Abstract
Aqueous phospholipid mixtures that form bilayered micelles (bicelles) have gained wide use by molecular biophysicists during the past 20 years for spectroscopic studies of membrane-bound peptides and structural refinement of soluble protein structures. Nonetheless, the utility of bicelle systems may be compromised by considerations of cost, chemical stability, and preservation of the bicelle aggregate organization under a broad range of temperature, concentration, pH, and ionic strength conditions. In the current work, 31P nuclear magnetic resonance (NMR) and atomic force microscopy (AFM) have been used to monitor the size and morphology of isotropically tumbling small bicelles formed by mixtures of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) or 1,2-di-O-tetradecyl-sn-glycero-3-phosphocholine (DIOMPC) with either 1,2-dihexanoyl-sn-glycero-3-phosphocholine (DHPC) or 1,2-di-O-hexyl-sn-glycero-3-phospho-choline (DIOHPC), testing their tolerance of variations in commonly used experimental conditions. 1H-15N 2D NMR has been used to demonstrate the usefulness of the robust DMPC-DIOHPC system for conformational studies of a fatty acid-binding protein that shuttles small ligands to and from biological membranes.
Keywords: bicelle, phospholipid, membrane mimetic, NMR, 31P NMR, 2D NMR, AFM
1. Introduction
Since the late 1980s, bilayered phospholipid micelles (bicelles) have attracted substantial interest in the molecular biophysics community as membrane mimetics for NMR structural studies of amphiphile assemblies and of membrane-bound peptides and proteins[1]. As compared with other model biological membranes such as micelles or small unilamellar vesicles, the principal advantage of bicelles lies in their ability to maintain stable planar bilayers that more closely mimic in vivo membranous structures and thus better preserve the native conformation of the polypeptide under study. Depending on the temperature, total aqueous phospholipid concentration (cL) and molar ratio of long- to short-chain components (q), these assemblies with disklike[2,3] or `Swiss cheese' morphologies [4,5] may tumble isotropically or become aligned with respect to a magnetic field. Whereas bicelles with q exceeding a value of 2 will orient in an NMR magnet regardless of whether cL is small (3–5%, w/v) [4] or large (15–25%, w/v) [6], disklike bicelles with q less than 2 can tumble isotropically.
Dilute preparations of the high-q assemblies have gained wide usage as magnetically aligned media for the refinement of NMR-based 3D structures of soluble proteins with measurements of residual dipolar couplings [1,4,6]. Solid-state NMR investigations [7–10] have typically made use of more concentrated preparations of these bicelles, for which the direction of magnetic alignment can be tuned by addition of lanthanide ions [11,12] that increase the spectral dispersion. In a number of cases the low-q, isotropically tumbling bicelles have made it possible to conduct high resolution solution-state NMR studies of peptides [13] and transmembrane proteins [14,15] without compromising their biochemical activity; the small size and rapid tumbling of these assemblies permits acquisition of well-resolved NMR spectra, comparable to those seen in micelles [14]. A bicelle organizational model has also been proposed for mixtures of long- and short-chain phospholipids, e.g. dimyristoylphosphatidylcholine (DMPC) and dihexanoylphosphatidylcholine (DHPC), respectively. In the bicelle thus produced, the two lipid species tend to segregate, with the long-chain phospholipids located in a central planar region and the short-chain phospholipids concentrated in a curved rim region [2,3,16] (Figure 1). One interesting feature of this system is that the two bicellar regions experience different magnetic environments and thus display distinct peaks in the 31P NMR spectrum [2].
Figure 1.
Schematic representation of DMPC-DIOHPC (or DMPC-DHPC) bicelles (see text for notation).
As a practical matter for the design of NMR structural studies using bicellar media, it is important to consider what factors influence their size, magnetic alignment behavior, and stability and how best to measure these properties. The critical micelle concentration (cmc) of each phospholipid controls the concentration of monomers that must be maintained in equilibrium with its associated aggregate. For a short-chain phospholipid such as DHPC with a large cmc of 14 ~ 15 mM [2,17], a significant portion will be present in monomeric form if the DMPC-DHPC bicelle solution is dilute, enhancing the molar ratio q in the aggregate and thus making it grow in size [2]. The larger head-group volume occupied by phospholipids spread out around the rim compared with those packed tightly in the bilayer is also expected to enlarge the size of the bicelle disk. The presence of salts in the bicelle solution can influence not only the size but also the magnetic alignment of a bicelle system. It is known, for instance, that trivalent lanthanide ions with positive magnetic anisotropy susceptibility (e.g. Er3+, Yb3+, Tm3+, and Eu3+) can change the bicelle orientation by 90° with respect to the magnetic field [11,12,18]. Bivalent ions such as Ca2+ and Mg2+ have been reported to increase bicelle sizes significantly and also to improve their magnetic alignment [19], whereas monovalent ions (e.g. K+ and Na+) only improve magnetic alignment [19–21]. Finally, the presence of chemically labile phospholipid linkages can compromise bicelle stability, especially at extremes of pH. Many investigators now prefer to use phospholipids such as 1,2-di-O-hexyl-sn-glycero-3-phosphocholine (6:0) (DIOHPC), in which ether (rather than ester) bonds link the glycerol backbone and the aliphatic chains, because ethers are less susceptible to hydrolysis under both acidic and alkaline conditions [22–24].
