Abstract
Elevated circulating levels of acute phase proteins (APP) are associated with inflammation and inflammatory disorders such as cardiovascular disease. APP are mainly synthesised by hepatocytes and their transcription is induced by pro-inflammatory cytokines such as interleukin-1 (IL-1). The molecular mechanisms underlying the IL-1-induced expression of key transcription factors implicated in the regulation of APP are poorly understood. We have investigated this aspect using the CCAAT/enhancer binding protein-δ (C/EBPδ) as a model gene. IL-1 induced the expression of C/EBPδ mRNA and protein in the human hepatoma Hep3B cell line, a widely employed model system for studies on cytokine signalling in relation to the expression of APP. The IL-1-mediated induction of C/EBPδ expression was attenuated in the presence of pharmacological inhibitors against c-Jun N-terminal kinase (JNK) (curcumin and SP600125), casein kinase 2 (CK2) (apigenin) and nuclear factor-κB (NF-κB) (NF-κB activation inhibitor). RNA interference assays showed significant attenuation of the IL-1-induced expression of C/EBPδ following knockdown of the p50 and p65 subunits of NF-κB. IL-1 induced NF-κB DNA binding and activation by this transcription factor and this was attenuated by curcumin and apigenin. Taken together, these results suggest a potentially crucial role for NF-κB in the IL-1-induced expression of C/EBPδ, and thereby downstream APP genes regulated by this transcription factor.
Keywords: Acute phase proteins, CCAAT/enhancer binding proteins, Hepatocytes, Interleukin-1, Cell signalling
1. Introduction
The acute phase response (APR) is the body's innate immune defence against infection, inflammation, stress, physical trauma and pathological conditions such as cardiovascular disease (Ramji et al., 1993a; Baumann and Gauldie, 1994). The APR is characterized by fever, leukocytosis, altered metabolism in many organs, and dramatic changes in the plasma concentrations of various acute phase proteins (APP) (Ramji et al., 1993a; Baumann and Gauldie, 1994). Numerous studies have suggested that such APP may act as biomarkers and possibly causal agents in the development of cardiovascular disease (Packard and Libby, 2008).
The APP can be distinguished into two major groups; the positive APP (e.g. hemopexin, C-reactive protein (CRP), fibrinogen, serum amyloid A (SAA)) whose plasma concentrations increase during the APR, and the negative APP (e.g. albumin, transferrin) whose levels decrease (Ramji et al., 1993a; Baumann and Gauldie, 1994). The main stimulators of APP production are inflammation-associated cytokines such as interleukin-6 (IL-6), interleukin-1 (IL-1), tumour necrosis factor-α and interferon-γ (IFN-γ) (Ramji et al., 1993a,b; Baumann and Gauldie, 1994; Moshage, 1997). The liver is the major site for APP production and cytokines regulate their expression mainly at the transcriptional level (Ramji et al., 1993a,b; Baumann and Gauldie, 1994; Moshage, 1997). It is essential that the molecular mechanisms underlying such regulation are fully understood, particularly how cytokines modulate the action of key transcription factors involved in this response. CCAAT/enhancer binding proteins (C/EBP) fall in this category (Ramji and Foka, 2002).
Transcription factors belonging to the C/EBP family contain a conserved basic-leucine zipper motif at the carboxyl terminus that is involved in dimerization and DNA binding (Ramji and Foka, 2002). There are six C/EBP family members (C/EBPα to ζ), most of which recognize similar DNA sequences, at least in vitro, and form heterodimers in intrafamilial combinations (Ramji and Foka, 2002). Binding sites for the C/EBPs have been identified in the regulatory regions of a large number of APP genes, including hemopexin, haptoglobin, CRP, α1-acid glycoprotein, α2-macroglobulin, SAA-1, -2 and -3, complement C3, plasminogen and plasminogen activator inhibitor-1 (Poli, 1998; Ramji and Foka, 2002; Bannach et al., 2004; Dong et al., 2005, 2007). The general trend that has emerged from these studies is that C/EBPα homodimers or C/EBPα:C/EBPβ heterodimers are mainly responsible for the constitutive transcription (Chen and Liao, 1993; Ray and Ray, 1994; Poli, 1998; Ramji and Foka, 2002). However, upon induction of the APR, C/EBPα dimers are rapidly replaced by C/EBP-β and -δ homo-/heterodimers, although the exact composition of these complexes varies with different gene promoters and depends on the experimental models used (Ramji et al., 1993b; Chen and Liao, 1993; Ray and Ray, 1994; Poli, 1998; Ramji and Foka, 2002).
