Abstract
Conjugated linoleic acids (CLA) are known to exert several isomer-specific biological effects, but their mechanisms of action are unclear. In order to determine whether the physicochemical effects of CLA on membranes play a role in their isomer-specific effects, we synthesized phosphatidylcholines (PCs) with 16:0 at sn-1 position and one of four CLA isomers (trans10 cis12 (A), trans9 trans11 (B), cis9 trans11 (C), and cis9 cis11 (D)) at sn-2, and determined their biophysical properties in monolayers and bilayers. The surface areas of the PCs with the two natural CLA (A and C) were similar at all pressures, but they differed significantly in presence of cholesterol, with PC-A condensing more than PC-C. Liposomes of PC-A similarly showed increased binding of cholesterol compared to PC-C liposomes. PC-A liposomes were less permeable to carboxyfluorescein compared to PC-C liposomes. The PC with two trans double bonds (B) showed the highest affinity to cholesterol and lowest permeability. The two natural CLA PCs (A and C) stimulated lecithin-cholesterol acyltransferase activity by 2-fold, whereas the unnatural CLA PCs (B and D) were inhibitory. These results suggest that the differences in the biophysical properties of CLA isomers A and C may partly contribute to the known differences in their biological effects.
1. Introduction
Conjugated linoleic acids (CLA) are the naturally occurring isomers of linoleic acid, which are present in the dairy products and ruminant meats, and which have been reported to have several beneficial effects, including protection against cancer, obesity, heart disease and immune dysfunction [1–3]. Of the 16 known isomers of CLA, two (cis 9 trans 11 or c9 t11, and trans 10 cis 12 or t10 c12) are predominant in the natural products, as well as in the synthetic supplements sold commercially. These two major isomers differ significantly in their biological effects, although their structural differences are subtle. Thus the c9 t11 isomer has been shown to be responsible for most of the anti-carcinogenic effects, whereas the t10 c12 isomer appears to be responsible for most of the anti-obesity effects in mice [1,3]. Despite extensive investigations from several laboratories, neither the exact mechanisms involved in the multiple biological effects of CLA, nor the basis for the isomer-specific effects are clear. The majority of the proposed mechanisms involve differential effects of CLA isomers on gene transcription, specifically as ligands or inducers of transcription factors PPARs, SREBPs, and NFκB [2,4]. However, CLA are relatively weak ligands compared to the other (more abundant) unsaturated fatty acids [3], and the studies with PPARα knockout mice in fact showed that the effects of CLA on body fat distribution are independent of this transcription factor [5]. CLA, like other polyunsaturated fatty acids (PUFA), have been shown to be incorporated into membrane lipids, the extent of incorporation ranging from <1% to >50% of total phospholipid fatty acids in various cells [6–11]. The enrichment of membrane lipids with dietary polyunsaturated fatty acids (PUFA) is known to affect the distribution and function of several membrane raft-associated proteins [12–14], and therefore we were interested in investigating whether the effects of dietary CLA on membrane functions of the cells would account for at least part of their biological effects. Since several receptors, signaling proteins, and enzymes are associated with the membranes, the pleiotropic effects of CLA may be explained by their effects on membrane structure and function. As a first step in testing this hypothesis, we synthesized phosphatidylcholines (PCs) containing palmitic acid at the sn-1 position and one of four isomers of CLA, c9t11, t10c12, cis 9 cis 11 (c9c11), or trans 9 trans 11, (t9t11) at the sn-2 position, and tested their physicochemical effects on model membranes, including the monolayer properties, fluidity, permeability, oxidizability, and the ability to bind cholesterol. In addition, we tested the effect of the presence of CLA isomers at the sn-2 position, on the activity of LCAT, the enzyme that transfers the sn-2 acyl group from PC to cholesterol, and is responsible for the synthesis of most of the cholesteryl esters present in plasma [15]. The results show significant differences in the physicochemical effects of various CLA isomers on the model membrane properties, as well as on the enzyme activity.
2. Materials and Methods
2.1. Materials
All isomers of CLA (t10c12, t9t11, c9t11, and c9c11) were purchased from Matreya, Inc. Lyso PC (1-palmitoyl), 16:0–18:2 PC (with unconjugated LA), dipalmitoyl PC (16:0–16:0 PC), and egg sphingomyelin were obtained from Avanti Polar Lipids. Carboxyfluorescein, methyl B cyclodextrin, and unlabeled cholesterol were obtained from Sigma Chemical Co (St. Louis, MO). Labeled cholesterol (4-14C) (53.0 mCi/mmol) was purchased from Perkin Elmer (Boston, MA) and dipalmitoyl-[1-14C] PC (55.0 mCi/mmol) was purchased from American Radiolabeled Chemicals Inc (St.Louis, MO). Sn-1-palmitoyl-2-diphenylhexatriene PC (DPH-PC) was obtained from Invitrogen (Carlsbad, CA). CLA-PCs, containing 16:0 at sn-1 and various isomers of CLA at sn-2 were synthesized by the procedure of Paltauf and Hermitter [16] using 1-palmitoyl lyso PC, and free CLA. The PCs were purified by silicic acid column chromatography, and the purity of the compounds assessed by TLC. Lipid phosphorus of the samples was determined by the modified Bartlett procedure [17]. More than 90% of CLA was present in the sn-2 position, as determined by snake venom PLA2 treatment [18].
2.2. Monolayer measurements
Force-area isotherms of CLA-PCs and cholesterol were obtained using a computer- controlled Nima 302M Langmuir-type surface balance (Nima Technologies, Coventry, England) on a subphase of purified water (Nanopure Infinity filtration system, Dubuque, IA; resistivity α17.5 MΩ cm). The PTFE trough and barriers were cleaned before and after each run with chloroform and isopropanol. Glassware was cleaned in base (ethanol-2 M KOH, 1/1) and acid (1 M HNO3) and rinsed thoroughly in purified water [19]. CLA-PC and cholesterol solutions were prepared by dissolving lipids in HPLC-grade chloroform at a concentration of ~1 mg ml−1. CLA- PC-cholesterol solutions were prepared by mixing appropriate volumes of stock lipids and were spread on the aqueous surface using a Hamilton (Reno, NV) digital syringe. Monolayer compression at a rate of 10 cm min−1 was initiated after a 10 min delay to allow for solvent evaporation. All compressions were done in triplicate to ensure reproducibility.
Compression data were collected using proprietary software from Nima and analyzed using Origin software (Northampton, MA). Condensation of CLA-PCs by cholesterol was determined as the deviation from ideal mixing according to the equation
| (Equation 1) |
where X1 is the mole fraction of component 1 and (A1)π and (A2)π are the mean molecular areas of components 1 and 2, respectively, at surface pressure π. Deviations from the linearity expressed by Eq. 1 represent attractive (negative deviations) or repulsive (positive deviations) interactions between the components of the binary films [20]. The degree of condensation reported herein was calculated using equation 2:
| [21] | (Equation 2) |
where Aideal is the mean molecular area calculated assuming ideal additivity and Aexp is that observed experimentally.
2.3. Cholesterol binding studies
The affinity of the liposomes prepared with each CLA-PC for cholesterol was determined by the rate of transfer of labeled cholesterol from the methyl β cyclodextrin (MBCD)-labeled cholesterol complex to the liposomes by a modification of the procedure of Niu and Litman [22]. Liposomes of each CLA-PC were prepared in 10 mM PIPES buffer by extrusion through 0.1 μm polycarbonate filters, and incubated with 0.5 mM 4-14C -cholesterol-10 mM MBCD complex for 2 h at 37 °C. The sample was diluted with an equal volume of 4M NaCl, and filtered through Microcon YM30 filter at 37 °C. The filter was washed 3 times with 2M NaCl and the radioactivity retained on the filter was determined in a liquid scintillation counter. The percent of labeled cholesterol bound to the liposomes was calculated and expressed as the % of the value obtained with control PC (16:0–18:2 PC, unconjugated LA). In the experiments where the effect of SM was studied, the liposomes contained 20 mol% of egg SM and 80 mol% of the test PC.
