Abstract
Brain asymmetries are thought to increase neural processing capacity and to prevent interhemispheric conflict. In order to develop asymmetrically, neurons must be specified along the left-right axis, assigned left-side versus right-side identities and differentiate appropriately. In C. elegans and zebrafish, the cellular and molecular mechanisms that lead to neural asymmetries have recently come to light. Here, we consider recent insights into the mechanisms involved in asymmetrical neural development in these two species. Although the molecular details are divergent, both organisms use iterative cell-cell communication to establish left-right neuronal identity.
Keywords: C. elegans, Left-right asymmetry, Zebrafish
Introduction
The phenomenon of left-right asymmetry in biological systems has piqued human curiosity for thousands of years. Brain asymmetry was once thought to be exclusive to humans, partly because two behaviors that we like to consider as typically human, namely hand usage and language, display sidedness. However, this notion has been dispelled over the last century by an accumulation of data that demonstrates asymmetric behaviors in many vertebrates and invertebrates (Sreng, 2003; Vallortigara, 2000), such as handedness in many mammals, task-specific eye use in chickens, fish and toads, sex pheromone perception in cockroaches, olfaction in honeybees and chemosensation in C. elegans. Albeit a blow to human exceptionalism, these discoveries have been a boon to researchers, as they have allowed model organisms to be employed in the investigation of asymmetric brain development. In this review, we highlight recent advances in two species, the nematode C. elegans and the zebrafish D. rerio, that have unveiled genetic pathways required for establishing brain asymmetry. We first discuss the specification of the left and right amphid wing `C' (AWC) neurons of the C. elegans olfactory system, which is influenced by calcium influx; this, in turn, is regulated by gap junction- and claudin-mediated cell-cell communication. We then discuss the development of asymmetry in the zebrafish epithalamus, where Nodal signaling initiates a series of reciprocal interactions between the left-sided parapineal organ and the left habenular nucleus.
Although there is a considerable evolutionary gulf between the 302 neurons of the nematode and the estimated 78,000 neurons of the larval zebrafish (Hill et al., 2003), some common themes arise, in particular the interaction of neurons across the midline during the formation of the lateralized nervous system and the inherently stochastic nature of some developmental pathways. However, the striking differences in the genetic and cellular pathways highlight the improbability that nematode and zebrafish brain asymmetry arose from a shared ancestral event. Rather, the advantages conferred upon neural networks by asymmetry suggest that left-right differences in the worm and zebrafish nervous systems have evolved convergently.
Left-right asymmetry in the C. elegans sensory system
Types of left-right asymmetry in C. elegans nerves
The left and right AWC neurons of the C. elegans olfactory system express different sets of odorant receptor genes and sense different attractive odors. The candidate odorant receptor gene str-2 is randomly expressed in only one of the two bilateral AWC neurons. These are designated as either AWCON, the neuron that expresses str-2, or as AWCOFF, the one that does not (Fig. 1A-C). srsx-3, a candidate odorant receptor gene that was initially identified through differential expression profiling between amphid wing `B' (AWB) olfactory and amphid finger cell (AFD) thermosensory neurons, is a marker for AWCOFF (Bauer Huang et al., 2007; Colosimo et al., 2004). Cell ablation and genetic studies suggest that AWCOFF is the default state and that the asymmetric expression of str-2 requires an interaction between the two AWC neurons (Troemel et al., 1999). Half of the animals in a population induce AWCON in the left side of the brain, whereas the other half induce AWCON in the right side.
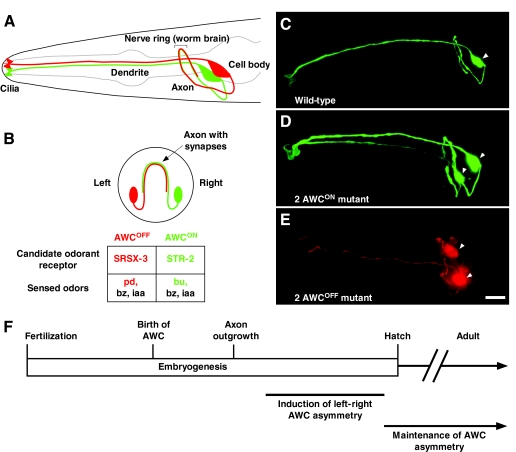
Fig. 1.
Left-right AWC neuronal asymmetry in C. elegans. (A,B) Amphid wing `C' (AWC) cell anatomy. Lateral (A) and transverse (B) views of a wild-type C. elegans head showing two bilateral AWC neurons. One AWC cell that expresses a GFP-tagged transgene of the candidate odorant receptor gene str-2 (str-2::GFP) (AWCON; right) is shown in green; the other AWC, with no str-2::GFP expression (AWCOFF; left), is shown in red. Within a population of worms, the left-right AWC asymmetry is stochastic, meaning that 50% of the animals display str-2 expression on the right, whereas the other 50% display str-2 expression on the left. The AWCON cell expresses the STR-2 candidate odorant receptor and senses butanone (bu), benzaldehyde (bz) and isoamyl alcohol (iaa). The AWCOFF cell expresses the SRSX-3 candidate odorant receptor and senses pentanedione (pd), bz and iaa (Bargmann et al., 1993; Wes and Bargmann, 2001). (C-E) Projections of micrograph stacks of wild-type and mutant animals expressing str-2::GFP (pseudo-colored green for GFP, red for DsRed). (C) Wild-type animals express str-2::GFP in one AWC neuron. (D) 2 AWCON animals express str-2::GFP in both AWC neurons. (E) Bilateral expression of the AWC marker transgene odr-1::DsRed in a 2 AWCOFF mutant showing the presence of both AWCs without str-2::GFP expression. (F) Time-line of developmental events in left-right AWC asymmetry. The AWC neurons are born at 300 minutes and their axons extend at 450 minutes after fertilization. AWC asymmetry is established during late embryogenesis and is maintained throughout the life of the animal. Arrowheads indicate the cell body of AWC neurons. Ventral is at the bottom in A-E, and anterior is to the left in A,C-E. Scale bar: 10 μm.
The stochastic nature of AWC asymmetry is classified as antisymmetry, and contrasts with the reproducible asymmetry of the left and right amphid exposed cell (ASE) taste neurons of C. elegans (known as ASEL and ASER, respectively), which is classified as directional asymmetry because of its stereotyped pattern (Hobert et al., 2002; Palmer, 1996; Sagasti, 2007; Vanvalen, 1962) (see Box 1). In both AWC and ASE neurons, the left and right neurons of a pair are functionally different from each other, with different patterns of gene expression. ASE neurons express multiple genes asymmetrically, including nuclear proteins, transcription factors, guanylyl cyclase sensory receptors and microRNAs (Chang et al., 2004; Chang et al., 2003; Etchberger et al., 2009; Johnston and Hobert, 2003; Johnston et al., 2005; Johnston et al., 2006; Johnston and Hobert, 2005; Ortiz et al., 2006; Ortiz et al., 2009; Pierce-Shimomura et al., 2001; Poole and Hobert, 2006; Sarin et al., 2009; Sarin et al., 2007; Uchida et al., 2003; Yu et al., 1997). In both cases, asymmetry contributes to being able to distinguish between attractive chemicals. ASE asymmetry allows the animal to discriminate between different salt ions and their relative concentrations (Ortiz et al., 2009; Pierce-Shimomura et al., 2001; Suzuki et al., 2008), whereas AWC asymmetry allows odor discrimination in the presence of different pervasive odors (Wes and Bargmann, 2001).
Box 1. Defining asymmetries
There are two important aspects to any lateralized feature: how the two sides differ from one another (asymmetry) and whether the vector of asymmetry always points in the same direction (directionality). An asymmetry in which the vector always points in the same direction (left or right) is called a directional asymmetry; if the direction of the vector is 50/50 left/right, it is called antisymmetry. It is thought that many directional asymmetries result from the addition of a genetic module for directionality to an ancestral antisymmetric pathway (Palmer, 2004).
At a developmental level, however, the mechanisms of ASE and AWC asymmetry are completely different. ASEL and ASER identities are hardwired by signaling and lineage mechanisms that act early in embryogenesis, unlike the variable AWC identities that are induced late in embryogenesis (Chuang and Bargmann, 2005; Poole and Hobert, 2006) (Fig. 1F). ASE asymmetry genes do not affect AWC asymmetry and, conversely, none of the AWC asymmetry genes known to date affect ASE asymmetry (Chang et al., 2003; Koga and Ohshima, 2004; Lanjuin et al., 2003; Lesch et al., 2009). Even the one gene known to be involved in the development of both ASE and AWE neurons, the Otx gene ceh-36, only affects asymmetry in ASE neurons, where mutations in ceh-36 cause the loss of the ASEL-specific gene gcy-7, but do not affect ASER genes. By contrast, these mutations cause the apparent loss of AWC identity, with mutant animals losing all AWC markers and failing to sense all odors recognized by AWC (Lanjuin et al., 2003). Furthermore, the ASEL and ASER neurons do not directly contact each other to generate asymmetry, in contrast to AWC neurons, which form chemical synapses at the nerve ring and require left-right interactions to generate asymmetry (Chuang et al., 2007; Poole and Hobert, 2006; Troemel et al., 1999; White et al., 1986). For reviews on ASE asymmetry, we refer the reader to several excellent reviews in the literature (Hobert, 2006; Hobert et al., 2002). In this review, we focus on AWC asymmetry and highlight the interactions and communication between a network of neurons in this unique system.
