Abstract
Background and Purpose
Some studies have suggested that the association between obesity and ischemic stroke differs for blacks versus whites. We explored race- and sex-specific incidence rates and hazard ratios (HRs) of ischemic stroke in relation to multiple obesity measures.
Methods
Body mass index (BMI), waist circumference, and waist-to-hip ratio (WHR) were obtained at baseline between 1987 and 1989 in the Atherosclerosis Risk in Communities Study for 13,549 black and white subjects aged 45 and 64 years without a history of cardiovascular disease or cancer. The incidence of ischemic stroke was ascertained from surveillance of hospital records, over a median follow-up 16.9 years.
Results
Although crude incidence rates of ischemic stroke varied more than three-fold by race and sex from 1.2 per 1,000 person-years in white women to 4.3 in black women in the lowest BMI category (< 23.9kg/m2), and 2.2 in white women to 8.0 in black men in the highest BMI category (≥ 32.0kg/m2), the associations of ischemic stroke incidence (n=598) with obesity measures were positive and linear in all race-sex groups. The HR for the highest versus lowest quintile ranged from 1.43-2.12 for BMI, 1.65-3.19 for waist circumference, and 1.69-2.55 for WHR in models adjusted for age, education, smoking status, pack years, usual ethanol consumption, and physical activity. Additional adjustment for potential mediating factors (e.g., hypertension and diabetes mellitus) significantly attenuated the associations, suggesting these factors explain much of the obesity-stroke associations.
Conclusions
Degree of obesity, defined either by BMI, waist circumference or WHR, was a significant risk factor for ischemic stroke regardless of sex or race.
Introduction
Stroke is the third leading cause of death and the leading cause of serious, long-term disability in the United States. Blacks have about twice the incidence of stroke as whites, and this black-white ratio is greater in middle-age.1 It has been reported that blacks have poorer rehabilitation outcomes after acute stroke compared to whites.2 Because of these disparities, further studies are warranted addressing possible stroke prevention strategies, especially in blacks.
Well-established risk factors for stroke include hypertension, diabetes mellitus, and current smoking.3, 4 Whether overweight is a risk factor for stroke is less clear. Overweight/obesity as measured by body mass index (BMI) was a risk factor for stroke in US male physicians,5 Swedish men,6 and in Korean men7 and women.8 Nevertheless, there are conflicting results, e.g., null9 or no independent association10 in predominantly white women, or an inverse association in black women.11 In general, few studies of obesity and stroke risk exist among blacks, as summarized in Table 1.
Table 1.
Cohort or population-based case-control studies reporting associations of obesity measures with stroke for blacks and whites separately
| Study | Design | Population | Findings |
|---|---|---|---|
| NHANES I Epidemiologic Follow-up Study (Gillum RF et al., 2001) | Prospective cohort | US, 5,961 white and 975 black men and women without a history of stroke, 45-74 years of age in 1971-75 | BMI significantly inversely associated with non-hemorrhagic stroke incidence in black women; insignificant positive associations in white men and women; and insignificant inverse association in black men.* |
| The Northern Manhattan Stroke Study (Suk SH et al., 2003) | Population-based case-control | US, 576 incident ischemic stroke cases and 1,142 controls recruited in 1993-97 | Obesity defined as WHR>0.93 for men and >0.86 for women was significantly positively associated with ischemic stroke incidence in both whites (OR: 3.3, 95% CI: 1.3-8.6) and blacks (OR: 2.4, 95% CI: 1.3-4.7).† BMI was not significantly associated with ischemic stroke. |
| The South London Stroke Register (Hajat C et al., 2004) | Population-based case-control | UK, 664 incident ischemic stroke cases and 716 controls recruited in 1995-99 | Obesity defined as WHR>0.98 for men and >0.88 for women did not differ between black African cases (39%) and controls (39%).‡ |
| The Black Pooling Project (Abell JE et al., 2008) | Meta-analysis of cohort studies using individual data | US, 27,691 white and 4,853 blacks, whose baseline information was collected between 1960-80 | Obesity defined as BMI 30.0-34.9 kg/m2, compared to normal weight (BMI: 18.5-24.9) was associated with significantly increased stroke mortality in white men (RR: 1.50, 95% CI: 1.05-2.14) and women (RR: 1.42, 95% CI: 1.07-1.60), but not in black men (RR: 0.74) and women (RR: 1.08) after adjustment for age and smoking. |
NHANES indicates National Health and Nutrition Examination Survey; US, the United States; BMI, body mass index; WHR, waist-to-hip ratio; OR, odds ratio; CI, confidence interval; RR, relative risk; UK, the United Kingdom
These patterns were observed in separate models adjusted for age, other confounders, and confounders and mediators. Confounders included age, smoking, history of heart disease, education, physical activity, alcohol, and hemoglobin. Mediators included blood pressure, medication use, diabetes, and serum total cholesterol.
Sex and age matched and adjusted for hypertension, diabetes mellitus, cardiac disease, smoking status, physical activity, alcohol, LDL cholestrol, HDL cholesterol, and education.
Unadjusted. Controls were seven years younger than the cases, and included more women (64% versus 52%)
In addition, there is still a debate about which measure of excess adiposity is most closely associated with disease risk. For example, waist-to-hip ratio (WHR), but not BMI, was related to stroke incidence in a study of US male health professionals aged 40 to 75.12 Similar findings were observed in multi-ethnic13 and German14 case-control studies and a prospective cohort of Swedish middle-aged women.15 In contrast, a study of Finnish men and women suggested that stroke risk was associated with the World Health Organization BMI classification, rather than with measures of abdominal obesity like waist circumference.16 Both BMI or waist circumference, or both, are often promoted as the best measures of obesity for defining health risks. However, BMI may be easier to standardize than waist circumference, the location of which is less-well defined. Further, these two measures are highly correlated.3 It is important to discover whether stroke risk varies on the basis of abdominal obesity and BMI categories within race groups.
