Abstract
Neural crest is a source of diverse cell types, including the peripheral nervous system. The transcription factor Sox10 is expressed throughout early neural crest. We exploited Sox10 reporter and selection markers created by homologous recombination to investigate the generation, maintenance and expansion of neural crest progenitors. Sox10-GFP-positive cells are produced transiently from mouse embryonic stem (ES) cells by treatment with retinoic acid in combination with Fgf8b and the cytokine leukaemia inhibitory factor (Lif). We found that expression of Sox10 can be maintained using noggin, Wnt3a, Lif and endothelin (NWLE). ES cell-derived Sox10-GFP-positive cells cultured in NWLE exhibit molecular markers of neural crest progenitors. They differentiate into peripheral neurons in vitro and are able to colonise the enteric network in organotypic gut cultures. Neural crest cells purified from embryos using the Sox10 reporter also survive in NWLE, but progressively succumb to differentiation. We therefore applied selection to eliminate differentiating cells. Sox10-selected cells could be clonally expanded, cryopreserved, and multiplied for over 50 days in adherent culture. They remained neurogenic in vitro and in foetal gut grafts. Generation of neural crest from mouse ES cells opens a new route to the identification and validation of determination factors. Furthermore, the ability to propagate undifferentiated progenitors creates an opportunity for experimental dissection of the stimuli and molecular circu that govern neural crest lineage progression. Finally, the demonstration of robust enteric neurogenesis provides a system for investigating and modelling cell therapeutic approaches to neurocristopathies such as Hirschsprung's disease.
Keywords: Embryonic stem cells, Enteric nervous system, Neural crest, Mouse
INTRODUCTION
Neural crest cells emerge from the dorsal margin of the neural plate and produce a wide variety of cell types and tissues, including melanocytes, neurons and glia of the peripheral nervous system, mesenchymal stromal cells, adipocytes, medullary cells, and bone and cartilage of the facial elements (Billon et al., 2007; Crane and Trainor, 2006; Huang and Saint-Jeannet, 2004; Le Douarin et al., 2004; Le Douarin and Dupin, 2003; Takashima et al., 2007). The founder population of neural crest may first be specified at mid-gastrulation (Basch et al., 2006). Upon subsequent receipt of inductive signals at the dorsal edge of the neural fold, definitive neural crest is established (Meulemans and Bronner-Fraser, 2004). Neural crest progenitor cells delaminate, proliferate and migrate extensively to their final destinations, where they differentiate into appropriate cell types. Some neural crest cells do not terminally differentiate but remain in an immature state in the post-natal skin (Fernandes et al., 2004; Sieber-Blum et al., 2004; Wong et al., 2006), enteric nervous system (Kruger et al., 2002), carotid body (Pardal et al., 2007), and as melanocyte stem cells in the bulge region of the hair follicle (Nishimura et al., 2002).
Their broad differentiation potential and extensive tissue colonisation pattern make neural crest cells a unique and interesting population for investigating mechanisms of lineage progression. However, experimental characterisation of neural crest cells is hampered by the difficulty of propagating them in vitro. Most of the information about neural crest precursor cells has come from transplantation and gain-of-function experiments in chick embryos (Le Douarin, 2004; Meulemans and Bronner-Fraser, 2004). Investigations of mammalian neural crest progenitor cells have relied primarily on descriptive and transgenic approaches in whole embryos. In vitro assays have largely been limited to short-term primary cultures, although a sphere culture system for enteric nervous system progenitor cells has been developed (Bondurand et al., 2003).
Mouse embryonic stem (ES) cells can be used to study early events in mammalian lineage commitment because of their pluripotency and ability to recapitulate sequential events of embryogenesis in vitro (Keller, 1995; Smith, 2001). ES cells have also been exploited to capture tissue-specific stem and progenitor cells by simulating the developmental process (Conti et al., 2005; Moretti et al., 2006; Yamashita et al., 2000). Isolation of somatic progenitors from ES cells enables investigation and manipulation of lineage commitment and holds promise for regenerative medicine (Keller, 2005; Smith, 2001). In this context, derivation of neural crest progenitors from ES cells will be a beneficial platform to gain insight into factors that govern their specification, expansion and differentiation. The knowledge obtained might eventually help us to develop cell-replacement therapies for neurocristopathies such as Hirschsprung's disease (Gershon, 1997; Heanue and Pachnis, 2007). Several reports have described the production of neural crest cells from human ES cells (Jiang et al., 2009; Lee et al., 2007; Pomp et al., 2005; Pomp et al., 2008). Neural crest derivatives, such as melanocytes, peripheral neurons, osteoblasts and chondrocytes, have also been reported from murine ES cells (Kawaguchi et al., 2005; Mizuseki et al., 2003; Motohashi et al., 2007).
In this study we focused on the purification of neural crest progenitors from differentiating mouse ES cells and from the developing embryo. Using a Sox10-GFP knock-in reporter, we have identified factors that support expansion of undifferentiated neural crest cells ex vivo with retention of peripheral neuron differentiation potential.
MATERIALS AND METHODS
Cell culture and differentiation
ES cells were maintained without feeders in Glasgow modification of Eagle medium (GMEM) supplemented with foetal calf serum (FCS, 10%), β-mercaptoethanol and leukaemia inhibitory factor (Lif), hereafter referred to as ES cell medium (Smith, 1991).
Embryoid bodies were formed by aggregation of 104 cells per 10 μl of medium in hanging drops for 24 hours (Kawaguchi et al., 2005). Aggregates were collected and cultured in suspension in serum-containing medium. Sox10-GFP-positive cells were purified by flow cytometry and cultured in a 1:1 mix of Neurobasal (Invitrogen) and DMEM/F12 (Invitrogen) media supplemented with N2 (Invitrogen), B27 (Invitrogen), l-glutamine and β-mercaptoethanol [N2B27 medium (Ying and Smith, 2003)] in the presence of Bmp4 (R&D systems; 20 ng/ml), Gdnf (R&D systems; 20 ng/ml) and bFgf (Sigma; 20 ng/ml) for neurogenesis, or in the presence of TGFβ3 (R&D systems; 10 ng/ml) and bFgf (Sigma; 20 ng/ml) for smooth muscle differentiation.
Sox10-GFP-positive cells were maintained in NCC medium [DMEM supplemented with N2, B27, 10–7 M retinoic acid, 15% chick embryo extract (CEE; a gift from V. Pachnis, National Institute for Medical Research, London, UK), l-glutamine, β-mercaptoethanol, Egf (Sigma; 20 ng/ml) and bFgf (20 ng/ml)], supplemented with noggin (R&D systems; 250 ng/ml), Wnt3a (R&D systems: 100 ng/ml), Lif (100 units/ml, prepared in-house) and endothelin 3 (Et-3) (Calbiochem; 100 nM).
Quantitative reverse transcription (qRT) PCR
Total RNA was extracted using the Absolutely RNA RT-PCR Miniprep Kit (Stratagene, La Jolla, CA, USA). First-strand cDNAs were prepared using the Superscript First-Strand Synthesis System for RT-PCR (Life Technologies, Rockville, MD, USA). cDNA was amplified with the primers shown in Table 1. T-PCR was carried out using either the Light Cycler 2000 (Roche) or 7900HT (Applied Biosystems). Cyber Green or Taqman probes were used for quantitation of amplicons.
Table 1.
Primers (5′ to 3′) and Taqman assays used in this study
Histochemical staining
For Oil Red O staining, cells were fixed with 10% formalin, equilibrated with 60% isopropyl alcohol (IPA) for 1 minute, incubated with 2% Oil Red O solution in 60% IPA and then washed in 60% IPA to eliminate excess staining. Samples were stored in 75% glycerol. Embryos were stained for β-galactosidase activity as described (Hogan et al., 1994).
Immunostaining
Cells were fixed in 4% paraformaldehyde (PFA) in PBS, permeabilised and blocked using 0.1% Triton X-100 and 3% serum of the primary antibody species. Primary antibodies used are shown in Table 2. Alexa Fluor-conjugated secondary antibodies (Invitrogen) were used at 1/200. Nuclei were visualised using DAPI.