In the current study we utilize both NMR and atomic force microscopy (AFM) methods to assess stability, aggregate size, and phospholipid organization for these versatile assemblies. We further describe a new isotropically tumbling DMPC-DIOHPC bicelle system that is cost effective, resistant to changes in size and morphology over a wide range of concentrations, temperatures, and physiological salt conditions, and stable enough to serve as a membranemimetic medium for NMR structural studies of peripheral membrane proteins. The utility and robustness of this DMPC-DIOHPC bicelle as a membrane mimetic for biological macromolecules is demonstrated by two means. Firstly, NMR of two intrinsically well-separated 31P NMR peaks from central planar and rim regions of the bilayer are shown to provide an accurate monitor of the bicelle morphological and chemical stability. Secondly, 2D 1H-15N NMR correlation data for the bicelle mixed with intestinal fatty acid binding protein (IFABP), a protein that transfers fatty acids to and from biological membranes, suggest the formation of a stable, rapidly tumbling bicellar complex that offers the potential to yield high-resolution spectra for structural studies of peripheral membrane proteins.
2. Materials and Methods
2.1. Preparation of bicelle samples
1,2-dimyristoyl-sn-glycero-3-phosphocholine (14:0) (DMPC, MW: 677.93), 1,2-dihexanoyl-sn-glycero-3-phosphocholine (6:0) (DHPC, MW: 453.25), 1,2-di-O-tetradecyl-sn-glycero-3-phosphocholine (14:0) (DIOMPC, MW: 649.97) and 1,2-di-O-hexyl-sn-glycero-3-phosphocholine (6:0) (DIOHPC, MW: 425.55) were purchased from Avanti Polar Lipids (Alabaster, AL) and used without further purification. Research grade NaCl, KCl, CaCl2 and MgCl2 were purchased from Fisher Scientific (Fair Lawn, NJ).
We compared properties of three bicellar systems, DMPC-DHPC, DIOMPC-DHPC, and DMPC-DIOHPC. To prepare a DMPC-DIOHPC bicelle stock solution with cL = 25% (w/v), the appropriate amount of DMPC was suspended in deionized water and then vortexed at room temperature to form a slurry. An aqueous DIOHPC stock solution (0.5g/ml in water) was mixed with the DMPC slurry to achieve q = 0.5, and water was added to maintain an overall lipid concentration of cL = 25%. The samples were vortexed, centrifuged and vortexed again, then heated briefly at 37 °C in a water bath and cooled down to 0°C in ice water. This protocol was repeated for at least 5 cycles until a clear, homogeneous bicelle solution was obtained. DIOMPC-DHPC and DMPC-DHPC bicelles were handled in the same fashion as DMPC-DIOHPC mixtures.
Samples with q = 0.5 and a series of cL values were prepared for NMR studies by adding appropriate amounts of the 25% (w/v) bicelle stock solution and D2O, then diluting with water to a final volume of 500 μl and 10% D2O. Selected samples contained various salts (NaCl, KCl, CaCl2 and MgCl2) that were added to yield final concentrations of 50, 100, 150, and 200 mM.
2.2 Preparation of Intestinal Fatty Acid-binding Protein
Rat intestinal fatty acid-binding protein (IFABP) was expressed in BL21(DE3) E. coli cells and purified by G-50 size exclusion chromatography, DEAE-52 ion exchange chromatography, and delipidation by a Lipidex column, as described previously [25,26]. A 0.3 mM solution of I-FABP was prepared in 50 mM NaH2PO4, 100 mM NaCl, 5μM EDTA, 0.02% NaN3, and 5% D2O at pH 7.0; 20% DMPC/DIOHPC stock bicelle solution in pure water was added directly to the protein solution to achieve a final bicelle concentration of 1% (w/v) in a total NMR sample volume of 500μl.
2.3. Nuclear Magnetic Resonance Spectroscopy
All data were acquired on Varian spectrometers at either the College of Staten Island or the City College of New York: a UNITYINOVA 600 instrument (Palo Alto, CA) equipped with an IDQG probe and operating at 1H and 31P frequencies of 599.497 and 242.856 MHz, respectively or a UNITYINOVA 600 spectrometer equipped with a cold probe and operating at 599.761 MHz. One-dimensional 31P spectra were recorded at various temperatures using a proton-decoupled single pulse experiment with 32 – 1024 scans for cL = 15% to 0.125%. The spectra were recorded with 4096 complex points and a sweep width of 4882.8Hz (~20 ppm), producing a digital resolution of < 0.01 ppm/point or 2.39 Hz/point. The carrier frequency was set to 0 ppm as the chemical shift reference. The data were processed with Varian VnmrJ software. Two-dimensional 1H-15N Heteronuclear Single Quantum Correlation NMR Spectroscopy (HSQC)[27] was conducted using a gradient-equipped HCN triple resonance probe. The spectra were recorded with 512×128 complex points and 64 scans per FID. The 1H chemical shift was referenced to the water peak (4.938 ppm at 37 °C).
2.4. Atomic force microscopy
A cL = 2% DMPC/DHPC bicelle solution (q = 0.5) was freshly diluted tenfold with distilled water; 200–300 μl were put on a cover slip in a liquid cell with a freshly isolated mica surface (Ted Pella, Inc., Redding, CA). The AFM measurements were performed at room temperature on an Agilent 5500 AFM/SPM Microscope (Agilent Technologies, Inc., Santa Clara, CA), using tapping mode with a scanning rate of 0.7 Hz and a tapping frequency of ~300 kHz. A cantilever (NSC15/no Al) with a spring constant of 45 N/m (MikroMasch USA, Wilsonville, OR) was used for these measurements.