Identification of AWC neuronal symmetry genes
Forward genetic screens were fruitful in isolating genes that affect AWC asymmetry, yielding various neuronal symmetry (nsy) mutant animals with two AWCON neurons (2 AWCON nsy mutants; Fig. 1D) or two AWCOFF neurons (2 AWCOFF nsy mutants; Fig. 1E), instead of having one of each kind. Most 2 AWCON nsy mutants, which express str-2 in both AWC cells, are defective in a calcium-regulated kinase cascade that is required for the specification of the AWCOFF default state (Chuang and Bargmann, 2005; Sagasti et al., 2001; Tanaka-Hino et al., 2002; Troemel et al., 1999) (Fig. 2A). Most 2 AWCOFF nsy mutants, which fail to express str-2 in either AWC cell, are defective in the induction of AWCON, with disturbed cell-cell contacts required for communication between the two AWC cells (Bauer Huang et al., 2007; Chuang et al., 2007; Troemel et al., 1999; VanHoven et al., 2006). One 2 AWCOFF nsy mutation, nsy-7, is defective in a transcription factor that is required for repressing the AWCOFF state and for maintaining the AWCON state once asymmetry has been established (Fig. 2B). cGMP signaling also contributes to the maintenance of both AWCON and AWCOFF genes (Lesch et al., 2009; Troemel et al., 1999). These mutations in the AWC asymmetry pathway have shed new light on how neuronal antisymmetry is established and maintained through neural network formation, multicellular interaction and feedback.
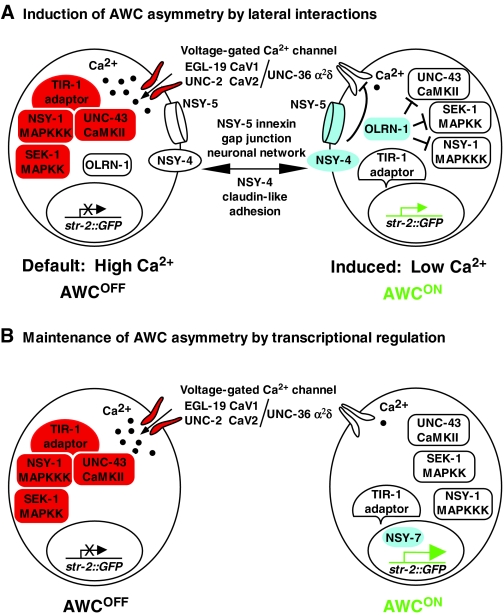
Fig. 2.
Left-right AWC asymmetry is controlled by calcium. (A) Induction of left-right AWC asymmetry by lateral interactions. The AWCOFF default state is specified by calcium-MAPK signaling. Before cell-cell communication is initiated, AWC cells undergo a calcium influx through voltage-gated calcium channels. Calcium signaling activates the CaMKII-MAPK pathway, mediated by the TIR-1 adaptor protein, resulting in the inactivity of the str-2 promoter and in the AWCOFF state in both AWC cells. After axonal outgrowth, the NSY-5 innexin gap junction neuronal network and the NSY-4 claudin-like protein activate the Raw repeat protein OLRN-1 to antagonize the calcium-mediated signaling pathway in the AWC that will induce str-2 promoter activity and become AWCON. NSY-4 and NSY-5 are more active in the future AWCON cell than the future AWCOFF cell (see Fig. 3 and text for details). (B) Maintenance of left-right AWC asymmetry by transcriptional regulation. The NSY-7 transcription factor maintains str-2 promoter activity in the induced AWCON cell and represses AWCOFF genes, such as srsx-3 (not shown).
Calcium-regulated signaling and the AWCOFF default state
Transient calcium release in the left side of vertebrates is known to initiate the asymmetric expression of the TGFβ ligand Nodal to coordinate visceral and brain asymmetry (Bisgrove et al., 2003; Liang et al., 2000; McGrath and Brueckner, 2003). In C. elegans AWC asymmetry, calcium also plays a role (Fig. 2). Prior to their interaction, the two AWC olfactory neurons have sufficient spontaneous activity to maintain a calcium-regulated signaling pathway and the default AWCOFF fate. Calcium influx into the AWC cells is mediated by the N/P/Q-type voltage-regulated calcium channel UNC-2 (the CaV2 pore-forming α1-subunit), by the L-type calcium channel EGL-19 (the CaV1 α1-subunit) and by UNC-36 (a regulatory α2δ-subunit) (Bauer Huang et al., 2007; Troemel et al., 1999). Mutations in unc-2 or unc-36 result in the loss of AWC asymmetry, with egl-19 functioning redundantly with unc-2, which indicates that calcium entry is essential for the specification of AWC asymmetry. The calcium channels act in cell-cell communication between the two AWCs to coordinate the decision to form one AWCON and one AWCOFF (Bauer Huang et al., 2007). By contrast, the calcium-regulated signaling cascade that comprises the unc-43 CaMKII, the tir-1 adaptor protein and the nsy-1 MAPKKK acts cell-autonomously to repress str-2 gene expression regardless of the state of the AWC on the contralateral side (Chuang and Bargmann, 2005; Sagasti et al., 2001). These results suggest that calcium channels act at the cell-cell interaction step to influence the coordinated AWCON/AWCOFF decision, whereas the downstream kinases act to execute the decision once it has been made.
Given that calcium-activated signaling pathways are very sensitive to the temporal pattern of calcium signals, transient differences in calcium influx between two cells have the potential to generate sustained differences in calcium-regulated signaling outputs. It is, however, not known whether calcium levels and fluctuations in the two AWC neurons are similar or different before they start to communicate with each other, as calcium signals have not been examined directly at these early time points.
Parallel functions and intrinsic biases of two signals
Both nsy-5, an innexin gap junction protein, and nsy-4, a claudin-like protein, function in parallel to inhibit the calcium-regulated signaling pathway in the neuron that becomes AWCON (Chuang et al., 2007; VanHoven et al., 2006). The communication across the midline appears to occur in axons because nsy-5 acts downstream of axon outgrowth in a genetic epistasis pathway for AWC asymmetry (Chuang et al., 2007; Troemel et al., 1999). This genetic pathway suggests that after the axons project to the midline, nsy-4 and nsy-5 are able to induce left-right asymmetry by inhibiting the calcium channels in one AWC, thereby activating olrn-1, which antagonizes calcium-related signaling in the AWC cell that will become AWCON (Bauer Huang et al., 2007) (Fig. 2A). nsy-4 and nsy-5 have parallel actions: the overexpression of either nsy-4 or nsy-5 can induce the AWCON state and the subcellular localization of NSY-4 or NSY-5 is unaffected when the other gene is mutated (Chuang et al., 2007).
Genetic mosaic analysis has proven to be an invaluable tool for dissecting nsy-4 and nsy-5 function. The somatic loss of unstable extrachromosomal arrays occurs naturally in C. elegans to generate mosaicism, according to the same principles, but without the complex genetic manipulations involved in making mosaic animals in fruit flies and mice, such as the FLP/FRT or Cre/Lox mitotic recombination systems (Golic, 1991; Golic and Lindquist, 1989; Herman, 1984; Liu et al., 2002). Following the loss of arrays with a transgenic marker allows the analysis of many cells in mosaic animals. Using this principle, the activity of nsy-4 and nsy-5 has been shown to have a side bias for the left and right AWC, respectively (Fig. 3A). In a nsy-5 mutant background, mosaic expression of nsy-5 in only the right AWC cell (AWCR) was sufficient to rescue the mutant phenotype in the majority of AWCR neurons. By contrast, the rescue efficiency of the left AWC (AWCL) was low when nsy-5 was only expressed in AWCL neurons. However, when nsy-5 was expressed in both AWB neurons in addition to AWCL, the majority of AWCL neurons were rescued. These results suggest that nsy-5 in AWCL needs to function in a network, but might function alone as a hemichannel in AWCR. In a similar rescue experiment for nsy-4, expressing nsy-4 in only AWCL was more efficient than expressing it in only AWCR in turning the respective cell into an AWCON cell (Chuang et al., 2007). These results suggest that random asymmetry is specified by the relative strengths of the nsy-4 and the nsy-5 signal when the two AWC cells interact. The stochastic expression of asymmetry suggests that nsy-4 and nsy-5 have an equal potential to cause either side to be AWCON in C. elegans wild-type animals. Interestingly, the intrinsic bias of nsy-4 and nsy-5 activity in the AWC neurons suggests the existence of an underlying directional asymmetry upon which AWC antisymmetry has been re-imposed.
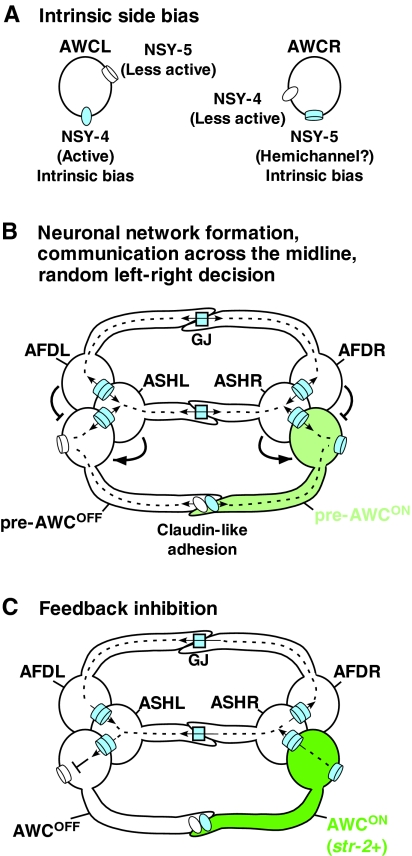
Fig. 3.
