Abstract
A dendrite grows by sprouting filopodia, some of which mature into stable dendrite branches that bear synapses and sprout filopodia of their own. Recent work has shown that a filopodium begins deciding to become a stable branch within one minute of contacting a presynaptic partner, but what triggers this decision remains unknown. We consider the evidence for three possible triggers: activity of neurotransmitter receptors, signaling through adhesion proteins, and heightened membrane tension as the filopodium attempts to retract but is held in place by adhesive contacts with the target. Of these, membrane tension-induced signaling is especially appealing, as it would serve as a general reporter of attachment, independent of which specific adhesion molecules are used.
Introduction
Building a dendrite is a monumental task. In a single day, an arborizing vertebrate neuron can add as much as 750 μm2 of new plasma membrane, three times the surface area of its cell body. This membrane addition is distributed over dozens – or, in some cases, hundreds – of dendrite branches. Meanwhile, postsynaptic machinery is trafficked into the arbor and directed along a maze of branches to sites of synapse formation. All this takes place in a tumultuous environment, with neighboring cells coordinating and competing for cell-cell contacts, while tissue growth and cell movement physically deform the surroundings.
In vivo, growing dendrites can be observed casting out fine filopodia, which are then gradually reeled back in. Long-term imaging of zebrafish tectal neurons showed that these filopodia extend over a period of ~20 min, and retract within ~1 h [1]. In special instances, a filopodium does not retract, but is stabilized and matures into a synapse-bearing dendritic branch [1]. A similar program of filopodium extension and retraction, with occasional stabilization, also underlies the growth of axon arbors in vivo [2, 3]. A major question in dendrite development is what prompts an unstable filopodium to mature into a stable dendritic branch.
Local calcium transients help stabilize filopodia
Recent work has converged on the generation of local calcium transients within the filopodium as a key event in its stabilization. Prior to their identification in filopodia, local calcium transients were seen in branches of growing dendrites of chick retinal ganglion cells, initiating ~1 h after the dendrite contacted a presynaptic cell [4]. These local calcium transients were linked to dendrite branch stabilization: pharmacologically blocking local calcium transients led to dendrite retraction, while focally uncaging calcium diminished retraction of nearby branches [4].
Imaging of hippocampal dendrites showed that calcium transients originate in individual filopodia and then spread to the nearby branch [5]. Filopodium calcium transients vary in frequency as the filopodium extends, reaching peak frequency as the filopodium attains its maximal length [5]. Uncaging calcium in the dendrite branch stabilized filopodia [5], as has been similarly shown for axonal filopodia [6, 7]. These experiments suggested that contact with a target in the environment – for example, a presynaptic partner – might increase the frequency of calcium transients and, in turn, stabilize the filopodium.
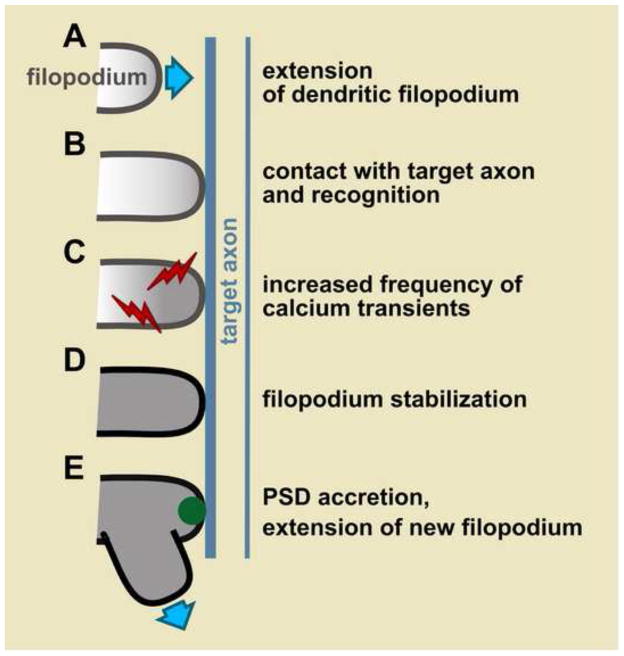
To test this idea, filopodia and target axons were imaged simultaneously [8]. Filopodia were seen to discriminate between target and non-target axons [8]. Within 10–40 sec of a filopodium contacting a target axon, the frequency of local calcium transients tripled [8]. The increased frequency of calcium transients was predictive of whether a filopodium-axon contact would be stable [8]. Thus, within 1 min of contacting a potential partner, a filopodium has begun to decide whether to stabilize (Fig. 1). What is the initiating event that provokes this decision?