The expression and the activity of C/EBP-β and -δ are also stimulated by IL-1 and IL-6 (Ramji et al., 1993b; Poli, 1998; Ramji and Foka, 2002). Unfortunately, the molecular mechanisms underlying such regulation are relatively poorly understood, particularly those relating to cytokine-induced expression, which are further restricted to the action of IL-6 (Ramji and Foka, 2002). The purpose of this study was therefore to investigate the mechanisms underlying the actions of IL-1 using C/EBPδ as a model gene.
2. Materials and methods
2.1. Reagents
The human hepatoma Hep3B cell line was from the European Collection of Animal Cell Cultures, whereas recombinant IL-1β (called IL-1 hereafter) was from Peprotech. Antibodies were from Cell Signalling Technology (p50/p105 NF-κB; p65 NF-κB; JNK; JNK pThr183/Tyr185; TAK1); Santa-Cruz Biotechnology [C/EBPδ (M17)X; c-Jun; CK2α and CK2α′); or Sigma (β-actin). The non-radioactive JNK kinase assay kit was from Cell Signalling Technology whereas small interfering RNA (siRNA) were from either Qiagen [p50 NF-κB (SI02654932); p65 NF-κB (SI02663094); TAK1 (SI02758756); JNK-1 (SI02758673); JNK2 (SI02758637); and CK2α′ (SI00605409)]; Invitrogen [CK2α, 5′-ACCAGACGUUAACAGACUAUGAUAU-3′ (duplex 1) and 5′-UCAUGAUUGAUCAUGAGCACAGAAA-3′ (duplex 2)]; or Ambion (Silencer® GAPDH siRNA control; NM_002046). The pharmacological inhibitors were from Alexis Biochemicals (apigenin) or Merck (curcumin, NF-κB activation inhibitor-NAI, SP600125).
2.2. Cell culture
Hep3B cells were maintained in Dulbecco's modified Eagle Medium (DMEM) with GlutaMAX™ containing 10% (v/v) heat-inactivated fetal calf serum (HI-FCS) (30 min at 56 °C), penicillin (100 U/ml) and streptomycin (100 μg/ml) (designated as complete medium). The cultures were maintained at 37 °C in a humid incubator with a 5% (v/v) CO2 atmosphere. Treatment with IL-1 or the different pharmacological agents was performed as previously described (Mead et al., 2003; Harvey et al., 2007; Harris et al., 2008; Foka et al., 2009).
2.3. RT-PCR
Total cellular RNA was isolated and subjected to RT-PCR and agarose gel electrophoresis essentially as described previously (Mead et al., 2003; Harvey et al., 2007; Foka et al., 2009) except that the total number of cycles and the annealing temperature varied: 25–29 cycles and 60 °C for C/EBPδ; 17 cycles and 60 °C for glyceraldehyde 3-phosphate dehydrogenase (GAPDH); and 10 cycles and 62 °C for 28SrRNA. The sequences of the primers were 5′-GCGCGAGCGCAACAACATC-3′ and 5′-CCAGGTCCCGCGTGAGCT-3′ for C/EBPδ; 5′-CCCTTCATTGACCTCAA CTACATGG-3′ and 5′-AGTCTTCTGGGTGGCAGTGATGG-3′ for GAPDH; and 5′-TGAACTATGCTTGGGCAGGG-3′ and 5′-AGCGCCATCCATTTTCAGGG-3′ for 28SrRNA (Mead et al., 2003; Harvey et al., 2007; Foka et al., 2009). The PCR products were size-fractionated on a 2% (w/v) agarose gel, photographed using a Syngene gel documentation system (GRI) and quantified using the Gene Tools computer package (Syngene). The signals for C/EBPδ were normalized to the constitutive control (28SrRNA or GAPDH).
