Abstract
PURPOSE
The purpose of this study was to investigate the significance of fascin expression in colorectal carcinoma.
METHODS
This is a retrospective study of 167 consecutive, well-documented cases of primary colorectal adenocarcinoma for which archival material of surgical specimens from primary tumor resections were available. We chose a representative tissue sample block and examined fascin expression by immunohistochemistry using a primary antibody against “fascin”. We calculated the “immunohistochemical score (IHS)” of fascin for each case, which was calculated from the multiplication of scores for the percentage of stained cells and the staining intensity.
RESULTS
Fascin immunoreactivity was observed in 59 (35.3%) of all cases with strong reactivity in 24 (14.4%), moderate reactivity in 25 (14.9%) and weak reactivity in 10 (6.0%) cases. Strong/moderate immunoreactivities were mostly observed in invasive fronts of the tumors or in both invasive and other areas. Fascin immunoreactivity scores were significantly higher in tumors with lymph node metastasis (p:0.002) and advanced stage presentation (p:0.007). There was no relation between fascin expression and age, gender, depth of invasion, distant metastasis or histological grade (p>0.05). There was a higher and statistically significant correlation between fascin immunoreactivity in the invasive borders of tumors and lymph node metastasis (r:0.747, p:0.005). In stage III/IV tumors, two-year survival was 92.2% in tumors without fascin immunoreactivity, and only 60.0% in tumors with a fascin IHS>10 (p:0.003).
CONCLUSION
These findings suggest that fascin is heterogeneously expressed in approximately one third of colorectal carcinomas with a significant association with lymph node metastasis, tumor stage and location. Moreover, these results indicate that fascin may have a role in the lymph node metastasis of colorectal carcinomas.
Keywords: Colorectal carcinoma, Fascin, Prognosis, Tumor
INTRODUCTION
Gaining motility and migratory capacity are important steps in the neoplastic transformation of a cell, enabling invasion and the metastatic potential of a tumor. Malignant cells have the ability to migrate, which requires rearrangements of the actin cytoskeleton facilitated by actin binding proteins. Fascin is a 55 kDa actin-binding globular protein that is responsible for aggregating F-actin into well-organized parallel bundles.1 Fascin binds beta-catenin, a molecule that is not only involved with cell-cell adhesion, but is also a component of the Wnt signaling pathway.2 The Wnt signaling pathway describes a complex network of proteins that are best known for their roles in embryogenesis and cancer. Moreover, this pathway is thought to play a key role in the occurrence of cancer stem cells. One regulator of the Wnt signaling pathway is sFRP1 (Secreted Frizzled Protein), which has the ability to bind Wnt proteins. When Wnt binds to sFRP, it cannot activate the Wnt signaling pathway. Beachy et al. found that sFRP expression is lost in colorectal and breast cancer.3 Fascin is expressed in normal mesenchymal, endothelial, dendritic and neuronal cells, but not in normal epithelia.4 Normal simple columnar epithelia of the biliary duct, colon, ovary, pancreas and stomach are all negative for fascin.5 The expression of fascin in epithelial neoplasia has recently been described, with the finding that fascin expression is often up-regulated in several types of human neoplasms such as ovarian,6 breast,7 pancreatic,8 lung,9 skin,10 brain,11 stomach12 and esophageal tumors.13
In a cell culture model developed to examine the effect of fascin expression on the adhesive interactions, invasiveness and differentiation of colonic epithelial cells, Jawhari et al. reported marked effects on the organization of the actin cytoskeleton, cell-surface protrusions and focal adhesions in the absence of alterations in the protein levels of the major components of these structures. These effects correlate with alterations in cell movements on a two-dimensional matrix and an increased invasiveness in a three-dimensional matrix. The cells also showed increased proliferation and a decreased capacity for normal glandular differentiation in collagen gels.1 The forced expression of fascin in colorectal cancer cells increased their migration and invasion in cell culture and caused cell dissemination and metastasis in vivo. In contrast, the suppression of fascin expression by small interfering RNA reduced cell invasion. Although the expression of fascin in primary tumors correlated with the presence of metastases, fascin was not expressed in metastases themselves.14
Few clinicopathologic studies have been conducted on fascin expression in colorectal carcinomas,15–17 and some were performed on tissue microarrays.15,17 In one study of syndecan-1 immunoreactivity in colon adenocarcinomas, loss of syndecan-1 expression was correlated with strong fascin staining in the stroma.18 In a study on fascin transcription, fascin up-regulation appeared to have multiple effects, with CREB and AhR playing roles in those effects.19 Recently, it was demonstrated that Rac regulates the interaction of fascin with protein kinase C in cell migration in colon cancer cell lines.20 The aim of this study was to evaluate the immunohistochemical expression of fascin in conventional tissue sections of colorectal carcinomas and to analyze the relationship between fascin expression and clinicopathological features, TNM stage and patient survival.