2.5. Modeling of Bicelle Size
The bicelle organizational models proposed previously [2,11] were used to relate the phospholipid ratio in the aggregate to its diameter, allowing us to assess the robustness of the DMPC-DIOHPC bicelle under various experimental conditions. Figure 1 shows a schematic representation of this bicelle, where the long-chain phospholipids occupy the central planar bilayer region and the short-chain phospholipids are present in the curved micelle-like rim [2,16]. Although DIOHPC could be partially miscible with the long-chain lipids in the planar region and undergo fast exchange between rim and planar sites [28–30], this effect was neglected in the absence of diagnostic 31P chemical shift changes (see Section 3.4). Straightforward geometrical considerations provide a relationship between the molar ratio of the two components and the dimensions of the bicelle assembly:
| (1), |
where R is the radius of the central planar region and r is the radius of the rim area. The thickness of the DMPC bilayer h has been estimated as about 4 nm [31]. As the radius of the DHPC micelles has been estimated to be 2 nm [16], we use this value as an approximation for the ether-linked DIOHPC case.
The model was refined by accounting for both the partitioning of free-state DIOHPC monomers ([DIOHPC]free) and DIOHPC molecules in the bicelle rim, and the distinctive head group areas of the differently located phospholipids, as in the DMPC-DHPC bicelle case. These considerations lead to an effective q value for our DMPC-DIOHPC bicelle:
| (2), |
where a is the ratio of DMPC to DIOHPC (or DHPC) head group areas in planar bilayers and rim micelles, respectively. The head group area of bilayered DMPC has been reported as 0.60 cm2 [31], whereas for micellar DHPC it has been reported variously as 0.66 cm2 [32] and 1.02 cm2 [33]. In conformance with other research reports [2,34], our calculations use the latter value and thus an a value of 0.6, making the assumption that DHPC and DIOHPC have the same head group area.
Finally, equation (2) yields a bicelle diameter as follows:
| (3) |
3. Results and Discussion
3.1. 31P NMR spectrum of DMPC-DIOHPC bicelle system
As noted above, environmental differences between the distinct bilayer and rim regions produce two peaks in the 31P NMR spectrum, described previously for the DMPC-DHPC bicelle system [2]. Identification of these peaks in our bicelle system is straightforward if the molar ratio (q) of the lipid constituents is known and unequal, in contrast to other small, isotropically tumbling low-q species that typically display some spectral overlap[2]. In the latter situation, integration of the two peaks to deduce the bicelle size may be unreliable unless lanthanide ions such as Pr3+ are added to enhance the separation [2].
Figure 2 illustrates the separation of 31P NMR resonances observed in three related mixed-phospholipid bicelle systems, without the addition of lanthanide salts. All three systems exhibit distinct peaks, presumably due to segregation of the two kinds of phospholipids into bilayer and rim regions of the aggregate. However, both bicelle mixtures containing ether-linked phospholipid groups (DMPC-DIOHPC, ester-ether and DIOMPC-DHPC, ether-ester) display improved separation over the exclusively ester-linked DMPC-DHPC system. The largest separation, observed for the DMPC-DIOHPC bicelles, indicates that the difference between chemical environments of the planar and rim areas is enhanced when the ether linkage is present in the molecular species that aggregates in the rim area. Thus, differences between ether- and ester-linked molecular structures combined with contrasting structural locations in terms of headgroup-headgroup and headgroup-water interactions accentuate the overall 31P chemical shift difference between long- and short-chain species. Finally, Figure 2d shows that the lipids retain their characteristic chemical shifts in a mixture of the four lipid species, indicating preservation of the bicellar morphology and segregation of the bilayer and rim regions. Together, these favorable spectral characteristics facilitate accurate measurements of the peak areas, chemical shifts, and linewidths that are diagnostic for bicellar component ratios, aggregate size, and chemical stability.
Figure 2.
Proton decoupled 242.856 MHz 31P NMR spectra of (a) DMPC-DIOHPC, (b) DMPC-DHPC, (c) DIOMPC-DHPC, and (d) a mixture of samples (a) and (c). All bicelle samples had values of q = 0.5, cL = 5% (w/v) and were dissolved in deionized water to which 10% D2O was added for NMR spectroscopy conducted at 37 °C. The sweep width of the spectra was 20.0 ppm; the carrier frequency was set to 0 ppm and placed at the center of the spectrum. Note the superior separation achieved for the DMPC-DIOHPC bicelle system.
3.2 Chemical stability of lipids in DMPC-DIOHPC mixtures
It has been reported that ether-linked DIOHPC is more stable than ester-linked DHPC against hydrolysis catalyzed by acid or base and can remain stable within a pH range from 2 to 10 [24]. This intrinsic resistance to chemical breakdown allows the DIOHPC component of our bicelle system to potentially confer stability on the bicelle aggregate: by virtue of its location in the aqueous-accessible rim region of the bicelle disk, the DIOHPC can shield the ester-linked DMPC in the central bilayer area from attack by acid or base. Based on these considerations, we expected the DMPC-DIOHPC bicelle system maintained at room temperature to be at least as stable as the DMPC-DHPC system against hydrolysis. Successive NMR examination of the q = 0.5, cL = 2 % w/v and q = 0.5, cL = 8% w/v DMPC-DIOHPC systems (kept at pH 6.3 for two years in a freezer at −20°C or ~3 months at room temperature) gave 31P spectra that were unchanged and free from degradation products, consistent with chemical stability that is at least comparable to the DMPC-DHPC bicelle system under similar conditions[4]. Moreover, this performance is achieved with significant cost savings, since retaining an ester-linked phospholipid as one of the bicelle components shaves a factor of > 10 off the costs of the chemicals.