Table 2.
Antibodies used in this study
Flow cytometry analysis and sorting
For immunolabelling, cultures were dissociated using Cell Dissociation Buffer (Gibco). They were incubated sequentially with primary and secondary antibody for 15 minutes each. Secondary antibody conjugated with APC was purchased from Jackson ImmunoResearch. For GFP analyses, cells were dissociated using trypsin. Cells from embryos were dissociated by incubation with 0.1 U/ml collagenase, 0.8 U/ml dispase and 0.026 mg/ml trypsin solution for 1 hour. Cell debris was eliminated using a strainer (30 μm) and dead cells were stained with 7-amino-actinomycin D (7AAD). After several washes with PBS containing 1% FCS, cells were analysed by flow cytometry using a BD Biosciences FACSCalibur or Dako CyAn ADP flow cytometer. Sorting was performed using a MoFlo high-speed cell sorter. Data were analysed with Summit v4.3 software.
Metaphase analyses
Cells were incubated with KaryoMax Colcemid (Invitrogen) for 40 minutes and dissociated with trypsin. After harvest, cells were incubated with 75 mM KCl and fixed in a final concentration of 1.5% methanol/0.5% acetic acid. Samples were spread on glass slides and chromosomes were stained with DAPI. Images were captured by fluorescence imaging for chromosome counting.
Gene targeting
Sox10-targeting vectors are detailed in Fig. S1 in the supplementary material. Exon 1 of Sox10 was replaced by a green fluorescent protein (GFP) or a GFP-IRES-BLS (blasticidin resistance) cassette. Following electroporation, hygromycin-resistant clones were characterised by Southern blotting. Targeted clones were transiently transfected with Cre, and marker deletion in ganciclovir-resistant clones was confirmed by Southern hybridisation.
Expression constructs for stable transfection
A Sox10 expression vector was generated by insertion of the Sox10 open reading frame between the CAG promoter and IRES-Puro cassette of the pPyFloxMTIPgfp plasmid derived from pPyFloxhNanogIPgfp (Chambers et al., 2003). The DsRedT3 expression vector (pPyCAGMSTIhph) carries a DsRed-IRES-Hygro cassette downstream of the CAG promoter. Transfection was carried out using Lipofectamine 2000 (Invitrogen) and transfectants were selected in either 1.5 μg/ml puromycin (for pPyFloxSox10IPgfp) or 150 μg/ml hygromycin (for pPyCAGMSTIhph).
Genotyping by Southern hybridisation
Gene targeting and Cre-mediated marker deletion were confirmed by genomic DNA hybridisation analysis using standard Southern transfer methods. For mouse genotyping, DNA was isolated and purified from biopsies or embryos and subjected to genomic PCR. Primers used in this study were (5′ to 3′): JK282, GTTGGGCTCTTCACGAGGAC; JK283, CTCTTGCTGGCACCGTTGAC; and JK286, TGAACAGCTCCTCGCCCTTG. The wild-type amplicon of JK283 and JK284 is 371 bp, whereas JK283 and JK286 give a 164 bp product from the targeted allele.
Grafting in ex vivo foetal gut culture
Grafting of cells into foetal gut was performed as described (Natarajan et al., 1999). The entire foetal gut tube was dissected intact from E11.5 embryos and 10-20 cells injected at multiple sites using glass capillary micropipettes. Guts were cultured in Opti-MEM (Invitrogen) supplemented with l-glutamine for 7-10 days, then fixed for immunostaining. For hindgut grafts, embryos were collected in the early morning at approximately E11.3 and the hindgut separated from the rest of the gut tube.
Chimaeras
Chimaeras were generated by aggregation with eight-cell F1 (C57BL/6 × CBA) embryos. Zonae were removed using acid tyrode solution and denuded embryos placed individually into small depressions (made using a Hungarian darning needle) in 10 μl drops of KSOM (Chemicon, MR-020P-5D) under mineral oil in a 30-mm plastic Petri dish, pre-equilibrated to 37°C and 7% CO2. Individual clumps of 10-20 ES cells that had been allowed to reaggregate in suspension for several hours following trypsinisation were placed in each well in contact with an embryo and incubated overnight. Blastocysts were then transferred to the uteri of pseudopregnant female F1 mice and embryos subsequently dissected at mid-gestation.
Mouse studies were authorised by a UK Home Office Project Licence and carried out in a Home Office designated facility.
RESULTS
Generation of Sox10-expressing neural crest progenitors from embryonic stem cells
The HMG-box transcription factor Sox10 is uniquely expressed throughout the neural crest at E10.5 (Britsch et al., 2001). Oligodendrocyte progenitor cells in the central nervous system also express Sox10, but this is observed only from E12.5. Previously, we noted that retinoic acid (RA) treatment of embryoid bodies in the presence of FCS leads to rapid activation of Sox10 mRNA expression, succeeded by other neural crest markers (Kawaguchi et al., 2005). To be able to detect those cells in which Sox10 was induced and thereby monitor the induction of putative neural crest from ES cells, we introduced a GFP reporter into the Sox10 locus (see Fig. S1 in the supplementary material). Following targeting and Cre recombinase-mediated excision of the neomycin selection cassette, GFP expression was undetectable in undifferentiated ES cells (data not shown). However, in chimaeras formed by morula aggregation, GFP was readily detectable throughout neural crest derivatives at mid-gestation and was absent from ectodermal, mesodermal and endodermal tissues (Fig. 1A). This Sox10-GFP reporter ES cell line is hereafter referred to as S10G.
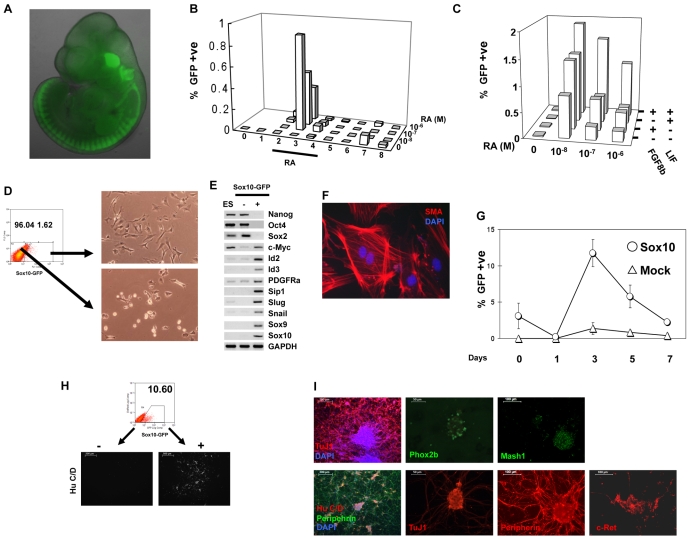
Fig. 1.
Characterisation of Sox10 reporter (S10G) and Sox10 constitutive expression (S10G-S) mouse ES cells. (A) Fluorescence image of E11.5 chimera produced by morula aggregation with S10G cells. (B) Flow cytometry analysis of Sox10-GFP-positive cells from embryoid bodies made with S10G cells. (C) Effect of Lif and Fgf8b on generation of Sox10-GFP-positive cells from embryoid bodies. Bars represent mean value of three independent plates. (D) Flow cytometry scatter plot for sorting Sox10-GFP-positive cells and bright-field images of sorted cells cultured overnight. (E) RT-PCR analysis of pluripotency and neural crest markers in undifferentiated ES cells, Sox10-GFP-negative, and Sox10-GFP-positive populations. (F) Sox10-GFP-positive purified cells cultured for 10 days in the presence of serum and immunostained for smooth muscle actin. (G) GFP-positive cells quantified by flow cytometry during differentiation of S10G-S cells and cells transfected with empty vector (Mock). RA was applied on day 2 at 10–8 M. (H) FACS purification of Sox10-GFP-positive S10G-S cells from day 3 embryoid bodies and subsequent neurogenesis in serum-free N2B27 medium supplemented with Bmp4. Images show immunostaining for HuC/D. (I) Sox10-GFP-positive cells purified as in H differentiate into neurons upon culture in Bmp4 and Gdnf for 4 days. Fixed cells were immunostained for the indicated markers. Scale bars: 500 μm in H; in I, 200 μm left, 50 μm centre, 100 μm right-hand columns.