2.4. Western blot analysis and c-Jun N-terminal kinase (JNK) activity assays
Cellular extracts were prepared either as described previously (Mead et al., 2003; Harvey et al., 2007; Harris et al., 2008; Foka et al., 2009) or using the Laemmli sample buffer [0.125 M Tris–HCl, pH 6.8; 4% (w/v) SDS; 10% (v/v) glycerol and 10% (v/v) 2-mercaptoethanol). For the latter, the cells, grown in 9.5 cm2 dishes, were washed with phosphate buffered saline, lysed using 75 μl of Laemmli sample buffer, and the cellular proteins collected by centrifugation. Western blot analysis was carried out as previously described (Mead et al., 2003; Harvey et al., 2007; Harris et al., 2008; Foka et al., 2009) whereas the JNK kinase activity assay on non-denatured proteins was performed according to the instructions of the manufacturers (Cell Signalling Technology). The signals from the western blots were normalized to the constitutive β-actin control.
2.5. Transient transfection assays
Transfection with plasmid DNA was carried out using polyethylenimine (PEI) as previously described (Harvey et al., 2007). The luciferase activity in cell extracts was determined using a commercially available kit (Promega) and normalized to total protein levels (determined using the microBCA protein assay kit from Pierce).
2.6. siRNA-mediated knockdown
Transfection with siRNA was carried out using DharmaFECT™ as recommended by the manufacturer (Dharmacon RNA Technologies). The cells were first incubated with the transfected siRNA for 65 h and, following aspiration of the medium, for the requisite time in complete medium containing the vehicle or the cytokine.
2.7. Electrophoretic mobility shift assays (EMSA)
Whole cell and nuclear extracts were prepared and used for EMSA as previously described (Mead et al., 2003; Harvey et al., 2007; Harris et al., 2008; Foka et al., 2009). The sequences of the oligonucleotides used were 5′-GGAGTTGAGGG GACTTTCCCAGGC-3′ and 5′-GGCCTGGGAAAGTCCCCTCAACT-3′ for NF-κB and 5′-CGCTTGATGAGTCAG-3′ and 5′-TTCCGGCTGACTCAT-3′ for AP-1. For antibody supershift/interference experiments, the antibody (0.5 μg) was added to the binding reaction and incubated on ice for 20 min prior to the addition of the radiolabelled probe. In competition assays, the binding reaction mixture was incubated for 10 min on ice with a 250-fold molar excess of unlabelled competitor oligonucleotides prior to the addition of the radiolabelled probe.
2.8. Statistical analysis
The intensity of bands from immunoblots and EMSA were analysed using GeneTools™(Syngene). Statistical analyses of the data was carried out using the Student's t test with P < 0.05 considered as statistically significant.
3. Results
3.1. IL-1 induces the expression of C/EBPδ mRNA and protein in Hep3B cells
The human hepatoma Hep3B cell line is used widely as a model for liver-specific gene expression and cytokine-mediated regulation of APP with demonstrated conservation to in vivo responses (Hiron et al., 1992; Ramji et al., 1993a; Foka et al., 2009; Coulouarn et al., 2004, 2005 and references therein). For example, the hepatocyte origin and pro-inflammatory cytokine responsiveness of mRNAs that were found to be upregulated in acute systemic inflammation, as judged by complete coverage of the human liver transcriptome, has been confirmed in these cells (Coulouarn et al., 2004). In addition, genome-wide studies in these cells in relation to the actions of pro-inflammatory cytokines have shown proper kinetics of changes in mRNA abundances for cytokines and their receptors, transcription factors and APPs, with the overall percentage of regulated mRNAs (∼7%) being similar to the number of liver mRNAs regulated during the APR in mouse or humans in vivo (Coulouarn et al., 2005).