3.3 Concentration of short-chain lipid monomers in bicelle solutions
The impact of DIOHPC partitioning between monomeric and bicellar locations on the ratio qeff and the corresponding aggregate size was assessed by determining the monomer concentration [DIOHPC]free from 31P NMR measurements as a function of the total phospholipid concentration cL in q = 0.5 DMPC-DIOHPC mixtures (Table 1) [2]. Taking 5.03 ppm as the chemical shift of free-state DIOHPC monomer, a plot of shift vs. [DIOHPC]−1 yielded a straight line (R2 = 0.999) and [DIOHPC]free = 6 mM at 37 °C (Figure 3). Analogous measurements for DMPC-DHPC bicelles (in pure water) give [DHPC]free = 7 mM at 37 °C (R2 = 0.997, chemical shift data not shown), in excellent agreement with the result obtained by Glover, et al. [2] in the presence of ~200 mM Pr+3. This consistency of [DHPC]free shows that neither the q value nor the aggregate size is altered by the presence of lanthanide ions. The comparative values of [DIOHPC]free and [DHPC]free suggest that the ether-linked lipid is slightly more hydrophobic than its ester-linked analog; for that reason it is also likely to have a cmc lower than the 14 mM value determined for DHPC [2] and should exert a smaller impact on the lipid ratio and bicelle size according to Eq. (2). Since [DHPC]free is approximately half as large as the cmc, it appears to be more favorable thermodynamically to partition into mixed-lipid bicelles than into short-chain lipid micelles. A similar argument should apply to DIOHPC.
Table 1.
31P Chemical shifts of DMPC-DIOHPC bicelles as a function of the total lipids concentrationa
| cL (%) | Total Lipid (mM) | DMPC (mM) | DMPC δ(ppm)b | DIOHPC (mM) | DIOHPC δ(ppm)b |
|---|---|---|---|---|---|
| 15 | 294.3 | 98.1 | 4.502 | 196.2 | 4.829 |
| 10 | 196.2 | 65.4 | 4.501 | 130.8 | 4.833 |
| 8 | 156.96 | 52.32 | 4.502 | 104.64 | 4.836 |
| 7 | 137.34 | 45.78 | 4.502 | 91.56 | 4.837 |
| 6 | 117.72 | 39.24 | 4.501 | 78.48 | 4.839 |
| 5 | 98.1 | 32.7 | 4.502 | 65.4 | 4.842 |
| 3 | 58.86 | 19.62 | 4.501 | 39.24 | 4.857 |
| 2 | 39.24 | 13.08 | 4.501 | 26.16 | 4.869 |
| 1.5 | 29.43 | 9.81 | 4.499 | 19.62 | 4.887 |
| 1 | 19.62 | 6.54 | 4.494 | 13.08 | 4.92 |
| 0.25 | 4.905 | 1.635 | 3.27 | 5.03 | |
| 0.125 | 2.4525 | 0.8175 | 1.635 | 5.03 |
All mixtures were prepared with a ratio [DMPC]/[DIOHPC]total = 0.5 in aqueous solutions containing 10% D2O and examined at 37 °C.
Spectra were referenced by setting the carrier frequency to 0 ppm.
Figure 3.
Dependence of 31P chemical shift of on the reciprocal of DIOHPC concentration in isotropically tumbling DMPC – DIOHPC bicellar mixtures. Following Glover et al. [2], the concentration of free DIOHPC was found to be 6 mM at 37°C from the equation δobs = {[DIOHPC]free ·(δfree − δbicelle ) ·[DIOHPC]−1tot} + δbicelle.
3.4. Variation of [DIOHPC]free with temperature and total lipid concentration
To evaluate whether the bicelle size is maintained over a range of temperatures that might be used in structural studies of biomacromolecules, the temperature dependence of [DIOHPC]free was evaluated by additional 31P NMR measurements made at 30 °C, 41 °C and 46 °C. The modest changes in 31P chemical shift (no more than 0.25 ppm) argued for complete segregation of DIOHPC at the rim rather than miscibility in the planar region [28,30,35], allowing us to determine the concentration of free short-chain lipid and bicelle size via Eqs. (2) and (3). The concentration of short-chain phospholipid monomers remained essentially constant at 5.7 – 6.5 mM over this temperature range (data not shown), in accord with prior reports for DHPC. For instance, dynamic light scattering has been used to show that the variation of hydrodynamic radii with cL for q = 0.5 bicelles is invariant to temperature, with a value of [DHPC]free at about 5 mM in the range of 25 to 37 °C[34]. Analogous temperature invariance of small angle neutron scattering (SANS) profiles for q = 0.5 bicelles has been reported in the temperature range of 10 to 40 °C [3].
It was also of interest to establish the range of total lipid concentration over which the bicelle size is retained. Subtracting [DIOHPC]free from [DIOHPC]total and calculating the effective q from equation (2), we find that qeff remains essentially constant at 0.5 when cL ≥ 98 mM (5% (w/w)). By comparison, cL must exceed 130 mM (7% (w/w)) for the DMPC/DHPC bicelle system in order to keep the qeff value constant at 0.5 [2]. This result demonstrates an augmented versatility for bicellar media containing an ether-linked short-chain phospholipid, since it becomes possible to work with smaller amounts of lipid and either peptide or protein while still maintaining a small, rapidly tumbling bicellar structure and high-resolution NMR spectra.