2.4. Permeability studies
The permeability property of the liposomes was measured by the rate of leakage of the trapped carboxyfluorescein, as described Roach et al [23]. Briefly, small unilamellar vesicles (SUV) of CLA-PCs were prepared by sonication in the presence of 60 mM carboxyfluorescein, and the unincorporated dye was removed by gel filtration on a Sepahdex G-50 column. The liposomes were spiked with a trace of dipalmitoyl[1-14C]-PC in order to monitor the column fractions for liposomes. An aliquot of the caroboxyfluorescein-labeled liposomes was incubated with a 30- fold excess of buffer (10 mM PO4, 90 mM KCl, pH 7.2) and the increase in fluorescence due to the leakage of the dye into the medium was monitored in a spectrofluorometer at 25 °C for 2 h (excitation 490 nm, emission 520 nm). At the end of this period, the total amount of carboxyfluorescein sequestered in the liposome (Fmax) was determined by disrupting the liposomes with 0.1% Triton X100 (final concentration). The rate of carboxyfluorescein leakage in the linear range of the graph was calculated by the formula
| (Equation 3) |
where F2 is the fluorescence value at the end of the linear range (t2), F1 is the fluorescence at the beginning of the linear range (t1), and Fmax is the total fluorescence in the sample. This value for each CLA PC was then expressed as % of the value obtained with control liposomes (16:0–18:2 PC, containing unconjugated LA).
2.5. Differential scanning calorimetry (DSC)
Calorimetric measurements were performed using a Microcal VP-DSC calorimeter. Chloroform solutions of dipalmitoyl PC (2 mg) alone or with 10 mol% or 25 mol% CLA-PC were evaporated under nitrogen, dissolved in 300 μl of ethanol, re-evaporated under nitrogen, and traces of the solvent were removed under vacuum. The lipid was dispersed in 1 ml of degassed distilled water by vortexing, and the sample was incubated at 50 °C for 20 min before loading into the sample chamber of DSC. Distilled water was used in the reference cell. At least 4 heating scans were performed for each sample, with scan rate of 60 °C/h. Scan 4 was used for the calculation of transition temperature (Tm), calorimetric enthalpy (ΔH), van’t Hoff enthalpy (ΔHvH), and cooperativity units (CU), with the software (Microcal Origin) provided by the manufacturer (non-two state Cursor Init model).
2.6. Oxidizability of CLA-PC
CLA-PC liposomes (0.1 μmol PC), prepared by extrusion, were incubated with 1 mM AAPH at 37 °C for 2 h. Aliquots of the reaction mixture were extracted at 0 h,1 h, and 2 h after adding 17:0–17:0 PC as the internal standard. The total lipid extract was methylated with methanolic HCl, and the fatty acid methyl esters analyzed by GC as described previously [24], except for the modification of the temperature programming. Initial temperature was set at 150 °C for 1.0 min, raised to 210 °C at the rate of 3 °C/min, and then to 225 °C at the rate of 2 °C/min and maintained at this temperature for 15 min. The percent of CLA remaining intact was calculated by taking the 0 h value as 100%. Control PC (16:0–18:2 PC, unconjugated LA) was oxidized under identical conditions for comparison.
2.7. Fluidity measurements
The fluidity of the liposomes was determined by measuring fluorescence polarization of DPH-PC incorporated into the CLA-PC liposomes. The liposomes containing test PC and DPH-PC at a molar ratio of 200:1 were prepared by sonication in 10 mM Tris-0.15 M NaCl-1mM EDTA, pH 7.4. The polarization values were determined at various temperatures in a spectrofluorometer (model PC1, ISI, Champagne, IL) (excitation 362 nm, emission 430 nm). The temperature of the cuvette was maintained with a circulating water bath, and measured with a probe. The sample was equilibrated for 10 min at each temperature before measuring the polarization values. The anisotropy values were calculated with the formula
| (Equation 4) |
where Iv is the intensity parallel to the excitation plane, Ih is the intensity perpendicular to the excitation plane, and g is the grating factor.
2.8. Lecithin-cholesterol acyltransferase (LCAT) assay
LCAT activity was assayed as described previously [25], using proteoliposome substrates containing the test PC: 4-14C cholesterol: apoprotein A-I at a molar ratio of 250:12: 0.8, prepared by the cholate dialysis procedure [26]. The enzyme preparation was the partially purified human plasma LCAT (Phenyl Sepharose eluate, prepared as described by Chen and Albers [27]). The reaction mixture contained 20 μl of proteoliposomes corresponding to 50 nmol of PC and 2.4 nmol of labeled cholesterol, 20 μg of enzyme of enzyme preparation, 10 mM mercaptoethanol, and 0.5 mg of human serum albumin in a total volume of 200 μl. The reaction was carried out for 30 min at 37 °C, and stopped by the addition of 800 μl of ethanol. The lipids were extracted with hexane containing 30 μg each of unlabeled cholesterol and cholesteryl oleate (carriers) and the radioactivity in cholesterol and cholesteryl ester was determined after TLC separation, as described previously [25]. The enzyme activity was calculated as % of cholesterol esterified per h. The activity obtained with each CLA-PC substrate was then expressed as % of the activity obtained with unconjugated 16:0–18:2 PC substrate (control).
3. Results
3.1. Monolayer properties of CLA-PCs
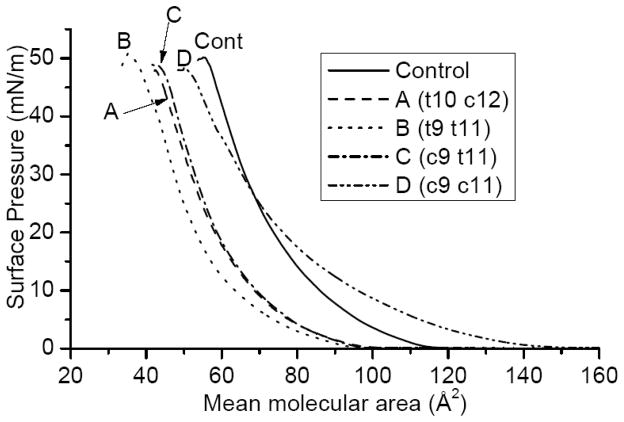
The surface-area isotherms of the four CLA-PCs and the control (unconjugated) 16:0–18:2 PC were determined in the absence and presence of increasing mol fraction of cholesterol. As shown in Fig. 1a, the molecular areas occupied by the PCs containing the two naturally occurring CLA (c9 t11, and t10 c12) were significantly lower than that of the unconjugated 16:0–18:2 PC. The conjugation of the double bonds alone appears to decrease the limiting surface area by about 10% (compare unconjugated control vs c9 c11 isomer), although this effect is not apparent at low surface pressure. The presence of a trans double bond alone decreases the molecular surface area significantly (compare the curve of c9 c11 CLA-PC with that of c9 t11 isomer). The presence of two trans double bonds (t9 t11 isomer) decreased the surface area further. The decrease in surface area due to trans unsaturation in the sn-2 acyl group has previously been reported for PCs with unconjugated fatty acids [28].
Fig. 1.
a) Surface area isotherms of CLA-PCs and control (unconjugated 18:2) PC. Compressions were performed at ambient temperature (~24 °C) on a surface of purified water at a rate of 10 cm2/min. Isotherms are representative of at least 3 runs; error ranges were ± 3 Å2 molecule−1.
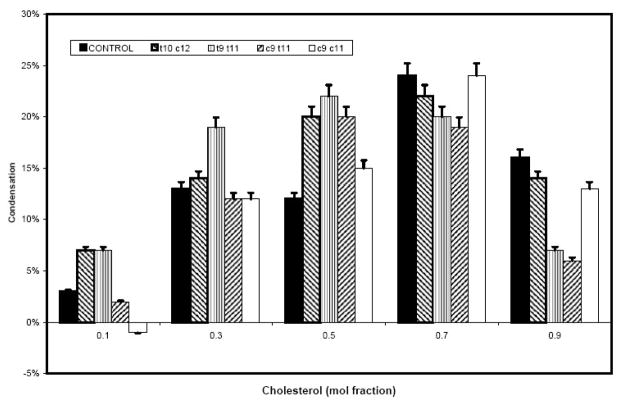
b) Condensation of CLA-PCs and control PC monolayers by cholesterol at 20 mN/m. Data for condensation were derived from the pressure-area curves similar to those shown in Fig. 1a, as described in Materials and Methods.