Upon aggregation of S10G cells and exposure to RA, we observed upregulation of Sox10-GFP (Fig. 1B). However, only 1% of cells became GFP positive and this effect was transient, peaking 24 hours after exposure to RA. Subsequent screening of a panel of growth factors and cytokines revealed that Lif and Fgf8b each increase the number of GFP-positive cells in the presence of RA. Their effects are additive, with the highest yield at 10–8 M RA (Fig. 1C). Even with both factors, however, Sox10-GFP-positive cells only persisted transiently.
Flow cytometric (FACS) purification was employed to characterise the Sox10-GFP-positive cells (Fig. 1D). The GFP+ population isolated 24 hours after RA treatment expressed neural crest markers including Sox10, Sox9, Id2, Id3, Slug (Snai2 – Mouse Genome Informatics) and Snail (Snai1) (Fig. 1E). These cells were negative for the pluripotency markers Oct4 (Pou5f1) and Nanog. They also lacked Sox2, which marks both pluripotent and neuroectodermal cells, and brachyury (data not shown), which marks early endoderm and mesoderm. The sorted GFP+ cells were cultured in the presence of FCS, and after 10 days differentiated into smooth muscle actin (SMA)-positive cells (Fig. 1F). Thus, the Sox10-GFP-expressing population exhibits molecular markers and a differentiation phenotype consistent with neural crest identity.
Forced expression of Sox10 enhances the generation of neural crest progenitors
Sox10 itself is implicated in neural crest commitment (Sauka-Spengler and Bronner-Fraser, 2008). We therefore engineered S10G cells to express Sox10 constitutively under control of the CAG promoter (S10G-S cells) (see Fig. S2 in the supplementary material). Forced expression of Sox10 in undifferentiated ES cells did not have an overt effect on pluripotent identity. Expression levels of the pluripotent factors Oct4 and Nanog were maintained (see Fig. S2 in the supplementary material; data not shown) and the cells contributed extensively to embryo chimaeras.
Upon aggregation and RA treatment, S10G-S cells gave an increased yield of Sox10-GFP-positive cells: from ∼2% to ∼10% (Fig. 1G). This result was reproduced in three independent clones of Sox10 transfectants.
To assess the identity of the induced Sox10-GFP-positive cells, we investigated differentiation potential. Neural crest progenitors should differentiate into autonomic neurons of the peripheral nervous system in response to Bmp and Gdnf (Bondurand et al., 2003; Shah et al., 1996). In line with this prediction, FACS-purified Sox10-GFP-positive cells produced clusters of neuron-like cells when exposed to Bmp4 and Gdnf (Fig. 1H). By contrast, GFP-negative cells, which are predominantly Sox2-positive persisting ES cells or neuroepithelial progenitors, did not produce neurons in these conditions, consistent with the potent effect of Bmp to suppress neural commitment (Ying et al., 2003a) and central nervous system neurogenesis (Nakashima et al., 1999).
Interestingly, the highest yield of neurons was obtained after induction with 10–6 M RA, even though more Sox10-GFP-positive cells were generated using lower concentrations of RA (10–8 M). This might suggest that neurogenic capacity is confined to a subset of neural crest precursors. It might also be related to the forced expression of Sox10, which is inhibitory to neuronal differentiation and maturation in the majority of neural crest progenitors (Kim et al., 2003). Nonetheless, immunofluorescence staining showed that Sox10-GFP cells differentiated in Gdnf and Bmp expressed the generic neuronal marker class III β-tubulin (TuJ1; Tubb3), and also Mash1 (Ascl1), Phox2b, c-Ret and peripherin (Fig. 1I). Quantitative RT-PCR confirmed mRNA expression for Mash1, Phox2b and peripherin (data not shown). This constellation of markers is characteristic of autonomic neurons.
We conclude that the combination of forced expression of Sox10 and a Sox10 reporter facilitate the production and purification of neural crest precursors from mouse ES cells.
Identification of extrinsic factors that maintain neural crest progenitors
In vitro expansion will facilitate experimental characterisation and future biomedical exploitation of neural crest cells. Rat embryo-derived neural crest cells can be maintained for some period in vitro in the presence of chick embryo extract (CEE) with Egf and Fgf (Stemple and Anderson, 1992). These conditions have not been reported to sustain mouse embryonic neural crest progenitors, however, and we found that they were not adequate to support ES cell-derived Sox10-GFP cells. Nor is Sox10-GFP expression maintained in the presence of foetal bovine serum. Therefore, we exploited S10G-S cells to screen for factors that might maintain undifferentiated neural crest cells.
We employed a culture medium developed for primary enteric nervous system progenitor cells (Bondurand et al., 2003) that contains CEE plus Egf and bFgf (Fgf2), hereafter called NCC medium. As shown in Fig. 2A, this supports initial survival of Sox10-GFP-positive cells. However, 3 days after plating only 5% of the cells retained Sox10-GFP expression (Fig. 2B), indicating relatively rapid differentiation. We examined candidate factors and conditions for their ability to prolong the presence of Sox10-GFP-positive cells.
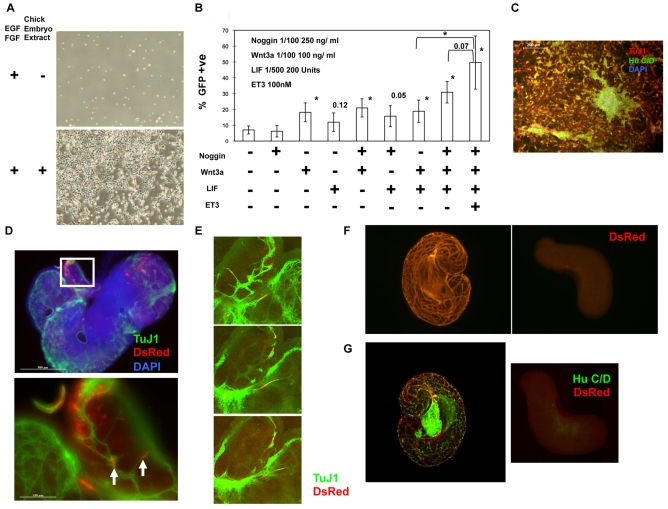
Fig. 2.
Maintenance of Sox10-GFP-positive neural crest progenitor cells in vitro. (A) Sox10-GFP-positive S10G-S cells cultured in neural crest cell (NCC) medium in the absence or presence of chick embryo extract (CEE) for 3 days. (B) Effect of cytokines on the maintenance of Sox10-GFP-positive cells. Sorted cells were cultured in NCC medium supplemented with the indicated combinations of cytokines for 3 days and analysed by flow cytometry. Bars represent mean ± s.d. of triplicate assays on three different S10G-S clones. The frequency of GFP-positive cells in NCC medium without any additive is ∼5%, 3 days after plating. Student's t-test was performed against untreated samples; *, P<0.05, with P-values greater than 0.05 shown. (C) Neuronal differentiation of purified Sox10-GFP-positive cells from S10G-S cells cultured in NCC medium containing noggin, Wnt3a, Lif and Et-3 for 7 days and subsequent culture in serum-free N2B27 medium containing Bmp4 and Gdnf for 7 days. Cells were immunostained for HuC/D and TuJ1. (D) Representative fluorescence images of injected E11.5 gut immunostained for DsRed and TuJ1. The boxed region is shown at higher magnification beneath. White arrows indicate DsRed (red) and TuJ1 (green) double-positive cells. Nuclei are stained with DAPI (blue). (E) Serial confocal images through a region of E11.5 gut graft. (F,G) Confocal images of E11.3 distal hindguts immunostained for DsRed (F) and merged DsRed with HuC/D (G). Right-hand panels show control hindgut cultured without grafting. Scale bars: 200 μm in C; in D, 500 μm above, 100 μm below.