3.5. Influence of salts on DMPC-DIOHPC isotropic bicelle size
Losonczi and Prestegard [20] first reported the beneficial effects of ionic strength on the stability and lifetime of phospholipid bicelle preparations. Both the results in Section 3.3 and prior experiments [11,12] suggest that trivalent lanthanide ions (e.g. Er3+, Yb3+, Tm3+, and Eu3+) do not influence the bicelle size despite their ability to change the orientation of liquid crystalline aggregates by 90° in the magnetic field. As noted above, both monovalent and divalent ions have been shown to impact the size and the magnetic field alignment properties of high-q bicelles [4,19,21]. The sensitivity of bicelle architecture to salt concentration may be important in designing structural studies to approximate particular physiological conditions.
31P NMR peak integrals and associated values of q were measured for DMPC-DIOHPC bicelle solutions to which four commonly used physiological salts (NaCl, KCl, CaCl2, MgCl2) were added in independent experiments. Table 2 summarizes the values of q measured at three different temperatures and four concentrations of each salt. The largest perturbation of bicelle size occurred upon addition of up to 200 mM NaCl to the cL = 2% DMPC-DIOHPC mixture at 25°C, for which the q value increased from 0.47 to 0.54 (namely 15% increments in q value) and the corresponding bicelle diameter increased from 92 to 98 Å (namely 6.5% increments in size). In other cases the changes in q were no greater than 0.03 (6% increments, KCl at 46 °C) and 0.04 (8% increments, NaCl 46 °C); thus none of these four salts had a significant influence on bicelle size over the concentration range 0–200 mM and the temperature range 25 °C to 46 °C.
Table 2.
Values of lipid ratio (q) measured for DMPC-DIOHPC bicelles at different temperatures and salt concentrationsa
| Salt concentration (mM) | KClb | NaClc | CalCl2b | MgCl2b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| q at T (°C) | q at T (°C) | q at T (°C) | q at T (°C) | |||||||||
| 25 | 37 | 46 | 25 | 37 | 46 | 25 | 37 | 46 | 25 | 37 | 46 | |
| 0 | 0.46 | 0.46 | 0.46 | 0.47 | 0.47 | 0.47 | 0.46 | 0.47 | 0.48 | 0.46 | 0.46 | 0.47 |
| 50 | 0.49 | 0.46 | 0.48 | 0.50 | 0.46 | 0.49 | 0.48 | 0.48 | 0.47 | 0.48 | 0.48 | 0.48 |
| 100 | 0.48 | 0.48 | 0.49 | 0.53 | 0.45 | 0.51 | 0.48 | 0.48 | 0.48 | 0.47 | 0.47 | 0.48 |
| 150 | --d | 0.54 | 0.45 | 0.52 | --d | --d | ||||||
| 200 | 0.48 | 0.48 | 0.49 | 0.54 | 0.47 | 0.51 | 0.46 | 0.47 | 0.47 | 0.48 | 0.48 | 0.48 |
The measured q value (defined as [DMPC]/[DIOHPC]) was derived from the ratio of normalized integrated areas for DMPC and DIOHPC peaks in 31P NMR spectra, with a precision of 2% estimated from duplicate measurements.
The mixture was prepared with cL = 10% (w/v), q = 0.5, 10% D2O.
The mixture was prepared with cL = 2% (w/v), q = 0.5, 10% D2O.
Not measured.
Our finding that the bicelle size is invariant to the monovalent ions K+ or Na+ was consistent with previous reports for DMPC-DHPC assemblies [19,21,34]. In contrast, the modest size changes we observed upon addition of the divalent ions Ca2+ and Mg2+ differed from the findings of Arnold et al., who used solid-state 31P NMR spectra to characterize high-q (cL = 20%) magnetically-aligned DMPC-DHPC bicelles made with a different sample preparation method [19]. They found that `depressed' qeff values of 2.6 could be restored to their stoichiometric values of 3.6 upon addition of 100 – 200 mM CaCl2 or MgCl2, corresponding to an increase in diameter from ~30 to ~50 nm and in accord with the known role of enhanced ionic strength in promoting bilayer and bicelle assembly [20].
3.6. Direct visualization of isotropic DMPC-DHPC bicelles by AFM
A direct assessment of bicelle size and shape was also obtained using atomic force microscopy in DMPC-DHPC and DMPC-DIOHPC bicelles. Previously, Arnold, et al. [19] used freeze-fracture electron microscopy to visualize disklike DMPC-DHPC bicelles with a q value of 3.5. Glover, et al. [2] also observed q = 0.5 DMPC-DHPC bicelles using negative staining electron microscopy, whereas Luchette, et al. [3] characterized such aggregates by SANS methods. As a more direct alternative that can be implemented under near-physiological conditions, we used AFM to obtain images of nominally similar q = 0.5 DMPC-DHPC bicelles, employing tapping mode to minimize destructive frictional forces [36,37]. Figures 4A–C show images acquired with scanning ranges of 3–5 μm; the corresponding cross-sectional plots (Figures 4D–F) provide disk thickness estimates of about 40 Å in each case, matching that expected for the lipid bilayer [2,34]. Figure 4A reveals at least three kinds of lipid assemblies: large disks, small disks, and worm-like structures. The worm-like structures, observed previously by SANS in high-q mesophases [38,39], may be cylindrical micelles of DHPC [34], whereas the large disks could be lamellar DMPC membranes. We identify the small bilayered disks, which are found most abundantly, as DMPC-DHPC bicelles.
Figure 4.
AFM images of DMPC/DHPC bicelles (10 folds diluted of cL = 2% w/v, q = 0.5) obtained with scanning ranges of 4.6 × 4.6 μm (A and B), and 3.25 × 3.25 μm (C). Panel C was scanned along an axis perpendicular to the ones in A and B, producing “shadows” of the individual disks that are oriented differently by 90°. Panels D, E and F are cross-sectional plots along the lines drawn in panels A, B, and C, respectively, yielding thickness estimates (heights) of about 40 Å for the bicelle planar area. The circled two aggregates were used to estimate 87 and 96 Å diameters, respectively.