In the present study, we describe incidence rates and hazard ratios of ischemic stroke according to race and sex for BMI, waist circumference and WHR. We tested the hypothesis that there are differences in the association for black versus white men and women. We also hypothesized that any associations of obesity measures with ischemic stroke would be mediated by known stroke predictors (particularly hypertension and diabetes). We also explored whether there is a graded increase in ischemic stroke incidence rates across National Institute of Health (NIH) BMI-waist circumference categories.17
Methods
Study population
The ARIC Study included a cohort of 15,792 persons between 45 and 64 years of age at recruitment in 1987 through 1989.18 Population samples were selected by probability sampling methods from Forsyth County, NC (n=4,035); Jackson, MS (black only, n=3,728); northwest suburbs of Minneapolis, MN (n=4,009); and Washington County, MD (n=4,020). Baseline response rates ranged from 46% in Jackson to 65-67% in the other three communities. Participants were subsequently contacted annually by telephone and three additional clinic visits. The retention rate was 93% through 2005, and the rates did not differ appreciably between races.
Obesity measures
Body mass index (BMI: kg/m2) was calculated from measurements of weight to the nearest pound and height to the nearest centimeter, with the participants wearing a scrub suit and no shoes. The ratio of waist (umbilical level) and hip (maximum buttocks) circumference (waist-to-hip ratio: WHR) was calculated as a measure of fat distribution in addition to waist circumference alone. The inter-technician reliability coefficients for waist and hip circumference, and WHR were all r > 0.94.19
Baseline assessment
Questionnaires were used to assess educational level, cigarette smoking, alcohol drinking, leisure time sports index, use of antihypertensive or diabetic medications, and histories of physician-diagnosed diabetes, cancer, CHD or stroke. The sports index was derived from questionnaire items on hours per week spent in up to four sports and the months per year each sport was done. By assuming a sport intensity level (light, moderate or heavy), a sport score was calculated ranging from 1 (lowest) to 5 (highest).20 Prevalent CHD at baseline was defined for exclusion as a reported history of a physician-diagnosed myocardial infarction, prior myocardial infarction detected by ECG, or prior cardiovascular surgery or coronary angioplasty. Three blood pressure measurements were taken with a random-zero sphygmomanometer; the last two measurements were averaged. Fasting blood levels of glucose as well as the levels of HDL cholesterol, albumin, and von Willebrand factor (vWF) were measured centrally by standard methods. Prevalent diabetes was defined a history of, or treatment for, diabetes, a fasting glucose level of 126 mg/dl or greater, or a casual blood glucose level of 200 mg/dl or greater.
Ascertainment of incident stroke
Ischemic strokes that occurred by December 31, 2005 (median follow-up 16.9 years) were included in the present study. During annual telephone contacts, interviewers asked each ARIC participant to list all hospitalizations during the past year; hospital records were obtained. In addition, all local hospitals annually provided lists of stroke discharges (International Classification of Diseases, Ninth Revision, Clinical Modification codes 430-438), which were scrutinized for ARIC participants' discharges. Details on quality assurance for ascertainment and classification of stroke are described elsewhere.21 Briefly, the stroke diagnosis was assigned according to criteria adapted from the National Survey of Stroke. A minimum criterion was sudden or rapid onset of neurological symptoms lasting for more than 24 hours or leading to death, not secondary to trauma, neoplasm, hematological abnormality, infection, or vasculitis. A stroke was classified as ischemic when a brain CT or MRI revealed acute infarction or showed no evidence of hemorrhage. Stroke that occurred during hospitalization for other condition or procedures (cardiac catheterization, open heart surgery, cerebral angiography, and carotid endarterectomy) were included (n=28). However, out-of-hospital stroke was not ascertained and validated. Along with a computer-based classification, cases were independently reviewed by a physician who was provided with a detailed report of the information abstracted from the medical record as well as the full discharge summary, the CT and MRI scan reports, reports from any neurological consults, and admission history. The final diagnosis was determined by agreement of computer and reviewer classification. In the rare occasion when there was a disagreement between computer and reviewer classifications, the diagnosis was adjudicated by a second physician-reviewer. CT or MRI was available for all the ischemic stroke cases except one cardioembolic stroke classified with carotid artery ultrasound and clinical information. The 92 hemorrhagic stroke cases identified were censored at the time of their occurrence.
Exclusions
Of the 15,744 blacks and whites in ARIC, we excluded 1,787 participants (blacks: 365, whites: 1,422) who at baseline had a prevalent stroke, CHD, or cancer since CVD treatment and associated behavioral change or cancer-induced weight loss could confound the association between obesity measures and stroke. Participants lacking baseline measurements of BMI, waist or hip circumference (n=32) were also excluded. Those with missing values of potential confounding variables, including leisure time sport index, smoking status and pack-years of smoking, usual ethanol intake, and educational level, were then excluded (n=376) leaving a final sample of 5,930 men and 7,619 women (n=13,549 in total).
Statistical analysis
Analyses were done separately for blacks and whites and men and women. Cox proportional hazards regression was used to calculate age- and multivariate-adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) of ischemic stroke incidence in relation to quintiles of the obesity measures. Quintile cutoff values of each obesity measure were created by averaging the four race-sex specific quintile cutoff values. The first model (model I) adjusted for age, smoking status (current, past, or never), pack-years of smoking, usual ethanol intake (grams/week), educational level (high school graduate or not) and leisure time sport index (1.0-1.9, 2.0-2.4, 2.5-2.9, 3.0-5.0) score. In a mediation model (Model II, n=288 more excluded), we further adjusted for systolic blood pressure, use of antihypertensive medication, prevalent diabetes, and blood levels of HDL cholesterol, vWF and albumin simultaneously in light of a previous ARIC paper that identified them as predictors of incident stroke.22
The assumption of hazard proportionality was tested by a model including follow-up time by obesity measure quintile interaction. The follow-up time was first treated as a continuous scale, and then dichotomized at year 10 in the model. Interactions for race by obesity measure quintile, sex by obesity measure quintile, and race by sex by obesity measure quintile were tested in Model I using Wald test at a significance level of 0.1.
Cubic spline analyses were performed to qualitatively evaluate any non-linear relationship between obesity measures and stroke incidence. Spline analyses were carried out using the truncated sample since extreme values would be over-influential.
Finally, we performed another analysis to estimate stroke incidence according to the NIH classification table of overweight and obesity based jointly on BMI and waist circumference. The reference group was normal weight and normal waist circumference -- 102 cm (40 inches) or less in men and 88 cm (35 inches) or less in women.