MATERIALS AND METHODS
Patients
This was a retrospective study of 167 consecutive, well-documented cases of primary colorectal adenocarcinoma for which archival material of surgical specimens from primary tumor resections were available in the Department of Pathology of Gulhane Military Medical Academy. The patient sample consisted of 106 men and 61 women with a median age of 64 years (range 21–88 years). None of the patients had received chemotherapy or radiation before surgery. All cases were reviewed for differentiation according to the World Health Organization classification of colonic carcinomas. There were 6 (3.6%) grade 1, 131 (78.4%) grade 2 and 19 (11.4%) grade 3 carcinomas. Ten cases (6%) were mucinous carcinomas and one (0.6%) was a signet ring cell carcinoma. According to the TNM system of colorectal cancer staging, tumors were classified as stage I (n =14), stage II [n =74, 21 stage IIA (T3N0M0) and 53 stage IIB (T4N0M0) cases], stage III [n =50, 3 stage IIIA (T1-T2N1M0), 27 stage IIIB (T3-T4N1M0) and 20 stage IIIC (anyTN2M0) cases] and stage IV (n =29, any T, any N M1 cases). The mean follow-up period for 151 patients was 28.29 months (range 1–67 months). The remaining 16 patients were lost to follow-up. By the time this study was undertaken, 36 patients died of their disease after a mean survival of 16.27 months (range 1–54 months).
Immunohistochemistry
We chose a representative block for each tumor for immunohistochemical analysis. A 4 μm thick section was cut from each formalin fixed and paraffin-embedded tumor tissue block. The sections were deparaffinized, for 30 min and heated rehydrated, immersed in 3% H2O2 in a microwave at 750 watts in citrate buffer (pH 6.0) for 20 min. The tissue sections were then incubated with Ultra V block (Labvision, Freemont, CA, USA) for 5 min at room temperature before applying the primary antibody. Then, sections were automatically (autostainer-480-LV1, Labvision, USA) immunostained using a primary antibody against “fascin” (FCN01, 1:80, Mouse Monoclonal Ab, Neomarkers, Fremont, CA, USA) with the avidin-biotin peroxidase detection technique (UltraVision Large Volume Detection System, Anti-Polyvalent, horseradish peroxidase; LabVision, Fremont, CA, USA). The internal positive control was vascular endothelial cell fascin staining for the same slide. A negative control was obtained by applying the same protocol without the primary antibody.
Evaluation of Fascin Expression by Immunohistochemistry
For each case, a minimum of five 400x magnification fields of all immunohistochemically stained slides were reviewed by two pathologists. Fascin immunoreactivity in each tumor was verified by the labeling of the endothelial cells of microvessels in each specimen. Cytoplasmic staining of fascin was recorded as a positive signal. The intensity of the immunohistochemical staining was categorized into three groups by comparing the staining intensity of tumor cells with the staining intensity of the vascular endothelial cells: 1+ (weak) = weaker than endothelial cells, 2+ (moderate) = same intensity as endothelial cells and 3+ (strong) = stronger than endothelial cells.13 The intensity score for each case was also characterized on the basis of the predominant area of intensity (invasive fronts of the tumor, other areas, or both). The percentage of positively stained cells was assessed semi-quantitatively and grouped from 1 to 4 as follows: 1=0–10%, 2=11–25%, 3=26–50% and 4=51–100%. We calculated the fascin “immunohistochemical score (IHS)” (0–12) for each case by multiplying the semi-quantitative staining intensity score (0–3) and the percentage of cells that were fascin positive (0–4). According to the IHS, we categorized cases into 3 groups as follows: 0 (negative), IHS≤10 and IHS>10.
Statistical analysis
SPSS software v.13.0 was used for the statistical analysis. p<0.05 was considered to be statistically significant. χ2 tests were used to measure the significance of the relationships between the nominalized immunoreactive fascin intensity, distribution percentages and clinicopathological characteristics. Survival analysis was carried out by the Kaplan-Meier method.
RESULTS
Fascin immunoreactivity was detected in 59 (35.3%) of all cases. Fascin immunoreactivity was observed in the cytoplasm of neoplastic cells in the tumor. No fascin immunoreactivity was present in the adjacent non-neoplastic epithelium. Immunoreactivity was also observed in endothelial cells of vessels in the tumor stroma and was used as an internal control. Fascin immunoreactivity was strong in 10 (6.0%), moderate in 25 (14.9%) and weak in 24 (14.4%) cases. While strong immunoreactivity was generally (60.0%) widespread (>50%) in the tumors, weak immunoreactivity was often (66.7%) focal (<10%) (p:0.0001) (Table 1). Strong or moderate immunoreactivities were mostly observed (90.0% and 80.0%, respectively) in the invasive fronts of the tumors or in both the invasive fronts and other tumor areas. However, weak immunoreactivity was generally (58.3%) observed in other areas of the tumor (p:0.0001) (Table 1).