Figure 4B shows that the small disks are not uniform in size, in agreement with prior freeze-fracture EM data on high-q aggregates [34]. This observation may reflect an intrinsic size variation, but it is also possible that the disks grow as a consequence of water evaporation from the bicelles. The “shadows” on the individual disks arise from the expected edge effects of the AFM tip; the cross-sectional plots allow us to estimate a 20Å thickness for these shadows, corresponding to a lipid monolayer and matching the expected height at the bicelle edge (Figure 1). Using the thickness measured perpendicular to the scanning plane for the “worm-like” cylindrical structures as a calibration distance, we find the diameters of the two smallest bicelles in Figures 4A and B to be 87 and 96 Å, respectively. These values are in excellent agreement with the calculated value of 96 Å obtained from Eq. (3) with q = 0.5, a = 0.6, and r = 2 nm; the AFM-derived dimensions are also in accord with fits of the SANS data [3]. Thus, the AFM images can provide a direct quantitative picture of the isotropically tumbling bicelle assemblies.
3.7. Isotropic DMPC-DIOHPC bicelles as membrane mimetics for NMR structural studies
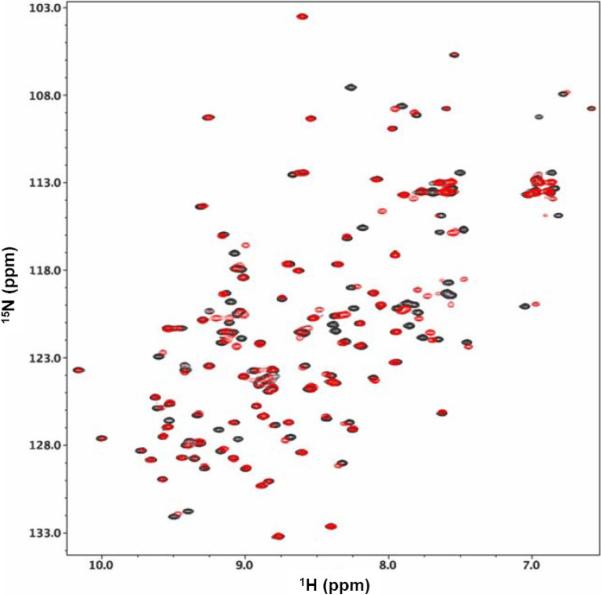
As noted above, DMPC-DHPC bicelles have been used widely as membrane mimetic media [1]. The utility of the new DMPC-DIOHPC bicelle system as a model membrane for structural biology research was evaluated using intestinal fatty acid-binding protein (IFABP), an intracellular protein that transfers hydrophobic ligands to and from biological membranes and may be viewed as a model peripheral membrane protein[26]. Figure 5 shows an overlay of 1H-15N HSQC spectra for IFABP alone (black) and upon addition of 1% w/v (~60:1 lipid:protein) aqueous DMPC-DIOHPC bicelles (red). It is clear that both spectra exhibit sharp resonances, excellent spectral resolution, and the high degree of chemical shift dispersion typical of β-sheet structures. These high-quality spectra, maintained without precipitation for more than 45 days at bicelle concentrations up to cL = 1%, indicate formation of small protein-bicelle assemblies that tumble rapidly and do not aggregate together. A single NH resonance appears for each observed backbone site, consistent with rapid exchange between bound and unbound states. Most of the NH peaks are coincident in the two samples, but a small subset showing changes in magnetic environment are likely to be diagnostic for interactions between particular regions of the IFABP protein and the DMPC-DIOHPC bicelles, respectively. Perhaps 10% of the NH resonances disappear upon addition of the bicelles, suggesting that protein-membrane interactions produce bound and unbound states of particular polypeptide sites that are interconverting at rates comparable to their respective chemical shift differences. These observations open the way for detailed studies of the structural and motional requisites of IFABP-facilitated transport to and from biological membranes, which are currently in progress.
Figure 5.
An overlay of the amide regions of 15N-1H HSQC NMR spectra for ~0.3 mM IFABP in 50 mM NaH2PO4, 100 mM NaCl, 5μM EDTA, 0.02% NaN3, and 5% D2O at pH 7.0, showing samples with (red) and without (black) a (cL = 1% w/v, q = 0.5) DMPC-DIOHPC bicelle solution in water.
Conclusions
A new DMPC-DIOHPC isotropic bicelle system has been evaluated as a membrane mimetic medium for biomolecular NMR and related structural biology research. Compared with other similar systems, DMPC-DIOHPC isotropic bicelles exhibit improved hydrolytic stability and superior 31P NMR peak separation, allowing more accurate measurements of integrals and associated sizes. Moreover, the new system extends the range of experimental conditions over which constant size and aggregate organization can be maintained, including overall phospholipid concentrations down to cL of 5%, temperatures from 25 °C to 46 °C, and the presence of physiologically relevant salts at concentrations up to 200 mM. AFM imaging experiments are demonstrated for direct visualization of bicellar aggregates under near-physiological conditions and comparison with prior EM data, confirming the predominance of the expected disklike assemblies but also revealing the presence of extended bilayers and DHPC micelles. Finally, the q = 0.5 DMPC-DIOHPC bicelle system shows potential as a useful medium for high-resolution NMR studies of peripheral membrane proteins.