Although the two PCs containing the natural CLA did not differ in their molecular surface areas, they differed significantly with respect to their interaction with cholesterol at 20 mN. (Fig 1b). Thus, at all cholesterol concentrations except 50 mol%, the t10 c12 CLA-PC was more condensed than the c9 t11 CLA-PC, suggesting a higher affinity of the former for cholesterol in monolayers. The maximum condensation occurred at 70 mol% cholesterol for the t10 c12 isomer, whereas it was at 50 mol% for the c9 t11 isomer. A cholesterol content of up to 50 mol% condensed the CLA-PC containing two trans double bonds (t9 t11) more than other isomers; above this cholesterol concentration, the double cis isomer was more condensed.
3.2. Differential scanning calorimetry
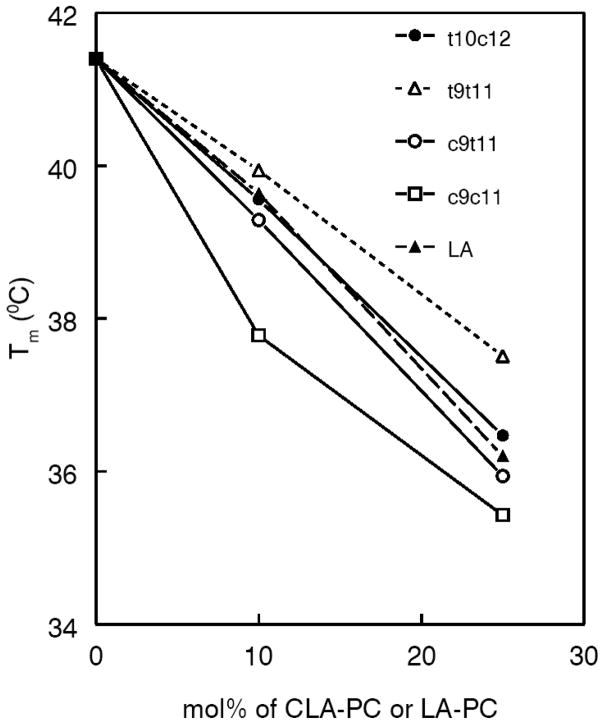
The effect of incorporating increasing amounts of CLA-PC on the phase transition temperature (Tm) of 16:0–16:0 PC (DPPC) is shown in Fig. 2. The main transition peak was broadened, and the Tm decreased in presence of all CLA-PCs and control PC. The two natural CLA-containing PCs differed in their effects, with the c9t11 CLA-PC showing a more ‘fluidizing’ effect than the t10c12 isomer. This is consistent with previous studies on free CLA, which showed that c9t11 CLA had lower Tm than that of t10c12 CLA [29]. The double cis CLA-PC showed the maximum decrease in Tm, while the double trans CLA-PC isomer showed the smallest change. The results show that the presence of a cis double bond at carbon 9 has the maximal ‘fluidizing’ effect. Furthermore, conjugation of double bonds increased the ‘fluidizing’ effect (compare the results for LA-PC vs c9c11 CLA-PC). The pre-transition peak (35.5 °C) exhibited by pure 16:0–16:0 PC disappeared in the presence of all CLA-PCs except the t9t11 CLA-PC, in which case it was diminished but not eliminated completely (not shown). In addition to the Tm, the cooperative unit (C.U.) value was calculated for each sample by the equation
Fig. 2. Effect of CLA-PC isomers on the transition temperatures (Tm) of dipalmitoyl (16:0–16:0) PC.
Multilamellar liposomes of 16:0–16:0 PC (2 mg/ml) alone or containing 10 mol% or 25 mol% of CLA-PC or LA-PC (control) were prepared in distilled water and at least 4 heating scans were performed in Microcal VP-DSC calorimeter. The transition temperatures (Tm) were determined from the fourth scan.
| (Equation 5) |
The C.U. value for 100% DPPC was 539. This value decreased significantly in the presence of 10 mol% and 25 mol% respectively, of LA-PC (33.8 and 23.9), t10c12 isomer (28.2 and 24.1), t9t11 isomer (54.8 and 48.6), c9t11 isomer (28.1 and 19.2), and c9c11 isomer (13.9 and 17.9). These results indicate that the c9t11 isomer interferes with phase transition cooperativity of disaturated PC more than the t10c12 isomer, at least at the higher concentration. As expected, the double-trans isomer has lower effect on C.U. of DPPC, than the double-cis isomer.
3.3. Cholesterol binding properties of CLA-PCs
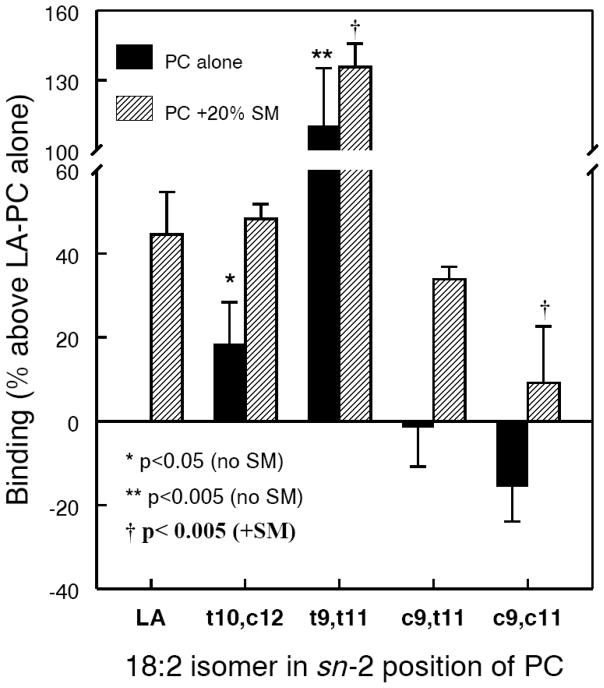
The affinity of various CLA-PCs to cholesterol was studied by the ability of the PC liposomes to extract cholesterol from cholesterol-cyclodextrin complex, as described previously [30], but employing radioactive cholesterol. In addition to the binding studies employing PC alone, we studied the effect of the presence of 20 mol% sphingomyelin (SM), which is a normal constituent of the plasma membrane, and which is known to influence the affinity of the membrane to cholesterol [31]. As shown in Fig. 3, substitution of normal (unconjugated) 18:2 at sn-2 of PC with t10 c12 CLA increased the affinity to cholesterol by 20%, whereas substitution with c9 t11 CLA had no effect. Substitution with the double cis CLA (c9 c11) decreased the affinity to cholesterol, whereas the substitution with a double trans CLA (t9 t11) more than doubled the binding of cholesterol compared to the control PC. These results suggest that the presence of a cis double bond at carbon 9 is important in maintaining the normal cholesterol binding property of PC. The presence of a trans double bond at carbon 9 or 10 significantly increases the cholesterol binding property, and the presence of two trans double bonds increases the binding more dramatically, as has been reported for the unconjugated trans fatty acid PCs [30]. Inclusion of 20 mol% SM increased the binding capacity of all PCs, but the magnitude of the SM effect was not equal. Maximal impact of SM was observed with the PCs that had a cis double bond at carbon 9 (c9 c11 and c9 t11).
Fig. 3. Cholesterol-binding properties of CLA-PCs.
The ability of the liposomes prepared from each of the CLA-PCs to extract labeled cholesterol from [14C]-cholesterol-β-methyl cyclodextrin complex was determined as described in the text, in the absence and presence of 20 mol% egg SM. The amount of cholesterol extracted by control LA-PC (16:0–18:2, unconjugated) was taken as the baseline, and all other values were expressed as % above or below that value. The values are shown are mean ± S.D of 3–5 experiments. * p< 0.05, and ** p<0.005 compared to LA-PC alone.
† p<0.005, compared to LA-PC + 20 mol% SM.