Table 1.
Comparison of fascin intensity with fascin distribution and location in colorectal carcinomas
| Fascin Intensity |
||||
|---|---|---|---|---|
| Weak (1+) (n=24) (%) | Moderate (2+) (n=25) (%) | Intense (3+) (n=10) (%) | p | |
| Fascin distribution in the tumor | ||||
| 0–9% | 16 (66.7) | 8 (32.0) | 1 (10.0) | 0.0001 |
| 10–25% | 4 (16.7) | 10 (40.0) | 1 (10.0) | |
| 26–50% | 3 (12.5) | 5 (20.0) | 2 (20.0) | |
| 51–100% | 1 (4.2) | 2 (8.0) | 6 (60.0) | |
| Fascin location in the tumor | ||||
| Invasive fronts or invasive fronts + other areas | 10 (41.7) | 20 (80.0) | 9 (90.0) | 0.0001 |
| Only other areas | 14 (58.3) | 5 (20.0) | 1 (10.0) | |
Both the intensity and distribution of fascin immunoreactivity were associated with lymph node metastasis and tumor stage (Table 2 and 3). The intensity of fascin immunoreactivity was also associated with tumor location. Tumors with fascin IHS>10 were larger than (4.9±3.3 vs. 4.5±1.7) those without fascin expression (p=0.005). The association between fascin expression and age, gender, depth of invasion, distant metastasis or histological grade was not significant (NS). Fascin immunoreactivity was present in 5 of 10 mucinous carcinomas, with weak positivity in 4 cases and moderate positivity in the remaining case. Moreover, one signet ring cell carcinoma was negative for fascin expression.
Table 2.
Relationship between fascin intensity and clinicopathological characteristics in 167 colorectal carcinomas
| Fascin Intensity | |||||
|---|---|---|---|---|---|
| Parameters | Absent (n=108) (%) | Weak (1+) (n=24) (%) | Moderate (2+) (n=25) (%) | Intense (3+) (n=10) (%) | p |
| Age | |||||
| ≤60 | 50 (69.4) | 7 (9.7) | 9 (12.5) | 6 (8.3) | 0.258 |
| >60 | 58 (61.1) | 17 (17.9) | 16 (16.8) | 4 (4.2) | |
| Gender | |||||
| M | 64 (72.1) | 16 (13.1) | 19 (9.8) | 7 (4.9) | 0.422 |
| F | 44 (60.4) | 8 (15.1) | 6 (17.9) | 3 (6.6) | |
| Location | |||||
| Rectum | 39 (69.6) | 9 (16.1) | 7 (12.5) | 1 (1.8) | 0.028 |
| Left colon | 50 (71.4) | 4 (5.7) | 11 (15.7) | 5 (7.1) | |
| Right colon | 19 (46.3) | 11 (26.8) | 7 (17.1) | 4 (9.8) | |
| T | |||||
| T1/T2 | 12 (70.6) | 5 (29.4) | 0 | 0 | 0.070 |
| T3/T4 | 96 (64.0) | 19 (12.7) | 25 (16.7) | 10 (6.7) | |
| N | |||||
| N0 | 68 (73.9) | 16 (17.4) | 4 (4.3) | 4 (4.3) | |
| N1 | 23 (57.5) | 2 (5.6) | 14 (35.0) | 1 (2.5) | < 0.0001 |
| N2 | 17 (48.6) | 6 (17.1) | 7 (20.0) | 5 (14.3) | |
| M | |||||
| M0 | 91 (66.4) | 20 (14.6) | 17 (12.4) | 9 (6.6) | 0.244 |
| M1 | 17 (56.7) | 4 (13.3) | 8 (26.7) | 1 (3.3) | |
| Stage | |||||
| I/II | 64 (72.7) | 14 (19.9) | 6 (6.8) | 4 (4.5) | 0.011 |
| III/IV | 44 (55.7) | 10 (12.7) | 19 (24.1) | 6 (7.6) | |
| Tumor grade | |||||
| G1 | 2 (33.3) | 3 (50.0) | 1 (16.7) | 0 | 0.168 |
| G2 | 87 (67.4) | 14 (10.9) | 20 (15.5) | 8 (6.2) | |
| G3 | 12 (63.2) | 2 (10.5) | 3 (15.8) | 2 (10.5) | |
Table 3.