Figure 1. Major steps in dendrite branch formation.
A, An unstable filopodium extends. B, It contacts a target axon where it receives a yet unidentified signal. C, This signal increases the frequency of filopodium calcium transients (red). D, The filopodium is then stabilized. E, Accretion of postsynaptic density (PSD) components (green) and extension of additional filopodia mark it as a mature dendrite branch.
Neurotransmitter-dependent signaling
One intriguing possibility is that neurotransmitter released at presynaptic sites binds to neurotransmitter receptors on the filopodium and leads to downstream signaling, including opening of neurotransmitter-gated calcium channels, which then promotes filopodium stabilization (Fig. 2A). In mature hippocampal neurons, filopodium dynamics are indeed sensitive to neurotransmitter receptor activity – electrical stimulation of dendrites increases filopodium growth and this effect is blocked by an NMDA receptor antagonist [9]. While these studies focused on filopodia of mature dendrites that give rise to dendritic spines, they raise the possibility that neurotransmitter-mediated signaling may also influence filopodia of growing dendrites as they give rise to new branches.
Figure 2. Possible initiating events triggering filopodium stabilization.
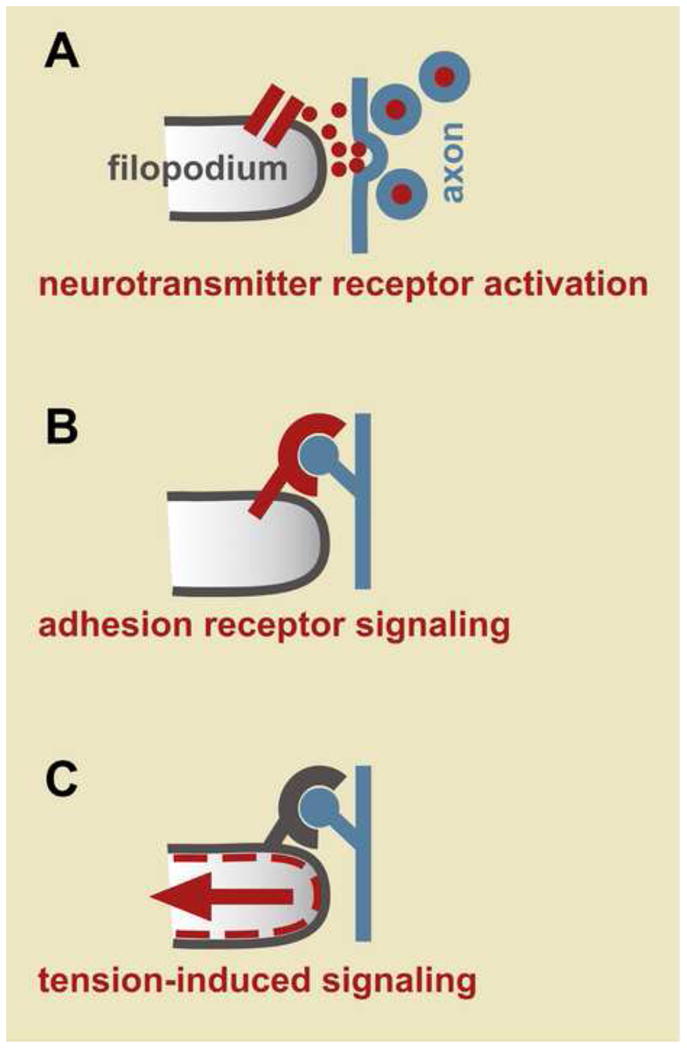
A, Release of presynaptic neurotransmitter may activate receptors on the filopodium. B, Binding of axonal ligands to adhesion receptors like integrins may induce downstream signaling. C, Adhesive attachments may resist retraction, increasing membrane tension and activating signaling, for example through stretch-activated calcium channels.
Experiments on growing dendrites showed that neurotransmitter receptor activity does indeed regulate dendrite growth, though not necessarily by altering filopodium stability. On growing dendrites of chick retinal ganglion cells, application of a nicotinic acetylcholine receptor antagonist led to reduced dendrite calcium transients and was followed by dendrite branch retraction, showing that neurotransmitter receptor activity helps maintain newly-established dendrite branches [4].