3.4. Binding of cholesterol to 16:0–18:1 PCs
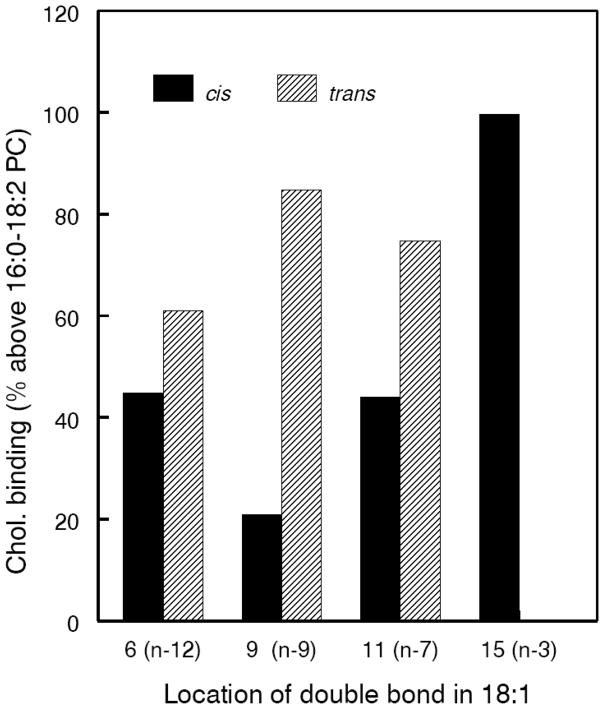
The importance of the double bond at carbon 9 for cholesterol binding is more clearly demonstrated with 16:0–18:1 PCs, where there is only one double bond. As shown in Fig. 4, the PC containing a cis double bond at carbon 9 showed the lowest affinity to cholesterol, among the PCs containing a cis double at various positions of sn-2 18:1. Furthermore, the presence of a trans double bond increases the affinity to cholesterol at all positions, compared to the cis double bond, with the effect being most evident at carbon 9. Previous studies showed that the introduction of a cis double bond at carbon 9 of an 18 carbon fatty acid has the maximal effect on the transition temperature of the phospholipids containing the fatty acid [32]. Our studies show that the substitution of a trans double bond for cis double bond at this position also increases the cholesterol binding property to the maximum.
Fig. 4. Cholesterol-binding properties of isomers of 16:0–18:1 PC.
PCs containing the different isomers of 18:1 at sn-2 position were chemically synthesized, and their affinity to cholesterol was determined as described in the text. The values are expressed as % of the value obtained with 16:0–18:2 (unconjugated) PC, and are average of two separate experiments. The n-3 trans isomer of 18:1 free fatty acid was not available, and therefore is not included in the study.
3.5. Membrane permeability
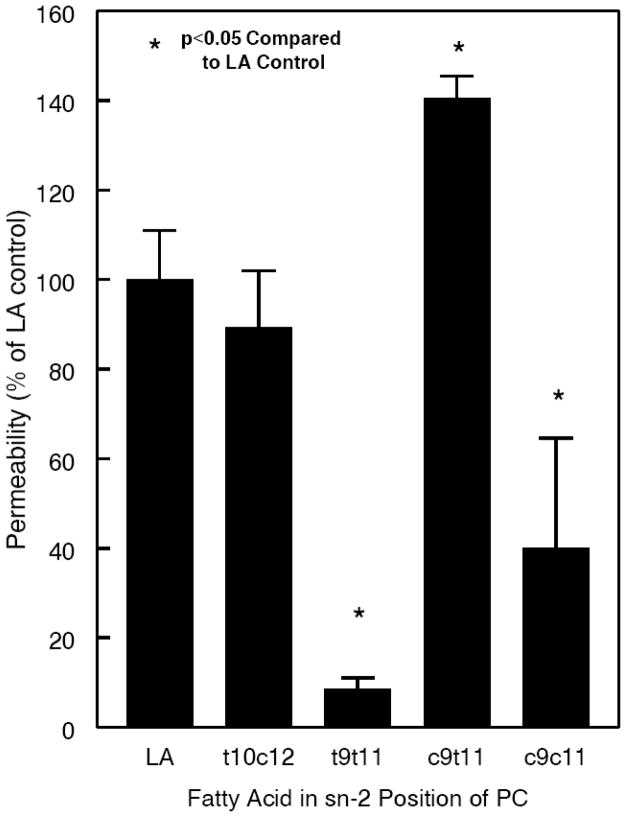
The permeability property of the membranes composed of various CLA-PCs was determined by the carboxyfluorescein leakage assay as described by Roach et al [23]. As shown in Fig. 5, the permeability was not affected by the presence of t10c12 CLA at sn-2, compared to the control PC containing c9c12 LA. However the presence of c9t11 CLA at sn-2 significantly increased the permeability, indicating a looser packing, whereas the presence of a double trans CLA (t9 t11) or double cis CLA (c9 c11) decreased the permeability indicating tighter packing of the bilayer.
Fig. 5. Carboxyfluorescein permeability of CLA-PC liposomes.
Small unilamellar vesicles of various CLA-PCs, containing the trapped carboxyflurescein were prepared by sonication, and the untrapped dye was removed by gel filtration chromatography. The leakage of the dye following its dilution with excess buffer was monitored in a flurometer as described in the text. The values shown are mean ± S.D of 4 separate experiments, and are expressed as % of the values obtained with unconjugated 16:0–18:2 PC.
3.6. Membrane fluidity
DPH-PC was incorporated into each CLA-PC liposome at the molar ratio of test PC: DPH-PC of 200:1, and the anisotropy values were determined at various temperatures in a spectrofluorometer. As shown in Fig. 6, the anisotropy values were similar for the two PCs with natural CLA (c9 t11 PC, and t10 c12 PC) and the control PC (with unconjugated 18:2) at most temperatures except at 15 °C, where the c9 t11 isomer showed a slightly lower anisotropy. The double trans isomer (t9 t11) showed the highest anisotropy values at low temperature, but as expected, decreased with increasing temperature, to give a value comparable to the control PC at 37°C. However, the double cis isomer (c9 c11) behaved anomalously, exhibiting high anisotropy values at all temperatures except at 50 °C. The melting point (Tm) for the free fatty acid form of this CLA is also unusually high for a fatty acid with two cis double bonds (42.5 °C), compared with unconjugated 18:2 with two cis double bonds (−5 °C), showing that the conjugation of double bonds has profound effect on the physical structure of the fatty acid. (Tm values for other free fatty acids are: c9 t11: 20 °C, t10 c12: 22.5 °C; c9 c11:42.5 °C; t9 t11: 54 °C, data supplied by Matreya Inc).
Fig. 6. Fluorescence anisotropy values of DPH-PC incorporated into various PC liposomes.
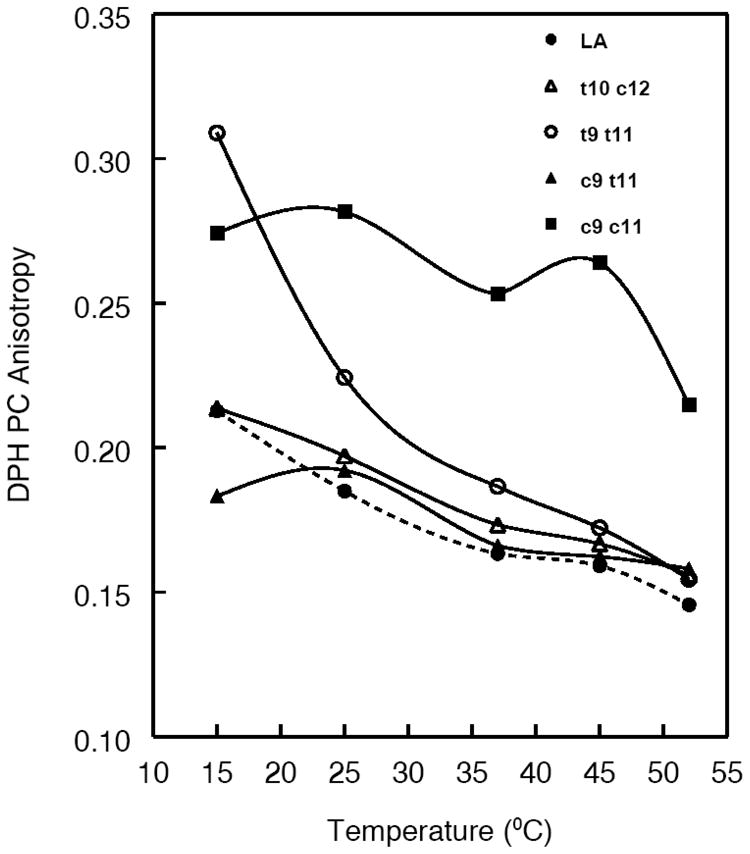
DPH-PC was incorporated into the liposomes at probe: PC ratio of 1:200, and fluorescence anisotropy was determined at various temperatures.