Relationship between fascin distribution and clinicopathological characteristics in 167 colorectal carcinomas
| Fascin Distribution | ||||||
|---|---|---|---|---|---|---|
| Parameters | 0 (n=108) | 0–9% (n=25) | 10–25% (n=15) | 26–50% (n=10) | 50–100% (n=9) | p |
| Age | ||||||
| <60 | 50 (69.4) | 7 (9.7) | 7 (9.7) | 3 (4.2) | 5 (6.9) | 0.390 |
| >60 | 58 (61.1) | 18 (18.9) | 8 (8.4) | 7 (7.4) | 4 (4.2) | |
| Gender | ||||||
| M | 64 (60.4) | 17 (16.0) | 12 (11.3) | 8 (7.5) | 5 (4.7) | 0.375 |
| F | 44 (72.1) | 8 (13.1) | 3 (4.9) | 2 (3.3) | 4 (6.6) | |
| Location | ||||||
| Rectum | 39 (69.6) | 10 (17.9) | 4 (7.1) | 2 (3.6) | 1 (1.8) | 0.146 |
| Left colon | 50 (71.4) | 8 (11.4) | 5 (7.1) | 3 (4.3) | 4 (5.7) | |
| Right colon | 19 (46.3) | 7 (17.1) | 6 (14.6) | 5 (12.2) | 4 (9.8) | |
| T | ||||||
| T1/T2 | 12 (70.6) | 4 (23.5) | 1 (5.9) | 0 | 0 | 0.496 |
| T3/T4 | 96 (64.0) | 21 (14.0) | 14 (9.3) | 10 (6.7) | 9 (6.0) | |
| N | ||||||
| 0 | 68 (73.9) | 15 (16.3) | 3 (3.3) | 3 (3.3) | 3 (3.3) | 0.010 |
| 1 | 23 (57.5) | 5 (12.5) | 7 (17.5) | 4 (10.0) | 1 (2.5) | |
| 2 | 17 (48.6) | 5 (14.3) | 5 (14.3) | 3 (8.6) | 5 (14.3) | |
| M | ||||||
| 0 | 91 (66.4) | 19 (13.9) | 12 (8.8) | 7 (5.1) | 8 (5.8) | 0.682 |
| 1 | 17 (56.7) | 6 (20.2) | 3 (10.0) | 3 (10.0) | 1 (3.3) | |
| Stage | ||||||
| I/II | 64 (72.7) | 14 (15.9) | 3 (3.4) | 4 (4.5) | 3 (3.4) | 0.034 |
| III/IV | 44 (55.7) | 11 (13.9) | 12 (15.2) | 6 (7.6) | 6 (7.6) | |
| Tumor grade | ||||||
| G1 | 2 (33.3) | 3 (50.0) | 1 (16.7) | 0 | 0 | 0.246 |
| G2 | 87 (67.4) | 18 (14.0) | 12 (9.39 | 5 (3.9) | 7 (5.4) | |
| G3 | 12 (63.2) | 1 (5.3) | 1 (5.3) | 3 (15.8) | 2 (10.5) | |
A fascin IHS >10 was statistically significantly more commonly observed in tumors with lymph node metastasis (p:0.005) and more often present in tumors located in the right colon (p:0.020) (Table 4). Similarly, higher stage (stage III/IV) tumors had significantly higher immunohistochemical scores (p:0.011). The percentage of cases with fascin IHS >10 was higher in the T3/T4 group (23.3%) compared to the T1/T2 group (5.9%). However, this difference was not statistically significant. Moreover, a mild to moderate correlation was observed between the number of positive lymph nodes and fascin immunoreactivity (r:0.373, p:0.001). There was a stronger correlation between fascin immunoreactivity in the invasive borders of tumors and lymph node metastasis (r:0.747, p:0.005).
Table 4.
