/nitrative stress. Finally, CXCR2+ neutrophils from the circulation also produce ROS. The interaction of mitochondrial impairment and oxidative/nitrative stress is widely studied in neurodegenerative diseases 41, and is considered likely to account in part for axonal pathology in MS 42. Our findings make it likely that this pathway also leads to demyelination in selected settings.
In cuprizone-induced demyelination, lesion formation follows loss of oligodendrocytes. The requirement for CXCR2+ neutrophils to produce both oligodendrocyte cell loss and demyelination confirms in vivo that direct cuprizone toxicity is necessary but not sufficient for this pathological process. In cuprizone-induced demyelination, the ‘second hit’ required for oligodendrocyte loss is contingent on CXCR2+ neutrophils. In CXCR2−/− mice, the neutrophil compartment is abnormally expanded and distributed aberrantly throughout tissues including the CNS. Signaling to CXCR2 governs a wide array of neutrophil effector functions including gene expression and degranulation. Therefore, we consider it likely that CXCR2 affects cuprizone-induced demyelination by promoting the effector functions of infiltrated neutrophils within the CNS.
Interestingly, recent EAE studies also showed that CXCR2+ neutrophils are required for disease pathogenesis 21. EAE is considered a useful model for dissecting immune/inflammatory mechanisms of MS lesions. The present findings establish a foundation for characterizing blood-derived CXCR2+ neutrophils implicated in cuprizone-induced demyelination and for determining either which neutrophil functions contribute to oligodendrocyte cell loss or possibly which protective functions are exerted by CXCR2−/− cells. By extension, this information should provide useful insights for limiting tissue damage in pattern III-type demyelinating diseases of the CNS.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by grants from the National MS Society (RG 3580 to RMR), the National Institutes of Health (NS32151 to RMR; NS36674 to RHM), the Myelin Repair Foundation (RHM) and the Nancy Davis Center Without Walls (RMR). We thank W. Stallcup (Burnham Institute for Medical Research, La Jolla, CA) for generously providing antibodies to PDGF receptor alpha and Dr. Grahame Kidd (Dept of Neurosciences, Lerner Research Institute, Cleveland Clinic) for assistance with imaging. We thank Glen Matsushima (UNC, Chapel Hill, NC) for helpful discussions regarding the cuprizone model. We thank Xiaoling Qu (Dept. of Stem cells, Lerner Research Institute, Cleveland Clinic) for help with TUNEL staining.
Abbreviations used
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- LPC
lysophosphatidylcholine
- MS
multiple sclerosis
- NOS
nitric oxide synthase
- OPC
oligodendrocyte progenitor cell
- PDGF
platelet-derived growth factor
Appendix
METHODS
Mice
The Cxcr2 deficient allele was backcrossed to C57BL/6 for 11 generations, and Cxcr2−/− mice were obtained from intercrossing Cxcr2 heterozygous mice. Genotyping was performed as described 19. Experiments were performed according to the Cleveland Clinic Animal Care and Use Committee guidelines.
Radiation bone marrow chimeras
Radiation bone marrow chimeras were generated as described 43 and reconstitution was analyzed after 6 weeks by flow cytometry and PCR genotyping. Cxcr2−/−→Cxcr2−/− were generated with difficulty and did not survive cuprizone feeding.
Cuprizone treatment
Eight-to-ten week old mice were fed cuprizone (0.2%,w/w, TD.06172; Harlan, WI) to induce demyelination 44.
Myelin staining and quantification
Black-gold staining was performed according to a protocol adapted from the manufacturer (Chemicon) with modification. Briefly, 2 to 3 free floating sections were stained in 2ml eppendorf tube with 300ul of 0.2% black-gold solution at 65°C water bath for 10 minutes. After staining with black-gold, brain and spinal cord sections were carefully matched and pictured by 3-CCD video camera interfaced with an Image-Pro Plus Analysis System (Version 4.1.0.0, MediaCybernetics) and analyzed with NIH Image J1.34s. Thresholding was set to black-gold staining within each gated corpus callosum or dorsal column and held constant for images obtained at equal objectives and light intensities, on slides that were processed in one session. Data represent mean demyelinated tissue areas (void of black-gold staining), expressed as percentages of total corpus callosum or dorsal column areas.