We found that the proportion of Sox10-GFP-expressing cells after 3 days was more than doubled in the presence of recombinant Wnt3a (Fig. 2B). Lif had a more modest effect that was statistically insignificant but observed in three independent experiments. We observed no positive effect of Gdnf and found that the presence of Bmp suppressed Sox10-GFP expression, consistent with its effect in promoting peripheral neuron differentiation. Although the combination of Bmp4 and Wnt3a has been reported to be beneficial for the maintenance of primary neural crest cells (Kleber et al., 2005), we found that addition of Bmp actually reduced the positive response to Wnt3a. We therefore tested the Bmp antagonist noggin. Alone noggin had no significant effect, but in combination with Lif and Wnt3a it increased the proportion of Sox10-GFP-positive cells to over 30% (Fig. 2B). We also found that endothelin 3 (Et-3; Edn3 – Mouse Genome Informatics), which is a supportive factor for enteric nervous system precursors (Bondurand et al., 2006), had a modest additive effect on the maintenance of Sox10-GFP cells (Fig. 2B). When Sox10-positive cells were cultured in NCC supplemented with noggin, Wnt3a, Lif and Et-3 (NWLE), ∼40% of cells remained positive for GFP 3 days after plating. Upon subsequent withdrawal of NWLE and treatment with Bmp4 and Gdnf, these cells differentiated into neurons (Fig. 2C).
These results indicate that in combination with forced expression of Sox10, NWLE enables short-term maintenance of Sox10-expressing cells generated from ES cells, with retention of neurogenic potential.
ES cell-derived Sox10-GFP-expressing neural crest progenitors can colonise the enteric nervous system
Grafting into cultured embryonic gut tube provides an assay system for the potential to colonise the enteric nervous system (Natarajan et al., 1999). In this system, the gut tissue maintains overall structure and viability for at least 14 days, providing an environment for introduced neural crest cells to migrate and differentiate (Natarajan et al., 1999). To enable tracing of grafted cells we introduced a constitutive CAG-DsRedT3 reporter into S10G-S ES cells. Sox10-GFP-positive cells were generated from the resultant S10G-SR ES cells and cultured in NWLE medium for 7 days. The Sox10-GFP-positive cells were then repurified by flow cytometry. After recovery overnight, cells were injected into embryonic gut cultures. Colonisation and differentiation were analysed 7 days later. Numerous DsRed-positive cells were observed to have survived and migrated from the injection sites. GFP expression from grafted cells was negligible, indicating downregulation of Sox10. Immunostaining revealed TuJ1 reactivity in a proportion of the DsRed-positive cells that also exhibited neuron-like morphologies (Fig. 2D,E and see Fig. S3 in the supplementary material).
The relatively low frequency of neuronal differentiation from donor cells might be due to competition from endogenous enteric nervous system progenitors. Furthermore, the presence of abundant host neurons might obscure a definitive contribution from grafted cells. We therefore isolated gut tubes from slightly earlier embryos, at E11.3, and separated the distal portion of the hindgut. Time-lapse studies have established that the wave front of neural crest cells has not reached the hindgut at this stage (Druckenbrod and Epstein, 2005). Consistent with this, we found that after culture for 5 days these hindguts exhibited very few, or no, HuC/D (Elavl3/4)-positive cells (Fig. 2F). By contrast, following injection of Sox10-GFP cells, we observed extensive DsRed expression throughout the tissue, with many cells exhibiting HuC/D (Fig. 2G).
These data indicate that ES cell-derived Sox10-positive neural crest progenitors are competent for extensive colonisation of the gut tube and contribution to enteric neurogenesis.
Propagation of embryo-derived neural crest cells
To test whether the ES cell differentiation system is representative of normal neural crest we compared neural crest precursors prepared from S10G-S ES cells with cells harvested from the developing embryo (Fig. 3A). We isolated Sox10-GFP-positive primary neural crest cells from E10.5 chimaeric embryos made with S10G ES cells. Importantly, these cells do not contain the Sox10 transgene. After purification by flow cytometry, GFP+ cells were cultured in NWLE medium for 5-7 days. We then analysed the expression of a panel of neural crest markers by qRT-PCR (Fig. 3B). With the exception of Sox9, which was noticeably lower in the ES cell derivatives, these markers were comparable between the two populations. This indicates that the ES cell-derived population is similar to embryonic neural crest and further suggests that forced expression of Sox10 does not induce any major transcriptional dysregulation.
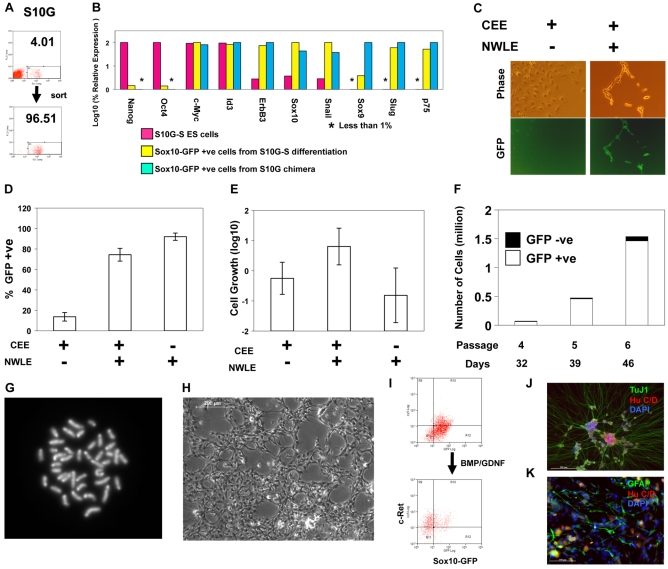
Fig. 3.
Culture of embryo-derived neural crest cells in the presence of noggin, Wnt3a, Lif and Et-3. (A) Flow cytometric purification of neural crest cells from E10.5 S10G chimaera. (B) qRT-PCR analysis of marker mRNA expression in: S10G-S ES cells; Sox10-GFP-positive cells purified from S10G-S embryoid bodies and cultured in NWLE for 5 days; Sox10-GFP-positive cells purified from E10.5 S10G chimaeras and cultured in NWLE for 7 days. The highest level of expression of each gene was defined as 100%. Bars represent means from duplicate PCR reactions. (C) Phase-contrast and fluorescence images of chimaera-derived neural crest cells in NCC medium with or without NWLE for 5 days. (D-K) Expansion of embryo-derived neural crest cells with blasticidin selection for Sox10 expression. (D,E) Proportion (D) and proliferative index (E) of Sox10-GFP-positive cells 6 days after purification from E10.5 S10G-B chimaeras. GFP-positive cells were detected by flow cytometry. Bars represent mean ± s.d. of six independent wells. Proliferation index was determined by dividing the number of GFP-positive cells by the initial cell number plated. (F) Long-term culture and growth of Sox10-GFP-positive neural crest cells purified from chimeric embryos and cultured in NWLE with blasticidin. Bars represent total number of cells at indicated days and passage number. The proportions of GFP-positive and -negative cells, as quantified by flow cytometry, are shown by white and black boxes, respectively. (G) Metaphase spread from neural crest progenitor culture in NWLE plus blasticidin. (H) Phase-contrast image of culture at passage 5 expanded for over one month. (I) Flow cytometry analysis of Sox10-GFP and c-Ret in cultured neural crest cells in NWLE and 5 days after transfer to N2B27 with Bmp and Gdnf. (J,K) Neuronal and glial differentiation of neural crest cells after 8 passages (over 50 days) in NWLE. Cells were transferred to medium containing bFgf/Bmp4/Gdnf for 5 days then fixed and immunostained for HuC/D, TuJ1 and Gfap. Scale bars: 200 μm in H,J; 100 μm in K.