Immunohistochemical scores of fascin expression in colorectal carcinomas
| Parameters | Immunohistochemical score of fascin expression | P | ||
|---|---|---|---|---|
| 0 n=108(%) | 1–10 n=23(%) | >10 n=36(%) | ||
| Age | ||||
| <60 | 50 (69.4) | 6 (8.3) | 16 (22.2) | 0.203 |
| >60 | 58 (61.1) | 17 (17.9) | 20 (21.1) | |
| Gender | ||||
| M | 44 (72.1) | 7 (11.5) | 10 (16.4) | 0.304 |
| F | 64 (60.4) | 16 (15.1) | 26 (24.5) | |
| T | ||||
| T1/T2 | 12 (70.6) | 4 (23.5) | 1 (5.9) | 0.168 |
| T3/T4 | 96 (64.0) | 19 (12.7) | 35 (23.3) | |
| N | ||||
| 0 | 68 (73.9) | 14 (14.3) | 10 (10.9) | 0.005 |
| 1 | 23 (57.5) | 5 (12.5) | 12 (30.0) | |
| 2 | 17 (48.6) | 4 (11.4) | 14 (40.0) | |
| M | ||||
| 0 | 91 (66.4) | 18 (13.1) | 28 (20.4) | 0.598 |
| 1 | 17 (56.7) | 5 (16.7) | 8 (26.7) | |
| Location | ||||
| Rectum | 39 (69.6) | 10 (17.9) | 7 (12.5) | 0.020 |
| Left colon | 50 (71.4) | 6 (8.6) | 14 (20.0) | |
| Right colon | 19 (46.3) | 7 (17.1) | 15 (36.6) | |
| Stage | ||||
| I/II | 64 (72.7) | 13 (14.8) | 11 (12.5) | 0.011 |
| III/IV | 44 (55.7) | 10 (12.7) | 25 (31.6) | |
| Tumor grade | ||||
| G1 | 2 (33.3) | 3 (50.0) | 1 (16.7) | 0.228 |
| G2 | 87 (67.4) | 16 (12.4) | 26 (20.2) | |
| G3 | 12 (63.2) | 1 (5.3) | 6 (31.6) | |
In stage III/IV tumors, while two-year survival was 92.2% in tumors without fascin immunoreactivity, survival was 68.2% in tumors with fascin IHS≤10 and only 60.0% in tumors with fascin IHS>10 (log rank:11.836, p:0.003).
DISCUSSION
Fascin is expressed in many types of transformed epithelial cell lines and in several solid neoplasms. Fascin expression has been correlated with tumor stage12, 13,21,22 and grade,9,12,22 pT class,12,13 lymph node involvement12,13,21,23 and recurrence.12 Furthermore, fascin expression is related to poor prognosis for some solid neoplasms because it facilitates invasion and metastasis.4,9,11–13,15,24,25
In the present study, no immunohistochemical staining was observed in the normal colonic epithelium adjacent to the tumor, in accordance with previous reports.1,15 Jawhari et al. demonstrated that fascin was not detectable in normal colonic epithelium, but was dramatically up-regulated in colorectal adenocarcinoma.1 Moreover, scattered fascin expression was demonstraed in control specimens of normal colonic glandular epithelia in the study by Tsai et al.17 Additionally, fascin was up-regulated in a proportion of adenomas, where its expression was often focal.15 Furthermore, only 16% of adenomas showed fascin expression in greater than 10% of the tumor cells. Finally, Tsai et al. found that higher fascin immunostaining scores were significantly associated with advanced dysplasia in colorectal adenomas.17
In this study, we observed fascin immunoreactivity in 35.3% of all cases and noted that fascin positivity was significantly higher in right colon adenocarcinomas. In a study by Hashimoto et al. using conventional tissue sections from 35 adenocarcinomas, 26% showed fascin expression in greater than 10% of tumor cells.15 When examining fascin expression in 228 advanced colonic adenocarcinoma patients, Puppa et al. found that fascin was detected in 71% of tumors.16 In both these studies, fascin immunoreactivity was more prevalent in tumors located in the proximal colon.15, 16
Interestingly, no association was identified between fascin expression and age or gender. This observation was in accordance with the clinically annotated tumors, where fascin immunoreactivity was not associated with age and gender.15 However, in a recent study, fascin correlated significantly with the female sex.16
Although immunohistochemical scores were higher for poorly differentiated tumors in the present study, this finding was not statistically significant. In a study by Tsai et al., fascin immunohistochemistry was performed in tissue microarrays of 91 surgical specimens. Tsai et al. found that higher fascin immunostaining scores were significantly associated with high-grade histopathological differentiation of colorectal adenocarcinomas.17 Similarly, another study found a significant correlation between fascin expression and tumor grade and mucinous differentiation.16 In the present study, no differences were observed in fascin expression between mucinous and nonmucinous adenocarcinomas. In mucinous adenocarcinomas, fascin immunoreactivity was generally weak in half of the cases. However, more studies with a large number of cases are needed to analyze the role of fascin in mucinous colorectal adenocarcinomas.