The population attributable fraction (PAF) was calculated as p multiplied by [(HR for a category being considered – HR for the reference category)/HR for the category being considered], where p is the proportion of cases that are exposed in whichever category is being considered.23
Sensitivity analyses were also performed after truncating the sample at the 1st and 99th percentile of each obesity measure, because extreme values had some impact on the association for blacks. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
At baseline, mean BMI, waist circumference and WHR were 30.8 kg/m2, 100.3 cm and 0.90 in black women; 27.6 kg/m2, 96.7 cm and 0.94 in black men; 26.6 kg/m2, 93.0 cm and 0.89 in white women; and 27.4 kg/m2, 99.5 cm and 0.97 in white men, respectively (Table 2).
Table 2.
Baseline characteristics of study sample according to race and sex, ARIC, 1987-89
| Black | White | |||
|---|---|---|---|---|
| variable | Women (n=2,330) |
Men (n=1,364) |
Women (n=5,289) |
Men (n=4,566) |
| Age (years) | 53.2 (5.7) | 53.5 (5.9) | 53.8 (5.7) | 54.4 (5.7) |
| Weight (kg) | 82.0 (17.7) | 85.7 (16.6) | 69.8 (14.9) | 85.2 (13.5) |
| Height (cm) | 162.7 (6.0) | 175.9 (6.7) | 161.6 (6.3) | 175.8 (6.9) |
| Body mass index (kg/m2) | 30.8 (6.5) | 27.6 (4.9) | 26.6 (5.5) | 27.4 (3.9) |
| Waist (cm) | 100.3 (16.2) | 96.7 (12.8) | 93.0 (14.9) | 99.5 (10.3) |
| Hip (cm) | 110.7 (12.4) | 102.7 (9.9) | 104.2 (10.8) | 102.7 (7.5) |
| Waist-hip ratio | 0.90 (0.08) | 0.94 (0.06) | 0.89 (0.08) | 0.97 (0.05) |
| High school graduate or more (%) | 37.9 | 38.1 | 41.6 | 53.9 |
| Current smoker (%) | 23.6 | 37.4 | 24.0 | 24.3 |
| Pack-years* | 356 (353) | 543 (479) | 462 (368) | 633 (456) |
| Usual ethanol intake (g/week) | 10.7 (40.1) | 70.2 (148.2) | 24.4 (53.4) | 71.4 (121.3) |
| Leisure time sports index (>=3) (%) | 13.1 | 19.8 | 26.3 | 39.4 |
| Hypertensive medication (%) | 46.6 | 32.8 | 25.5 | 19.9 |
| Diabetes mellitus (%) | 18.9 | 16.1 | 7.6 | 9.0 |
| Systolic blood pressure (mmHg) | 127.8 (21.2) | 129.4 (20.5) | 117.0 (17.6) | 120.0 (15.9) |
| Diastolic blood pressure (mmHg) | 77.9 (11.4) | 82.3 (12.7) | 69.9 (9.8) | 73.8 (9.9) |
| High density lipoprotein cholesterol (mg/dl) | 58.1 (17.1) | 50.7 (16.8) | 57.7 (17.1) | 43.1 (12.5) |
| von Willebrand factor (%) | 134.9 (58.7) | 130.1 (55.3) | 110.6 (41.7) | 111.9 (42.6) |
| Albumin (g/dl) | 3.8 (0.3) | 3.9 (0.3) | 3.9 (0.3) | 3.9 (0.2) |
Values are mean (standard deviation) or otherwise as indicated.
: Mean pack years of smoking was calculated among current and former smokers.
During a median of 16.9 years of follow-up (max=19.1 y), 598 incident ischemic strokes were identified. Crude incidence rates of ischemic stroke varied more than three-fold by race and sex from 1.2 per 1,000 person-years in white women to 4.3 in black women in the lowest BMI category, and 2.2 in white women to 8.0 in black men in the highest BMI category (Table 3). The absolute difference in incidence rates for being in the highest BMI quintile compared to the lowest quintile ranged from 1.9 per 1,000 person-years in white women to 5.6 per 1,000 person-years in black women. The HRs of ischemic stroke in relation to BMI quintiles were generally linear in black men and white men and women (all trend p<0.05), with HRs for the highest versus lowest quintile ranging 1.43–2.12 (Model 1). Although the trend p across BMI quintiles for black women was also significant, the HR estimate for the highest quintile was only 1.43 (95%CI: 0.81-2.53). Nevertheless, interaction testing suggested no significant effect modification by race or sex. Continuous BMI showed a significant linear positive association with ischemic stroke only in black men (HR1 in Table 3), but exclusion of extreme observations indicated significant positive associations in all race and sex subgroups (HR2 in Table 3, Figure 1-a, b, c, d). Adjustment for potential mediating factors (model II, Table 3), particularly hypertension and diabetes, as expected, significantly attenuated most BMI associations with ischemic stroke.
Table 3.