Extensive in vivo studies of Xenopus tectal neurons have shown that growing dendrites are stunted by application of an NMDA receptor antagonist [10], NMDA receptor misexpression [11], or genetic constructs that interfere with activity of the AMPA [12] or GABA [13] receptors (in this system, early GABA-receptor-dependent activity helps promote excitatory synapse formation later [14]). Taken together, these experiments show that neurotransmitter receptor activity can sculpt dendrite growth, possibly by stabilizing individual filopodia, but also potentially by maintaining already-formed branches or increasing overall arborization.
Indirect evidence that neurotransmitter receptors may help stabilize filopodia comes from time-lapse imaging of zebrafish tectal neurons, which showed that the postsynaptic density protein PSD-95, a marker of neurotransmitter receptor sites, accretes into stable puncta on some filopodia ~20 min after filopodium extension [1]. When a filopodium bearing these puncta undergoes retraction, distal regions retract normally but retraction is halted when a PSD-95 punctum is encountered [1]. Stabilized filopodia then become dendritic branches, serving as initiation points for new filopodial growth [1]. Thus, PSD-95 accretion strongly correlates with stabilization of a filopodium and its maturation into a dendrite branch. A similar accretion of nascent synaptic puncta – in this case, containing the presynaptic marker synaptophysin – is seen during stabilization of axon filopodia [2, 3].
In dendrites, the assembly of PSD-95-containing puncta is suggestive of a gathering point for neurotransmitter receptors, but these puncta may also be home to adhesion molecules involved in presynaptic partner recognition. Indeed, application of NMDA receptor antagonists did not prevent a filopodium from recognizing its target axon or becoming stabilized, arguing that neurotransmitter signaling is not required for these early steps. Furthermore, the slow accretion of postsynaptic puncta (20–40 min, [1]) relative to the rapidity of the filopodium calcium response (10–40 sec, [8]) suggests that punctum formation takes place after the initial decision to stabilize has been made. Therefore, postsynaptic puncta accretion and neurotransmitter-dependent signaling likely lie downstream of the initiating recognition event, and help to execute the stabilization program and to maintain the dendrite branch.
Cell adhesion molecules
There are numerous candidates for the adhesion receptors that could kick off the initiating recognition event (Fig. 2B). In principle, these include any molecules involved at early steps of synapse formation (reviewed in [15]). One synaptic protein that has been shown to affect filopodia in particular is agrin, which induces filopodium formation when subjected to antibody-induced clustering or overexpression [16, 17]. In mature neurons, a proteolytically processed form of agrin induces filopodium formation when pre- and postsynaptic partners are stimulated simultaneously [18].
Another set of synaptic proteins linked to filopodia are the leucine-rich repeat (LRR) family proteins, which have emerged from two independent functional screens for postsynaptic regulators of synapse formation [19, 20]. In Drosophila, the LRR protein Capricious localizes to the tips of postsynaptic muscle filopodia, called myopodia, as they extend in search of a presynaptic motor axon [21]. Like dendrite filopodia, myopodia undergo rounds of extension and retraction with occasional stabilization, and can distinguish target from non-target axons [21]. Loss of Capricious and its close relative Tartan decreases the number of myopodium-axon contacts but not the overall number of myopodia, suggesting that these LRR proteins are required to stabilize a myopodium after it contacts an appropriate presynaptic partner [21]. LRR proteins are thus strong candidates to serve a similar function in the postsynaptic filopodia of dendrites.
Significant attention has also been paid to integrins, which are widely involved in cell adhesion. In fibroblasts, integrin ligand binding leads to calcium influx that mediates cell adhesion [22]. In cortical neurons, calcium influx peaks within 1–2 sec of addition of integrin ligand peptides containing RGD sequences, consistent with the rapid time scale of filopodium calcium transients [23]. Likewise, in axon growth cones, filopodium calcium transients increase in frequency within seconds of exposure to soluble RGD peptides [6]. These transients also increase in frequency when growth cones are cultured on the RGD-containing matrix protein tenascin, although a similar increase is seen on polylysine, which does not specifically activate integrins [6]. Thus, integrin activation may be a special instance of a general mechanism for increasing filopodium calcium transients in response to cell adhesion.