We also noted an association between fascin expression and the N stage of colorectal carcinomas. Specifically, strong immunoreactivity in the invasive front of tumors was correlated with the number of metastatic lymph nodes. The association of fascin expression with lymph node involvement has also been documented for other gastrointestinal cancers such as oesophageal13 and gastric carcinomas,12 as well as pulmonary neoplasms.21, 26 Tsai et al. reported that higher fascin immunostaining scores were significantly associated with an advanced T stage of colorectal carcinomas.17 In their study, higher fascin scores were also related with more advanced M and N stages of colorectal carcinomas. However, in the study by Hashimoto et al. with clinically annotated colorectal carcinomas, fascin immunoreactivity was not associated with TNM stage.15 In a recent study, fascin expression was found to be significantly correlated with the number of metastatic lymph nodes, extranodal tumor extensions and the occurrence of distant metastases.16
In the present study, although the mean follow-up was not long; overall survival in stage III/IV tumors was shorter for cases with fascin expression compared to those without. In the study of Hashimoto et al., fascin expression was investigated using a tissue microarray containing cores of 158 colorectal adenocarcinomas with a mean of 38 months of clinical follow-up.15 Patients with stage III/ IV adenocarcinomas with strong fascin immunoreactivity had a worse prognosis than patients with low or no fascin immunoreactivity (3-year overall survival of 11% vs. 43% for fascin-negative patients; p = 0.023). Similarly, a study of fascin expression in 228 advanced colonic adenocarcinoma patients with a long follow-up period revealed that patients with fascin-expressing tumors had a shorter disease-free and overall survival compared to patients with fascin-negative tumors. Moreover, fascin immunoreactivity emerged as an independent prognostic factor in the multivariate analysis.16 Moreover, patients with the same tumor stages could be stratified in different risk categories for relapse and progression according to fascin expression.
The contribution of fascin to the migration of cancer cells and its relationship with beta-catenin were recently analyzed.14,27 Vignjevic et al. identified fascin as a target of beta-catenin-TCF signaling in colorectal cancer cells. Fascin mRNA and protein expression were increased in primary cancers in a stage-dependent manner. Fascin was exclusively localized at the invasive front of tumors that also displayed the nuclear localization of beta-catenin, which is a hallmark of activated Wnt signaling. Their studies show that fascin expression is tightly regulated during the development of colon cancer metastases and that it is a novel target of beta-catenin-TCF signaling. They proposed that transient up-regulation of fascin in colorectal cancer promotes the acquisition of migratory and invasive phenotypes that lead to metastasis. Moreover, they also found that the expression of fascin is down-regulated when tumor cells reach their metastatic destination, where migration ceases and proliferation is enhanced.14 To understand the functional and mechanistic contributions of fascin, Hashimoto et al. used inducible short hairpin RNA (shRNA) to knockdown fascin expression in human colon carcinoma cells derived from an aggressive primary tumor. Fascin depletion led to decreased numbers of filopodia and an altered morphology of cell protrusions, decreased Rac-dependent migration on laminin, decreased turnover of focal adhesions, and decreased xenograft tumor development and metastasis in vivo. They further showed that both the actin bundling and active PKC-binding activities of fascin were required for the organization of filopodial protrusions and tumor metastasis. Thus, these authors concluded that fascin contributes to carcinoma migration and metastasis through parallel pathways that have an impact on multiple subcellular structures that are needed for cell migration.27
Over the past 10 years, our knowledge of the genes and genetic pathways associated with cancer development and progression has been greatly expanded. From the perspective of colorectal carcinomas, one of the important carcinogenetic factors is epidermal growth factor receptor (EGFR), which contributes to the development and progression of several carcinomas and is overexpressed in 50–80% of colorectal cancers.28 Gain-of-function KRAS mutations lead to EGFR-independent activation of intracellular signaling pathways, resulting in tumor cell proliferation, protection against apoptosis, increased invasion and metastasis.29 For EGFR-targeted therapy, standardized testing for KRAS mutation status has been recommended.30 Another example is CD133, the expression of which correlates with liver metastasis and has a prognostic impact in colorectal carcinomas.31 The combination of CD133 and nuclear beta-catenin expression was shown to be a powerful tool to identify high-risk cases of stage IIA colon cancer.32
CONCLUSION
Fascin expression is present in approximately one third of colorectal adenocarcinomas and is more prevalent in right colon tumors. In this study, we noted an association between fascin expression and the N stage of colorectal carcinomas. Specifically, we observed strong immunoreactivity in the invasive front of tumors that was correlated with the number of metastatic lymph nodes. Moreover, we found that in stage III/IV tumors, fascin immunoreactivity was a poor prognostic factor. These findings suggest a possible role of fascin in the process of invasion and lymph node metastasis. More studies are needed to further characterize this possible role of fascin and should use molecular and experimental approaches to do so.