Body mass index and incidence and hazard ratios (95%CI) of ischemic stroke by race and sex, ARIC, 1987-2005
| Quintile | Q1 | Q2 | Q3 | Q4 | Q5 | Trend p | HR1 | HR2 |
|---|---|---|---|---|---|---|---|---|
| Range (median) (kg/m2) | 14.4- <23.9 (22.2) | 23.9- <26.2 (25.1) | 26.2- <28.6 (27.3) | 28.6- <32.0 (30.1) | 32.0- <65.9 (35.1) | |||
| Black women | ||||||||
| n of cases/N | 15/253 | 13/322 | 25/393 | 35/487 | 73/875 | |||
| incidence rate | 3.9 | 2.6 | 4.1 | 4.6 | 5.5 | |||
| Age-adjusted | 1 (Reference) | 0.62 (0.30-1.30) | 0.99 (0.52-1.88) | 1.09 (0.60-2.00) | 1.38 (0.79-2.40) | 0.013 | 1.10 (0.98-1.25) | 1.19 (1.02-1.39) |
| Model I | 1 (Reference) | 0.66 (0.32-1.40) | 1.02 (0.53-1.94) | 1.15 (0.62-2.13) | 1.43 (0.81-2.53) | 0.016 | 1.10 (0.97-1.24) | 1.17 (1.00-1.38) |
| Model II | 1 (Reference) | 0.67 (0.30-1.48) | 0.95 (0.48-1.89) | 0.93 (0.48-1.81) | 0.88 (0.47-1.65) | 0.99 | 0.91 (0.79-1.05) | 0.92 (0.77-1.10) |
| Black men | ||||||||
| n of cases/N | 17/298 | 16/262 | 23/296 | 22/290 | 25/218 | |||
| incidence rate | 4.3 | 4.1 | 5.1 | 4.9 | 8.0 | |||
| Age-adjusted | 1 (Reference) | 1.00 (0.50-1.99) | 1.18 (0.63-2.20) | 1.16 (0.61-2.19) | 1.89 (1.02-3.50) | 0.029 | 1.28 (1.05-1.55) | 1.29 (1.04-1.61) |
| Model I | 1 (Reference) | 1.15 (0.57-2.29) | 1.36 (0.72-2.57) | 1.33 (0.70-2.55) | 2.12 (1.13-4.00) | 0.015 | 1.34 (1.10-1.62) | 1.35 (1.08-1.69) |
| Model II | 1 (Reference) | 1.07 (0.52-2.24) | 1.11 (0.56-2.18) | 1.00 (0.49-2.06) | 1.19 (0.59-2.41) | 0.68 | 1.05 (0.85-1.31) | 1.08 (0.85-1.37) |
| White women | ||||||||
| n of cases/N | 39/1,928 | 25/1,035 | 19/776 | 24/731 | 29/819 | |||
| incidence rate | 1.2 | 1.5 | 1.5 | 2.0 | 2.2 | |||
| Age-adjusted | 1 (Reference) | 1.11 (0.67-1.83) | 1.13 (0.66-1.96) | 1.44 (0.87-2.40) | 1.70 (1.05-2.75) | 0.019 | 1.22 (1.05-1.42) | 1.22 (1.02-1.44) |
| Model I | 1 (Reference) | 1.15 (0.69-1.90) | 1.16 (0.67-2.02) | 1.49 (0.89-2.50) | 1.78 (1.08-2.93) | 0.015 | 1.25 (1.07-1.46) | 1.23 (1.03-1.47) |
| Model II | 1 (Reference) | 0.97 (0.58-1.62) | 0.83 (0.47-1.46) | 0.93 (0.54-1.61) | 0.78 (0.45-1.38) | 0.41 | 0.94 (0.78-1.13) | 0.91 (0.74-1.12) |
| White men | ||||||||
| n of cases/N | 28/769 | 38/1,104 | 51/1,210 | 54/956 | 27/527 | |||
| incidence rate | 2.3 | 2.2 | 2.6 | 3.6 | 3.3 | |||
| Age-adjusted | 1 (Reference) | 0.93 (0.57-1.52) | 1.13 (0.72-1.80) | 1.61 (1.02-2.54) | 1.51 (0.89-2.57) | 0.010 | 1.30 (1.09-1.56) | 1.31 (1.08-1.58) |
| Model I | 1 (Reference) | 1.01 (0.62-1.65) | 1.26 (0.79-2.01) | 1.85 (1.17-2.94) | 1.85 (1.08-3.17) | <0.001 | 1.39 (1.16-1.66) | 1.41 (1.16-1.71) |
| Model II | 1 (Reference) | 1.04 (0.63-1.73) | 1.26 (0.77-2.06) | 1.64 (1.00-2.69) | 1.26 (0.70-2.25) | 0.15 | 1.17 (0.96-1.41) | 1.17 (0.96-1.44) |
HR denotes hazard ratio; CI, confidence interval.
Model I: Adjusted for age, education, smoking status, pack years, usual ethanol consumption, and physical activity.
Model II: Model I + systolic blood pressure, hypertension medication, diabetes, and blood levels of high density lipoprotein cholesterol, von Willebrand factor and albumin.
Trend test was performed by assigning the median value of each quintile to corresponding individuals and treating it as a continuous variable in the model.
HR1: HR per 1 standard deviation (5.4 kg/m2) increment of body mass index.
HR2: HR per 5.4 kg/m2 increment of body mass index with sample truncated at the 1st and 99th percentile (n=13,024)
Figure 1.
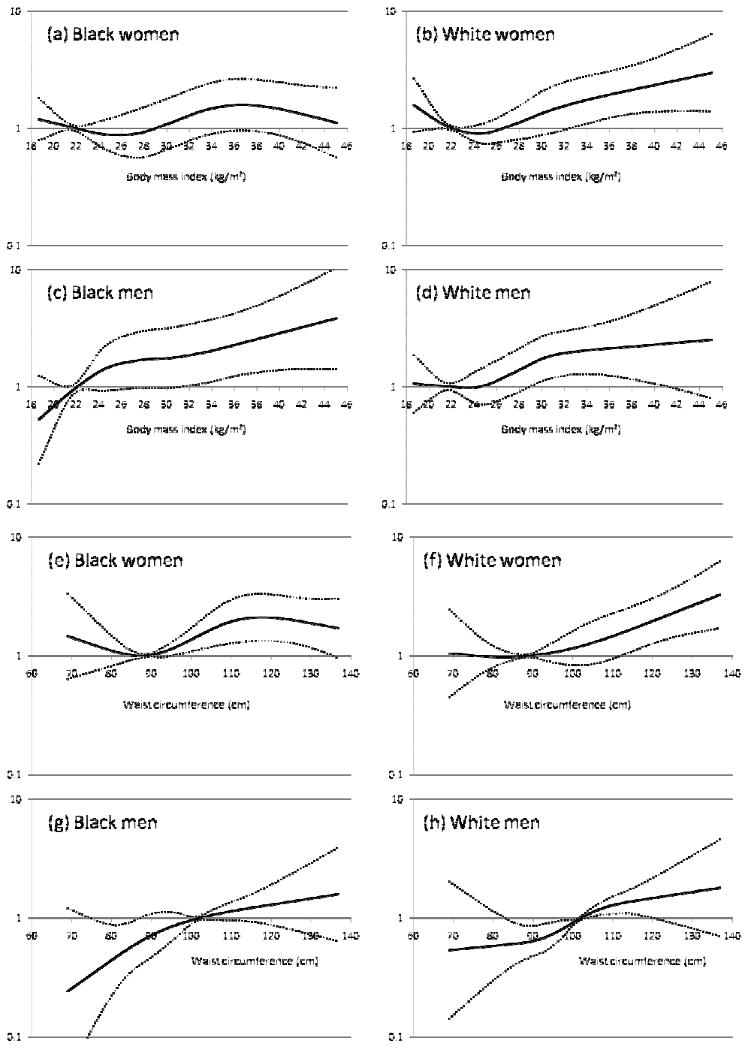
Hazard ratio of ischemic stroke in relation to body mass index (a: black women, b: white women, c: black men, d: white men), and in relation to waist circumference (e: black women, f: white women, g: black men, h: white men) by cubic spline regression analysis, ARIC
The solid line represents the hazard ratio; dotted line, 95% confidence intervals. The reference values were set at 22 kg/m2 (a, b, c, d), and at 88 cm in women and 102 cm in men (e, f, g, h). The hazard ratios (HRs) were adjusted for age, education, smoking status, pack years, usual ethanol consumption, and physical activity. The sample for the spline analysis was truncated at the 1st and 99th percentile of body mass index.