Membrane tension
Another mechanism for sensing cell adhesion comes from recent studies showing that fibroblasts exhibit local calcium transients at their leading edge during cell migration [24]. These local calcium transients are stimulated by addition of integrin ligand peptides, but also by applying shear stress across the leading edge of the fibroblast or by physically deforming the substrate with a glass needle [24]. Thus, these calcium transients likely respond directly to membrane tension and, indeed, they are mediated by the stretch-activated calcium channel TRPM7 [24]. Axon growth cones also bear stretch-activated calcium channels that can control axon extension [25]. If these channels are present on dendrites, they would suggest an elegant mechanism to sense attachment to a presynaptic partner. As the filopodium attempts to retract, it would pull against its adhesive attachment and experience an increase in membrane tension (Fig. 2C). As seen in migrating fibroblasts, this membrane tension could open stretch-activated calcium channels to generate the increased calcium transients that lead to filopodium stabilization.
In support of a role for tension in dendrite growth, we recently found that a stretching mechanism is responsible for the growth of the major class of sensory dendrites in C. elegans. In mature animals, these neurons each display a single unbranched dendrite extending to the nose where it collects information from the environment. Using time-lapse imaging, we watched single sensory dendrites as they formed in the embryo [26]. The cell bodies were born near the nose, anchored a short projection there, and then migrated away, stretching their dendrites out behind them as they crawled, in a phenomenon we termed retrograde extension [26]. Anchoring at the nose is required for dendrite growth: we isolated two mutants, dyf-7 and dex-1, in which neurons do not anchor at the nose, instead dragging their nascent dendrites with them, resulting in adult neurons with short dendrite stubs that fail to reach the nose [26]. DYF-7 and DEX-1 are extracellular matrix proteins produced by the neurons themselves and by neighboring cells, respectively, and act at the time and place of dendrite anchoring [26]. DYF-7 resembles zona pellucida (ZP) proteins that form a matrix around the vertebrate egg, while DEX-1 resembles the sperm protein zonadhesin which binds the ZP [26]. Together, they resemble tectorins, matrix-forming proteins of the vertebrate inner ear that anchor the tips of sensory hair cells used for hearing (reviewed in [27]). It is intriguing to think that DYF-7 and DEX-1 might similarly play a role in mechanosensation, possibly helping stretch-activated channels in the dendrites respond to membrane tension, much as the tectorial matrix helps mechanosensory channels in hair cells respond to the force of sound waves.
There is also evidence from axon studies that tension can promote neurite maturation. In one study, hippocampal neurons were plated in culture conditions under which they normally sprout neurites and then stochastically specify one neurite as the axon [28]. Using a force-calibrated glass needle, experimenters could stretch any single neurite and artificially induce it to take on characteristics of a mature axon, including long-term stability, development of a growth cone, and the localization of axon-specific markers like dephospho-tau [28]. In separate experiments, severing a growing Drosophila motor axon in vivo resulted in the severed distal segment continuing to migrate towards its target muscle and making contact with it, but failing to generate tension or to accumulate presynaptic vesicles at the nerve terminal [29]. Remarkably, using a glass needle to apply tension to the severed axon restored presynaptic vesicle clustering [29].
Mature axons have been shown to remain under resting tension in vitro [30] and in vivo [29, 31] and, in several systems, developing axons appear to grow by stretch-mediated mechanisms: oculomotor axons first contact their targets and then are stretched out as their cell bodies migrate across the midline [32, 33]; vestibulo-acoustic efferent axons make contacts to a ganglion and then are stretched as their cell bodies cross the floor plate [34]; C. elegans pharyngeal axons [35, 36] and Drosophila visual system axons [37] make contacts with neighboring cells which then are displaced, stretching the axons with them; and finally, zebrafish lateral line axons contact their targets and then stretch to keep up with them as the target migrates away, a phenomenon called axon towing [38]. Each of these examples bears strong similarities to dendrite growth by retrograde extension.
The hypothesis that membrane tension is the initiating event in filopodium stabilization, while speculative, is attractive because it depends only on the formation of a cell-cell adhesive junction, and thus could encompass the nearly infinite variety of adhesion molecules thought to control dendrite-axon recognition. It would also unite dendrite development with other areas of biology that involve tension sensing (see Box 1).
Box 1. Sensing tension.