Acknowledgements
The IFABP plasmid was a generous gift of Dr. Judith Storch, Rutgers University Department of Nutritional Sciences. The NMR and AFM Facilities used in this work are operated by the College of Staten Island, The City College, and the CUNY Institute for Macromolecular Assemblies, a Center of Excellence of the Generating Employment through New York State Science Program. Additional infrastructural support was provided at The City College of New York by NIH 5G12 RR03060 from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prosser RS, Evanics F, Kitevski JL, Al-Abdul-Wahid MS. Current applications of bicelles in NMR studies of membrane-associated amphiphiles and proteins. Biochemistry. 2006;45:8453. doi: 10.1021/bi060615u. [DOI] [PubMed] [Google Scholar]
- 2.Glover KJ, Whiles JA, Wu G, Yu N, Deems R, Struppe JO, Stark RE, Komives EA, Vold RR. Structural evaluation of phospholipid bicelles for solution-state studies of membrane-associated biomolecules. Biophys. J. 2001;81:2163. doi: 10.1016/s0006-3495(01)75864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luchette PA, Vetman TN, Prosser RS, Hancook RE, Nieh M, Glinkar CJ, Krueger S, Katsaras J. Morphology of fast-tumbling bicelles: a small angle neutron scattering and NMR study. Biochimica Biophysica Acta. 2001;1513:83. doi: 10.1016/s0005-2736(01)00358-3. [DOI] [PubMed] [Google Scholar]
- 4.Ottiger M, Bax A. Characterization of magnetically oriented phospholipid micelles for measurement of dipolar couplings in macromolecules. J. Biomol. NMR. 1998;12:361. doi: 10.1023/a:1008366116644. [DOI] [PubMed] [Google Scholar]
- 5.Gaemers S, Bax A. Morphology of three lyotropic liquid crystalline biological NMR media studied by translational diffusion anisotropy. J Am Chem Soc. 2001;123:12343. doi: 10.1021/ja011967l. [DOI] [PubMed] [Google Scholar]
- 6.Whiles JA, Deems R, Vold RR, Dennis EA. Bicelles in structure- function studies of membrane-associated proteins. Bioorg. Chem. 2002;30:431. doi: 10.1016/s0045-2068(02)00527-8. [DOI] [PubMed] [Google Scholar]
- 7.Durr U, Waskell L, Ramamoorthy A. The cytochromes P450 and b5 and their reductases–Promising targets for structural studies by advanced solid-state NMR spectroscopy. Biochimica Biophysica Acta. 2007;1768:3235. doi: 10.1016/j.bbamem.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Durr U, Yamamoto K, Im S, Waskell L, Ramamoorthy A. Solid-State NMR Reveals Structural and Dynamical Properties of a Membrane-Anchored Electron-Carrier Protein, Cytochrome b5. J. Am. Chem. Soc. 2007;129:6670. doi: 10.1021/ja069028m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith PES, Brender JR, Ramamoorthy A. Induction of Negative Curvature as a Mechanism of Cell Toxicity by Amyloidogenic Peptides: The Case of Islet Amyloid Polypeptide. J Am Chem Soc. 2009;131:4470. doi: 10.1021/ja809002a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Durr U, Im S, Gan Z, Waskell L, Ramamoorthy A. Bicelle-Enabled Structural Studies on a Membrane-Associated Cytochrome b5 by Solid-State MAS NMR Spectroscopy. Angew. Chem. Intl. 2008;47:7864. doi: 10.1002/anie.200801338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prosser RS, Hunt SA, DiNatale JA, Vold RR. Magnetically Aligned Membrane Model Systems with Positive Order Parameter: Switching the Sign of Szz with Paramagnetic Ions. J. Am. Chem. Soc. 1996;118:269. [Google Scholar]
- 12.Prosser RS, Volkov VB, Shiyanovskaya IV. Novel chelate-induced magnetic alignment of biological membranes. Biophys J. 1998;75:2163. doi: 10.1016/S0006-3495(98)77659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vold RR, Prosser RS, Deese AJ. Isotropic solutions of phospholipid bicelles: a new membrane mimetic for high-resolution NMR studies of polypeptides. J. Biomol. NMR. 1997;9:329. doi: 10.1023/a:1018643312309. [DOI] [PubMed] [Google Scholar]
- 14.Poget SF, Girvin ME. Solution NMR of membrane proteins in bilayer mimics: Small is beautiful, but sometimes bigger is better. Biochim Biophys Acta. 2007;1768:3098. doi: 10.1016/j.bbamem.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poget SF, Cahill SM, Girvin ME. Isotropic bicelles stabilize the functional form of a small multidrug-resistance pump for NMR structural studies. J. Am. Chem. Soc. 2007;129:1232. doi: 10.1021/ja0679836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vold RR, Prosser RS. Magnetically oriented phospholipid bilayered micelles for structural studies of polypeptides. Does the ideal bicelle exist? J. Magn. Reson. 1996;B 113:267. [Google Scholar]
- 17.Burns RA, Jr., Roberts MF, Dluhy R, Mendelsohn R. Monomer-to-micelle transition of dihexanoylphosphatidylcholine: carbon-13 NMR and Raman studies. J. Am. Chem. Soc. 1982;104:430. [Google Scholar]