As shown in Table 4, quintiles of waist circumference were positively and strongly associated with the risk of ischemic stroke in all demographic groups, and there was no interaction by race or sex. In most race-sex groups, continuous waist circumference was also related positively to ischemic stroke incidence (HR1 and HR2 in Table 4, Figure 1-e, f, g, h). The association of waist circumference with ischemic stroke incidence was attenuated with adjustment for mediating factors (e.g., hypertension and diabetes) but incompletely in white men (trend p=0.021 in Model II).
Table 4.
Waist circumference and incidence and hazard ratios (95%CI) of ischemic stroke by race and sex, ARIC, 1987-2005
| Quintile | Q1 | Q2 | Q3 | Q4 | Q5 | Trend p | HR1 | HR2 |
|---|---|---|---|---|---|---|---|---|
| Range (median) (cm) | 52-86 (81) | 87-92 (90) | 93-99 (96) | 100-107 (103) | 108-178 (115) | |||
| Black women | ||||||||
| n of cases/N | 25/456 | 16/331 | 22/425 | 31/435 | 67/683 | |||
| incidence rate | 3.5 | 3.1 | 3.3 | 4.7 | 6.6 | |||
| Age-adjusted | 1 (Reference) | 0.86 (0.46-1.61) | 0.88 (0.49-1.56) | 1.27 (0.75-2.15) | 1.75 (1.11-2.78) | 0.0011 | 1.21 (1.06-1.37) | 1.28 (1.10-1.48) |
| Model I | 1 (Reference) | 0.84 (0.45-1.57) | 0.84 (0.47-1.50) | 1.22 (0.71-2.09) | 1.65 (1.03-2.65) | 0.0029 | 1.19 (1.04-1.35) | 1.26 (1.08-1.46) |
| Model II | 1 (Reference) | 0.76 (0.39-1.49) | 0.73 (0.40-1.31) | 0.79 (0.45-1.39) | 0.83 (0.50-1.38) | 0.74 | 0.94 (0.81-1.09) | 0.96 (0.82-1.14) |
| Black men | ||||||||
| n of cases/N | 10/272 | 20/254 | 22/333 | 25/278 | 26/227 | |||
| incidence rate | 2.7 | 5.3 | 4.4 | 6.0 | 8.2 | |||
| Age-adjusted | 1 (Reference) | 1.99 (0.93-4.25) | 1.58 (0.75-3.35) | 2.18 (1.05-4.55) | 2.95 (1.42-6.11) | 0.0030 | 1.33 (1.11-1.61) | 1.46 (1.18-1.82) |
| Model I | 1 (Reference) | 2.29 (1.07-4.91) | 1.84 (0.87-3.91) | 2.37 (1.13-4.97) | 3.19 (1.53-6.67) | 0.0026 | 1.36 (1.12-1.65) | 1.48 (1.19-1.84) |
| Model II | 1 (Reference) | 2.63 (1.16-5.96) | 1.62 (0.71-3.68) | 1.89 (0.81-4.40) | 2.07 (0.90-4.73) | 0.31 | 1.07 (0.86-1.34) | 1.17 (0.91-1.50) |
| White women | ||||||||
| n of cases/N | 37/1,978 | 21/869 | 24/898 | 17/686 | 37/858 | |||
| incidence rate | 1.1 | 1.5 | 1.6 | 1.5 | 2.7 | |||
| Age-adjusted | 1 (Reference) | 1.17 (0.68-1.99) | 1.21 (0.73-2.03) | 1.09 (0.61-1.94) | 1.95 (1.23-3.09) | 0.0078 | 1.28 (1.10-1.48) | 1.17 (0.98-1.38) |
| Model I | 1 (Reference) | 1.17 (0.68-2.00) | 1.27 (0.75-2.12) | 1.08 (0.60-1.93) | 1.97 (1.23-3.15) | 0.0091 | 1.29 (1.10-1.50) | 1.17 (0.98-1.39) |
| Model II | 1 (Reference) | 0.98 (0.57-1.69) | 0.91 (0.53-1.55) | 0.69 (0.37-1.26) | 0.90 (0.53-1.54) | 0.57 | 0.99 (0.83-1.19) | 0.88 (0.72-1.08) |
| White men | ||||||||
| n of cases/N | 12/369 | 22/715 | 49/1,361 | 61/1,234 | 54/887 | |||
| incidence rate | 2.1 | 1.9 | 2.2 | 3.2 | 4.0 | |||
| Age-adjusted | 1 (Reference) | 0.91 (0.45-1.84) | 1.05 (0.56-1.98) | 1.49 (0.80-2.77) | 1.85 (0.99-3.47) | <0.001 | 1.38 (1.15-1.65) | 1.39 (1.14-1.69) |
| Model I | 1 (Reference) | 0.95 (0.47-1.92) | 1.14 (0.61-2.15) | 1.66 (0.89-3.09) | 2.15 (1.14-4.03) | <0.001 | 1.45 (1.21-1.75) | 1.48 (1.21-1.81) |
| Model II | 1 (Reference) | 0.95 (0.45-1.99) | 1.14 (0.58-2.23) | 1.54 (0.79-3.01) | 1.61 (0.81-3.21) | 0.021 | 1.21 (1.00-1.47) | 1.25 (1.01-1.54) |
HR denotes hazard ratio; CI, confidence interval.