Electronic microscopy
For electron microscopy, we anesthetized and perfused at least 3 mice per time point with 1x Sorenson’s buffer followed by solution containing 4% PFA and 2.5% glutararaldehyde. Brains were sliced into 1-2 mm sections, and the sections containing the hippocampus area were trimmed, and cross-sections of the corpus callosum were obtained. Sections were postfixed in the same solution overnight at 4°C, after which sections were embedded in Araldite resin. Semithin sections were stained with 1% Toluidine blue staining at 65°C for a few seconds, washed several times with water, and then dehydrated and mounted with Permount. Thin sections were cut, stained with uranyl acetate and lead citrate, and analyzed. Using ImageJ software, G-ratios (defined as the diameter of the axon divided by the diameter of the axon and myelin) were calculated from the outer perimeter of the axon divided by the total perimeter of the axon and myelin. Ten pictures were randomly chosen for each mouse and 50-100 fibers per picture were calculated. At least 3 mice/strain/time point was analyzed. The data are shown as G-ratio and total number of myelinated fibers.
TUNEL assay
Sections were processed for the terminal deoxynucleotidyl transferase biotin-dUTP nick-end labeling (TUNEL) kit using a protocol adapted from the manufacturer (Chemicon Int., S7110) with modification. Briefly, free floating tissue sections from controls or cuprizone-treated mice were incubated with Target Retrieval Solution pH6.0 (DAKO) for 30 minutes in an 85°C water bath, then stained with TUNEL kit following the manufacturer’s manual except for the incubation with enzyme at 37°C for 2 hours. Then sections were co-labeled with DAPI (4′,6′-diamidino-2-phenylindole). TUNEL-positive cells were quantified by counting within the corpus callosum. Only those TUNEL+ cells with an observable nucleus by DAPI staining were counted. Cell counts are presented as averages from at least three mice per time point.
Histological staining and analysis
Fixed CNS (4% PFA) sections were stained with the following antibodies as described 45: CD45 (MCA1388; Serotec); PDGFRα (Dr. W. Stallcup, La Jolla, CA); PCNA (PC10, Sigma); PSA-NCAM (Chemicon); Olig2 (Chemicon); GST-π (Assay Designs).
Flow cytometry
Expression of CXCR2 on neutrophils in freshly isolated peripheral leukocytes was performed using rabbit anti-CXCR2 antibodies 21 followed by Alexa fluor ® 488 goat anti-rabbit IgG (Invitrogen) or CXCR2-PE (R&D), Gr1-PE (BD) and Ly6G-FITC (BD) as described 21, 45. Expression of CXCR2 on microglia cells in freshly isolated brain tissues 46 was performed using the same rabbit anti-CXCR2 antibodies 21, with CD45APC (BD). The combination of F4/80PE, CD45PerCP, Ly6GFITC and Gr1APC (BD) antibodies was used in kinetic analysis of myeloid cells in cuprizone-fed mice.
Real-time quantitative RT-PCR (qPCR)
After sacrifice by perfusion with PBS and dissection of the corpus callosum, RNA was isolated using Trizol reagent (Invitrogen), and 1μg RNA was analyzed by qPCR on a LightCycler 47. Primers for CNPase, MBP and GAPDH were as described 48.
Gr1 antibody depletion assay
For depletion of neutrophils in vivo, supernatant of Gr1 antibodies from cultures of hybridoma (RB6-8C5, 200μg/mouse) were used. Mice were injected with either Gr1 antibodies or rat IgG (Jackson ImmunoResearch) every other day for 1week beginning two days before feeding with cuprizone. The complete depletion of neutrophils was analyzed by flow cytometry two days after injection. At 3weeks after cuprizone feeding, mice were sacrified for TUNEL staining.