Model of a neuronal signaling network for AWC asymmetry. (A) Intrinsic side bias of NSY-4 claudin-like protein and NSY-5 innexin gap juction protein in AWC neurons. NSY-4 activity has a bias toward the left AWC cell (AWCL), whereas NSY-5 activity, perhaps present as hemichannels, has a bias toward the right AWC cell (AWCR). (B) Random left-right AWC asymmetry decisions via neuronal network formation and communication across the midline. Gap junctions (GJ) and claudin-like adhesions connect bilateral AWC neurons and their neighboring neurons, including AFD and ASH neurons, in a network. Gap junctions mediate direct contact between the cell bodies of the respective neurons on each side, whereas axonal contacts through gap junctions and claudin-like adhesions mediate signaling across the midline between left and right neurons. AFD cells have a negative influence on the ipsilateral cell becoming AWCON, and ASH cells have a positive influence on the ipsilateral cell becoming AWCON. Both NSY-4 and NSY-5 are required to induce AWCON and the relative strengths of NSY-4 and NSY-5 activity between the two AWC cells specify random left-right asymmetry. The pre-AWCON cell indicates the AWC cell that turns on str-2 expression. (C) Feedback inhibition of NSY-4 and NSY-5 across the midline from the AWCON cell to the contralateral AWCOFF cell coordinates and strengthens str-2 gene expression in only one AWC cell.
A gap junction network coordinates AWC asymmetry
Gap junctions have been implicated in the initial asymmetric gene expression that ultimately leads to the asymmetry of the heart, gut and gall bladder in the early embryos of frogs and chickens (Levin and Mercola, 1998; Levin and Mercola, 1999). Satisfyingly, genetic results implicated nsy-5 gap junctions in AWC asymmetry, providing a further example of nature employing similar mechanisms in different contexts in developmental processes. The analysis of worm embryos by electron microscopy showed that the innexin gap junction protein NSY-5 links the cell bodies of AWC neurons and their surrounding neurons, including ASH sensory neurons and AFD thermosensory neurons, to form a transient gap junction network (Chuang et al., 2007). This gap junction ultrastructure is visible during embryogenesis but has disappeared by the time the worms hatch. Gap junctions are also present in the axons of neurons that surround AWC neurons; these axons meet their contralateral homologs at the midline to link the left and right sides of the nervous system (White et al., 1986). Approximately 15% of all C. elegans neurons, including AWC, ASH, AFD and AWB sensory neurons, as well as others, express nsy-5 and are likely to be linked as part of this network (Chuang et al., 2007).
nsy-5 activity in non-AWC neurons of the network can promote or inhibit the AWCON fate. For example, ASH sensory neurons form direct gap junctions with AWC neurons and have a positive influence toward the cell becoming AWCON. By contrast, AFD thermosensory neurons do not form direct gap junctions with AWC neurons, but have a negative influence toward a cell becoming AWCON. Interestingly, AFD neurons do form gap junctions with ASH neurons (Fig. 3B). AWB neurons, which are closely related to AFD neurons by lineage, also inhibit the AWCON state. It is still unknown whether nsy-5 is required for the communication across the midline because no expression has been detected in the axons. However, there seems to be a mechanism by which nsy-5 activity in neurons on one side can influence the AWC fate on the contralateral side, as determined by mosaic analysis, possibly through other gap junctions in the network (Fig. 3B,C). For example, AWCL can be turned into AWCON by the influence of AWB neurons and cell lineages related to AWB on the right side of the network. Once the AWC cells sense each other's presence, the presumptive AWCON must produce a negative-feedback signal that prevents the contralateral AWC from becoming AWCON (Fig. 3C). This feedback signal appears to be mediated by the nsy-5 network through its action in the lineage-related AFD and AWB neurons. The expression of nsy-5 in AWC is a prerequisite to rescue the nsy-5 2 AWCOFF mutant phenotype, but when nsy-5 is expressed in both AWC and ASH neurons, the animal produces a 2 AWCON gain-of-function phenotype, which indicates a lack of feedback coordination. However, when nsy-5 is expressed in AFD neurons, in addition to AWC and ASH neurons, the 2 AWCON gain-of-function phenotype is suppressed (Chuang et al., 2007). Furthermore, when AWC, ASH and AFD express nsy-5, it causes a predominant 2 AWCOFF non-rescued phenotype, which can be rescued to a wild-type phenotype by adding additional nsy-5 neurons back into the network. Similar conclusions were obtained in experiments that involved nsy-5 overexpression. These lines of evidence indicate that both signaling and feedback require multiple cells within the nsy-5 gap junction network for normal AWC asymmetry.
These results suggest that individual neighboring neurons in the gap junction network communicate to help AWC neurons fine-tune the signaling for the establishment of precise asymmetry. However, the identity of the actual signal that crosses the gap junction, and how the neighboring cells in the network influence AWC asymmetry, remains unknown.
Maintenance of left-right AWC asymmetry
Once AWC asymmetry is induced and specified, it must be maintained throughout the life of the worm (Fig. 1F). cGMP signaling appears to be required for maintaining AWCON and AWCOFF fate, as cGMP signaling mutants have normal AWC asymmetry at early larval stages but fail to express either str-2 or srsx-3 as adults (Lesch et al., 2009; Troemel et al., 1999).
nsy-7 is also required for the maintenance of AWC left-right asymmetry (Fig. 2B). Like cGMP signaling mutants, nsy-7 mutants show normal AWCON induction in young animals, with str-2 expression in one neuron, but they fail to maintain str-2 expression, leading to a 2 AWCOFF phenotype as adults (Lesch et al., 2009). Unlike cGMP signaling mutants, nsy-7 mutants exhibit srsx-3 expression in both AWC neurons, which suggests that nsy-7 is specifically required to maintain the asymmetric AWCON identity. nsy-7 encodes a DNA binding transcription factor with a distant resemblance to engrailed homeobox proteins, is asymmetrically expressed in AWCON, binds to an element in the srsx-3 promoter that is required for both activation and repression and directly inhibits srsx-3 in the AWCON cell. The altered expression of nsy-7 reporters in nsy-5 and nsy-1 mutants suggests that the AWC asymmetry pathway regulates nsy-7 transcription.
In summary, the C. elegans AWC neurons, which are bilaterally symmetric with regard to their morphological and anatomical features, develop asymmetry at the molecular and functional level. AWC asymmetry is determined in a stochastic manner through the modulation of calcium signaling by gap junctions and claudin-mediated cell-cell communication across the midline. Once AWC asymmetry is established, it is maintained through transcriptional regulation and cGMP signaling. AWC asymmetry seems to be evolutionarily advantageous because it allows the worm to sense multiple odors using a limited number of olfactory sensory neurons.
Similar to the establishment of AWC asymmetry in C. elegans, the zebrafish epithalamus uses multiple rounds of cell-cell communication to establish asymmetry. However, in the zebrafish epithalamus, the asymmetry is directional and is developed at the neuroanotomical level with global asymmetries in gene expression. In the following section, we review the current knowledge on the lateralization of the zebrafish epithalamus.
Left-right asymmetry in the zebrafish nervous system
The zebrafish epithalamus: an accessible model of vertebrate brain asymmetry
Although the molecular and cellular strategies that result in asymmetric neurogenesis differ significantly between invertebrate and vertebrate models, signaling events between neurons play a crucial role in specifying asymmetric fate in both systems. Much of the work on the development of vertebrate central nervous system (CNS) laterality has centered on the zebrafish, both because of its well-documented advantages in embryological and genetic experiments and because of the presence of robust left-right asymmetries in the dorsal diencephalon, or epithalamus. The vertebrate epithalamus comprises the so-called pineal complex and the bilateral habenular nuclei (Butler and Hodos, 1996). The pineal complex of zebrafish includes a pineal organ, the stalk of which emerges from just left of the midline (Liang et al., 2000) and a small sister organ called the parapineal, which is found on the left side of the brain (Concha et al., 2000). The pineal organ in fish is a photosensitive clock organ that secretes melatonin (Cahill, 1996); the function of the parapineal organ, however, is not well understood, although it also produces at least one melatonin-biosynthetic enzyme (Gothilf et al., 1999).
The habenular nuclei, or habenulae, flank the pineal complex and are central to an evolutionarily ancient conduction system, the dorsal diencephalic conduction pathway (Fig. 4A). Both habenulae receive input from the forebrain via the stria medullaris and send outputs to the midbrain through the fasciculus retroflexus (Sutherland, 1982). The habenulae serve as a funnel for descending impulses. As such, sources of afferents are diverse and include neurons of the pallium, the eminentia thalami (the future entopeduncular nucleus) and the posterior tuberculum, all of which contact both habenulae by crossing at the habenular commissure (Hendricks and Jesuthasan, 2007). Only the left habenula receives input from parapineal axons (Aizawa et al., 2005; Concha et al., 2000; Gamse et al., 2005), whereas the right habenula is unilaterally contacted by axons from the olfactory bulb (Miyasaka et al., 2009). The habenulae are asymmetric in size, in the number of subnuclei present and in terms of gene expression in many vertebrates, with the most striking asymmetries found in amphibians, lizards and fish (Concha and Wilson, 2001). In zebrafish, each nucleus is divided into a lateral and a medial subnucleus. This nomenclature describes their position in the adult animal, and it should be noted that in embryonic and larval stages, the lateral subnuclei actually lie closer to the midline than the medial subnuclei. Lateral and medial subnuclei can be identified by their expression of different members of the potassium channel tetramerization domain-containing (KCTD) gene family, kctd12.1/leftover (lov) and kctd12.2/right on (ron), respectively (Aizawa et al., 2005; Gamse et al., 2005) (Fig. 4B). The lateral subnucleus is larger in the left habenula and sends efferents to both the dorsal and the ventral interpeduncular nucleus (IPN), whereas the medial subnucleus is larger on the right side and exclusively projects to the ventral IPN (Aizawa et al., 2005; Gamse et al., 2005) (Fig. 4C).