Model I: Adjusted for age, education, smoking status, pack years, usual ethanol consumption, and physical activity.
Model II: Model I + systolic blood pressure, hypertension medication, diabetes, and blood levels of high density lipoprotein cholesterol, von Willebrand factor and albumin.
Trend test was performed by assigning the median value of each quintile to corresponding individuals and treating it as a continuous variable in the model.
HR1: HR per 1 standard deviation (13.9 cm) increment of waist circumference.
HR2: HR per 13.9 cm increment of waist circumference with sample truncated at the 1st and 99th percentile (n=13,059).
The analyses using WHR showed similar results to those for waist circumference (Table 5). The HR of ischemic stroke for the highest quintile of WHR ranged 1.69-2.55 across race-sex groups and there was no interaction by race or sex.
Table 5.
Waist-to-hip ratio and incidence and hazard ratios (95%CI) of ischemic stroke by race and sex, ARIC, 1987-2005
| Quintile | Q1 | Q2 | Q3 | Q4 | Q5 | Trend p | HR1 | HR2 |
|---|---|---|---|---|---|---|---|---|
| Range (median) | 0.49- <0.87 (0.82) | 0.87- <0.91 (0.89) | 0.91- <0.94 (0.93) | 0.94- <0.98 (0.96) | 0.99- <1.39 (1.01) | |||
| Black women | ||||||||
| n of cases/N | 33/781 | 29/398 | 26/359 | 27/393 | 46/399 | |||
| incidence rate | 2.6 | 4.7 | 4.7 | 4.5 | 8.2 | |||
| Age-adjusted | 1 (Reference) | 1.71 (1.04-2.81) | 1.61 (0.96-2.70) | 1.61 (0.97-2.67) | 2.75 (1.75-4.33) | <0.001 | 1.34 (1.15-1.57) | 1.38 (1.16-1.63) |
| Model I | 1 (Reference) | 1.64 (1.00-2.71) | 1.46 (0.87-2.45) | 1.46 (0.88-2.44) | 2.45 (1.55-3.87) | <0.001 | 1.29 (1.10-1.51) | 1.32 (1.11-1.57) |
| Model II | 1 (Reference) | 1.20 (0.71-2.02) | 1.09 (0.64-1.85) | 0.88 (0.52-1.51) | 1.17 (0.72-1.91) | 0.87 | 0.96 (0.81-1.14) | 0.99 (0.83-1.20) |
| Black men | ||||||||
| n of cases/N | 5/126 | 13/279 | 30/345 | 31/341 | 24/273 | |||
| incidence rate | 2.7 | 3.1 | 5.9 | 6.2 | 6.4 | |||
| Age-adjusted | 1 (Reference)* | 1.92 (1.07-3.45) | 2.00 (1.12-3.58) | 1.98 (1.07-3.68) | 0.027 | 1.42 (1.08-1.88) | 1.39 (1.04-1.87) | |
| Model I | 1 (Reference) | 1.91 (1.06-3.44) | 1.92 (1.07-3.46) | 1.69 (0.91-3.15) | 0.098 | 1.32 (1.00-1.74) | 1.28 (0.96-1.72) | |
| Model II | 1 (Reference) | 1.65 (0.88-3.09) | 1.28 (0.66-2.50) | 1.03 (0.51-2.06) | 0.80 | 0.98 (0.72-1.35) | 0.97 (0.69-1.36) | |
| White women | ||||||||
| n of cases/N | 38/2,071 | 20/971 | 23/800 | 24/779 | 31/668 | |||
| incidence rate | 1.1 | 1.3 | 1.7 | 1.9 | 3.0 | |||
| Age-adjusted | 1 (Reference) | 1.04 (0.61-1.77) | 1.27 (0.75-2.13) | 1.25 (0.74-2.10) | 1.90 (1.17-3.10) | 0.013 | 1.30 (1.09-1.55) | 1.31 (1.09-1.57) |
| Model I | 1 (Reference) | 1.00 (0.58-1.70) | 1.19 (0.71-2.02) | 1.20 (0.71-2.02) | 1.76 (1.08-2.88) | 0.029 | 1.27 (1.06-1.52) | 1.28 (1.06-1.54) |
| Model II | 1 (Reference) | 0.85 (0.50-1.47) | 0.83 (0.48-1.44) | 0.77 (0.44-1.33) | 0.93 (0.54-1.60) | 0.68 | 1.01 (0.83-1.23) | 1.01 (0.82-1.24) |
| White men | ||||||||
| n of cases/N | 3/129 | 10/424 | 22/895 | 62/1,455 | 101/1,663 | |||
| incidence rate | 1.4 | 1.5 | 1.5 | 2.7 | 4.0 | |||
| Age-adjusted | 1 (Reference)* | 1.06 (0.53-2.10) | 1.82 (1.00-3.30) | 2.48 (1.39-4.41) | <0.001 | 1.69 (1.41-2.04) | 1.87 (1.47-2.38) | |
| Model I | 1 (Reference) | 1.09 (0.55-2.16) | 1.87 (1.03-3.41) | 2.55 (1.42-4.57) | <0.001 | 1.70 (1.41-2.06) | 1.88 (1.47-2.40) | |
| Model II | 1 (Reference) | 1.04 (0.52-2.08) | 1.62 (0.88-2.99) | 1.96 (1.07-3.60) | 0.0036 | 1.49 (1.21-1.84) | 1.61 (1.24-2.10) | |
HR denotes hazard ratio; CI, confidence interval.
Model I: Adjusted for age, education, smoking status, pack years, usual ethanol consumption, and physical activity.
Model II: Model I + systolic blood pressure, hypertension medication, diabetes, and blood levels of high density lipoprotein cholesterol, von Willebrand factor and albumin.
Trend test was performed by assigning the median value of each quintile to corresponding individuals and treating it as a continuous variable in the model.
HR1: HR per 1 standard deviation (0.078) increment of waist-to-hip ratio.