LPC microinjection
LPC-induced demyelination of the spinal cord was performed as described 49, with local modification to adapt to mice, which were anesthetized with 5 mL/kg of 0.2 mL Ketamine (Fort Dodge, IA)/0.1 mL Xylazine (Lloyd Laboratories, IA)/0.7 mL of distilled water. After shaving and cleaning with ethanol and Betadine, a T10 laminectomy was performed in a stereotactic apparatus (Stoelting, Wood Dale, IL). Using a 30 mm glass-micropipette attached to a Nano-injector (World Precision Instruments), 1.5 μL of 1% freshly-prepared LPC (Sigma) solution was infused at 15 mL/hour. The needle was kept in place for 5 min after, to prevent reflux; skin was closed and treated with Betagen (Med-Pharmex, CA).
Statistical analysis
Data expressed as means ± standard deviation (SD) were evaluated with an unpaired Student’s t-test assuming unequal variances. *P<0.05, **P<0.01.
Footnotes
The authors have no conflicting financial interests.
Reference List
- 1.Lucchinetti C, et al. A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain. 1999;122:2279–2295. doi: 10.1093/brain/122.12.2279. [DOI] [PubMed] [Google Scholar]
- 2.Lucchinetti C, et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.Storch MK, et al. Autoimmunity to myelin oligodendrocyte glycoprotein in rats mimics the spectrum of multiple sclerosis pathology. Brain Pathol. 1998;8:681–694. doi: 10.1111/j.1750-3639.1998.tb00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trapp BD. Pathogenesis of multiple sclerosis: the eyes only see what the mind is prepared to comprehend. Ann. Neurol. 2004;55:455–457. doi: 10.1002/ana.20087. [DOI] [PubMed] [Google Scholar]
- 5.Lassmann H. Experimental models of multiple sclerosis. Rev Neurol (Paris) 2007;163:651–655. doi: 10.1016/s0035-3787(07)90474-9. [DOI] [PubMed] [Google Scholar]
- 6.Lassmann H, Bruck W, Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol Med. 2001;7:115–121. doi: 10.1016/s1471-4914(00)01909-2. [DOI] [PubMed] [Google Scholar]
- 7.Wagner T, Rafael J. Biochemical properties of liver megamitochondria induced by chloramphenicol or cuprizone. Exp Cell Res. 1977;107:1–13. doi: 10.1016/0014-4827(77)90379-2. [DOI] [PubMed] [Google Scholar]
- 8.Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ludwin SK, Johnson ES. Evidence for a “dying-back” gliopathy in demyelinating disease. Ann. Neurol. 1981;9:301–305. doi: 10.1002/ana.410090316. [DOI] [PubMed] [Google Scholar]
- 10.Mahad DJ, et al. Expression of chemokine receptors CCR1 and CCR5 reflects differential activation of mononuclear phagocytes in pattern II and pattern III multiple sclerosis lesions. J Neuropathol. Exp. Neurol. 2004;63:262–273. doi: 10.1093/jnen/63.3.262. [DOI] [PubMed] [Google Scholar]
- 11.Remington LT, Babcock AA, Zehntner SP, Owens T. Microglial recruitment, activation, and proliferation in response to primary demyelination. Am. J Pathol. 2007;170:1713–1724. doi: 10.2353/ajpath.2007.060783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cammer W. The neurotoxicant, cuprizone, retards the differentiation of oligodendrocytes in vitro. J Neurol Sci. 1999;168:116–120. doi: 10.1016/s0022-510x(99)00181-1. [DOI] [PubMed] [Google Scholar]
- 13.Pasquini LA, et al. The neurotoxic effect of cuprizone on oligodendrocytes depends on the presence of pro-inflammatory cytokines secreted by microglia. Neurochem. Res. 2007;32:279–292. doi: 10.1007/s11064-006-9165-0. [DOI] [PubMed] [Google Scholar]
- 14.Gao X, et al. Interferon-gamma protects against cuprizone-induced demyelination. Mol. Cell Neurosci. 2000;16:338–349. doi: 10.1006/mcne.2000.0883. [DOI] [PubMed] [Google Scholar]