We then examined the effect of NWLE on the maintenance of primary neural crest precursors. Sorted cells were plated with or without NWLE in NCC medium. Without NWLE, cultures rapidly became heterogeneous. Many of the cells acquired a flattened morphology and lost expression of Sox10-GFP within 5 days (Fig. 3C). By contrast, in the presence of NWLE, the majority of cells retained immature morphology and Sox10-GFP expression during the initial 5-7 days in culture. However, 3 weeks after plating, flow cytometry analysis revealed that the number of Sox10-GFP-positive cells was reduced to 11% in NCC medium and was only modestly higher, at 18%, in the presence of NWLE (data not shown). NWLE medium is therefore beneficial for short-term maintenance of Sox10-positive neural crest cells, but only partially suppresses differentiation over the long term.
Sox10 selection facilitates expansion of neural crest progenitors
A recurrent theme in stem cell biology is the paracrine influence of differentiating progeny to promote further differentiation (Tada et al., 2005). To eliminate the influence of differentiated cells in neural crest progenitor cultures, we created a selectable Sox10 allele. We replaced the Sox10 coding sequence with GFP-IRES-BLS (blasticidin resistance). Gene targeting was confirmed by Southern blotting (see Fig. S1 in the supplementary material). The targeted cell line (S10G-B) contributed to term chimeras and yielded germline transmission, confirming essential ES cell identity and integrity.
Chimeric embryos formed by morula aggregation with S10G-B cells were dissociated and total cell populations cultured in NWLE medium for 3 days in the presence or absence of blasticidin. Over 70% of cells surviving in the presence of blasticidin expressed GFP, showing effective selection of Sox10-GFP-positive cells. We then purified Sox10-GFP-positive cells from E10.5 chimeric embryos and cultured them in various combinations of NWLE or CEE without blasticidin (Fig. 3D,E). Three days after plating, the percentage of Sox10-GFP-positive cells was less than 20% in CEE only, whereas the proportion was greater than 70% in the presence of NWLE plus CEE. Interestingly, ∼95% of cells remained positive for Sox10-GFP in the NWLE(+) and CEE(–) condition. However, cells did not expand in this condition. By contrast, in the presence of NWLE plus CEE, the number of Sox10-positive cells increased up to 10-fold. Cryopreserved Sox10-positive cells can be recovered in this medium. Furthermore, clonogenic expansion is possible (see below). These results indicate that NWLE is crucial for maintenance of Sox10-positive cells, whereas CEE supports their multiplication.
Sox10-GFP-positive cells grown in NWLE medium plus blasticidin differentiated into neurons when transferred to N2B27 medium supplemented with bFgf/Bmp4/Gdnf and in the absence of blasticidin. Occasionally, adipocytes were observed in these cultures. However, adipogenic differentiation was invariably lost after the second passage (data not shown), whereas neurogenic and gliogenic potential was retained.
We attempted to culture embryo-derived neural crest cells in adherent conditions under low oxygen tension (Morrison et al., 2000) with continuous blasticidin selection. Under selection, cells expanded continuously and the percentage of Sox10-GFP-positive cells remained ∼90% or more over multiple passages (Fig. 3F). Over a 2-week period, the total number of Sox10-GFP-positive cells increased more than 10-fold. They retained a diploid chromosome complement (Fig. 3G; see Fig. S4 in the supplementary material). The Sox10-GFP-positive cells exhibited relatively immature morphology (Fig. 3H) and were c-Ret low or negative (Fig. 3I). If medium was switched from CEE/NWLE to N2B27 containing Bmp4 and Gdnf, the majority of cells shifted into a Sox10-GFP-negative and c-Ret-high population preceding overt differentiation of neurons and glia (Fig. 3J,K).
Clonal analysis of Sox10-GFP-positive cells in NWLE medium
Limiting dilution analysis indicated that the cloning efficiency of Sox10-positive cells acutely isolated from E10.5 chimeric embryos was ∼2% in medium supplemented with CEE/NWLE in 5% oxygen (data not shown). After flow cytometry with single-cell deposition, three colonies grew up from 96 wells, consistent with the limiting dilution study. These colonies were initially adherent (Fig. 4A), but eventually detached and grew as aggregates in suspension, remaining GFP positive. After culturing for 30 days, the aggregates were dissociated, replated on fibronectin/poly-l-ornithine-coated dishes and cultured in various conditions. They exhibited the typical undifferentiated morphology of neural crest precursors in NWLE medium, differentiated into neurons in the presence of Bmp4/Gdnf, and gave SMA-positive cells with spread morphology in the presence of TGFβ3 (Fig. 4B).
Fig. 4.
Clonal expansion and differentiation of Sox10-positive neural crest progenitors. (A) Colony derived from expansion of a single Sox10-GFP-positive cell purified by flow cytometry from E10.5 S10G-B chimaera. Cells were cultured in NWLE medium for 30 days with periodic blasticidin selection. (B) Phase-contrast and immunofluorescent images of clonal culture after transfer to adherent culture in (left to right) NWLE medium, bFgf/Bmp4/Gdnf, or TGFβ3. Scale bars: 200 μm in left-hand panels; 500 μm in centre and right-hand panels.
Grafting of cultured neural crest cells into foetal gut cultures
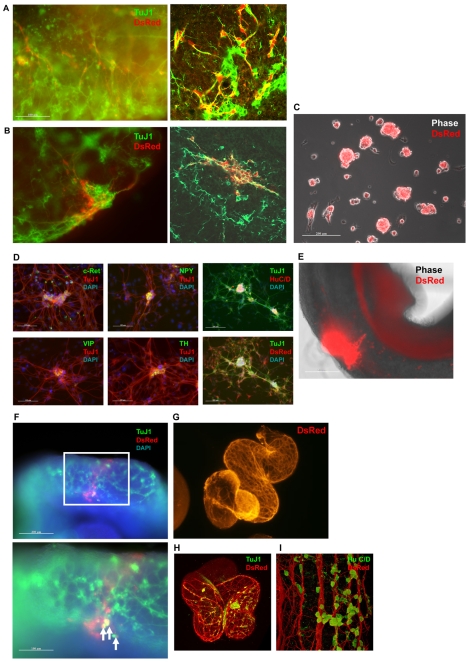
Traceable S10G-B cells were generated by stable transfection with CAG-DsRedT3. These S10G-BR ES cells yielded DsRed expression throughout chimaeric embryos after morula aggregation, while GFP was confined to neural crest cells. Double-positive cells were purified by flow cytometry and grown in NWLE medium in adherent culture for various periods. Cells were then introduced into cultured embryonic gut at the level of either intestine or caecum. Generation of neurons in the foetal gut cultures was detected by TuJ1 staining of DsRed-positive cells after culture in NWLE medium for 7 or 30 days (Fig. 5A,B).
Fig. 5.
Grafting of culture-expanded embryo-derived neural crest cells into foetal gut ex vivo. (A,B) Immunostaining for DsRed and TuJ1 of guts grafted with Sox10-positive cells after culture in NWLE medium for 7 (A) or 30 (B) days. Right-hand images are confocal sections. (C) Aggregate formation from neural crest cells cultured in the presence of Bmp4/Gdnf. Merge of phase-contrast and fluorescence (DsRed) images. (D) Immunostaining for DsRed, HuC/D, c-Ret, Npy, Vip and Th in TuJ1-positive neurons differentiated in vitro from aggregates shown in C. (E) Migration of neural crest cells and extension of axon-like structure from grafted aggregate after 24 hours. Merged phase-contrast and fluorescence images. (F) Immunostaining for TuJ1 and DsRed showing contribution of grafted neural crest aggregate after 9 days. The boxed region is shown at higher magnification beneath. Arrows indicate DsRed (red) and TuJ1 (green) double-positive cells. (G-I) Immunostaining of neuronal network formed in the distal hindgut by grafted cells. Embryo-derived Sox10-GFP cells were cultured for 12 days in NWLE medium prior to grafting. Shown are a confocal image of DsRed immunostaining (G), a merged image of DsRed and TuJ1 immunostaining (H), and a high-magnification image of TuJ1 and HuC/D immunostaining (I). Scale bars: 100 μm in A,B; 200 μm in C,E; in D, 100 μm left and middle, 200 μm right; in F, 200 μm above, 100 μm below.