HR2: HR per 0.078 increment of waist-to-hip ratio with sample truncated at the 1st and 99th percentile (n=13,012).
: The lowest two categories were collapsed.
The NIH risk classification table performed well predicting stroke risk in both blacks and whites: the risk categories paralleled the observed incidence rates of ischemic stroke (online only Table 6).
Hypertension did not modify the associations of any obesity measure with ischemic stroke incidence in a race-sex collapsed sample (p for interaction>0.10, online only Table 7). BMI, waist circumference and WHR were positively associated (p<0.05) with the incidence of ischemic stroke in subjects both with and without hypertension.
The PAF represents the proportion that might be prevented by eliminating a risk factor. The PAFs of ischemic stroke for being in top 40% of BMI, waist circumference, or WHR, compared with the lower 60%, were 17.7%, 21.2%, and 21.7%, respectively.
Discussion
In this ARIC analysis, blacks had about two to three times higher incidence rates of ischemic stroke compared to whites in each obesity quintile. However, obesity, regardless of the measure, was a risk factor for ischemic stroke, without statistical evidence for differences by race or sex.
For whites, ARIC findings were consistent with most previous studies. Prior studies in blacks have shown less association of obesity with stroke, except for the Northern Manhattan Stroke Study which found that obesity defined as a high sex-specific WHR was significantly positively associated with ischemic stroke incidence.13 The Black Pooling Project, a meta-analysis of cohort studies using individual data, did not find association between BMI and stroke mortality in blacks (incidence not examined).24 The unadjusted prevalence of obesity defined by sex-specific WHR did not differ among black ischemic stroke cases and controls in the South London Stroke Register Study25 though the controls were seven years younger than the cases, and included more women (64% versus 52%). Finally, the lowest BMI quartile was associated with a surprisingly higher non-hemorrhagic stroke incidence in the NHANES Epidemiologic Follow-up Study (NHEFS).11
One of the reasons for the discrepancy between ARIC and NHEFS, a cohort study like ours, may be differences in the average degree of obesity. The median BMI values of black women (29.8 kg/m2) and men (27.1 kg/m2) at baseline (1987-89) in ARIC were 2.5 kg/m2 greater than those at baseline (1971-75) in NHEFS (27.4 kg/m2 in women and 24.5 kg/m2 in men). The rightward shift of the BMI distribution in blacks over the decades, which was greater than in whites (0.2 and 1.3 kg/m2 increase in white women and men, respectively) may account for significant positive association between BMI and ischemic stroke incidence in ARIC blacks. Further, NHEFS included only 955 blacks compared to 3,694 in the present study, which made our analysis more powerful. In addition, about half of NHEFS participants were aged 65 to 74 at baseline in contrast to ours that included only 45 to 64 years old at baseline. The older age distribution, with possible impact of weight loss in the elderly, might have distorted BMI-stroke association in NHEFS.26
Based on the fact that we consistently found positive associations between obesity measures and ischemic stroke incidence in blacks in the present study, we believe that obesity, however it is measured, significantly increases ischemic stroke risk in blacks as well as in whites. From a public health point of view, the estimated PAF values suggested that 18-20% of ischemic stroke occurrence may be accounted for by BMI >=28.1kg/m2, waist circumference >=100 cm, or WHR >=0.95. We are aware of debates on PAF calculation methods.23, 27, 28 In the present study we defined the obese group as top 40% of each obesity-measure and calculated the PAF using a multivariate (Model I) adjusted HR23. Since disease risk in relation to obesity measure is expected to be continuous, misclassification due to the selection of a certain cutoff points is likely to underestimate PAF.29 Although there might be possible inaccuracies related to the method employed to calculate PAF, we considered such errors would not be critical.
In all race-sex groups, significant positive associations of obesity measures with ischemic stroke incidence were largely explained by mediators related to obesity. In fact, either blood pressure or diabetes mellitus alone in Model II could have eliminated significant associations between obesity measure quintiles and ischemic stroke incidence. Yet, obesity did not fully account for the higher blood pressure and stroke risk of blacks compared to whites. For example, the mean systolic blood pressure in the lowest quintile of BMI in blacks was comparable to the systolic blood pressure in the highest quintile in whites (125.2 versus 126.3 mmHg).
Hypertension did not modify the associations of obesity measures with ischemic stroke incidence. In other words, in subjects both with or without hypertension, there were significant positive associations between obesity measures and ischemic stroke incidence. These associations were, however, significantly attenuated with additional adjustment for systolic blood pressure. Given the strong association between obesity and hypertension and other risk factors including diabetes mellitus, obesity would be an important target for the prevention of ischemic stroke.
Strengths of the present study included the prospective design; large sample size and number of ischemic strokes which allowed race-sex specific analyses; the long duration of follow-up, systematic surveillance and confirmation of outcome events; the detailed assessment of potential confounding and mediating variables; and the standardized recording of multiple obesity measures. As a limitation, although our goal was a careful description of ischemic stroke incidence using anthropometric measurements relevant in the field of public health, formal comparison among these measures for the prediction of ischemic stroke should be considered. Second, most of the blacks were from one field center and the whites from three other centers, limiting the generalizability of our findings to other cultural or socio-economic contexts.
In conclusion, the degree of obesity defined either by BMI, waist circumference or WHR was a significant risk factor for ischemic stroke incidence regardless of sex or race. Prevention and control of obesity has a potential to reduce the incidence of ischemic stroke.