3.7. Oxidizability of CLA-PCs
The oxidative susceptibility of CLA containing PCs was tested by treating the PC liposomes with 1 mM AAPH at 37 °C, and analyzing the fatty acid composition at 0, 1 and 2 h time points. As shown in Fig. 7, all CLA-PCs showed increased susceptibility to oxidation, compared to the unconjugated control PC. In contrast to the previous studies with PCs containing unconjugated linoleic acids which showed that the trans double bond renders the fatty acid more resistant to oxidation [33], the double-trans CLA-PC, (t9 t11 PC) was actually oxidized at the highest rate, whereas the double-cis CLA-PC (c9c11 PC) showed the highest resistance among the conjugated species.
Fig. 7. Oxidation of various PC species in presence of 1 mM AAPH.
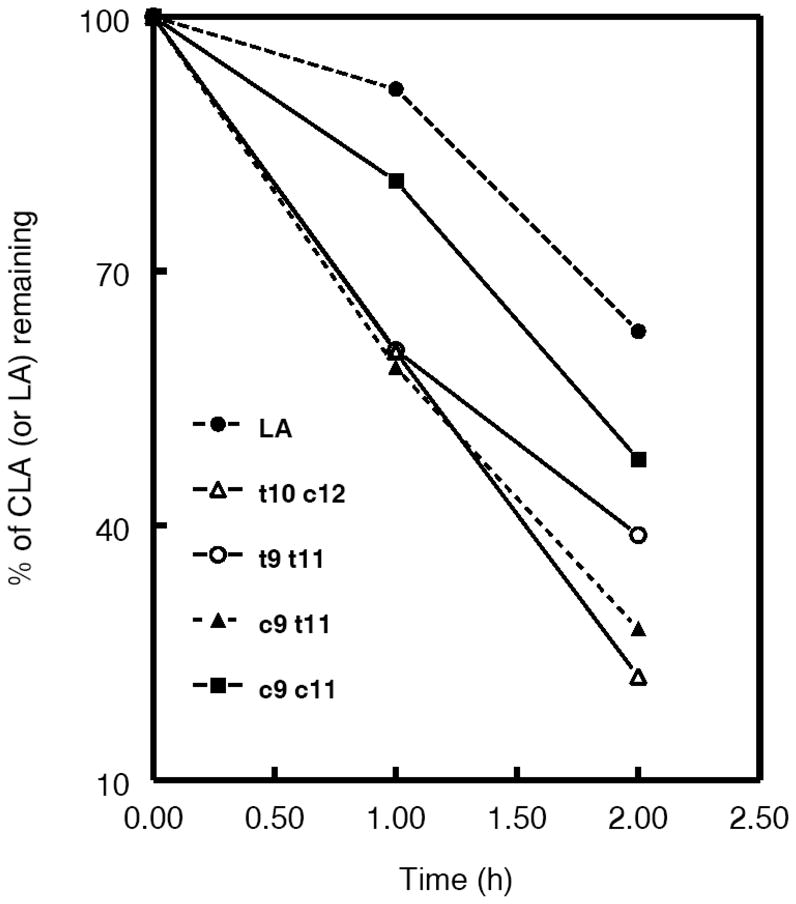
PC liposomes (0.1 μmol PC) were incubated at 37 °C in presence of 1 mM AAPH. At the indicated period of time the total lipids were extracted and the fatty acid composition analyzed by GC, after adding 17:0–17:0 PC as internal standard. The % CLA remaining was calculated, relative to 17:0. The data shown are averages of 3 separate experiments.
3.8. Effect of CLA on LCAT activity
The effect of various CLA isomers at sn-2 position of PC on the LCAT reaction, which predominantly transfers the sn-2 acyl group to cholesterol, is shown in Fig. 8. Compared to the control PC substrate containing the unconjugated 18:2, the PCs containing the two natural CLA isomers (t10 c12 and c9 t11 isomers) were superior substrates for LCAT, showing on average about 2-fold higher activity. On the other hand, the presence of a double trans CLA (t9 t11) inhibited the enzyme activity by 85%, while the presence of a double cis isomer (c9 c11) inhibited the activity by about 20%. Previous studies from our laboratory showed that the presence of trans unsaturation in sn-2 fatty acids (unconjugated) was inhibitory to human LCAT [34]. Interestingly, the present studies show that the naturally occurring conjugated fatty acids promote LCAT activity, although they also contain a trans double bond.
Fig. 8. Effect of CLA on LCAT activity.
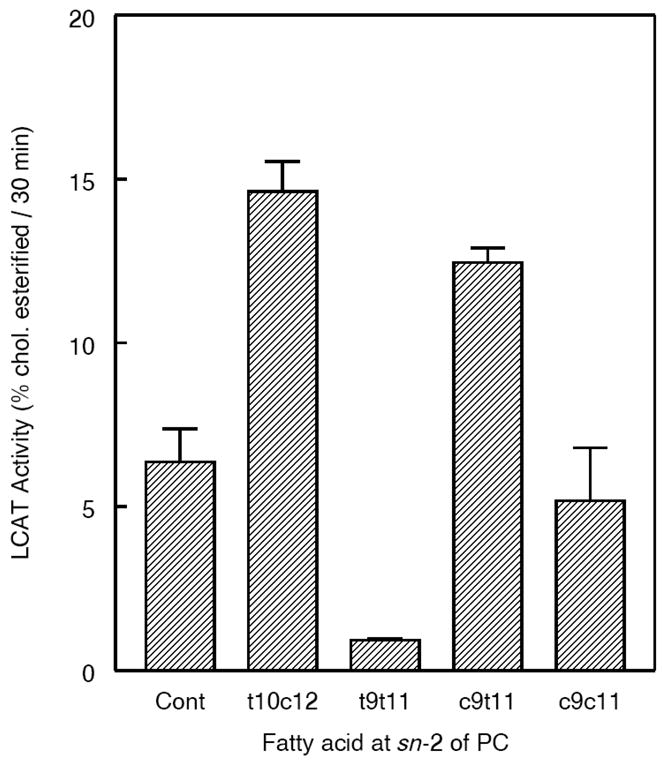
Proteoliposome substrates containing the respective PCs and labeled cholesterol were prepared as described in the text, and incubated with a semi-purified preparation of human LCAT for 30 min. The percent of labeled cholesterol esterified was determined after TLC separation of the lipid extract. The values shown are mean ± S.D of 3 separate assays.
4. Discussion
Trans unsaturated fatty acids (TUFA) produced by partial hydrogenation of vegetable oils are well known to be harmful to human health because of their adverse effects on the lipoprotein profile and on atherogenic risk. In addition, TUFA consumption has been associated with inflammatory disease, increased insulin resistance, impaired endothelial function, and increases in certain types of cancer [35,36]. A significant part of the adverse effects of TUFA is apparently due to their effects on membrane properties and consequent influence on receptor and signaling functions. In contrast to the deleterious effects of the manufactured TUFA, the naturally occurring TUFA in dairy products, namely CLA, have been shown to exhibit several beneficial effects in experimental animals including anti-obesity and anti-carcinogenic effects, and amelioration of cardiovascular risk. The major structural difference between the harmful and beneficial forms of TUFA in the diet is the presence of conjugated double bonds in the latter, although it is not known how this explains their differential biological effects. Like other unsaturated fatty acids in the diet, CLA are incorporated into cell membranes to varying extent, depending upon the experimental conditions. Thus, while some feeding experiments in humans and experimental animals showed that only about 1% of the phospholipid fatty acids were replaced by CLA [9,37], cell culture experiments showed that CLA could replace up to 50% of phospholipid fatty acids in leukemic cells [6], up to 32% in human breast cancer cells [11], and up to 13% in macrophages [10]. Although some dietary trans unsaturated fatty acids have been shown to be incorporated partly into the sn-1 position of membrane phospholipids [38], the positional distribution of CLA in phospholipids is unknown. Our recent studies showed that the natural isomers of CLA were incorporated predominantly, but not exclusively into sn-2 position of PC and PE in Chinese Hamster Ovary cells (unpublished data). In this study we tested the effect of incorporation of the two major naturally occurring CLA isomers in the sn-2 position of PC, on the physicochemical properties of model membranes including surface area, fluidity, permeability, cholesterol binding property and oxidizability. To determine the relative importance of the double bond geometry and conjugation on the membrane properties, we included two unnatural CLA that contained either two cis or two trans double bonds, and compared the effects with the unconjugated double cis 18:2 isomer (linoleic acid). A summary of various parameters measured in this study is presented in Table 1.
Table 1.