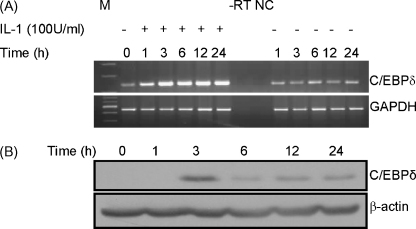
We have previously analysed the effect of IL-1 on C/EBPδ expression in J774.2 macrophages and glomerular mesangial cells (Tengku-Muhammad et al., 2000; Granger et al., 2000) but not in Hep3B cells. This was therefore investigated by time course RT-PCR and western blot analysis. For RT-PCR, sequence analysis confirmed the specific amplification of C/EBPδ. Fig. 1A shows that IL-1 induces C/EBPδ mRNA expression that peaks at 3 h and remains at similar or slightly reduced levels throughout the 24 h incubation period. Such an induction was due to IL-1 and not because of a non-specific effect of harvesting the cells at the various time-points. The expression of the C/EBPδ protein was also induced by IL-1 at 3 h post-treatment and was then expressed at reduced levels over the rest of the 24 h period (Fig. 1B). Statistical analysis of the data from three independent experiments showed that the IL-1-induced expression of the C/EBPδ protein (normalized to β-actin) was significant (P < 0.01 at 3 h, 6 h and 12 h and <0.05 at 24 h). Overall, these data show that IL-1 induces C/EBPδ mRNA and protein expression in Hep3B cells, which is consistent with the previously noted activation of C/EBPδ expression in the liver during experimentally induced inflammation in rodents (Alam et al., 1992; Magalini et al., 1995). However, the induced levels of C/EBPδ mRNA were maintained for a more prolonged period than the corresponding protein. This suggests that, similar to the actions of several extracellular mediators on the C/EBPs (Ramji and Foka, 2002; Magalini et al., 1995), multiple mechanisms are involved in the IL-1-mediated induction of C/EBPδ expression.
Fig. 1.
IL-1 increases the expression of C/EBPδ mRNA and protein. (A) Hep3B cells were either left untreated or incubated with IL-1 (100 U/ml) (− and +, respectively) for the indicated period of time and total cellular RNA was subjected to RT-PCR using primers for C/EBPδ or GAPDH. The amplification products were size-fractionated by agarose gel electrophoresis along with molecular size markers (M). −RT shows the outcome of a reaction in which the reverse transcriptase step was omitted (RNA from untreated cells at 0 h was used) and NC indicates a reaction that contained water instead of cDNA. (B) Western blot analysis was performed on extracts from cells that were either left untreated (0 h) or incubated with IL-1 (100 U/ml) for the indicated period of time, and antiserum against C/EBPδ or β-actin. The results shown in both panels A and B are representative of three independent experiments.
An IL-1-mediated induction of C/EBPδ promoter activity was not observed when transient transfection assays were carried out using luciferase-based plasmids containing 1.6 kB of human C/EBPδ promoter (Sanford and DeWille, 2005) or 2.2 kB of mouse C/EBPδ promoter (Hutt et al., 2000) (data not shown). As expected from previous studies (Yamada et al., 1997; Cantwell et al., 1998; Sanford and DeWille, 2005), both promoters were activated by IL-6 (data not shown). IL-1 was functional in this system as it could activate the pNF-κB-Luc plasmid (Clontech) which contains multiple binding site of this transcription factor linked to a minimal promoter upstream of the luciferase reporter gene (data not shown). Although the precise reason(s) for the non-responsiveness of the C/EBPδ promoter to IL-1 is currently unclear, it is possible that either the regulatory sequences for IL-1 actions reside outside those present in the DNA constructs used or a specific chromatin context is required for the response that is not present in the transfected plasmids. Our further studies therefore focused on the signalling pathways underlying IL-1 actions.