Acknowledgments
Financial Support: The ARIC Study was funded by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Ottenbacher KJ, Campbell J, Kuo YF, Deutsch A, Ostir GV, Granger CV. Racial and ethnic differences in postacute rehabilitation outcomes after stroke in the United States. Stroke. 2008;39:1514–1519. doi: 10.1161/STROKEAHA.107.501254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folsom AR, Prineas RJ, Kaye SA, Munger RG. Incidence of hypertension and stroke in relation to body fat distribution and other risk factors in older women. Stroke. 1990;21:701–706. doi: 10.1161/01.str.21.5.701. [DOI] [PubMed] [Google Scholar]
- 4.Folsom AR, Rasmussen ML, Chambless LE, Howard G, Cooper LS, Schmidt MI, Heiss G. Prospective associations of fasting insulin, body fat distribution, and diabetes with risk of ischemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Diabetes Care. 1999;22:1077–1083. doi: 10.2337/diacare.22.7.1077. [DOI] [PubMed] [Google Scholar]
- 5.Kurth T, Gaziano JM, Berger K, Kase CS, Rexrode KM, Cook NR, Buring JE, Manson JE. Body mass index and the risk of stroke in men. Arch Intern Med. 2002;162:2557–2562. doi: 10.1001/archinte.162.22.2557. [DOI] [PubMed] [Google Scholar]
- 6.Jood K, Jern C, Wilhelmsen L, Rosengren A. Body mass index in mid-life is associated with a first stroke in men: A prospective population study over 28 years. Stroke. 2004;35:2764–2769. doi: 10.1161/01.STR.0000147715.58886.ad. [DOI] [PubMed] [Google Scholar]
- 7.Song YM, Sung J, Davey Smith G, Ebrahim S. Body mass index and ischemic and hemorrhagic stroke: A prospective study in Korean men. Stroke. 2004;35:831–836. doi: 10.1161/01.STR.0000119386.22691.1C. [DOI] [PubMed] [Google Scholar]
- 8.Park JW, Lee SY, Kim SY, Choe H, Jee SH. Bmi and stroke risk in Korean women. Obesity (Silver Spring) 2008;16:396–401. doi: 10.1038/oby.2007.67. [DOI] [PubMed] [Google Scholar]
- 9.Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 10.Kurth T, Gaziano JM, Rexrode KM, Kase CS, Cook NR, Manson JE, Buring JE. Prospective study of body mass index and risk of stroke in apparently healthy women. Circulation. 2005;111:1992–1998. doi: 10.1161/01.CIR.0000161822.83163.B6. [DOI] [PubMed] [Google Scholar]
- 11.Gillum RF, Mussolino ME, Madans JH. Body fat distribution, obesity, overweight and stroke incidence in women and men--the NHANES I Epidemiologic Follow-up Study. Int J Obes Relat Metab Disord. 2001;25:628–638. doi: 10.1038/sj.ijo.0801590. [DOI] [PubMed] [Google Scholar]
- 12.Walker SP, Rimm EB, Ascherio A, Kawachi I, Stampfer MJ, Willett WC. Body size and fat distribution as predictors of stroke among US men. Am J Epidemiol. 1996;144:1143–1150. doi: 10.1093/oxfordjournals.aje.a008892. [DOI] [PubMed] [Google Scholar]
- 13.Suk SH, Sacco RL, Boden-Albala B, Cheun JF, Pittman JG, Elkind MS, Paik MC. Abdominal obesity and risk of ischemic stroke: The Northern Manhattan Stroke Study. Stroke. 2003;34:1586–1592. doi: 10.1161/01.STR.0000075294.98582.2F. [DOI] [PubMed] [Google Scholar]
- 14.Winter Y, Rohrmann S, Linseisen J, Lanczik O, Ringleb PA, Hebebrand J, Back T. Contribution of obesity and abdominal fat mass to risk of stroke and transient ischemic attacks. Stroke. 2008;39:3145–3151. doi: 10.1161/STROKEAHA.108.523001. [DOI] [PubMed] [Google Scholar]
- 15.Lu M, Ye W, Adami HO, Weiderpass E. Prospective study of body size and risk for stroke amongst women below age 60. J Intern Med. 2006;260:442–450. doi: 10.1111/j.1365-2796.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 16.Hu G, Tuomilehto J, Silventoinen K, Sarti C, Mannisto S, Jousilahti P. Body mass index, waist circumference, and waist-hip ratio on the risk of total and type-specific stroke. Arch Intern Med. 2007;167:1420–1427. doi: 10.1001/archinte.167.13.1420. [DOI] [PubMed] [Google Scholar]
- 17.Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158:1855–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 18.The Atherosclerosis Risk in Communities (ARIC) Study: Design and objectives. The ARIC Investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 19.Ferrario M, Carpenter MA, Chambless LE. Reliability of body fat distribution measurements. The ARIC Study baseline cohort results. Atherosclerosis Risk in Communities Study. Int J Obes Relat Metab Disord. 1995;19:449–457. [PubMed] [Google Scholar]
- 20.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 21.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 22.Chambless LE, Heiss G, Shahar E, Earp MJ, Toole J. Prediction of ischemic stroke risk in the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2004;160:259–269. doi: 10.1093/aje/kwh189. [DOI] [PubMed] [Google Scholar]
- 23.Greenland S. Re: “Confidence limits made easy: Interval estimation using a substitution method”. Am J Epidemiol. 1999;149:884. doi: 10.1093/oxfordjournals.aje.a009906. author reply 885-886. [DOI] [PubMed] [Google Scholar]
- 24.Abell JE, Egan BM, Wilson PW, Lipsitz S, Woolson RF, Lackland DT. Differences in cardiovascular disease mortality associated with body mass between black and white persons. Am J Public Health. 2008;98:63–66. doi: 10.2105/AJPH.2006.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hajat C, Tilling K, Stewart JA, Lemic-Stojcevic N, Wolfe CD. Ethnic differences in risk factors for ischemic stroke: A European case-control study. Stroke. 2004;35:1562–1567. doi: 10.1161/01.STR.0000131903.04708.b8. [DOI] [PubMed] [Google Scholar]
- 26.Park HS, Song YM, Cho SI. Obesity has a greater impact on cardiovascular mortality in younger men than in older men among non-smoking Koreans. Int J Epidemiol. 2006;35:181–187. doi: 10.1093/ije/dyi213. [DOI] [PubMed] [Google Scholar]
- 27.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: Examples and software. Cancer Causes Control. 2007;18:571–579. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 28.Flegal KM, Graubard BI. Estimates of excess deaths associated with body mass index and other anthropometric variables. Am J Clin Nutr. 2009;89:1213–1219. doi: 10.3945/ajcn.2008.26698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wacholder S, Benichou J, Heineman EF, Hartge P, Hoover RN. Attributable risk: Advantages of a broad definition of exposure. Am J Epidemiol. 1994;140:303–309. doi: 10.1093/oxfordjournals.aje.a117252. [DOI] [PubMed] [Google Scholar]