All biological structures experience tension but, as tension is difficult to observe, it is easy to overlook. Tensegrity models have attempted to draw on architectural principles of tension and compression to understand the mechanical forces that influence many processes in cell biology [41].
One cell biological process in which tension has been proposed to play a major role is mitotic spindle assembly, in which kinetochores are thought to sense the tension generated as microtubules pull in opposite directions. Glass needle micromanipulations demonstrated that kinetochores respond to artificially-induced tension [42], although the physiological roles of attachment and tension have remained difficult to separate (reviewed in [43]). Recent experiments show that intrakinetochore stretch is an important step in sensing spindle attachment [44, 45]. Similarly, stretching or tension could play an important role in licensing a filopodium to mature into a dendrite branch.
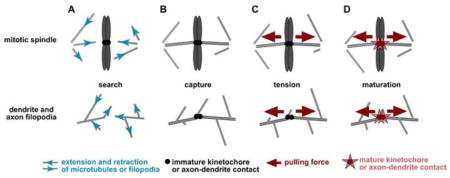
This figure highlights possible parallels in the use of tension during mitotic spindle assembly (left) and dendrite branch development (right). A, Microtubules and filopodia both use cycles of extension and retraction (blue arrows) to search a volume of space. B and C, Attachment to kinetochores or a target axon (black circles) may cause resistance to retractive force, generating tension (red arrows). D, This tension may signal the kinetochore or nascent synaptic contact to mature (red stars).
Conclusions
Immediately upon encountering a presynaptic partner, a filopodium receives a signal and initiates its decision to become a dendrite. Since axon arbors also grow by filopodium extension and retraction, it is possible that a similar signal controls the stabilization of an axon filopodium. Interestingly, contact-dependent signals also help trigger regeneration after neurite damage. For example, when a C. elegans axon is cut by a laser, its regrowth is inhibited by the persistence of a segment that remains in contact with the postsynaptic partner [39]. Conversely, in mutants unable to form proper contacts with postsynaptic partners during development, axons fail to stabilize and instead sprout ectopic neurites [40]. Given the widespread importance of sensing axon-dendrite contacts throughout the life of a neurite, a better understanding of how the budding twig of a filopodium is transformed into the sturdy branch of a dendrite is bound to bear abundant fruit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–260. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- 2.Meyer MP, Smith SJ. Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. J Neurosci. 2006;26:3604–3614. doi: 10.1523/JNEUROSCI.0223-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruthazer ES, Li J, Cline HT. Stabilization of axon branch dynamics by synaptic maturation. J Neurosci. 2006;26:3594–3603. doi: 10.1523/JNEUROSCI.0069-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohmann C, Myhr KL, Wong RO. Transmitter-evoked local calcium release stabilizes developing dendrites. Nature. 2002;418:177–181. doi: 10.1038/nature00850. [DOI] [PubMed] [Google Scholar]
- 5.Lohmann C, Finski A, Bonhoeffer T. Local calcium transients regulate the spontaneous motility of dendritic filopodia. Nat Neurosci. 2005;8:305–312. doi: 10.1038/nn1406. [DOI] [PubMed] [Google Scholar]
- 6.Gomez TM, Robles E, Poo M, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–1987. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- 7.Robles E, Huttenlocher A, Gomez TM. Filopodial calcium transients regulate growth cone motility and guidance through local activation of calpain. Neuron. 2003;38:597–609. doi: 10.1016/s0896-6273(03)00260-5. [DOI] [PubMed] [Google Scholar]
- 8••.Lohmann C, Bonhoeffer T. A role for local calcium signaling in rapid synaptic partner selection by dendritic filopodia. Neuron. 2008;59:253–260. doi: 10.1016/j.neuron.2008.05.025. Simultaneous time-lapse imaging of dendrite filopodia and their target axons shows that a filopodium begins deciding to stabilize within 1 min of contacting an appropriate presynaptic partner. [DOI] [PubMed] [Google Scholar]