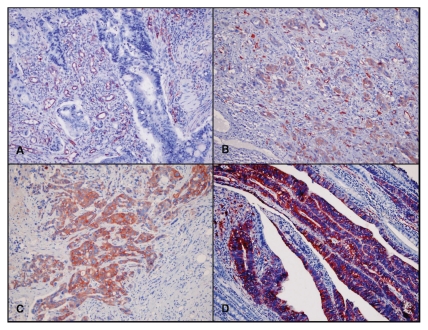
Figure 1.
Negative control (A), weak (B), moderate (C) and strong (D) staining.
REFERENCES
- 1.Jawhari AU, Buda A, Jenkins M, Shehzad K, Sarraf C, Noda M, et al. Fascin, an actin-bundling protein, modulates colonic epithelial cell invasiveness and differentiation in vitro. Am J Pathol. 2003;162:69–80. doi: 10.1016/S0002-9440(10)63799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tao YS, Edwards RA, Tubb B, Wang S, Bryan J, McCrea PD. beta-Catenin associates with the actin-bundling protein fascin in a noncadherin complex. J Cell Biol. 1996;134:1271–81. doi: 10.1083/jcb.134.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–31. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 4.Kureishy N, Sapountzi V, Prag S, Anilkumar N, Adams JC. Fascins, and their roles in cell structure and function. Bioessays. 2002;24:350–61. doi: 10.1002/bies.10070. [DOI] [PubMed] [Google Scholar]
- 5.Zhang FR, Tao LH, Shen ZY, Lv Z, Xu LY, Li EM. Fascin expression in human embryonic, fetal, and normal adult tissue. J Histochem Cytochem. 2008;56:193–9. doi: 10.1369/jhc.7A7353.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu W, McCrea PD, Deavers M, Kavanagh JJ, Kudelka AP, Verschraegen CF. Increased expression of fascin, motility associated protein, in cell cultures derived from ovarian cancer and in borderline and carcinomatous ovarian tumors. Clin Exp Metastasis. 2000;18:83–8. doi: 10.1023/a:1026596609969. [DOI] [PubMed] [Google Scholar]
- 7.Grothey A, Hashizume R, Ji H, Tubb BE, Patrick CW, Jr, Yu D, et al. C-erbB-2/ HER-2 upregulates fascin, an actin-bundling protein associated with cell motility, in human breast cancer cell lines. Oncogene. 2000;19:4864–75. doi: 10.1038/sj.onc.1203838. [DOI] [PubMed] [Google Scholar]
- 8.Maitra A, Iacobuzio-Donahue C, Rahman A, Sohn TA, Argani P, Meyer R, et al. Immunohistochemical validation of a novel epithelial and a novel stromal marker of pancreatic ductal adenocarcinoma identified by global expression microarrays: sea urchin fascin homolog and heat shock protein 47. Am J Clin Pathol. 2002;118:52–9. doi: 10.1309/3PAM-P5WL-2LV0-R4EG. [DOI] [PubMed] [Google Scholar]
- 9.Pelosi G, Pastorino U, Pasini F, Maissoneuve P, Fraggetta F, Iannucci A, et al. Independent prognostic value of fascin immunoreactivity in stage I nonsmall cell lung cancer. Br J Cancer. 2003;88:537–47. doi: 10.1038/sj.bjc.6600731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goncharuk VN, Ross JS, Carlson JA. Actin-binding protein fascin expression in skin neoplasia. J Cutan Pathol. 2002;29:430–8. doi: 10.1034/j.1600-0560.2002.290708.x. [DOI] [PubMed] [Google Scholar]
- 11.Gunal A, Onguru O, Safali M, Beyzadeoglu M. Fascin expression [corrected] in glial tumors and its prognostic significance in glioblastomas. Neuropathology. 2008;28:382–6. doi: 10.1111/j.1440-1789.2008.00889.x. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto Y, Shimada Y, Kawamura J, Yamasaki S, Imamura M. The prognostic relevance of fascin expression in human gastric carcinoma. Oncology. 2004;67:262–70. doi: 10.1159/000081327. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto Y, Ito T, Inoue H, Okumura T, Tanaka E, Tsunoda S, et al. Prognostic significance of fascin overexpression in human esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11:2597–605. doi: 10.1158/1078-0432.CCR-04-1378. [DOI] [PubMed] [Google Scholar]
- 14.Vignjevic D, Schoumacher M, Gavert N, Janssen KP, Jih G, Lae M, et al. Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res. 2007;67:6844–53. doi: 10.1158/0008-5472.CAN-07-0929. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto Y, Skacel M, Lavery IC, Mukherjee AL, Casey G, Adams JC. Prognostic significance of fascin expression in advanced colorectal cancer: an immunohistochemical study of colorectal adenomas and adenocarcinomas. BMC Cancer. 2006;6:241. doi: 10.1186/1471-2407-6-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puppa G, Maisonneuve P, Sonzogni A, Masullo M, Chiappa A, Valerio M, et al. Independent prognostic value of fascin immunoreactivity in stage III-IV colonic adenocarcinoma. Br J Cancer. 