3.2. The IL-1-induced expression of C/EBPδ is attenuated in the presence of inhibitors of JNK, casein kinase 2 (CK2) and nuclear factor-κ B (NF-κB)
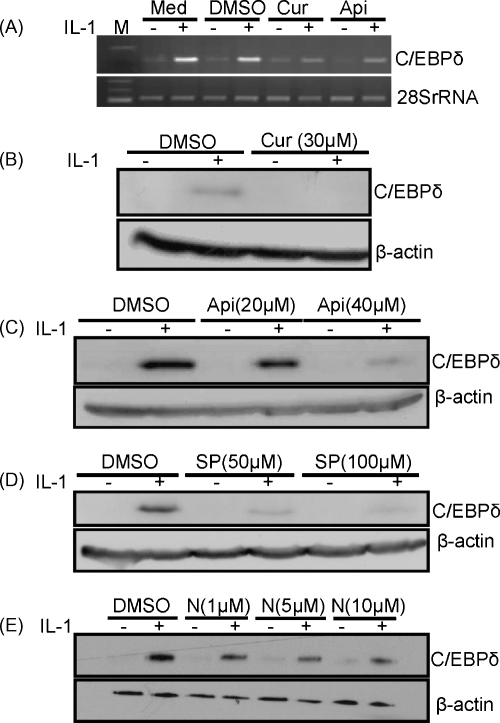
An initial screen analysing the effects of a single concentration of pharmacological inhibitors against different signal transduction pathways showed that the IL-1-induced expression of C/EBPδ mRNA was significantly attenuated by 30 μM curcumin and 20 μM apigenin, inhibitors of JNK and CK2, respectively (Mead et al., 2003; Li et al., 2005; Yamada et al., 2005; Singh and Ramji, 2006, 2008; Parhar et al., 2007; Harris et al., 2008) (Fig. 2A). The action of these two inhibitors was therefore investigated in more detail along with SP600125, another JNK inhibitor (Han et al., 2001), and the NF-κB activation inhibitor (NAI) (Tobe et al., 2003) because of the important role of this transcription factor in IL-1 signalling (Dunne and O’Neill, 2003). All four inhibitors attenuated the IL-1-induced expression of the C/EBPδ protein (Fig. 2B–E) (P < 0.001 for curcumin, 40 μM apigenin and 50 μM SP600125; <0.05 for 5 μM and 10 μM NAI; and <0.01 for 20 μM apigenin and 100 μM SP600125). A similar attenuation by the inhibitors was observed at the level of IL-1-induced C/EBPδ mRNA expression (data not shown). The marked inhibition of the IL-1-mediated induction of C/EBPδ expression by these pharmacological agents was not due to cellular toxicity as judged by the specific inhibition of expression of C/EBPδ but not 28SrRNA or β-actin (Fig. 2) and trypan blue exclusion assays using different concentrations of all four inhibitors (data not shown).
Fig. 2.
The effect of pharmacological inhibitors on the IL-1-induced expression of C/EBPδ. (A) Hep3B cells were pre-treated for 1 h with curcumin (Cur; 30 μM), apigenin (Api; 20 μM) or DMSO as a vehicle control, or left for this period of time in normal medium (Med). They were then incubated for 3 h in the absence or the presence of IL-1 (100 U/ml) (− and +, respectively) and total RNA was subjected to RT-PCR using primers against C/EBPδ or 28SrRNA. The products were resolved by agarose gel electrophoresis along with molecular size markers (M). (B–E) The cells were pre-treated for 1 h with DMSO or the indicated concentration of curcumin (Cur), apigenin (Api), SP600125 (SP) or NAI (N), and then incubated for 3 h in the absence or the presence of IL-1 (− and +, respectively). Total cellular proteins were subjected to western blot analysis using antiserum against C/EBPδ or β-actin. The data shown are representative of three to four independent experiments (3 for SP600125 and curcumin, and 4 for the others).
3.3. IL-1 activates JNK in Hep3B cells
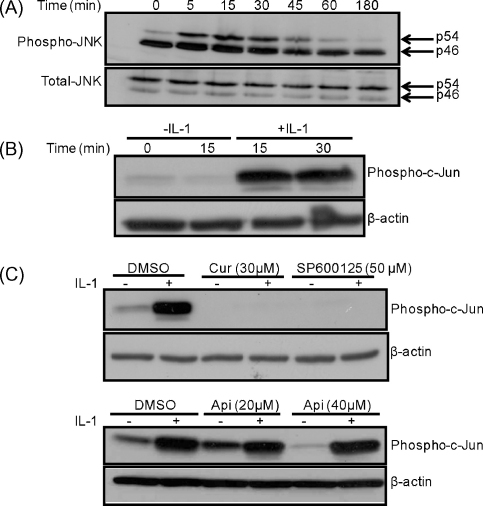
Differential splicing of the JNK genes leads to the generation of two predominant forms of this enzyme, p54 and p46, and these can be activated by phosphorylation at threonine 183 and tyrosine 185. The action of IL-1 on the phosphorylation of JNKs at these two sites was therefore investigated by western blot analysis. IL-1 increased the levels of phosphorylated p54 without affecting the total levels of the protein (Fig. 3A). Further experiments showed that such an increase in JNK phosphorylation was due to IL-1 and not because of incubation of the cells for the time period (Fig. I in supplementary data).