Summary of physicochemical properties of isomers of CLA-PC compared to LA-PC.
| Property | Control (LA-PC) | t10 c12 CLA-PC | t9 t11 CLA-PC | c9 t11 CLA-PC | c9 c11 CLA-PC |
|---|---|---|---|---|---|
| Area at 20 mN/m (Å2/mole) | 73.8 | 58.2 | 53.2 | 58.8 | 76.2 |
| Area condensation with 10% chol. at 20 mN/m | 3% | 7% | 7% | 2% | −1% |
| Chol. binding (% of Cont) | 100 | 118.5 | 210.3 | 98.6 | 84.5 |
| Tm of DPPC (+25% test PC) | 36.2 °C | 36.5 °C | 37.5 °C | 34.7 °C | 35.4 °C |
| Cooperative unit1 (DPPC +25% test PC) | 23.9 | 24.1 | 48.6 | 19.2 | 17.9 |
| DPH-PC anisotropy (37 °C) (% of Cont.) | 100 | 106.1 | 114.3 | 101.7 | 154.9 |
| Permeability (% of Cont.) | 100 | 89.3 | 8.5 | 140.6 | 40.0 |
| Oxidizability (% of Cont.) | 100 | 336 | 312 | 339 | 184 |
| LCAT activity (% of Cont.) | 100 | 230 | 14 | 195 | 81 |
Cooperative unit (C.U) was obtained by dividing the van’t Hoff enthalpy (ΔHVH) with the calorimetric enthalpy (ΔH). The C.U value for pure DPPC was 539.
The monolayer properties of the CLA PCs showed that the molecular surface areas of the two natural CLA-containing PCs were similar at various surface pressures, but lower than that of the unconjugated LA-PC. They also differed from each other significantly in presence of cholesterol, especially at very low or very high sterol/PC molar ratios. Thus the PC containing t10c12 CLA was condensed more than the PC that contains c9t11 CLA, in the presence of various concentrations of cholesterol, indicating that the former interacts with the sterol more avidly than the latter. These results were supported by the direct binding experiments with labeled sterol and PC bilayers, which showed that the t10c12 CLA-PC bound 20% more cholesterol compared to either c9t11 isomer or the unconjugated 18:2 isomer (Fig. 3). Previous studies showed that the presence of a trans double bond in the fatty acid increases its affinity to cholesterol, compared to the cis double bond at the same position [30]. Furthermore, feeding TUFA to experimental animals increases the cholesterol content of cell membranes [39]. However, since both c9t11 and t10c12 contain one cis and one trans double bond, the difference in their cholesterol affinity is most probably be due to the location of the double bonds in the acyl chain. In this context, it is interesting to note that Stillwell et al [40] showed that cholesterol binds weakly to the PCs containing a fatty acid with a cis double bond prior to the carbon 9 position, and binds more efficiently to PCs that contain no cis double bond at carbon 9. Since the t10c12 CLA does not have a cis double bond up to carbon 12, it may provide a deeper pocket to accommodate the sterol ring as depicted schematically in Fig. 9. Since the trans double bond does not induce a ‘kink’ in the chain as much as the cis double bond, it is similar to a single bond with respect to the overall shape and cholesterol interaction. The importance of the position of the double bond, as well as its configuration is also evident from the studies of the cholesterol binding properties of 16:0–18:1 PC species. The presence of a cis double bond at carbon 9 results in much weaker binding of cholesterol compared to a trans double bond at the same position. Previous studies showed that feeding t10c12 CLA increases insulin resistance in mice [41], although the authors proposed a mechanism that involved the effect of CLA on nuclear transcription factors. An alternative explanation is that an increase in membrane cholesterol may be responsible, because such an increase leads to a decrease in membrane fluidity and impaired function of insulin receptors [42].
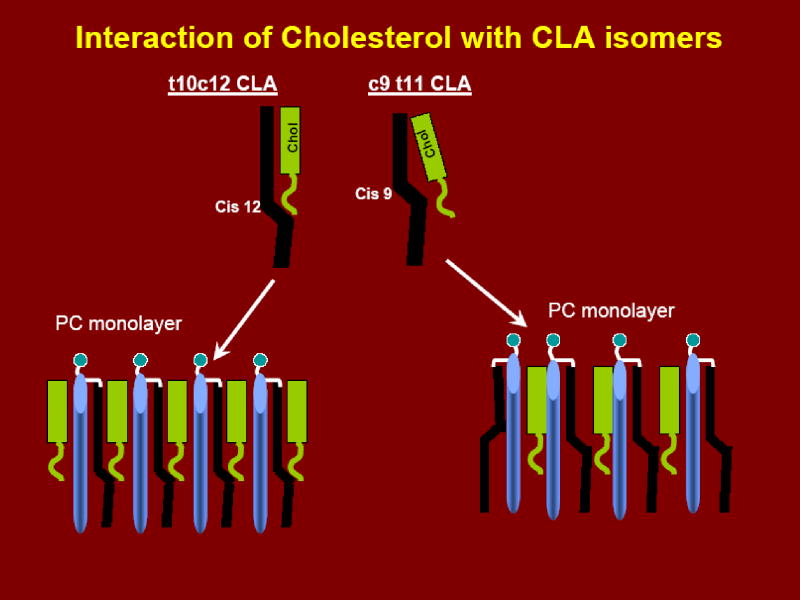
Fig. 9. A postulated mechanism for the increased binding of cholesterol by t10c12 CLA-PC.
It is proposed that position of the first cis double bond (starting from the carboxy end) is critical in cholesterol binding. Because of the kink in the acyl chain generated by the presence of the cis double bond, the pocket formed by the cis 12 double bond in t10c12 CLA-PC is larger than the pocket formed by the cis 9 double bond in c9t11 CLA-PC, and therefore more easily accommodates the bulky and rigid sterol ring. Furthermore, in a membrane that contains a saturated fatty acid at sn-1 and t10c12 at sn-2, the cholesterol may interact with both sides of the PC molecule, whereas it interacts only with the saturated acyl group in the PC containing c9t11 CLA. Since the trans double bond does not introduce a kink in the acyl chain, it is considered to be equivalent to a single bond in this model.
Conflicting reports have appeared on the oxidizability of CLA and their potential antioxidant effects. The free radical scavenging property of CLA was reported by many laboratories [43,44], with the t10c12 CLA showing stronger scavenging capacity than the c9t11 isomer [44]. This property may be responsible for the antioxidant effects reported in vivo [45] [46]. However, Basu et al [47] reported that CLA have pro-oxidant effects in vitro. Whereas some studies reported that CLA methyl esters of CLA are less oxidizable than methyl linoleate [48], others reported a greater oxidative susceptibility [49,50]. Although most of the in vitro studies have been performed with free CLA or methyl esters, the physiological form of CLA that is relevant to membrane function is the phospholipid that contains esterified CLA. Therefore we focused on the oxidizability of PC species containing the different isomers of CLA at sn-2 and a saturated fatty acid at sn-1, a most likely configuration that occurs in vivo. Our results show that all the tested CLA-PCs are more susceptible to oxidative degradation compared to the PC with unconjugated 18:2. Furthermore, unlike the unconjugated LA, where the presence of trans double bonds decreases the oxidizability by up to six-fold [33], the presence of even two trans double bonds in CLA actually increased its oxidizability, suggesting that the conjugated structure in the fatty acid dramatically alters the physicochemical properties of the phospholipids.
Another important finding of the present study is that the presence of either c9t11 CLA or t10c12 CLA in the sn-2 position of PC activates the LCAT-mediated cholesterol esterification reaction by 2-fold, indicating a potentially beneficial effect on the reverse cholesterol transport pathway. In contrast to this, our previous studies showed that the presence of (unconjugated) TUFA at the sn-2 position of PC inhibits the LCAT reaction [34], indicating that the conjugated structure overcomes the negative effects of trans unsaturation with respect to some biological activities. Although the effect of CLA on LCAT has not been reported previously, the study of Burdge et al [51] showed that following the feeding of a CLA-enriched diet to humans, there was a disproportionate increase in the CLA content of plasma CE, compared to plasma PC. This indicates a preferential incorporation of CLA into CE by the plasma LCAT activity, since the LCAT reaction is the predominant source of all cholesteryl esters in human plasma [15] and since the CLA feeding is known to actually inhibit cholesterol esterification in liver by the acyl CoA: cholesterol acyltransferase (ACAT) reaction [52], the other source of plasma cholesteryl esters.