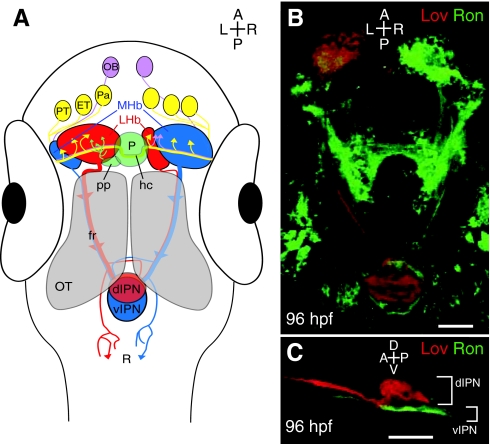
Fig. 4.
The asymmetric zebrafish habenular nuclei are central to the dorsal diencephalic conduction pathway. (A) The dorsal diencephalic conduction system. The ratio of lateral (red) to medial (blue) subnuclei is greater in the left habenula, but both sides receive input from both ipsilateral and contralateral neurons of the pallium, the eminentia thalami and the posterior tuberculum. The parapineal only sends projections to the left habenula, whereas the right habenula receives additional afferents from both olfactory bulbs. Habenular efferents course ventrally to the optic tecta in converging bundles known as the fasciculus retroflexi. Axons originating in the medial subnuclei innervate the ventral region of the interpeduncular nucleus, whereas those of lateral origin can target the dorsal interpeduncular nucleus. Some habenular axons also contact the raphe, just posterior to the interpeduncular nucleus. (B) Dorsal perspective confocal z-projection of Leftover (Lov)-positive (Lov+; red) and Right on (Ron)-positive (Ron+; green) neurons coursing from the habenular nuclei to the interpeduncular nucleus via the fasciculus retroflexi. (C) Lateral view of Lov+ and Ron+ axons, segregated into the dorsal and ventral domains of the interpeduncular nucleus. dIPN, dorsal interpeduncular nucleus; ET, eminentia thalami; fr, fasciculus retroflexus; hc, habenular commissure; LHb, lateral habenular subnuclei; MHb, medial habenular subnuclei; OB, olfactory bulb; OT, optic tectum; P, pineal organ; Pa, pallium; pp, parapineal organ; PT, posterior tuberculum; R, raphe nucleus; vIPN, ventral interpeduncular nucleus. Scale bars: 50 μm.
The embryos of another fish species, the medaka, develop parapineal and habenular asymmetries precociously relative to the zebrafish (although the sequence of events is similar). In addition, in medaka, the parapineal innervation of the habenula is more spatially restricted than in zebrafish and it is unclear if the same habenular subnucleus is innervated (Signore et al., 2009). Whether the development of medaka brain asymmetry is fundamentally identical or substantially different from zebrafish awaits the additional characterization of the medaka using molecular markers of the parapineal and habenula.
In the following sections, we trace the developmental steps and signals that transform an initially symmetric neuroepithelium into the asymmetric epithalamus (as shown schematically in Fig. 5A). We focus on the interaction between the parapineal and the left habenula; the formation and migration of the parapineal has been reviewed recently (Concha et al., 2009; Snelson and Gamse, 2009).
Fig. 5.
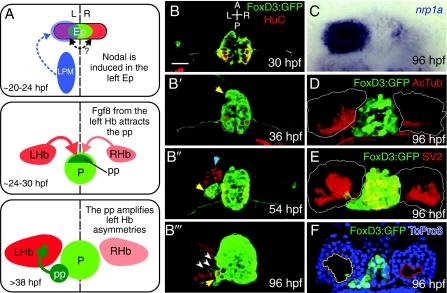
Asymmetric development of the zebrafish epithalamus. The zebrafish epithalamus begins as a symmetrical cell population and develops a suite of asymmetries. (A) Model of asymmetric epithalamic development. First, around 20 hours post-fertilization (hpf), Nodal expression (blue) in the left lateral plate mesoderm (LPM) activates Nodal expression in the left epithalamus (Ep). There might be communication across the midline in the epithalamus (double-headed arrow; see text for discussion). At around 24 hpf, a slight left-sided bias of Fgf8 from the habenulae (Hb) attracts the parapineal (pp). By 38 hpf, the parapineal induces the elaboration of asymmetries only in the left habenula. (B-B″′) Timecourse of epithalamic development. Tg(foxd3:GFP) (green) marks the pineal and parapineal, and mature neurons are marked with antibodies against HuC (red). (B) At 30 hpf, the pineal is a symmetrical group of neurons centered on the midline. (B′) By 36 hpf, the parapineal (yellow arrow) begins to migrate away from the left anterior border of the pineal. (B″) At 54 hpf, the parapineal (yellow arrow) is a distinct accessory organ, and more neurons in the left habenula (blue arrow) have differentiated (i.e. are HuC-positive) than in the right. (B″′) The 96 hpf epithalamus is robustly lateralized, with parapineal axons (white arrows) contacting only the left habenula, which is made up of many more HuC-positive neurons than the right. (C-F) Asymmetrical features of the habenular nuclei at 96 hpf. (C) The transcript of the gene encoding the semaphorin co-receptor neuropilin1a (nrp1a) (blue) is expressed predominantly on the left side in the lateral habenular subnucleus. (D) Antibodies against acetylated tubulin (red) reveal all neuronal processes and show a greater density of neuropil in the left habenula (habenular borders outlined in white). (E) Antibodies against the presynaptic component SV2 (red) demonstrate a greater density of synaptic contacts in the left habenula. (F) An optical slice through the left and right habenulae at the level of the parapineal stained with the nuclear marker To-Pro3 (blue) demonstrates that the soma-free core of the left habenula (yellow outline) is larger than that in the right (red outline) and is invaded by parapineal axons. Scale bar: 50 μm.
Nodal provides a directional asymmetry cue to the parapineal and habenulae
The Nodal signaling pathway, which directs and coordinates visceral asymmetry in all vertebrates (Shiratori and Hamada, 2006), also plays a crucial role in the directionality of brain asymmetry in fish (Ahmad et al., 2004). The Nodal proteins are a subset of the TGFβ family of secreted growth factors. In medaka and zebrafish, the expression of Nodal genes in the anterior lateral plate mesoderm (LPM) precedes a transient pulse of expression in the left epithalamus (Bisgrove et al., 2000; Concha et al., 2000; Liang et al., 2000; Soroldoni et al., 2007). The parapineal and pineal are derived from a single group of cells in the future epithalamus, located at the dorsal midline and identified by their expression of the transcription factor flh at the end of gastrulation (Concha et al., 2003; Gamse et al., 2003). Left-sided Nodal signaling in the epithalamus sets the direction in which the parapineal organ migrates and where the pineal stalk emerges.
To form the parapineal, it is clear that cells from both the left and right sides migrate leftward, although the mechanism that coordinates this migration across the midline is yet to be fully uncovered. One hypothesis is that a left-biased signal, such as Fgf8 (see below), can attract both left- and right-sided cells by crossing the dorsal midline. Another idea is that parapineal cells are extremely cohesive prior to migration; in this scenario, left-sided cells responding to an attractive signal could drag their right-sided companions across the midline. Consistent with this idea, in the tbx2b mutant, where parapineal cells remain in the dorsal midline, there is an apparent loss of parapineal cohesion (see below). Of course, a midline-crossing signal and the dragging of right-sided cells by left-sided cells could also occur simultaneously.
Asymmetry in the habenular nuclei, like parapineal migration, is affected by Nodal signaling. A subtle asymmetry in habenular neurogenesis sets in just after Nodal signaling ends in the epithalamus. More cxcr4b-positive habenular precursor cells are found in the left side of the brain than in the right at 28 hours post-fertilization (hpf); subsequently, approximately 25% more left-sided neurons exit the cell cycle (as indicated by the presence of the neuronal marker HuC) by 34-38 hpf (Roussigne et al., 2009) (Fig. 5B). An earlier onset for left-sided neurogenesis is supported by birthdating experiments using a BrdU pulse administered at 32 hpf (Aizawa et al., 2007).
Mutants or drugs that cause either absent or bilateral Nodal signaling result in symmetric HuC labeling (Roussigne et al., 2009). It remains to be seen whether Nodal signaling affects the timing of cell cycle exit or the size of the neurogenic region in the habenular precursors, and whether it acts directly on precursor cells or indirectly by affecting the precursor cell environment.
Surprisingly, either bilateral or absent Nodal expression seems to reduce the number of habenular precursor cells on the left side (Roussigne et al., 2009), which indicates that the proliferative response of the habenular precursors relies on the relative levels of Nodal on each side rather than on absolute levels. An important, unanswered question is if this apparently coordinated response results from communication between the two sides, perhaps via a lateral inhibition mechanism.
Just such a mechanism is hinted at by the experimental manipulation of the Notch pathway. The hyperactivation of Notch signaling between 28 hpf and 48 hpf results in symmetric neurogenesis irrespective of Nodal signaling (Aizawa et al., 2007). The left and right habenular precursors lie adjacent to one another in the subventricular zone prior to dorsal migration and differentiation (Aizawa et al., 2007; Concha et al., 2003) and therefore could signal across the midline via the Notch juxtacrine pathway. In addition, Notch pathway components are expressed in both habenular precursors and adjacent tissues (Aizawa et al., 2007). A thorough understanding of the role of Notch in cross-midline communication, however, awaits the production of genetic mosaics with Notch loss-of-function or hyperactivated cells on only the left or right side of the brain.
Interaction of the parapineal and habenular nuclei
Recent work has more fully detailed the reciprocal nature of the interactions between parapineal and habenular neurons. What was once thought to be a linear cascade of events, beginning with parapineal specification and ending with the elaboration of habenular asymmetries, has now been appreciated as a much more complex interplay initiated by Nodal signaling and subsequently reinforced by reciprocal signaling interactions.