- 15.Linares D, et al. Neuronal nitric oxide synthase plays a key role in CNS demyelination. J Neurosci. 2006;26:12672–12681. doi: 10.1523/JNEUROSCI.0294-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon EJ, Cook DN, Suzuki K, Matsushima GK. Absence of macrophage-inflammatory protein-1alpha delays central nervous system demyelination in the presence of an intact blood-brain barrier. J. Immunol. 2001;167:2964–2971. doi: 10.4049/jimmunol.167.5.2964. [DOI] [PubMed] [Google Scholar]
- 17.Iocca HA, et al. TNF superfamily member TWEAK exacerbates inflammation and demyelination in the cuprizone-induced model. J Neuroimmunol. 2008;194:97–106. doi: 10.1016/j.jneuroim.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 19.Tsai HH, et al. The chemokine receptor CXCR2 controls positioning of oligodendrocyte precursors in developing spinal cord by arresting their migration. Cell. 2002;110:373–383. doi: 10.1016/s0092-8674(02)00838-3. [DOI] [PubMed] [Google Scholar]
- 20.Miller RH, Mi S. Dissecting demyelination. Nat Neurosci. 2007;10:1351–1354. doi: 10.1038/nn1995. [DOI] [PubMed] [Google Scholar]
- 21.Carlson T, Kroenke M, Rao P, Lane TE, Segal B. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp. Med. 2008;205:811–823. doi: 10.1084/jem.20072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindner M, et al. The chemokine receptor CXCR2 is differentially regulated on glial cells in vivo but is not required for successful remyelination after cuprizone-induced demyelination. Glia. 2008;56:1104–1113. doi: 10.1002/glia.20682. [DOI] [PubMed] [Google Scholar]
- 23.Lindner M, et al. Erratum. Glia. 2008 doi.10.1002/glia.20833. [Google Scholar]
- 24.Jurevics H, et al. Alterations in metabolism and gene expression in brain regions during cuprizone-induced demyelination and remyelination. J Neurochem. 2002;82:126–136. doi: 10.1046/j.1471-4159.2002.00954.x. [DOI] [PubMed] [Google Scholar]
- 25.Kerstetter AE, Padovani-Claudio DA, Bai L, Miller RH. Inhibition of CXCR2 signaling promotes recovery in models of multiple sclerosis. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 27.Cacalano G, et al. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 28.Ransohoff RM. Microgliosis: the questions shape the answers. Nat. Neurosci. 2007;10:1507–1509. doi: 10.1038/nn1207-1507. [DOI] [PubMed] [Google Scholar]
- 29.McMahon EJ, Suzuki K, Matsushima GK. Peripheral macrophage recruitment in cuprizone-induced CNS demyelination despite an intact blood-brain barrier. J Neuroimmunol. 2002;130:32–45. doi: 10.1016/s0165-5728(02)00205-9. [DOI] [PubMed] [Google Scholar]
- 30.Shea-Donohue T, et al. Mice deficient in the CXCR2 ligand, CXCL1 (KC/GRO-alpha), exhibit increased susceptibility to dextran sodium sulfate (DSS)-induced colitis. Innate. Immun. 2008;14:117–124. doi: 10.1177/1753425908088724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuller ML, et al. Bone morphogenetic proteins promote gliosis in demyelinating spinal cord lesions. Ann. Neurol. 2007;62:288–300. doi: 10.1002/ana.21179. [DOI] [PubMed] [Google Scholar]
- 32.Lin W, et al. Interferon-gamma inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain. 2006;129:1306–1318. doi: 10.1093/brain/awl044. [DOI] [PubMed] [Google Scholar]