Fig. 3.
IL-1 activates JNK activity in Hep3B cells. (A) The cells were treated with IL-1 (100 U/ml) for the indicated period of time and whole cell extracts were subjected to western blot analysis using antiserum against phospho- and total-JNK. (B and C) The cells were treated in the absence or the presence of IL-1 (100 U/ml) for the indicated period of time (panel B), or pre-treated for 1 h with the DMSO vehicle control or the indicated concentration of curcumin (Cur), SP600125 or apigenin (Api) and then incubated for 15 min in the absence or the presence of this cytokine (− and +, respectively) (panel C). Whole cell extracts were subjected to the JNK kinase assay followed by western blot analysis with antiserum against phospho-c-Jun. Western blots probed with the β-actin antibody were used as a control for the amount of proteins in the extracts. The results shown are representative of two (A) to three (B–C) independent experiments.
The activity of JNK can be monitored using an in vitro kinase assay kit in which the ability of immunoprecipitated JNKs to phosphorylate c-Jun, its key downstream target, is determined. This assay was therefore employed to determine the action of IL-1 on JNK activity in the absence or the presence of pharmacological inhibitors. Consistent with the pattern of phospho-JNK levels (Fig. 3A), IL-1 induced the activity of the enzyme (Fig. 3B) (P < 0.05 at 15 min and 30 min). In addition, curcumin and SP600125, but not apigenin, attenuated the IL-1-induced JNK activity (Fig. 3C) (P < 0.001 for both curcumin and SP600125).
3.4. siRNA-mediated knockdown of NF-κB attenuates the IL-1-induced expression of C/EBPδ
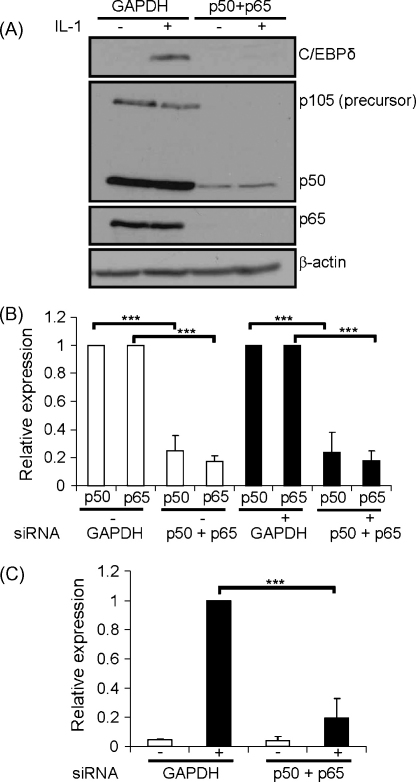
siRNA-mediated knockdown of JNK-1 and -2, the two prominent isoforms expressed in hepatocytes, or its downstream target c-Jun did not attenuate the IL-1-induced expression of the C/EBPδ protein (Figs. IIA-IIB in supplementary data). Similarly, siRNA-mediated knockdown of CK2-α and -α′, two of the three catalytic subunits of this enzyme (Singh and Ramji, 2008) had no effect (Fig. IIC in supplementary data). For JNK and CK2, the findings were confirmed at the level of C/EBPδ mRNA expression (data not shown). Although the precise reason(s) for these results are currently unclear, it is possible that this could be because of functional redundancy between the different pathways and/or sufficient amount of residual activity being present following siRNA-mediated knockdown. Because it was not possible to simultaneously knockdown all the JNK and CK2 isoforms due to potential toxicity effects from the use of large quantities of siRNA that would be required, the role of the downstream transcription factor NF-κB was investigated. The expression of both the p50 and p65 NF-κB subunits, the two most abundant isoforms that play a key role in IL-1 signalling (Pereira and Oakley, 2008), was knocked down by transfection of Hep3B cells with siRNA against these two subunits but not GAPDH (Fig. 4A and B). The IL-1-induced expression of the C/EBPδ protein was attenuated by knockdown of p50 and p65 expression but not by GAPDH (Fig. 4A and C). Knockdown had no effect on the expression of the β-actin protein (Fig. 4A). Similar results were obtained when the C/EBPδ mRNA expression was monitored by RT-PCR (data not shown).