For its part, the left habenular nucleus attracts the parapineal. Both the left and the right habenular nuclei express the secreted signaling ligand Fgf8, whereas parapineal cells express the fgfr4 receptor (Regan et al., 2009). Parapineal cells in the fgf8 zebrafish mutant acerebellar (ace) fail to migrate properly toward the left habenula, which indicates that a subtle asymmetry in Fgf8 signaling could act to tip the balance toward the left habenula (Regan et al., 2009). Regan and colleagues found that in ace mutants, a local source of Fgf8 was sufficient to restore leftward parapineal migration, regardless of the left-right placement of the source. In the absence of asymmetric Nodal signaling and fgf8 expression, however, the parapineal migrates towards the source of the exogenous Fgf8 protein (Regan et al., 2009). Thus, a right-sided Fgf8 bead is insufficient to attract the parapineal to the right in embryos with intact Nodal signaling. The left-sided initiation of neurogenesis by Nodal might bias the parapineal to the left side, perhaps because of the earlier or higher expression of a chemoattractant, such as Fgf8. Consistent with this idea, the ablation of habenular precursors can produce antisymmetry in parapineal placement (Concha et al., 2003). Alternatively, Nodal or some other secreted molecule could act as a chemoattractant.
The transcription factor Tbx2b is also required for the asymmetric placement of the parapineal (Snelson et al., 2008a). tbx2b is expressed within parapineal cells prior to their migration and probably acts in a cell-autonomous fashion. In tbx2b mutants, parapineal cells fail to cohere into an anterior cluster; subsequently, they migrate posteriorly and ventrally as they do in wild-type fish, but remain at the midline (Snelson et al., 2008a). By contrast, parapineal cells in fgf8 mutants do form a cluster just anterior to the pineal, but never move away from this position (Regan et al., 2009). This subtle difference in the phenotypes of tbx2b and fgf8 mutants suggests that the two molecules either act in different steps of parapineal migration or in parallel pathways. Although the analysis of double mutants has not yet been reported, the clustering of parapineal precursors might be a prerequisite for cells to leave the midline in response to chemoattractants from the left habenula.
The left-sided placement of the parapineal organ amplifies the asymmetrical features of the left habenula: when the parapineal is ablated by laser surgery, habenular gene expression takes on a more bilaterally symmetric pattern (Bianco et al., 2008; Gamse et al., 2003). Also, in the absence of a functional parapineal, almost all habenular axons, regardless of left-right origin, innervate the ventral targeting domain of the IPN (Bianco et al., 2008; Snelson et al., 2008b). The slight asymmetry in habenular development following parapineal ablation is probably due to asymmetric neurogenesis initiated by Nodal signaling. By stark phenotypic contrast, loss of Nodal signaling has no effect on the total number of habenular neurons, asymmetric dendritogenesis or subnucleus formation at two days post-fertilization (dpf) and later (Concha et al., 2003; Gamse et al., 2003), although antisymmetry, rather than directional laterality, is observed. It seems that some habenular asymmetry can be initiated in a parapineal-independent manner but that the full array of left-right differences between the habenulae requires an asymmetrically placed parapineal organ.
Signaling between the parapineal and the habenular nuclei is a vertebrate example of an asymmetrically defined system that, in the absence of Nodal signaling from the lateral plate mesoderm, is stochastically oriented, much like the randomly asymmetric specification of the C. elegans AWC neurons. Fgf signaling has been identified as at least one mode of communication from the habenulae to the parapineal, but the nature of parapineal signaling to the left habenula remains unknown, although it is crucial for the establishment of mature habenular asymmetries. Good candidates for the signal (or signals) mediating parapineal-habenular communication include canonical Wnt signaling (Carl et al., 2007) and direct synaptic contact.
Differentiation of the habenular nuclei
Although habenular asymmetries have been described in many vertebrate classes (Concha and Wilson, 2001), the left-right features of the zebrafish habenulae are certainly the most thoroughly studied. Asymmetries have been described in nearly all aspects of habenular neuroarchitecture, including the size ratio of medial and lateral subnuclei, gene expression patterns, process morphology and axonal targeting pattern.
The left and right habenulae each comprise a medial and a lateral subnucleus, terms that describe their position in the adult epithalamus. In the larval brain, these structures have not yet undergone the morphogenic movements that shift the lateral subnucleus from its initial, primarily medial, location. In the left habenula, the lateral subnucleus is much larger than the medial, whereas in the right habenula, cells of the medial subnucleus greatly outnumber those of the lateral. This feature persists into adulthood and might be the primary laterality from which other asymmetrical features follow (Aizawa et al., 2007).
Although some genes are expressed asymmetrically in a transient fashion due to asymmetrical neurogenesis rates (cxcr4b/HuC, see above), other expression patterns have proven to be robustly lateralized into adulthood. Members of the KCTD gene family are expressed asymmetrically from the onset. lov is expressed in neurons of the lateral subnuclei, and consequently in more cells in the left habenula, whereas ron and kctd8/dexter are predominantly expressed in medial subnuclei, giving them predominantly right-sided expression patterns (Gamse et al., 2005) (Fig. 4B). The habenular expression of KCTD genes has been used as a direct readout for the direction of asymmetry and for relative subnuclear size, although the function of KCTD proteins in habenular neurons remains unknown.
Several other gene expression patterns serve as useful molecular markers of habenular asymmetry. By expressing GFP under brn3a regulatory elements, Aizawa and colleagues were able to mark the late-proliferating cells of the right habenula in a pattern inverse to that of lov (Aizawa et al., 2005). Members of the acid-sensing ion channel (ASIC) gene family are also useful markers of habenular laterality, as asic1.1 and asic1.3 are both expressed more strongly in the left habenula than in the right (Paukert et al., 2004). The semaphorin co-receptor neuropilin1a (nrp1a) (Fig. 5C) is expressed in a domain that largely overlaps that of lov. This robustly lateralized gene was shown to have a role in directing most Lov+ axons to a targeting domain that is distinct from that of Ron+ axons (Kuan et al., 2007), as discussed further below.
Neuronal morphology also differs a great deal between the left and the right habenulae. The left lateral subnucleus is the most conspicuous: by 3 dpf, a density of neuropil develops at the core of this nucleus to a degree that greatly exceeds the other habenular subnuclei (Concha and Wilson, 2001) (Fig. 5D). The asymmetric nature of habenular afferents is revealed by an examination of synaptic vesicle SV2 labeling (Hendricks and Jesuthasan, 2007), as the left lateral subnucleus is contacted by a much greater density of afferents in a central, soma-free core (Fig. 5E,F). Interestingly, it is this very core of dense neuropil and synapses that is contacted by parapineal axons, which supports the concept of a left-sided specialization with a unique function possibly related to circadian input from the pineal complex.
Further support for the functional specialization of the left lateral subnucleus is provided by the striking segregation of habenular axons that originate from different left-right positions. Zebrafish habenular neurons project strongly to the IPN, and to a lesser extent to the anterior raphe, in the ventral midbrain (Aizawa et al., 2005) (Fig. 4A,C). As in other vertebrates, habenular axons reaching the IPN form a series of `en passant' synaptic connections (i.e. synapses formed along the shaft of axons rather than at their terminals) with the IPN (Hamill and Lenn, 1984) and, strikingly, cross the midline multiple times. Medial subnuclear axons exclusively innervate a flat domain in the ventral aspect of the IPN, which causes mature axons to create a spiraling morphology while restricting themselves to a single dorsoventral plane. Conversely, many Lov+ axons that originate from the large left lateral subnucleus adopt a crown-shaped morphology that arc dorsally as they cross and recross the midline (Bianco et al., 2008). Thus, differential neural information encoded by the left-right position of habenular neurons is preserved by dorsoventral target segregation within a midline structure; however, a well-founded speculation as to the consequences of this segregation awaits a better understanding of IPN function.
The epithalamus in behavioral asymmetries
It is increasingly clear that most vertebrates display some level of behavioral asymmetry, and in many cases, these behavioral lateralities can be stereotyped across the vertebrate lineage. In general, the vertebrate left hemisphere seems to be responsible for so-called considered responses, such as approach and manipulation of unfamiliar objects and vocalization recognition, whereas the right hemisphere is more involved in rapid responses, like escape and aggression (Vallortigara and Rogers, 2005). Behavioral similarities across vertebrates suggest a common evolutionary origin of brain asymmetry within the vertebrate clade, although few of these behaviors have been linked to neuroanatomical asymmetries.
The involvement of habenular neurons in diverse circuits and their interesting laterality in zebrafish has led several researchers to investigate the behavioral consequences of reversed brain laterality, using the prominent epithalamic asymmetries as a marker for neural sidedness. Barth and colleagues studied behavioral reversals in an ENU mutagenized line, frequent situs inversus (fsi), and found a reversal of some behaviors: eye usage when fry approach their own reflection for the first time and side of bias when adults attack an object (Barth et al., 2005). Other lateralized behaviors, such as the direction of turning when entering a new environment or when startled, were not reversed. However, Facchin et al. found no eye use reversals in spaw morpholino (MO)-generated situs inversus larvae (Facchin et al., 2009). Both studies are in accord that brain laterality reversal reduced exploratory behavior and increased the latency of emergence into a novel environment. Thus, the global reversal of brain laterality might even affect some behaviors that are not overtly lateralized.