- 33.Mason JL, Ye P, Suzuki K, D’Ercole AJ, Matsushima GK. Insulin-like growth factor-1 inhibits mature oligodendrocyte apoptosis during primary demyelination. J Neurosci. 2000;20:5703–5708. doi: 10.1523/JNEUROSCI.20-15-05703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelchtermans H, Billiau A, Matthys P. How interferon-gamma keeps autoimmune diseases in check. Trends Immunol. 2008;29:479–486. doi: 10.1016/j.it.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Glabinski AR, Krakowski M, Han Y, Owens T, Ransohoff RM. Chemokine expression in GKO mice (lacking interferon-gamma) with experimental autoimmune encephalomyelitis. J. Neurovirol. 1999;5:95–101. doi: 10.3109/13550289909029750. [DOI] [PubMed] [Google Scholar]
- 36.Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur. J. Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 37.Tran EH, Prince EN, Owens T. IFN-gamma shapes immune invasion of the central nervous system via regulation of chemokines. J. Immunol. 2000;164:2759–2768. doi: 10.4049/jimmunol.164.5.2759. [DOI] [PubMed] [Google Scholar]
- 38.Savarin C, Bergmann CC, Hinton DR, Ransohoff RM, Stohlman SA. Memory CD4+ T-cell-mediated protection from lethal coronavirus encephalomyelitis. J Virol. 2008;82:12432–12440. doi: 10.1128/JVI.01267-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aboul-Enein F, Lassmann H. Mitochondrial damage and histotoxic hypoxia: a pathway of tissue injury in inflammatory brain disease? Acta Neuropathol. 2005;109:49–55. doi: 10.1007/s00401-004-0954-8. [DOI] [PubMed] [Google Scholar]
- 40.Mahad D, Ziabreva I, Lassmann H, Turnbull D. Mitochondrial defects in acute multiple sclerosis lesions. Brain. 2008;131:1722–1735. doi: 10.1093/brain/awn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaturvedi RK, Beal MF. Mitochondrial approaches for neuroprotection. Ann. N. Y Acad Sci. 2008;1147:395–412. doi: 10.1196/annals.1427.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith KJ. Sodium channels and multiple sclerosis: roles in symptom production, damage and therapy. Brain Pathol. 2007;17:230–242. doi: 10.1111/j.1750-3639.2007.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardona AE, et al. Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood. 2008;112:256–263. doi: 10.1182/blood-2007-10-118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armstrong RC, Le TQ, Frost EE, Borke RC, Vana AC. Absence of fibroblast growth factor 2 promotes oligodendroglial repopulation of demyelinated white matter. J Neurosci. 2002;22:8574–8585. doi: 10.1523/JNEUROSCI.22-19-08574.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L, et al. Severe disease, unaltered leukocyte migration, and reduced IFN-{gamma} production in CXCR3−/−mice with experimental autoimmune encephalomyelitis. J. Immunol. 2006;176:4399–4409. doi: 10.4049/jimmunol.176.7.4399. [DOI] [PubMed] [Google Scholar]
- 46.Cardona AE, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 47.Schreiber RC, et al. Monocyte chemoattractant protein (MCP)-1 is rapidly expressed by sympathetic ganglion neurons following axonal injury. Neuroreport. 2001;12:601–606. doi: 10.1097/00001756-200103050-00034. [DOI] [PubMed] [Google Scholar]
- 48.Pernet V, Joly S, Christ F, Dimou L, Schwab ME. Nogo-A and myelin-associated glycoprotein differently regulate oligodendrocyte maturation and myelin formation. J Neurosci. 2008;28:7435–7444. doi: 10.1523/JNEUROSCI.0727-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waxman SG, Kocsis JD, Nitta KC. Lysophosphatidyl choline-induced focal demyelination in the rabbit corpus callosum. Light-microscopic observations. J Neurol Sci. 1979;44:45–53. doi: 10.1016/0022-510x(79)90221-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.