2007;96:1118–26. doi: 10.1038/sj.bjc.6603690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai WC, Chao YC, Sheu LF, Chang JL, Nieh S, Jin JS. Overexpression of fascin-1 in advanced colorectal adenocarcinoma: tissue microarray analysis of immunostaining scores with clinicopathological parameters. Dis Markers. 2007;23:153–60. doi: 10.1155/2007/685163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto Y, Skacel M, Adams JC. Association of loss of epithelial syndecan-1 with stage and local metastasis of colorectal adenocarcinomas: an immunohistochemical study of clinically annotated tumors. BMC Cancer. 2008;8:185. doi: 10.1186/1471-2407-8-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto Y, Loftis DW, Adams JC. Fascin-1 promoter activity is regulated by CREB and the aryl hydrocarbon receptor in human carcinoma cells. PLoS One. 2009;4:e5130. doi: 10.1371/journal.pone.0005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsons M, Adams JC. Rac regulates the interaction of fascin with protein kinase C in cell migration. J Cell Sci. 2008;121(Pt 17):2805–13. doi: 10.1242/jcs.022509. [DOI] [PubMed] [Google Scholar]
- 21.Choi PJ, Yang DK, Son CH, Lee KE, Lee JI, Roh MS. Fascin immunoreactivity for preoperatively predicting lymph node metastases in peripheral adenocarcinoma of the lung 3 cm or less in diameter. Eur J Cardiothorac Surg. 2006;30:538–42. doi: 10.1016/j.ejcts.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 22.Yoder BJ, Tso E, Skacel M, Pettay J, Tarr S, Budd T, et al. The expression of fascin, an actin-bundling motility protein, correlates with hormone receptor-negative breast cancer and a more aggressive clinical course. Clin Cancer Res. 2005;11:186–92. [PubMed] [Google Scholar]
- 23.Zhang H, Xu L, Xiao D, Xie J, Zeng H, Cai W, et al. Fascin is a potential biomarker for early-stage oesophageal squamous cell carcinoma. J Clin Pathol. 2006;59:958–64. doi: 10.1136/jcp.2005.032730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Pinilla SM, Sarrio D, Honrado E, Hardisson D, Calero F, Benitez J, et al. Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin Cancer Res. 2006;12:1533–9. doi: 10.1158/1078-0432.CCR-05-2281. [DOI] [PubMed] [Google Scholar]
- 25.Roma AA, Prayson RA. Fascin expression in 90 patients with glioblastoma multiforme. Ann Diagn Pathol. 2005;9:307–11. doi: 10.1016/j.anndiagpath.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Pelosi G, Pasini F, Fraggetta F, Pastorino U, Iannucci A, Maisonneuve P, et al. Independent value of fascin immunoreactivity for predicting lymph node metastases in typical and atypical pulmonary carcinoids. Lung Cancer. 2003;42:203–13. doi: 10.1016/s0169-5002(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto Y, Parsons M, Adams JC. Dual actin-bundling and protein kinase C-binding activities of fascin regulate carcinoma cell migration downstream of Rac and contribute to metastasis. Mol Biol Cell. 2007;18:4591–602. doi: 10.1091/mbc.E07-02-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spano JP, Lagorce C, Atlan D, Milano G, Domont J, Benamouzig R, et al. Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann Oncol. 2005;16:102–8. doi: 10.1093/annonc/mdi006. [DOI] [PubMed] [Google Scholar]
- 29.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–74. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 30.van Krieken JH, Jung A, Kirchner T, Carneiro F, Seruca R, Bosman FT, et al. KRAS mutation testing for predicting response to anti-EGFR therapy for colorectal carcinoma: proposal for an European quality assurance program. Virchows Arch. 2008;453:417–31. doi: 10.1007/s00428-008-0665-y. [DOI] [PubMed] [Google Scholar]
- 31.Horst D, Scheel SK, Liebmann S, Neumann J, Maatz S, Kirchner T, et al. The cancer stem cell marker CD133 has high prognostic impact but unknown functional relevance for the metastasis of human colon cancer. J Pathol. 2009;219:427–34. doi: 10.1002/path.2597. [DOI] [PubMed] [Google Scholar]
- 32.Horst D, Kriegl L, Engel J, Jung A, Kirchner T. CD133 and nuclear beta-catenin: the marker combination to detect high risk cases of low stage colorectal cancer. Eur J Cancer. 2009;45:2034–40. doi: 10.1016/j.ejca.2009.04.004. [DOI] [PubMed] [Google Scholar]