- 9.Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 10.Rajan I, Cline HT. Glutamate receptor activity is required for normal development of tectal cell dendrites in vivo. J Neurosci. 1998;18:7836–7846. doi: 10.1523/JNEUROSCI.18-19-07836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ewald RC, Van Keuren-Jensen KR, Aizenman CD, Cline HT. Roles of NR2A and NR2B in the development of dendritic arbor morphology in vivo. J Neurosci. 2008;28:850–861. doi: 10.1523/JNEUROSCI.5078-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas K, Li J, Cline HT. AMPA receptors regulate experience-dependent dendritic arbor growth in vivo. Proc Natl Acad Sci USA. 2006;103:12127–12131. doi: 10.1073/pnas.0602670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen W, Da Silva JS, He H, Cline HT. Type A GABA-receptor-dependent synaptic transmission sculpts dendritic arbor structure in Xenopus tadpoles in vivo. J Neurosci. 2009;29:5032–5043. doi: 10.1523/JNEUROSCI.5331-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akerman CJ, Cline HT. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J Neurosci. 2006;26:5117–5130. doi: 10.1523/JNEUROSCI.0319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colón-Ramos DA. Synapse formation in developing neural circuits. Curr Top Dev Biol. 2009;87:53–79. doi: 10.1016/S0070-2153(09)01202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Annies M, Bittcher G, Ramseger R, Löschinger J, Wöll S, Porten E, Abraham C, Rüegg MA, Kröger S. Clustering transmembrane-agrin induces filopodia-like processes on axons and dendrites. Mol Cell Neurosci. 2006;31:515–524. doi: 10.1016/j.mcn.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 17.McCroskery S, Chaudhry A, Lin L, Daniels MP. Transmembrane agrin regulates filopodia in rat hippocampal neurons in culture. Mol Cell Neurosci. 2006;33:15–28. doi: 10.1016/j.mcn.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto-Miyai K, Sokolowska E, Zurlinden A, Gee CE, Lüscher D, Hettwer S, Wölfel J, Ladner AP, Ster J, Gerber U, et al. Coincident pre- and postsynaptic activation induces dendritic filopodia via neurotrypsin-dependent agrin cleavage. Cell. 2009;136:1161–1171. doi: 10.1016/j.cell.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 19.Kurusu M, Cording A, Taniguchi M, Menon K, Suzuki E, Zinn K. A screen of cell-surface molecules identifies leucine-rich repeat proteins as key mediators of synaptic target selection. Neuron. 2008;59:972–985. doi: 10.1016/j.neuron.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linhoff MW, Laurén J, Cassidy RM, Dobie FA, Takahashi H, Nygaard HB, Airaksinen MS, Strittmatter SM, Craig AM. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61:734–749. doi: 10.1016/j.neuron.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Kohsaka H, Nose A. Target recognition at the tips of postsynaptic filopodia: accumulation and function of Capricious. Development. 2009;136:1127–1135. doi: 10.1242/dev.027920. A leucine-rich-repeat (LRR) protein rides the tips of postsynaptic muscle filopodia and is required for their stable contact with a presynaptic axon. [DOI] [PubMed] [Google Scholar]
- 22.Coppolino MG, Woodside MJ, Demaurex N, Grinstein S, St-Arnaud R, Dedhar S. Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature. 1997;386:843–847. doi: 10.1038/386843a0. [DOI] [PubMed] [Google Scholar]
- 23.Watson PM, Humphries MJ, Relton J, Rothwell NJ, Verkhratsky A, Gibson RM. Integrin-binding RGD peptides induce rapid intracellular calcium increases and MAPK signaling in cortical neurons. Mol Cell Neurosci. 2007;34:147–154. doi: 10.1016/j.mcn.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 24•.Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H. Calcium flickers steer cell migration. Nature. 2009;457:901–905. doi: 10.1038/nature07577. This paper shows that membrane tension in migrating fibroblasts produces calcium transients through opening of the stretch-activated calcium channel TRPM7, raising the possibility that membrane tension could explain the calcium transients that accompany filopodium stabilization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacques-Fricke BT, Seow Y, Gottlieb PA, Sachs F, Gomez TM. Ca2+ influx through mechanosensitive channels inhibits neurite outgrowth in opposition to other influx pathways and release from intracellular stores. J Neurosci. 2006;26:5656–5664. doi: 10.1523/JNEUROSCI.0675-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Heiman MG, Shaham S. DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell. 2009;137:344–355. doi: 10.1016/j.cell.2009.01.057. This paper provides evidence for a stretch-based mechanism of dendrite extension, and identifies two extracellular proteins that help mediate dendrite stretch. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodyear R, Richardson G. Extracellular matrices associated with the apical surfaces of sensory epithelia in the inner ear: molecular and structural diversity. J Neurobiol. 2002;53:212–227. doi: 10.1002/neu.10097. [DOI] [PubMed] [Google Scholar]