Single-cell suspensions are difficult to graft in a controlled manner and many cells are lost through dispersion. We therefore also examined grafting of aggregates and pretreated these with Bmp4 and Gdnf to encourage neuronal commitment. After expansion in adherent culture for 4 days in NWLE, aggregates were formed by suspension culture for 2 days in N2B27 supplemented with bFgf/Bmp4/Gdnf (Fig. 5C). In vitro attachment and outgrowth from these aggregates yielded abundant neurons immunopositive for c-Ret, neuropeptide Y (Npy), vasoactive intestinal peptide (Vip) and tyrosine hydroxylase (Th) (Fig. 5D). These neurons retained expression of visible levels of DsRed. We then grafted aggregates into the stomach wall and intestine of gut tubes isolated from E11.5 embryos. Fig. 5E shows migration of the grafted cells and extension of axons from the centre of grafting. Seven days after grafting, specimens were fixed and stained with anti-DsRed and TuJ1 antibodies. DsRed-positive cells were detected away from the graft site and intermingled with the enteric neuronal network. Some of the donor cells acquired neuron-like morphology and stained for TuJ1 (Fig. 5F).
Finally, we injected suspension cells into the distal portion of E11.3 hindgut. Similar to results using ES cell-derived Sox10-positive cells (Fig. 2F), embryo-derived Sox10-positive cells cultured in NWLE medium for 12 days before injection migrated throughout the tissue. They differentiated to produce a striking network of interconnected processes within 5 days (Fig. 5G). Many of the DsRed-positive donor-derived cells were immunopositive for the neuronal markers HuC/D and TuJ1 (Fig. 5H,I). Similar results were obtained with frozen and thawed Sox10-positive cells.
We conclude that neural crest progenitor cells with a capacity for extensive enteric neurogenesis can be expanded as relatively pure adherent populations using NWLE with Sox10 selection.
DISCUSSION
To date, efforts to understand and control the commitment of pluripotent stem cells into embryonic progenitors have largely overlooked the neural crest. Here, we have confirmed that authentic neural crest progenitor cells can be generated from mouse ES cells in vitro. We show that Sox10-expressing cells with molecular features of neural crest are produced in embryoid bodies treated with RA. Retinoic acid has previously been shown to suppress mesoderm formation (Kawaguchi et al., 2005) and promote neuroectodermal fate (Aubert et al., 2002; Bain et al., 1995). Sox10 is induced within 24 hours of exposure to RA, but only in ∼1% of cells. This frequency doubles in response to Fgf8b plus Lif. Fgf8 has been identified as a neural crest inducer in Xenopus (Monsoro-Burq et al., 2003). We found no positive effect of Wnt or Bmp, which are implicated in embryonic neural crest induction at the neural border (Garcia-Castro et al., 2002; Marchant et al., 1998). This might be because the embryoid bodies produce antagonists, such as the Wnt-binding protein Sfrp2 (Aubert et al., 2002). Unfortunately, our attempts to develop an adherent culture system for neural crest commitment, as achieved for other germ layers (Nishikawa et al., 1998; Tada et al., 2005; Ying et al., 2003b), have so far proved unsuccessful for neural crest. This remains an important challenge for future studies.
Class E Sox factors are implicated in the specification of definitive neural crest in the embryo (Sauka-Spengler and Bronner-Fraser, 2008). Consistent with this, we found that forced expression of Sox10 significantly enhanced the yield of neural crest progenitors from the embryoid body differentiation system. This kick-start to neural crest commitment appears to bypass the embryonic sequence in which Sox9 expression precedes that of Sox10. This effect is unlikely to be due simply to autoregulation because although a small fraction of GFP-positive cells was apparent in ES cell culture conditions, aggregation and retinoic acid treatment induced a significant increase. Furthermore, the GFP-positive population is only transiently induced. This transience emphasizes the vulnerability of nascent neural crest to the cellular environment. The ability to generate consistently ∼10% Sox10-GFP-positive cells via forced expression of Sox10 allowed routine isolation and analysis of this population. A potential drawback of this strategy is that constitutive expression of Sox10 can suppress neuronal differentiation (Bondurand et al., 2006; Kim et al., 2003). Indeed, using a doxycycline-regulatable Sox10 transgene we find that the frequency of neuronal differentiation is increased if the transgene is repressed after isolation of Sox10-GFP-positive cells (data not shown).
The consistent production of Sox10-GFP cells by ES cell differentiation enabled us to determine conditions for the maintenance of undifferentiated progenitors. Fgf, Egf and CEE did not sustain appreciable numbers of Sox10-positive cells for more than a few days, but by empirical trial we found additive effects of noggin, Wnt, Lif and Et-3 (NWLE). Importantly, the maintenance of Sox10 expression in these conditions is neither specific to ES cell differentiation, nor dependent on Sox10 transgene expression, as neural crest precursors isolated from E10.5 chimaeric embryos responded similarly. The requirement for noggin can be rationalised as antagonising the differentiation-inducing effects of Bmp, whereas Wnt3 has been shown to contribute to neural crest cell progenitor maintenance (Kleber et al., 2005). A role for Lif or other gp130 cytokines in neural crest progenitors has not previously been described and it would be of interest to evaluate the pleiotropic developmental phenotypes of Lif receptor and gp130 mutants from this perspective (Li et al., 1995; Ware et al., 1995; Yoshida et al., 1996). Endothelins have been implicated in a variety of physiopathologies and diseases such as pulmonary hypertension, renal disease, diabetes and cancer (Barton and Yanagisawa, 2008). Et-3 is expressed in endothelial cells, neurons, renal tubular epithelial cells and intestinal epithelial cells. Gene targeting and natural mutations in mice have revealed a crucial requirement for Et-3 and the endothelin-B receptor in development of the enteric nervous system (Baynash et al., 1994; Gershon, 1995; Hosoda et al., 1994; Puffenberger et al., 1994). Subsequent studies showed that Et-3 affects the survival, proliferation and migration of enteric nervous system progenitor cells (Barlow et al., 2003; Lahav et al., 1998; Shin et al., 1999; Taraviras et al., 1999), and mutations in the Et-3 receptor gene EDNRB cause Hirschsprung's disease (Heanue and Pachnis, 2007). Et-3 has been reported to enhance the maintenance of enteric nervous system progenitors in sphere cultures (Bondurand et al., 2006), but is not known at what stage neural crest cells first become responsive to Et-3.
Even in NWLE, however, a pure Sox10-GFP-expressing population could not be expanded and cultures were overtaken by differentiation. Opportunity remains, therefore, to improve this culture system and better define the requirements of the neural crest progenitors. For the present study, we adopted a selection approach and found that ablation of differentiating cells dramatically improves the expansion of Sox10-expressing progenitors, enabling both clonal proliferation and continuous multiplication for over 50 days. It is important to emphasize that this result was obtained without forced expression of Sox10. Indeed, we used Sox10-heterozygous cells, which could even have reduced self-renewal capabilities. Although heterozygosity for Sox10 does not cause any major perturbation of neural crest development and pups are viable, migration into the caudal hindgut appears delayed (see Fig. S5 in the supplementary material), which is indicative of partial haploinsufficiency. Nonetheless, NWLE-expanded Sox10-GFP-expressing cells were competent for neuronal differentiation in vitro and able to differentiate in the enteric network when introduced into foetal gut cultures. Their potency is confirmed by robust colonisation of the E11.3 hindgut, which was isolated prior to colonisation by endogenous neural crest progenitors. This modification of the established foetal gut graft protocol (Natarajan et al., 1999) provides a convenient assay system and might be useful for modelling cell therapy approaches to Hirschsprung's disease.