- 18.Prosser RS, Hwang JS, Vold RR. Magnetically Aligned Phospholipid Bilayers with Positive Ordering: A New Model Membrane System. Biophys. J. 1998;74:2405. doi: 10.1016/S0006-3495(98)77949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold A, Labrot T, Oda R, Dufourc EJ. Cation Modulation of Bicelle Size and Magnetic Alignment as Revealed by Solid-State NMR and Electron Microscopy. Biophys. J. 2002;83:2667. doi: 10.1016/S0006-3495(02)75276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Losonczi JA, Prestegard JH. Improved dilute bicelle solutions for highresolution NMR of biological macromolecules. J. Biomol. NMR. 1998;12:447. doi: 10.1023/a:1008302110884. [DOI] [PubMed] [Google Scholar]
- 21.Raffard G, Steinbruckner S, Arnold A, Davis JH, Dufourc EJ. Temperature-Composition Diagram of Dimyristoylphosphatidylcholine-Dicaproylphosphatidylcholine “Bicelles” Self-Orienting in the Magnetic Field. A Solid State 2H and 31P NMR Study. Langmuir. 2000;16:7655. 2000. [Google Scholar]
- 22.Aussenac F, Lavigne B, Dufourc EJ. Toward bicelle stability with ether-linked phospholipids: temperature, composition, and hydration diagrams by 2H and 31P solid-state NMR. Langmuir. 2005;21:7129. doi: 10.1021/la050243a. [DOI] [PubMed] [Google Scholar]
- 23.Cavagnero S, Dyson HJ, Wright PE. Improved low pH bicelle system for orienting macromolecules over a wide temperature range. J Biomol NMR. 1999;13:387. doi: 10.1023/a:1008360022444. [DOI] [PubMed] [Google Scholar]
- 24.Ottiger M, Bax A. Bicelle-based liquid crystals for NMR-measurement of dipolar couplings at acidic and basic pH values. J. Biomol. NMR. 1999;13:187. doi: 10.1023/a:1008395916985. [DOI] [PubMed] [Google Scholar]
- 25.He Y, Yang X, Wang H, Estephan R, Francis F, Kodukula S, Storch J, Stark RE. Solution-state molecular structure of apo and oleate-liganded liver fatty acid-binding protein. Biochemistry. 2007;46:12543. doi: 10.1021/bi701092r. [DOI] [PubMed] [Google Scholar]
- 26.Hsu KT, Storch J. Fatty acid transfer from liver and intestinal fatty acid-binding proteins to membranes occurs by different mechanisms. J Biol Chem. 1996;271:13317. doi: 10.1074/jbc.271.23.13317. [DOI] [PubMed] [Google Scholar]
- 27.Cavanagh J, Fairbrother WJ, Palmer AG, III, Rance M, Skelton NJ. Protein NMR Spectrascopy Principles and Practice. Elsevier; New York: 2007. [Google Scholar]
- 28.Soong R, Macdonald PM. Water Diffusion in Bicelles and the Mixed Bicelle Model. Langmuir. 2009;25:380. doi: 10.1021/la801739a. [DOI] [PubMed] [Google Scholar]
- 29.Triba MN, Warschawski DE, Devaux PF. Reinvestigation by phosphorus NMR of lipid distribution in bicelles. Biophys. J. 2005;88:1887. doi: 10.1529/biophysj.104.055061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto K, Soong R, Ramamoorthy A. Comprehensive Analysis of Lipid Dynamics Variation with Lipid Composition and Hydration of Bicelles Using Nuclear Magnetic Resonance (NMR) Spectroscopy. Langmuir. 2009;25:7010. doi: 10.1021/la900200s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagle JF, Tristam-Nagle S. Structure of lipid bilayers. Biochim. Biophys. Acta. 2000;1469:159. doi: 10.1016/s0304-4157(00)00016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tausk RJM, Karmiggelt J, Oudshoorn C, Overbeek JTG. Physical chemical studies of short-chain lecithin homologues. I. Influence of the chain length of the fatty acid ester and of electrolytes on the critical micelle concentration. Biophys. Chemist. 1974;1:175. doi: 10.1016/0301-4622(74)80004-9. [DOI] [PubMed] [Google Scholar]
- 33.Lin TL, Chen SH, Gabriel NE, Roberts MF. Use of small-angle neutron scattering to determine the structure of dihexanoylphosphatidylcholine micelles. J. Am. Chem. Soc. 1986;108:3499. [Google Scholar]
- 34.Van Dam L, Karlsson G, Edwards K. Direct observation and characterization of DMPC/DHPC aggregates under conditions relevant for biological solution NMR, Biochim. Biophys. Acta. 2004;1664:241. doi: 10.1016/j.bbamem.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Triba MN, Warschawski DE, Devaux PF. Reinvestigation by Phosphorus NMR of Lipid Distribution in Bicelles. Biophys J. 2005;88:1887. doi: 10.1529/biophysj.104.055061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heinz WF, Hoh JH. Getting physical with your chemistry: Mechanically investigating local structure and properties of surfaces with the atomic force microscope. J. Chem. Educ. 2005;82:695. [Google Scholar]
- 37.Santos NC, Castanho MARB. An overview of the biophysical applications of atomic force microscopy. Biophys. Chem. 2004;107:133. doi: 10.1016/j.bpc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Harroun TA, Koslowsky M, Nieh M, de Lannoy C, Raghunathan VA, Katsaras J. Comprehensive Examination of Mesophases Formed by DMPC and DHPC Mixtures. Langmuir. 2005;21:5356. doi: 10.1021/la050018t. [DOI] [PubMed] [Google Scholar]
- 39.Nieh M-P, Raghunathan VA, Glinka CJ, Harroun TA, Pabst G, Katsaras J. Magnetically Alignable Phase of Phospholipid “Bicelle” Mixtures Is a Chiral Nematic Made Up of Wormlike Micelles. Langmuir. 2004;20:7893. doi: 10.1021/la048641l. [DOI] [PubMed] [Google Scholar]