In summary, our results show that the two natural CLA, when incorporated into PC, exhibit significant differences in their physicochemical properties in model membranes. Compared to the c9t11 CLA-PC, the t10c12 CLA-PC was found to interact more strongly with cholesterol, and to decrease membrane permeability. Since the latter isomer is known cause insulin resistance in some animal models, this property may be relevant to its biological effect. Both the CLA-PCs, however, have similar surface areas and fluidity properties, and activate the LCAT reaction to a similar extent. The presence of two cis or two trans double bonds in the CLA, on the other hand, affected the physical properties of PC more strongly. Interestingly, the conjugation of double bonds alone appears to profoundly affect the physicochemical properties of the PC, as evident from the differences between c9c12 PC (unconjugated) and c9 c11 PC (conjugated), both with two cis double bonds. Thus, the c9c11 PC binds cholesterol less efficiently, shows decreased permeability, and increased oxidizability, compared to c9c12 PC. Furthermore, the effects of trans unsaturation appear to be neutralized to some extent by the presence of conjugation, as evident form the increased activation of LCAT reaction by the t10c12 PC and c9t11 PC, compared to the previously reported inhibition of LCAT by the unconjugated trans fatty acid PCs [34].
Acknowledgments
This work was supported by award # R21DK078165 from NIDDK and Office of Dietary Supplements, NIH, and award # R01 HL68585 from NHLBI, NIH (PVS). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDDK, NHLBI, or NIH.. We wish to thank Mr. Sameer Gopal for assistance in the fluidity studies, Dr. Peter Horvath for performing the LCAT assays, and Mr. Rakesh Marreddy for assistance in the oxidation studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pariza MW. Perspective on the safety and effectiveness of conjugated linoleic acid. Am J Clin Nutr. 2004;79:1132S–1136S. doi: 10.1093/ajcn/79.6.1132S. [DOI] [PubMed] [Google Scholar]
- 2.Wahle KWJ, Heys SD, Rotondo D. Conjugated linoleic acids: are they beneficial or detrimental to health? Progress in Lipid Research. 2004;43:553–587. doi: 10.1016/j.plipres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Belury MA. Dietary conjugated linoleic acid in health: Physiological Effects and Mechanisms of Action. Annual Review of Nutrition. 2002;22:505–531. doi: 10.1146/annurev.nutr.22.021302.121842. [DOI] [PubMed] [Google Scholar]
- 4.House RL, Cassady JP, Eisen EJ, McIntosh MK, Odle J. Conjugated linoleic acid evokes de-lipidation through the regulation of genes controlling lipid metabolism in adipose and liver tissue. Obesity Reviews. 2005;6:247–258. doi: 10.1111/j.1467-789X.2005.00198.x. [DOI] [PubMed] [Google Scholar]
- 5.Peters JM, Park Y, Gonzalez FJ, Pariza MW. Influence of conjugated linoleic acid on body composition and target gene expression in peroxisome proliferator-activated receptor [alpha]-null mice. Biochim Biophys Acta. 2001;1533:233–242. doi: 10.1016/s1388-1981(01)00155-x. [DOI] [PubMed] [Google Scholar]
- 6.Agatha G, Voigt A, Kauf E, Zintl F. Conjugated linoleic acid modulation of cell membrane in leukemia cells. Cancer Lett. 2004;209:87–103. doi: 10.1016/j.canlet.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Banni S, Carta G, Angioni E, Murru E, Scanu P, Melis MP, Bauman DE, Fischer SM, Ip C. Distribution of conjugated linoleic acid and metabolites in different lipid fractions in the rat liver. J Lipid Res. 2001;42:1056–1061. [PubMed] [Google Scholar]
- 8.Sebedio JL, Angioni E, Chardigny JM, Gregoire S, Juaneda P, Berdeaux O. The effect of conjugated linoleic acid isomers on fatty acid profiles of liver and adipose tissues and their conversion to isomers of 16:2 and 18:3 conjugated fatty acids in rats. Lipids. 2001;36:575–582. doi: 10.1007/s11745-001-0759-8. [DOI] [PubMed] [Google Scholar]
- 9.Burdge GC, Lupoli B, Russell JJ, Tricon S, Kew S, Banerjee T, Shingfield KJ, Beever DE, Grimble RF, Williams CM, Yaqoob P, Calder PC. Incorporation of cis-9,trans-11 or trans-10,cis-12 conjugated linoleic acid into plasma and cellular lipids in healthy men. J Lipid Res. 2004;45:736–741. doi: 10.1194/jlr.M300447-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Ringseis R, Wen G, Saal D, Eder K. Conjugated Linoleic Acid Isomers Reduce Cholesterol Accumulation in Acetylated LDL-Induced Mouse RAW264.7 Macrophage-Derived Foam Cells. Lipids. 2008;43:913–923. doi: 10.1007/s11745-008-3226-x. [DOI] [PubMed] [Google Scholar]
- 11.Amaru DL, Field CJ. Conjugated linoleic acid decreases mcf-7 human breast cancer cell growth and insulin-like growth factor-1 receptor levels. Lipids. 2009;44:449–458. doi: 10.1007/s11745-009-3288-4. [DOI] [PubMed] [Google Scholar]
- 12.Stulnig TM, Huber J, Leitinger N, Imre EM, Angelisova P, Nowotny P, Waldhausl W. Polyunsaturated Eicosapentaenoic Acid Displaces Proteins from Membrane Rafts by Altering Raft Lipid Composition. J Biol Chem. 2001;276:37335–37340. doi: 10.1074/jbc.M106193200. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Baracos VE, Quinney HA, Clandinin MT. Dietary omega-3 and polyunsaturated fatty acids modify fatty acyl composition and insulin binding in skeletal-muscle sarcolemma. Biochem J. 1994;299:831–837. doi: 10.1042/bj2990831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Wang M, Tan L, Wang C, Ma J, Li N, Li Y, Xu G, Li J. Docosahexaenoic acid changes lipid composition and interleukin-2 receptor signaling in membrane rafts. J Lipid Res. 2005;46:1904–1913. doi: 10.1194/jlr.M500033-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Glomset JA. Lecithin: cholesterol acyltransferase. An exercise in comparative biology. Prog Biochem Pharmacol. 1979;15:41–66. [PubMed] [Google Scholar]
- 16.Paltauf F, Hermetter A. Preparation of alkyl ether and vinyl ether substrates for phospholipases. Methods Enzymol. 1991;197:134–148. doi: 10.1016/0076-6879(91)97140-t. [DOI] [PubMed] [Google Scholar]
- 17.Marinetti GV. Chromatographic separation, identification, and analysis of phosphatides. J Lipid Res. 1962;3:1–20. [Google Scholar]
- 18.Subbaiah PV, Liu M, Bolan PJ, Paltauf F. Altered positional specificity of human plasma lecithin-cholesterol acyltransferase in the presence of sn-2 arachidonoyl phosphatidyl cholines. Mechanism of formation of saturated cholesteryl esters. Biochim Biophys Acta. 1992;1128:83–92. doi: 10.1016/0005-2760(92)90261-s. [DOI] [PubMed] [Google Scholar]
- 19.Kauffman JM, Westerman PW, Carey MC. Fluorocholesterols, in contrast to hydroxycholesterols, exhibit interfacial properties similar to cholesterol. J Lipid Res. 2000;41:991–1003. [PubMed] [Google Scholar]
- 20.Phillips MC, Ladbrooke BD, Chapman D. Molecular interactions in mixed lecithin systems. Biochim Biophys Acta. 1970;196:35–44. doi: 10.1016/0005-2736(70)90163-x. [DOI] [PubMed] [Google Scholar]
- 21.Ali S, Smaby JM, Brockman HL, Brown RE. Cholesterol’s interfacial interactions with galactosylceramides. Biochemistry. 2002;33:2900–2906. doi: 10.1021/bi00176a020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu SL, Litman BJ. Determination of membrane cholesterol partition coefficient using a lipid vesicle-cyclodextrin binary system: effect of phospholipid acyl chain unsaturation and headgroup composition. Biophys J. 2002;83:3408–3415. doi: 10.1016/S0006-3495(02)75340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roach C, Feller SE, Ward JA, Shaikh SR, Zerouga M, Stillwell W. Comparison of Cis and Trans Fatty Acid Containing Phosphatidylcholines on Membrane Properties. Biochemistry. 2004;43:6344–6351. doi: 10.1021/bi049917r. [DOI] [PubMed] [Google Scholar]