The zebrafish epithalamus is initially competent to develop molecular and neuroanatomical sidedness in either direction by amplification of initially subtle asymmetries via reciprocal signaling events. Left-sided Nodal signaling overlays reproducible directionality on an otherwise stochastically oriented developmental program. Asymmetry in paired structures could allow for the specialization of function between neuron types and consequently enhance the processing capacity of the system by freeing each structure to take on separate roles. As discussed above, such a model is supported by studies in C. elegans, where proper odorant processing as a consequence of left-right asymmetry has been demonstrated directly. Although a great deal has been discovered concerning asymmetric neurogenesis in both invertebrates and vertebrates, many basic questions remain open in the field, as discussed below.
Conclusions
In C. elegans, stochastic AWC asymmetry is established by the interaction and communication between the bilateral AWC cells to produce a single AWCON cell and a single AWCOFF cell. Initially, a calcium-regulated CaMKII/MAPK signaling cascade maintains the default state of AWCOFF in both AWC cells. Following the formation of a neuronal network mediated by gap junctions and claudin-like adhesion, the symmetry between the two AWC cells is broken: the AWCON cell expresses str-2 and the AWCOFF cell expresses srsx-3. Feedback through the network ensures that only one AWC cell turns on str-2, represses srsx-3 and becomes AWCON. This asymmetry is then reinforced by cGMP signaling and through transcriptional regulation by nsy-7 to allow odor discrimination and proper functioning of the olfactory system throughout the life of the animal. However, it is unclear what transient signal mediates intercellular communication across the NSY-5 gap junction network and whether the network could potentially have a broad impact on other neuron pairs in setting up their left-right asymmetries.
In the zebrafish epithalamus, asymmetries in morphology and gene expression are robust and directional. It is clear that parapineal and habenular neurons must reciprocally interact to establish the mature suite of asymmetrical features observed in the developing larvae. Additionally, Nodal signaling drives epithalamic asymmetries in a particular direction. When Nodal signaling is disrupted, asymmetries develop normally, but with randomized directionality. This highlights the intrinsically stochastic nature of neural asymmetric development when the overlaid directionality cues are removed. The major remaining questions include identifying whether (and if so, how) the left and right sides communicate across the midline to prevent symmetrical development, discovering how the parapineal reinforces asymmetric habenular identity, and understanding the behavioral consequences of asymmetric innervation of the habenula and the IPN.
The theme that emerges when the worm olfactory system and the fish epithalamus are compared is that reciprocal interactions, rather than a simple linear pathway, elaborate and reinforce left-right asymmetry in the nervous system, although the outcomes and molecular mechanisms are very different. In the worm olfactory system, gap-junctional communication and calcium influxes result in functional differences in morphologically identical neurons. By contrast, in the fish epithalamus, secreted signaling molecules regulate morphogenic changes that result in neuroanatomical left-right differences. Therefore, it appears that neuroanatomical asymmetry has arisen more than once in animals; presumably the further study of model systems across phyla will reveal both conserved and novel strategies for establishing neural laterality. Asymmetry has also been implicated as an important aspect of normal brain development and function in humans, as deviations from normal asymmetry have been observed in several pathologies (see Boxes 2 and 3). Although the molecular mechanisms that underpin human cortical asymmetry are not yet understood, the identification and examination of more model systems that exhibit left-right differences in the brain could someday illuminate these aspects of human brain development and dysfunction.
Box 2. Anatomical asymmetries in the human brain
The existence of functional brain lateralization in humans has long been appreciated, and several anatomical asymmetries have also been described.
Petalias. Cortical protrusions that deform the inner surface of the skull, which occur on the left occipital and the right frontal lobes. The increased width of the right frontal and left occipital lobes causes a hemispheric shift across the midline known as the Yakovlevian Torque (LeMay, 1976; Lyttelton et al., 2009). Petalia asymmetries are reversed in situs inversus patients (a condition in which all visceral organ asymmetries are concordantly reversed).
Sulcus. A large inward fold that marks the division between the frontal and parietal lobes. The left central sulcus is deeper than the right central sulcus in right-handers (Amunts et al., 1996; Sun and Walsh, 2006).
Sylvian fissures. Cortical clefts that separate the frontal lobes and temporal lobes. They are related to language ability. The posterior end of the Sylvian fissure in the right hemisphere is higher than in the left (Rubens et al., 1976; Van Essen, 2005).
Temporal lobe. Geschwind and Levitsky identified a marked asymmetry of the planum temporale, a region on the dorsal surface of the superior temporal gyrus involved in speech production (Geschwind and Levitsky, 1968). The left planum was larger in 65% of individuals, the right planum in 11%. Temporal lobe asymmetries are unaffected in situs inversus patients (Kennedy et al., 1999).
Box 3. Clinical implications of human brain asymmetry
Unilateral polymicrogyria. Excessive cortical folding and abnormal lamination in one hemisphere, causing seizures. Chang and colleagues have examined several familial cases of unilateral, right-sided polymicrogyria and speculate that, like the bilateral form, the unilateral form of this disease could have a heritable basis (Chang et al., 2006).
Dyslexia. Many studies have reported a reduction of asymmetry in the brains of dyslexics (Duara et al., 1991; Galaburda et al., 1985; Humphreys et al., 1990; Kushch et al., 1993), although symmetrical features are thought to be necessary but insufficient to cause reading disorders, as up to one-third of healthy individuals can display similar deviations from normal asymmetry (Steinmetz, 1996).
Schizophrenia. Schizophrenic patients show more highly symmetrical traits in trials that directly measure neural activity (Jeon and Polich, 2001) or that use task-based approaches to assess laterality (Asai et al., 2009). A reduction in functional asymmetry of language production areas seems to involve increased activity in the right hemisphere, rather than reduced activity in the left (Bleich-Cohen et al., 2009).
Depression. One recent study described a unilateral, right-sided reduction in habenular volume, cell number and cell area in depressed patients (Ranft et al., 2009). This is of particular interest as it suggests a subtle but functionally relevant asymmetry in the human habenular nuclei, structures that have been extensively studied in the zebrafish (see text).
Autism. The loss of overall asymmetry in the cerebral cortex has been observed in autism, as measured by grey-white segmentation (Herbert et al., 2005).
Acknowledgments
We thank Cori Bargmann, Masato Nakafuku, Bibi Lesch, Miri VanHoven and Jim Patton for comments on the manuscript. C.-F.C. is supported by a Whitehall Foundation Research Award. J.T.G. is supported by the NIH. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
References
- Ahmad N., Long S., Rebagliati M. (2004). A southpaw joins the roster: the role of the zebrafish nodal-related gene southpaw in cardiac LR asymmetry. Trends Cardiovasc. Med. 14, 43-49 [DOI] [PubMed] [Google Scholar]
- Aizawa H., Bianco I. H., Hamaoka T., Miyashita T., Uemura O., Concha M. L., Russell C., Wilson S. W., Okamoto H. (2005). Laterotopic representation of left-right information onto the dorso-ventral axis of a zebrafish midbrain target nucleus. Curr. Biol. 15, 238-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa H., Goto M., Sato T., Okamoto H. (2007). Temporally regulated asymmetric neurogenesis causes left-right difference in the zebrafish habenular structures. Dev. Cell 12, 87-98 [DOI] [PubMed] [Google Scholar]
- Amunts K., Schlaug G., Schleicher A., Steinmetz H., Dabringhaus A., Roland P. E., Zilles K. (1996). Asymmetry in the human motor cortex and handedness. Neuroimage 4, 216-222 [DOI] [PubMed] [Google Scholar]
- Asai T., Sugimori E., Tanno Y. (2009). Schizotypal personality traits and atypical lateralization in motor and language functions. Brain Cogn. 71, 26-37 [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Hartwieg E., Horvitz H. R. (1993). Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74, 515-527 [DOI] [PubMed] [Google Scholar]
- Barth K. A., Miklosi A., Watkins J., Bianco I. H., Wilson S. W., Andrew R. J. (2005). fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Curr. Biol. 15, 844-850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer Huang S. L., Saheki Y., VanHoven M. K., Torayama I., Ishihara T., Katsura I., van der Linden A., Sengupta P., Bargmann C. I. (2007). Left-right olfactory asymmetry results from antagonistic functions of voltage-activated calcium channels and the Raw repeat protein OLRN-1 in C. elegans. Neural Dev. 2, 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco I. H., Carl M., Russell C., Clarke J. D., Wilson S. W. (2008). Brain asymmetry is encoded at the level of axon terminal morphology. Neural Dev. 3, 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgrove B. W., Essner J. J., Yost H. J. (2000). Multiple pathways in the midline regulate concordant brain, heart and gut left-right asymmetry. Development 127, 3567-3579 [DOI] [PubMed] [Google Scholar]