Neural crest development may proceed through a succession of increasingly restricted progenitors (Crane and Trainor, 2006). Future studies will address whether NWLE selects for, or induces, progression to restricted enteric nervous system progenitor cells, or is sufficient to maintain broader neural crest potency. The loss of adipogenic differentiation after initial passages is suggestive of lineage segregation, although more detailed studies are required to establish this definitively and to determine whether any loss of potency can be modulated by extrinsic cues.
Further opportunities afforded by the Sox10-GFP reporter/selection system include screening for additional neural crest commitment signals and factors, and identification of cell surface markers for purification of precursors without genetic manipulation. Such studies might facilitate the harvesting of neural crest progenitors from human embryo-derived or induced pluripotent stem cells. It will also be of interest to determine whether the NWLE culture system can be applied to enteric nervous system progenitors isolated from human gut, which could have potential for therapeutic application in Hirschsprung's disease.
Supplementary Material
Acknowledgments
We thank Carmen Birchmeier, Vassilis Pachnis, Dipa Natarajan, Hideki Enomoto and Val Wilson for advice, discussion and provision of materials; Hitoshi Niwa for support; David Anderson for generously providing Sox10 and c-Ret antibodies; Christo Goridis for the Phox2b antibody; Rachael Walker, Nigel Miller and Jan Vrana for flow cytometric sorting; William Mansfield, Ken Jones and Mary Chol for chimaera production; and Sameera Patel for genotyping. This research was supported by the Biotechnology and Biological Sciences Research Council, the Medical Research Council, the Wellcome Trust and the European Commission Project EuroStemCell. A.S. is a Medical Research Council Professor. Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.046896/-/DC1
References
- Aubert J., Dunstan H., Chambers I., Smith A. (2002). Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat. Biotechnol. 20, 1240-1245 [DOI] [PubMed] [Google Scholar]
- Bain G., Kitchens D., Yao M., Huettner J. E., Gottlieb D. I. (1995). Embryonic stem cells express neuronal properties in vitro. Dev. Biol. 168, 342-357 [DOI] [PubMed] [Google Scholar]
- Barlow A., de Graaff E., Pachnis V. (2003). Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron 40, 905-916 [DOI] [PubMed] [Google Scholar]
- Barton M., Yanagisawa M. (2008). Endothelin: 20 years from discovery to therapy. Can. J. Physiol. Pharmacol. 86, 485-498 [DOI] [PubMed] [Google Scholar]
- Basch M. L., Bronner-Fraser M., Garcia-Castro M. I. (2006). Specification of the neural crest occurs during gastrulation and requires Pax7. Nature 441, 218-222 [DOI] [PubMed] [Google Scholar]
- Baynash A. G., Hosoda K., Giaid A., Richardson J. A., Emoto N., Hammer R. E., Yanagisawa M. (1994). Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell 79, 1277-1285 [DOI] [PubMed] [Google Scholar]
- Billon N., Iannarelli P., Monteiro M. C., Glavieux-Pardanaud C., Richardson W. D., Kessaris N., Dani C., Dupin E. (2007). The generation of adipocytes by the neural crest. Development 134, 2283-2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondurand N., Natarajan D., Thapar N., Atkins C., Pachnis V. (2003). Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development 130, 6387-6400 [DOI] [PubMed] [Google Scholar]
- Bondurand N., Natarajan D., Barlow A., Thapar N., Pachnis V. (2006). Maintenance of mammalian enteric nervous system progenitors by SOX10 and endothelin 3 signalling. Development 133, 2075-2086 [DOI] [PubMed] [Google Scholar]
- Britsch S., Goerich D. E., Riethmacher D., Peirano R. I., Rossner M., Nave K. A., Birchmeier C., Wegner M. (2001). The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 15, 66-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I., Colby D., Robertson M., Nichols J., Lee S., Tweedie S., Smith A. (2003). Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113, 643-655 [DOI] [PubMed] [Google Scholar]
- Conti L., Pollard S. M., Gorba T., Reitano E., Toselli M., Biella G., Sun Y., Sanzone S., Ying Q. L., Cattaneo E., et al. (2005). Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 3, e283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane J. F., Trainor P. A. (2006). Neural crest stem and progenitor cells. Annu. Rev. Cell Dev. Biol. 22, 267-286 [DOI] [PubMed] [Google Scholar]
- Druckenbrod N. R., Epstein M. L. (2005). The pattern of neural crest advance in the cecum and colon. Dev. Biol. 287, 125-133 [DOI] [PubMed] [Google Scholar]
- Fernandes K. J., McKenzie I. A., Mill P., Smith K. M., Akhavan M., Barnabe-Heider F., Biernaskie J., Junek A., Kobayashi N. R., Toma J. G., et al. (2004). A dermal niche for multipotent adult skin-derived precursor cells. Nat. Cell Biol. 6, 1082-1093 [DOI] [PubMed] [Google Scholar]
- Garcia-Castro M. I., Marcelle C., Bronner-Fraser M. (2002). Ectodermal Wnt function as a neural crest inducer. Science 297, 848-851 [DOI] [PubMed] [Google Scholar]
- Gershon M. D. (1995). Neural crest development. Do developing enteric neurons need endothelins? Curr. Biol. 5, 601-604 [DOI] [PubMed] [Google Scholar]
- Gershon M. D. (1997). Genes and lineages in the formation of the enteric nervous system. Curr. Opin. Neurobiol. 7, 101-109 [DOI] [PubMed] [Google Scholar]
- Heanue T. A., Pachnis V. (2007). Enteric nervous system development and Hirschsprung's disease: advances in genetic and stem cell studies. Nat. Rev. Neurosci. 8, 466-479 [DOI] [PubMed] [Google Scholar]
- Hogan B., Beddington R., Costantini F., Lacy E. (1994). Manipulating the Mouse Embryo: a Laboratory Manual Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Hosoda K., Hammer R. E., Richardson J. A., Baynash A. G., Cheung J. C., Giaid A., Yanagisawa M. (1994). Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell 79, 1267-1276 [DOI] [PubMed] [Google Scholar]
- Huang X., Saint-Jeannet J. P. (2004). Induction of the neural crest and the opportunities of life on the edge. Dev. Biol. 275, 1-11 [DOI] [PubMed] [Google Scholar]
- Jiang X., Gwye Y., McKeown S. J., Bronner-Fraser M., Lutzko C., Lawlor E. R. (2009). Isolation and characterization of neural crest stem cells derived from in vitro-differentiated human embryonic stem cells. Stem Cells Dev. 18, 1059-1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi J., Mee P. J., Smith A. G. (2005). Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone 36, 758-769 [DOI] [PubMed] [Google Scholar]
- Keller G. M. (1995). In vitro differentiation of embryonic stem cells. Curr. Opin. Cell Biol. 7, 862-869 [DOI] [PubMed] [Google Scholar]
- Keller G. (2005). Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 19, 1129-1155 [DOI] [PubMed] [Google Scholar]
- Kim J., Lo L., Dormand E., Anderson D. J. (2003). SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron 38, 17-31 [DOI] [PubMed] [Google Scholar]
- Kleber M., Lee H. Y., Wurdak H., Buchstaller J., Riccomagno M. M., Ittner L. M., Suter U., Epstein D. J., Sommer L. (2005). Neural crest stem cell maintenance by combinatorial Wnt and BMP signaling. J. Cell Biol. 169, 309-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger G. M., Mosher J. T., Bixby S., Joseph N., Iwashita T., Morrison S. J. (2002). Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron 35, 657-669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav R., Dupin E., Lecoin L., Glavieux C., Champeval D., Ziller C., Le Douarin N. M. (1998). Endothelin 3 selectively promotes survival and proliferation of neural crest-derived glial and melanocytic precursors in vitro. Proc. Natl. Acad. Sci. USA 95, 14214-14219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. M. (2004). The avian embryo as a model to study the development of the neural crest: a long and still ongoing story. Mech. Dev. 121, 1089-1102 [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M., Dupin E. (2003). Multipotentiality of the neural crest. Curr. Opin. Genet. Dev. 13, 529-536 [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M., Creuzet S., Couly G., Dupin E. (2004). Neural crest cell plasticity and its limits. Development 131, 4637-4650 [DOI] [PubMed] [Google Scholar]