- 28.Lamoureux P, Ruthel G, Buxbaum RE, Heidemann SR. Mechanical tension can specify axonal fate in hippocampal neurons. The Journal of Cell Biology. 2002;159:499–508. doi: 10.1083/jcb.200207174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Siechen S, Yang S, Chiba A, Saif T. Mechanical tension contributes to clustering of neurotransmitter vesicles at presynaptic terminals. Proc Natl Acad Sci USA. 2009;106:12611–12616. doi: 10.1073/pnas.0901867106. This paper provides in vivo evidence that axons are under resting tension, and shows that axon tension is required for neurite maturation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anava S, Greenbaum A, Ben Jacob E, Hanein Y, Ayali A. The regulative role of neurite mechanical tension in network development. Biophys J. 2009;96:1661–1670. doi: 10.1016/j.bpj.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Condron BG, Zinn K. Regulated neurite tension as a mechanism for determination of neuronal arbor geometries in vivo. Curr Biol. 1997;7:813–816. doi: 10.1016/s0960-9822(06)00343-5. [DOI] [PubMed] [Google Scholar]
- 32.Puelles L. A Golgi-study of oculomotor neuroblasts migrating across the midline in chick embryos. Anat Embryol. 1978;152:205–215. doi: 10.1007/BF00315925. [DOI] [PubMed] [Google Scholar]
- 33.Puelles L, Privat A. Do oculomotor neuroblasts migrate across the midline in the fetal rat brain? Anat Embryol. 1977;150:187–206. doi: 10.1007/BF00316650. [DOI] [PubMed] [Google Scholar]
- 34.Simon H, Lumsden A. Rhombomere-specific origin of the contralateral vestibulo-acoustic efferent neurons and their migration across the embryonic midline. Neuron. 1993;11:209–220. doi: 10.1016/0896-6273(93)90179-u. [DOI] [PubMed] [Google Scholar]
- 35.Axäng C, Rauthan M, Hall DH, Pilon M. Developmental genetics of the C. elegans pharyngeal neurons NSML and NSMR. BMC Dev Biol. 2008;8:38. doi: 10.1186/1471-213X-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mörck C, Axäng C, Pilon M. A genetic analysis of axon guidance in the C elegans pharynx. Dev Biol. 2003;260:158–175. doi: 10.1016/s0012-1606(03)00238-0. [DOI] [PubMed] [Google Scholar]
- 37.Schmucker D, Jäckle H, Gaul U. Genetic analysis of the larval optic nerve projection in Drosophila. Development. 1997;124:937–948. doi: 10.1242/dev.124.5.937. [DOI] [PubMed] [Google Scholar]
- 38.Gilmour D, Knaut H, Maischein HM, Nüsslein-Volhard C. Towing of sensory axons by their migrating target cells in vivo. Nat Neurosci. 2004;7:491–492. doi: 10.1038/nn1235. [DOI] [PubMed] [Google Scholar]
- 39.Wu Z, Ghosh-Roy A, Yanik MF, Zhang JZ, Jin Y, Chisholm AD. Caenorhabditis elegans neuronal regeneration is influenced by life stage, ephrin signaling, and synaptic branching. Proc Natl Acad Sci USA. 2007;104:15132–15137. doi: 10.1073/pnas.0707001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaefer AM, Hadwiger GD, Nonet ML. rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans. Neuron. 2000;26:345–356. doi: 10.1016/s0896-6273(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 41.Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003;116:1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Nicklas RB. Mitotic forces control a cell-cycle checkpoint. Nature. 1995;373:630–632. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- 43.Pinsky BA, Biggins S. The spindle checkpoint: tension versus attachment. Trends Cell Biol. 2005;15:486–493. doi: 10.1016/j.tcb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Maresca TJ, Salmon ED. Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. The Journal of Cell Biology. 2009;184:373–381. doi: 10.1083/jcb.200808130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchida KS, Takagaki K, Kumada K, Hirayama Y, Noda T, Hirota T. Kinetochore stretching inactivates the spindle assembly checkpoint. The Journal of Cell Biology. 2009;184:383–390. doi: 10.1083/jcb.200811028. [DOI] [PMC free article] [PubMed] [Google Scholar]