- 24.Subbaiah PV, Kaufman D, Bagdade JD. Incorporation of dietary n-3 fatty acids into molecular species of phosphatidyl choline and cholesteryl ester in normal human plasma. Am J Clin Nutr. 1993;58:360–368. doi: 10.1093/ajcn/58.3.360. [DOI] [PubMed] [Google Scholar]
- 25.Subbaiah PV, Horvath P, Achar SB. Regulation of the Activity and Fatty Acid Specificity of Lecithin-Cholesterol Acyltransferase by Sphingomyelin and Its Metabolites, Ceramide and Ceramide Phosphate. Biochemistry. 2006;45:5029–5038. doi: 10.1021/bi0600704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CH, Albers JJ. Characterization of proteoliposomes containing apoprotein AI: a new substrate for the measurement of lecithin: cholesterol acyltransferase activity. J Lipid Res. 1982;23:680–691. [PubMed] [Google Scholar]
- 27.Chen CH, Albers JJ. Further characterization of purified lecithin:cholesterol acyltransferase from human plasma. Biochem Med. 1981;25:215–226. doi: 10.1016/0006-2944(81)90078-8. [DOI] [PubMed] [Google Scholar]
- 28.Evans FAURW, Williams FAUMA, Tinoco J. Surface areas of 1-palmitoyl phosphatidylcholines and their interactions with cholesterol. doi: 10.1042/bj2450455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uehara H, Suganuma T, Negishi S, Uda Y, Furukawa Y, Ueno S, Sato K. Physical Properties of Two Isomers of Conjugated Linoleic Acid. Journal of the American Oil Chemists’ Society. 2008;85:29–36. [Google Scholar]
- 30.Niu SL, Mitchell DC, Litman BJ. Trans fatty Acid derived phospholipids show increased membrane cholesterol and reduced receptor activation as compared to their cis analogs. Biochemistry. 2005;44:4458–4465. doi: 10.1021/bi048319+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halling KK, Ramstedt B, Nystrom JH, Slotte JP, Nyholm TKM. Cholesterol interactions with fluid phase phospholipids: effect on the lateral organization of the bilayer. Biophys J. 2008;95:3861–3871. doi: 10.1529/biophysj.108.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stubbs CD, Smith AD. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984;779:89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- 33.Sargis RM, Subbaiah PV. Trans unsaturated fatty acids are less oxidizable than cis unsaturated fatty acids and protect endogenous lipids from oxidation in lipoproteins and lipid bilayers. Biochemistry. 2003;42:11533–11543. doi: 10.1021/bi034927y. [DOI] [PubMed] [Google Scholar]
- 34.Subbaiah PV, Subramanian VS, Liu M. Trans unsaturated fatty acids inhibit lecithin: cholesterol acyltransferase and alter its positional specificity. J Lipid Res. 1998;39:1438–1447. [PubMed] [Google Scholar]
- 35.Mozaffarian D, Aro A, Willett WC. Health effects of trans-fatty acids: experimental and observational evidence. Eur J Clin Nutr. 0 AD;63:S5–S21. doi: 10.1038/sj.ejcn.1602973. [DOI] [PubMed] [Google Scholar]
- 36.Thompson AK, Shaw DI, Minihane AM, Williams CM. Trans-fatty acids and cancer: the evidence reviewed. Nutrition Research Reviews. 2008;21:174–188. doi: 10.1017/S0954422408110964. [DOI] [PubMed] [Google Scholar]
- 37.de Deckere EA, van Amelsvoort JM, Mcneill GP, Jones P. Effects of conjugated linoleic acid (CLA) isomers on lipid levels and peroxisome proliferation in the hamster. Br J Nutr. 1999;82:309–317. [PubMed] [Google Scholar]
- 38.Emken EA. Do Trans Acids Have Adverse Health Consequence? In: Nelson GJ, editor. Health Effects of Dietary Fatty Acids. AOCS; Champagne, IL: 1990. pp. 245–262. [Google Scholar]
- 39.Awad AB, Chattopadhyay JP. Alteration of rat heart sarcolemma lipid composition by dietary elaidic acid. J Nutr. 1983;113:913–920. doi: 10.1093/jn/113.4.913. [DOI] [PubMed] [Google Scholar]
- 40.Stillwell W, Dallman T, Dumaual AC, Crump FT, Jenski LJ. Cholesterol versus αTocopherol: Effects on Properties of Bilayers Made from Heteroacid Phosphatidylcholines. Biochemistry. 1996;35:13353–13362. doi: 10.1021/bi961058m. [DOI] [PubMed] [Google Scholar]
- 41.Taylor CG, Zahradka P. Dietary conjugated linoleic acid and insulin sensitivity and resistance in rodent models. Am J Clin Nutr. 2004;79:1164S–1168. doi: 10.1093/ajcn/79.6.1164S. [DOI] [PubMed] [Google Scholar]
- 42.Vainio S, Bykov I, Hermansson M, Jokitalo E, Somerharju P, Ikonen E. Defective insulin receptor activation and altered lipid rafts in Niemann-Pick type C disease hepatocytes. Biochem J. 2005;391:465–472. doi: 10.1042/BJ20050460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leung YH, Liu RH. trans-10, cis-12-Conjugated Linoleic Acid Isomer Exhibits Stronger Oxyradical Scavenging Capacity than cis-9, trans-11-Conjugated Linoleic Acid Isomer. J Agr Food Chem. 2000;48:5469–5475. doi: 10.1021/jf991163d. [DOI] [PubMed] [Google Scholar]
- 44.Yu L. Free Radical Scavenging Properties of Conjugated Linoleic Acids. Journal of Agricultural and Food Chemistry. 2001;49:3452–3456. doi: 10.1021/jf010172v. [DOI] [PubMed] [Google Scholar]
- 45.Ha YL, Storkson J, Pariza MW. Inhibition of Benzo(a)pyrene-induced Mouse Forestomach Neoplasia by Conjugated Dienoic Derivatives of Linoleic Acid. Canc Res. 1990;50:1097–1101. [PubMed] [Google Scholar]
- 46.Ip C, Briggs SP, Haegele AD, Thompson HJ, Storkson J, Scimeca JA. The efficacy of conjugated linoleic acid in mammary cancer prevention is independent of the level or type of fat in the diet. Carcinogenesis. 1996;17:1045–1050. doi: 10.1093/carcin/17.5.1045. [DOI] [PubMed] [Google Scholar]
- 47.Basu S, Smedman A, Vessby B. Conjugated linoleic acid induces lipid peroxidation in humans. FEBS Lett. 2000;468:33–36. doi: 10.1016/s0014-5793(00)01193-5. [DOI] [PubMed] [Google Scholar]
- 48.Luna P, de la Fuente M, Salvador D, Marquez-Ruiz G. Differences in Oxidation Kinetics Between Conjugated and Non-Conjugated Methyl Linoleate. Lipids. 2007;42:1085–1092. doi: 10.1007/s11745-007-3113-x. [DOI] [PubMed] [Google Scholar]
- 49.Van den Berg JJ, Cook NE, Tribble DL. Reinvestigation of the antioxidant properties of conjugated linoleic acid. Lipids. 1995;30:599–605. doi: 10.1007/BF02536996. [DOI] [PubMed] [Google Scholar]
- 50.Yang L, Leung LK, Huang Y, Chen ZY. Oxidative Stability of Conjugated Linoleic Acid Isomers. Journal of Agricultural and Food Chemistry. 2000;48:3072–3076. doi: 10.1021/jf0003404. [DOI] [PubMed] [Google Scholar]
- 51.Burdge GC, Tricon S, Morgan R, Kliem KE, Childs C, Jones E, Russell JJ, Grimble RF, Williams CM, Yaqoob P, Calder PC. Incorporation of cis-9, trans-11 conjugated linoleic acid and vaccenic acid (trans-11 18:1) into plasma and leucocyte lipids in healthy men consuming dairy products naturally enriched in these fatty acids. British Journal of Nutrition. 2005;94:237–243. doi: 10.1079/bjn20051506. [DOI] [PubMed] [Google Scholar]
- 52.Navarro V, Macarulla M, Fernandez-Quintela A, Rodriguez VM, Simon E, Portillo MP. Effects of trans -10, cis -12 conjugated linoleic acid on cholesterol metabolism in hypercholesterolaemic hamsters. Eur J Nutr. 2007;46:213–219. doi: 10.1007/s00394-007-0653-z. [DOI] [PubMed] [Google Scholar]