- Lee G., Kim H., Elkabetz Y., Al Shamy G., Panagiotakos G., Barberi T., Tabar V., Studer L. (2007). Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat. Biotechnol. 25, 1468-1475 [DOI] [PubMed] [Google Scholar]
- Li M., Sendtner M., Smith A. (1995). Essential function of LIF receptor in motor neurons. Nature 378, 724-727 [DOI] [PubMed] [Google Scholar]
- Marchant L., Linker C., Ruiz P., Guerrero N., Mayor R. (1998). The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev. Biol. 198, 319-329 [PubMed] [Google Scholar]
- Meulemans D., Bronner-Fraser M. (2004). Gene-regulatory interactions in neural crest evolution and development. Dev. Cell 7, 291-299 [DOI] [PubMed] [Google Scholar]
- Mizuseki K., Sakamoto T., Watanabe K., Muguruma K., Ikeya M., Nishiyama A., Arakawa A., Suemori H., Nakatsuji N., Kawasaki H., et al. (2003). Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells. Proc. Natl. Acad. Sci. USA 100, 5828-5833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsoro-Burq A. H., Fletcher R. B., Harland R. M. (2003). Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development 130, 3111-3124 [DOI] [PubMed] [Google Scholar]
- Moretti A., Caron L., Nakano A., Lam J. T., Bernshausen A., Chen Y., Qyang Y., Bu L., Sasaki M., Martin-Puig S., et al. (2006). Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 127, 1151-1165 [DOI] [PubMed] [Google Scholar]
- Morrison S. J., Csete M., Groves A. K., Melega W., Wold B., Anderson D. J. (2000). Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J. Neurosci. 20, 7370-7376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi T., Aoki H., Chiba K., Yoshimura N., Kunisada T. (2007). Multipotent cell fate of neural crest-like cells derived from embryonic stem cells. Stem Cells 25, 402-410 [DOI] [PubMed] [Google Scholar]
- Nakashima K., Yanagisawa M., Arakawa H., Kimura N., Hisatsune T., Kawabata M., Miyazono K., Taga T. (1999). Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science 284, 479-482 [DOI] [PubMed] [Google Scholar]
- Natarajan D., Grigoriou M., Marcos-Gutierrez C. V., Atkins C., Pachnis V. (1999). Multipotential progenitors of the mammalian enteric nervous system capable of colonising aganglionic bowel in organ culture. Development 126, 157-168 [DOI] [PubMed] [Google Scholar]
- Nishikawa S. I., Nishikawa S., Hirashima M., Matsuyoshi N., Kodama H. (1998). Progressive lineage analysis by cell sorting and culture identifies FLK1+ VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 125, 1747-1757 [DOI] [PubMed] [Google Scholar]
- Nishimura E. K., Jordan S. A., Oshima H., Yoshida H., Osawa M., Moriyama M., Jackson I. J., Barrandon Y., Miyachi Y., Nishikawa S. (2002). Dominant role of the niche in melanocyte stem-cell fate determination. Nature 416, 854-860 [DOI] [PubMed] [Google Scholar]
- Pardal R., Ortega-Saenz P., Duran R., Lopez-Barneo J. (2007). Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell 131, 364-377 [DOI] [PubMed] [Google Scholar]
- Pomp O., Brokhman I., Ben-Dor I., Reubinoff B., Goldstein R. S. (2005). Generation of peripheral sensory and sympathetic neurons and neural crest cells from human embryonic stem cells. Stem Cells 23, 923-930 [DOI] [PubMed] [Google Scholar]
- Pomp O., Brokhman I., Ziegler L., Almog M., Korngreen A., Tavian M., Goldstein R. S. (2008). PA6-induced human embryonic stem cell-derived neurospheres: a new source of human peripheral sensory neurons and neural crest cells. Brain Res. 1230, 50-60 [DOI] [PubMed] [Google Scholar]
- Puffenberger E. G., Hosoda K., Washington S. S., Nakao K., deWit D., Yanagisawa M., Chakravart A. (1994). A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung's disease. Cell 79, 1257-1266 [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T., Bronner-Fraser M. (2008). A gene regulatory network orchestrates neural crest formation. Nat. Rev. Mol. Cell Biol. 9, 557-568 [DOI] [PubMed] [Google Scholar]
- Shah N. M., Groves A. K., Anderson D. J. (1996). Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell 85, 331-343 [DOI] [PubMed] [Google Scholar]
- Shin M. K., Levorse J. M., Ingram R. S., Tilghman S. M. (1999). The temporal requirement for endothelin receptor-B signalling during neural crest development. Nature 402, 496-501 [DOI] [PubMed] [Google Scholar]
- Sieber-Blum M., Grim M., Hu Y. F., Szeder V. (2004). Pluripotent neural crest stem cells in the adult hair follicle. Dev. Dyn. 231, 258-269 [DOI] [PubMed] [Google Scholar]
- Smith A. G. (1991). Culture and differentiation of embryonic stem cells. J. Tiss. Cult. Meth. 13, 89-94 [Google Scholar]
- Smith A. G. (2001). Embryo-derived stem cells: of mice and men. Ann. Rev. Cell Dev. Biol. 17, 435-462 [DOI] [PubMed] [Google Scholar]
- Stemple D. L., Anderson D. J. (1992). Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell 71, 973-985 [DOI] [PubMed] [Google Scholar]
- Tada S., Era T., Furusawa C., Sakurai H., Nishikawa S., Kinoshita M., Nakao K., Chiba T., Nishikawa S. (2005). Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development 132, 4363-4374 [DOI] [PubMed] [Google Scholar]
- Takashima Y., Era T., Nakao K., Kondo S., Kasuga M., Smith A. G., Nishikawa S. (2007). Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell 129, 1377-1388 [DOI] [PubMed] [Google Scholar]
- Taraviras S., Marcos-Gutierrez C. V., Durbec P., Jani H., Grigoriou M., Sukumaran M., Wang L. C., Hynes M., Raisman G., Pachnis V. (1999). Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development 126, 2785-2797 [DOI] [PubMed] [Google Scholar]
- Ware C. B., Horowitz M. C., Renshaw B. R., Hunt J. S., Liggit D., Koblar S. A., Gliniak B. C., McKenna H. J., Papayannopoulou T., Thoma B., et al. (1995). Targeted disruption of the low-affinity leukemia inhibitory factor receptor gene causes placental, skeletal, neural and metabolic defects and results in perinatal death. Development 121, 1283-1299 [DOI] [PubMed] [Google Scholar]
- Wong C. E., Paratore C., Dours-Zimmermann M. T., Rochat A., Pietri T., Suter U., Zimmermann D. R., Dufour S., Thiery J. P., Meijer D., et al. (2006). Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J. Cell Biol. 175, 1005-1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita J., Itoh H., Hirashima M., Ogawa M., Nishikawa S., Yurugi T., Naito M., Nakao K. (2000). Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408, 92-96 [DOI] [PubMed] [Google Scholar]
- Ying Q. L., Smith A. G. (2003). Defined conditions for neural commitment and differentiation. Methods Enzymol. 365, 327-341 [DOI] [PubMed] [Google Scholar]
- Ying Q. L., Nichols J., Chambers I., Smith A. (2003a). BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115, 281-292 [DOI] [PubMed] [Google Scholar]
- Ying Q. L., Stavridis M., Griffiths D., Li M., Smith A. (2003b). Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 21, 183-186 [DOI] [PubMed] [Google Scholar]
- Yoshida K., Taga T., Saito M., Suematsu S., Kumanogoh A., Tanaka T., Fujiwara H., Hirata M., Yamagami T., Nakahata T., et al. (1996). Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc. Natl. Acad. Sci. USA 93, 407-411 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.