- Bisgrove B. W., Morelli S. H., Yost H. J. (2003). Genetics of human laterality disorders: insights from vertebrate model systems. Annu. Rev. Genomics Hum. Genet. 4, 1-32 [DOI] [PubMed] [Google Scholar]
- Bleich-Cohen M., Hendler T., Kotler M., Strous R. D. (2009). Reduced language lateralization in first-episode schizophrenia: an fMRI index of functional asymmetry. Psychiatry Res. 171, 82-93 [DOI] [PubMed] [Google Scholar]
- Butler A. B., Hodos W. (1996). Comparative vertebrate neuroanatomy: evolution and adaptation New York: Wiley-Liss; [Google Scholar]
- Cahill G. M. (1996). Circadian regulation of melatonin production in cultured zebrafish pineal and retina. Brain Res. 708, 177-181 [DOI] [PubMed] [Google Scholar]
- Carl M., Bianco I. H., Bajoghli B., Aghaallaei N., Czerny T., Wilson S. W. (2007). Wnt/Axin1/beta-catenin signaling regulates asymmetric nodal activation, elaboration, and concordance of CNS asymmetries. Neuron 55, 393-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B. S., Apse K. A., Caraballo R., Cross J. H., McLellan A., Jacobson R. D., Valente K. D., Barkovich A. J., Walsh C. A. (2006). A familial syndrome of unilateral polymicrogyria affecting the right hemisphere. Neurology 66, 133-135 [DOI] [PubMed] [Google Scholar]
- Chang S., Johnston R. J., Jr, Hobert O. (2003). A transcriptional regulatory cascade that controls left/right asymmetry in chemosensory neurons of C. elegans. Genes Dev. 17, 2123-2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Johnston R. J., Jr, Frokjaer-Jensen C., Lockery S., Hobert O. (2004). MicroRNAs act sequentially and asymmetrically to control chemosensory laterality in the nematode. Nature 430, 785-789 [DOI] [PubMed] [Google Scholar]
- Chuang C. F., Bargmann C. I. (2005). A Toll-interleukin 1 repeat protein at the synapse specifies asymmetric odorant receptor expression via ASK1 MAPKKK signaling. Genes Dev. 19, 270-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang C. F., VanHoven M. K., Fetter R. D., Verselis V. K., Bargmann C. I. (2007). An innexin-dependent cell network establishes left-right neuronal asymmetry in C. elegans. Cell 129, 787-799 [DOI] [PubMed] [Google Scholar]
- Colosimo M. E., Brown A., Mukhopadhyay S., Gabel C., Lanjuin A. E., Samuel A. D., Sengupta P. (2004). Identification of thermosensory and olfactory neuron-specific genes via expression profiling of single neuron types. Curr. Biol. 14, 2245-2251 [DOI] [PubMed] [Google Scholar]
- Concha M. L., Wilson S. W. (2001). Asymmetry in the epithalamus of vertebrates. J. Anat. 199, 63-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha M. L., Burdine R. D., Russell C., Schier A. F., Wilson S. W. (2000). A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron 28, 399-409 [DOI] [PubMed] [Google Scholar]
- Concha M. L., Russell C., Regan J. C., Tawk M., Sidi S., Gilmour D. T., Kapsimali M., Sumoy L., Goldstone K., Amaya E., et al. (2003). Local tissue interactions across the dorsal midline of the forebrain establish CNS laterality. Neuron 39, 423-438 [DOI] [PubMed] [Google Scholar]
- Concha M. L., Signore I. A., Colombo A. (2009). Mechanisms of directional asymmetry in the zebrafish epithalamus. Semin. Cell Dev. Biol. 20, 498-509 [DOI] [PubMed] [Google Scholar]
- Duara R., Kushch A., Gross-Glenn K., Barker W. W., Jallad B., Pascal S., Loewenstein D. A., Sheldon J., Rabin M., Levin B., et al. (1991). Neuroanatomic differences between dyslexic and normal readers on magnetic resonance imaging scans. Arch. Neurol. 48, 410-416 [DOI] [PubMed] [Google Scholar]
- Etchberger J. F., Flowers E. B., Poole R. J., Bashllari E., Hobert O. (2009). Cis-regulatory mechanisms of left/right asymmetric neuron-subtype specification in C. elegans. Development 136, 147-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchin L., Burgess H. A., Siddiqi M., Granato M., Halpern M. E. (2009). Determining the function of zebrafish epithalamic asymmetry. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 1021-1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda A. M., Sherman G. F., Rosen G. D., Aboitiz F., Geschwind N. (1985). Developmental dyslexia: four consecutive patients with cortical anomalies. Ann. Neurol. 18, 222-233 [DOI] [PubMed] [Google Scholar]
- Gamse J. T., Thisse C., Thisse B., Halpern M. E. (2003). The parapineal mediates left-right asymmetry in the zebrafish diencephalon. Development 130, 1059-1068 [DOI] [PubMed] [Google Scholar]
- Gamse J. T., Kuan Y. S., Macurak M., Brosamle C., Thisse B., Thisse C., Halpern M. E. (2005). Directional asymmetry of the zebrafish epithalamus guides dorsoventral innervation of the midbrain target. Development 132, 4869-4881 [DOI] [PubMed] [Google Scholar]
- Geschwind N., Levitsky W. (1968). Human brain: left-right asymmetries in temporal speech region. Science 161, 186-187 [DOI] [PubMed] [Google Scholar]
- Golic K. G. (1991). Site-specific recombination between homologous chromosomes in Drosophila. Science 252, 958-961 [DOI] [PubMed] [Google Scholar]
- Golic K. G., Lindquist S. (1989). The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell 59, 499-509 [DOI] [PubMed] [Google Scholar]
- Gothilf Y., Coon S. L., Toyama R., Chitnis A., Namboodiri M. A., Klein D. C. (1999). Zebrafish serotonin N-acetyltransferase-2: marker for development of pineal photoreceptors and circadian clock function. Endocrinology 140, 4895-4903 [DOI] [PubMed] [Google Scholar]
- Hamill G. S., Lenn N. J. (1984). The subnuclear organization of the rat interpeduncular nucleus: a light and electron microscopic study. J. Comp. Neurol. 222, 396-408 [DOI] [PubMed] [Google Scholar]
- Hendricks M., Jesuthasan S. (2007). Asymmetric innervation of the habenula in zebrafish. J. Comp. Neurol. 502, 611-619 [DOI] [PubMed] [Google Scholar]
- Herbert M. R., Ziegler D. A., Deutsch C. K., O'Brien L. M., Kennedy D. N., Filipek P. A., Bakardjiev A. I., Hodgson J., Takeoka M., Makris N., et al. (2005). Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain 128, 213-226 [DOI] [PubMed] [Google Scholar]
- Herman R. K. (1984). Analysis of genetic mosaics of the nematode Caneorhabditis elegans. Genetics 108, 165-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A., Howard C. V., Strahle U., Cossins A. (2003). Neurodevelopmental defects in zebrafish (Danio rerio) at environmentally relevant dioxin (TCDD) concentrations. Toxicol. Sci. 76, 392-399 [DOI] [PubMed] [Google Scholar]
- Hobert O. (2006). Architecture of a microRNA-controlled gene regulatory network that diversifies neuronal cell fates. Cold Spring Harb. Symp. Quant. Biol. 71, 181-188 [DOI] [PubMed] [Google Scholar]
- Hobert O., Johnston R. J., Jr, Chang S. (2002). Left-right asymmetry in the nervous system: the Caenorhabditis elegans model. Nat. Rev. Neurosci. 3, 629-640 [DOI] [PubMed] [Google Scholar]
- Humphreys P., Kaufmann W. E., Galaburda A. M. (1990). Developmental dyslexia in women: neuropathological findings in three patients. Ann. Neurol. 28, 727-738 [DOI] [PubMed] [Google Scholar]
- Jeon Y. W., Polich J. (2001). P300 asymmetry in schizophrenia: a meta-analysis. Psychiatry Res. 104, 61-74 [DOI] [PubMed] [Google Scholar]
- Johnston R. J., Jr, Hobert O. (2003). A microRNA controlling left/right neuronal asymmetry in Caenorhabditis elegans. Nature 426, 845-849 [DOI] [PubMed] [Google Scholar]
- Johnston R. J., Jr, Hobert O. (2005). A novel C. elegans zinc finger transcription factor, lsy-2, required for the cell type-specific expression of the lsy-6 microRNA. Development 132, 5451-5460 [DOI] [PubMed] [Google Scholar]
- Johnston R. J., Jr, Chang S., Etchberger J. F., Ortiz C. O., Hobert O. (2005). MicroRNAs acting in a double-negative feedback loop to control a neuronal cell fate decision. Proc. Natl. Acad. Sci. USA 102, 12449-12454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. J., Jr, Copeland J. W., Fasnacht M., Etchberger J. F., Liu J., Honig B., Hobert O. (2006). An unusual Zn-finger/FH2 domain protein controls a left/right asymmetric neuronal fate decision in C. elegans. Development 133, 3317-3328 [DOI] [PubMed] [Google Scholar]
- Kennedy D. N., O'Craven K. M., Ticho B. S., Goldstein A. M., Makris N., Henson J. W. (1999). Structural and functional brain asymmetries in human situs inversus totalis. Neurology 53, 1260-1265 [DOI] [PubMed] [Google Scholar]
- Koga M., Ohshima Y. (2004). The C. elegans ceh-36 gene encodes a putative homemodomain transcription factor involved in chemosensory functions of ASE and AWC neurons. J. Mol. Biol. 336, 579-587 [DOI] [PubMed] [Google Scholar]
- Kuan Y. S., Gamse J. T., Schreiber A. M., Halpern M. E. (2007). Selective asymmetry in a conserved forebrain to midbrain projection. J. Exp. Zoolog. B Mol. Dev. Evol. 308, 669-678 [DOI] [PubMed] [Google Scholar]
- Kushch A., Gross-Glenn K., Jallad B., Lubs H., Rabin M., Feldman E., Duara R. (1993). Temporal lobe surface area measurements on MRI in normal and dyslexic readers. Neuropsychologia 31, 811-821 [DOI] [PubMed] [Google Scholar]
- Lanjuin A., VanHoven M. K., Bargmann C. I., Thompson J. K., Sengupta P. (2003). Otx/otd homeobox genes specify distinct sensory neuron identities in C. elegans. Dev. Cell 5, 621-633 [DOI] [PubMed] [Google Scholar]
- LeMay M. (1976). Morphological cerebral asymmetries of modern man, fossil man, and nonhuman primate. Ann. New York Acad. Sci. 280, 349-366 [DOI] [PubMed] [Google Scholar]
- Lesch B. J., Gehrke A. R., Bulyk M. L., Bargmann C. I. (2009). Transcriptional regulation and stabilization of left-right neuronal identity in C. elegans. Genes Dev. 23, 345-358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Mercola M. (1998). Gap junctions are involved in the early generation of left-right asymmetry. Dev. Biol. 203, 90-105 [DOI] [PubMed] [Google Scholar]
- Levin M., Mercola M. (1999). Gap junction-mediated transfer of left-right patterning signals in the early chick blastoderm is upstream of Shh asymmetry in the node. Development 126, 4703-4714 [DOI] [PubMed] [Google Scholar]
- Liang J. O., Etheridge A., Hantsoo L., Rubinstein A. L., Nowak S. J., Izpisua Belmonte J. C., Halpern M. E. (2000). Asymmetric nodal signaling in the zebrafish diencephalon positions the pineal organ. Development 127, 5101-5112 [DOI] [PubMed] [Google Scholar]
- Liu P., Jenkins N. A., Copeland N. G. (2002). Efficient Cre-loxP-induced mitotic recombination in mouse embryonic stem cells. Nat. Genet. 30, 66-72 [DOI] [PubMed] [Google Scholar]
- Lyttelton O. C., Karama S., Ad-Dab'bagh Y., Zatorre R. J., Carbonell F., Worsley K., Evans A. C. (2009). Positional and surface area asymmetry of the human cerebral cortex. Neuroimage 46, 895-903 [DOI] [PubMed] [Google Scholar]
- McGrath J., Brueckner M. (2003). Cilia are at the heart of vertebrate left-right asymmetry. Curr. Opin. Genet. Dev. 13, 385-392 [DOI] [PubMed] [Google Scholar]
- Miyasaka N., Morimoto K., Tsubokawa T., Higashijima S., Okamoto H., Yoshihara Y. (2009). From the olfactory bulb to higher brain centers: genetic visualization of secondary olfactory pathways in zebrafish. J. Neurosci. 29, 4756-4767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz C.O., Etchberger J.F., Posy S.L., Frokjaer-Jensen C., Lockery S., Honig B., Hobert O. (2006). Searching for neuronal left/right asymmetry: genomewide analysis of nematode receptor-type guanylyl cyclases. Genetics 173, 131-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz C. O., Faumont S., Takayama J., Ahmed H. K., Goldsmith A. D., Pocock R., McCormick K. E., Kunimoto H., Iino Y., Lockery S., et al. (2009). Lateralized gustatory behavior of C. elegans is controlled by specific receptor-type guanylyl cyclases. Curr. Biol. 19, 996-1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A. R. (1996). From symmetry to asymmetry: phylogenetic patterns of asymmetry variation in animals and their evolutionary significance. Proc. Natl. Acad. Sci. USA 93, 14279-14286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A. R. (2004). Selection for asymmetry. Science 306, 812-813 [DOI] [PubMed] [Google Scholar]
- Paukert M., Sidi S., Russell C., Siba M., Wilson S. W., Nicolson T., Grunder S. (2004). A family of acid-sensing ion channels from the zebrafish: widespread expression in the central nervous system suggests a conserved role in neuronal communication. J. Biol. Chem. 279, 18783-18791 [DOI] [PubMed] [Google Scholar]
- Pierce-Shimomura J. T., Faumont S., Gaston M. R., Pearson B. J., Lockery S. R. (2001). The homeobox gene lim-6 is required for distinct chemosensory representations in C. elegans. Nature 410, 694-698 [DOI] [PubMed] [Google Scholar]
- Poole R. J., Hobert O. (2006). Early embryonic programming of neuronal left/right asymmetry in C. elegans. Curr. Biol. 16, 2279-2292 [DOI] [PubMed] [Google Scholar]
- Ranft K., Dobrowolny H., Krell D., Bielau H., Bogerts B., Bernstein H. G. (2009). Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychol. Med. 1-11 [DOI] [PubMed] [Google Scholar]
- Regan J. C., Concha M. L., Roussigne M., Russell C., Wilson S. W. (2009). An Fgf8-dependent bistable cell migratory event establishes CNS asymmetry. Neuron 61, 27-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussigne M., Bianco I. H., Wilson S. W., Blader P. (2009). Nodal signalling imposes left-right asymmetry upon neurogenesis in the habenular nuclei. Development 136, 1549-1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens A. B., Mahowald M. W., Hutton J. T. (1976). Asymmetry of the lateral (sylvian) fissures in man. Neurology 26, 620-624 [DOI] [PubMed] [Google Scholar]
- Sagasti A. (2007). Three ways to make two sides: genetic models of asymmetric nervous system development. Neuron 55, 345-351 [DOI] [PubMed] [Google Scholar]
- Sagasti A., Hisamoto N., Hyodo J., Tanaka-Hino M., Matsumoto K., Bargmann C. I. (2001). The CaMKII UNC-43 activates the MAPKKK NSY-1 to execute a lateral signaling decision required for asymmetric olfactory neuron fates. Cell 105, 221-232 [DOI] [PubMed] [Google Scholar]
- Sarin S., O'Meara M. M., Flowers E. B., Antonio C., Poole R. J., Didiano D., Johnston R. J., Jr, Chang S., Narula S., Hobert O. (2007). Genetic screens for Caenorhabditis elegans mutants defective in left/right asymmetric neuronal fate specification. Genetics 176, 2109-2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin S., Antonio C., Tursun B., Hobert O. (2009). The C. elegans Tailless/TLX transcription factor nhr-67 controls neuronal identity and left/right asymmetric fate diversification. Development 136, 2933-2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori H., Hamada H. (2006). The left-right axis in the mouse: from origin to morphology. Development 133, 2095-2104 [DOI] [PubMed] [Google Scholar]
- Signore I. A., Guerrero N., Loosli F., Colombo A., Villalon A., Wittbrodt J., Concha M. L. (2009). Zebrafish and medaka: model organisms for a comparative developmental approach of brain asymmetry. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 991-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelson C. D., Gamse J. T. (2009). Building an asymmetric brain: development of the zebrafish epithalamus. Semin. Cell Dev. Biol. 20, 491-497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelson C. D., Burkart J. T., Gamse J. T. (2008a). Formation of the asymmetric pineal complex in zebrafish requires two independently acting transcription factors. Dev. Dyn. 237, 3538-3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelson C. D., Santhakumar K., Halpern M. E., Gamse J. T. (2008b). Tbx2b is required for the development of the parapineal organ. Development 135, 1693-1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroldoni D., Bajoghli B., Aghaallaei N., Czerny T. (2007). Dynamic expression pattern of Nodal-related genes during left-right development in medaka. Gene. Expr. Patterns 7, 93-101 [DOI] [PubMed] [Google Scholar]
- Sreng L. (2003). Sensory asymmetries in the olfactory system underlie sexual pheromone communication in the cockroach Nauphoeta cinerea. Neurosci. Lett. 351, 141-144 [DOI] [PubMed] [Google Scholar]
- Steinmetz H. (1996). Structure, functional and cerebral asymmetry: in vivo morphometry of the planum temporale. Neurosci. Biobehav. Rev. 20, 587-591 [DOI] [PubMed] [Google Scholar]
- Sun T., Walsh C. A. (2006). Molecular approaches to brain asymmetry and handedness. Nat. Rev. Neurosci. 7, 655-662 [DOI] [PubMed] [Google Scholar]
- Sutherland R. J. (1982). The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci. Biobehav. Rev. 6, 1-13 [DOI] [PubMed] [Google Scholar]
- Suzuki H., Thiele T. R., Faumont S., Ezcurra M., Lockery S. R., Schafer W. R. (2008). Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature 454, 114-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka-Hino M., Sagasti A., Hisamoto N., Kawasaki M., Nakano S., Ninomiya-Tsuji J., Bargmann C. I., Matsumoto K. (2002). SEK-1 MAPKK mediates Ca2+ signaling to determine neuronal asymmetric development in Caenorhabditis elegans. EMBO Rep. 3, 56-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel E. R., Sagasti A., Bargmann C. I. (1999). Lateral signaling mediated by axon contact and calcium entry regulates asymmetric odorant receptor expression in C. elegans. Cell 99, 387-398 [DOI] [PubMed] [Google Scholar]
- Uchida O., Nakano H., Koga M., Ohshima Y. (2003). The C. elegans che-1 gene encodes a zinc finger transcription factor required for specification of the ASE chemosensory neurons. Development 130, 1215-1224 [DOI] [PubMed] [Google Scholar]
- Vallortigara G. (2000). Comparative neuropsychology of the dual brain: a stroll through animals' left and right perceptual worlds. Brain Lang. 73, 189-219 [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Rogers L. J. (2005). Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 28, 575-589; discussion 589-633 [DOI] [PubMed] [Google Scholar]
- Van Essen D. C. (2005). A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage 28, 635-662 [DOI] [PubMed] [Google Scholar]
- VanHoven M. K., Bauer Huang S. L., Albin S. D., Bargmann C. I. (2006). The claudin superfamily protein nsy-4 biases lateral signaling to generate left-right asymmetry in C. elegans olfactory neurons. Neuron 51, 291-302 [DOI] [PubMed] [Google Scholar]
- Vanvalen L. (1962). Study of fluctuating asymmetry. Evolution 16, 125-142 [Google Scholar]
- Wes P. D., Bargmann C. I. (2001). C. elegans odour discrimination requires asymmetric diversity in olfactory neurons. Nature 410, 698-701 [DOI] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N., Brenner S. (1986). The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314, 1-340 [DOI] [PubMed] [Google Scholar]
- Yu S., Avery L., Baude E., Garbers D. L. (1997). Guanylyl cyclase expression in specific sensory neurons: a new family of chemosensory receptors. Proc. Natl. Acad. Sci. USA 94, 3384-3387 [DOI] [PMC free article] [PubMed] [Google Scholar]