Fig. 4.
Effect of siRNA-mediated knockdown of p50 and p65 NF-κB subunits on the IL-1-induced expression of C/EBPδ. Hep3B cells were transfected with siRNA against GAPDH or the p50 and p65 NF-κB subunits together (the final concentration of siRNA was the same at 12.5 nM). They were then either left untreated (−) or incubated for 3 h with 100 U/ml IL-1 (+). Protein extracts were subjected to western blot analysis with antiserum against p50 (also detects the p105 precursor), p65 and β-actin as shown (panel A). Panel B shows the p50:β-actin or the p65:β-actin ratios (mean ± SD), as determined by densitometric scanning of signals from three independent experiments, with the ratio from GAPDH siRNA transfected cells being arbitrarily assigned as 1. Panel C displays the C/EBPδ:β-actin ratio with that from IL-1 treated, GAPDH siRNA transfected cells being arbitrarily assigned as 1 with the others represented to this value. Asterisks indicate significant attenuation of expression following siRNA-mediated knockdown (***P < 0.001).
The IL-1-mediated activation of NF-κB is known to occur via a TAK-dependent or -independent manner (Yao et al., 2007). siRNA-mediated knockdown of TAK1, however, failed to attenuate the IL-1-induced expression of C/EBPδ (Fig. III in supplementary data), thereby suggesting a potential TAK1-independent pathway.
3.5. The action of pharmacological inhibitors on the DNA binding activity and the trans-activation potential of NF-κB
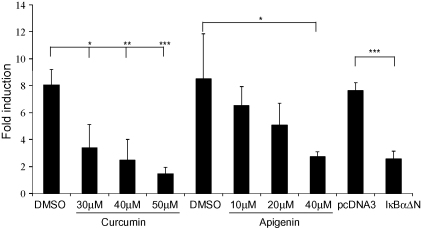
Because the IL-1-induced expression of C/EBPδ was attenuated by inhibitors of JNK and CK2 (Fig. 2), the action of these on NF-κB-mediated transcriptional activation along with its DNA binding activity was investigated. Transfection assays using the pNF-κB-Luc plasmid, which contains multiple NF-κB binding sites linked to a luciferase reporter gene, showed that IL-1 induced its activity and this was attenuated in a statistically significant manner by both apigenin and curcumin (Fig. 5). The IL-1-mediated activation of pNF-κB-Luc was specific to this transcription factor because it could be attenuated by expression of a superrepressor (IκBαΔN) that inhibits NF-κB action (Heissmeyer et al., 1999) (Fig. 5).
Fig. 5.
The effect of apigenin and curcumin on NF-κB-mediated trans-activation in Hep3B cells. The cells were transfected with the pNF-κB-Luc plasmid either alone (experiments involving curcumin or apigenin where DMSO was used as a vehicle control), or along with pcDNA3 control vector or the NF-κB superrepressor (IκBαΔN). The cells were then either left untreated or incubated for 3 h with 100 U/ml IL-1 (the inhibitors were added 1 h before the addition of IL-1). The luciferase activity in cell extracts was then determined and normalized to the total protein concentration. In each case, the normalized luciferase activity in untreated cells was arbitrarily assigned as 1 (not shown) with those from IL-1 treated cells represented as Fold induction. The results are mean ± SD from three independent experiments (*P < 0.05; **P < 0.01, ***P < 0.001).
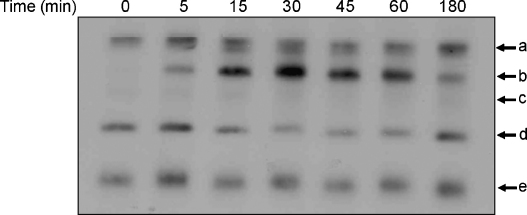
Time course EMSA using whole cell extracts and a radiolabelled consensus NF-κB binding site oligonucleotide as a probe (called κB oligonucleotide hereafter) showed the formation of at least five DNA–protein complexes (labelled a–e in Fig. 6). From these, four of the complexes (a, c, d and e) were present in untreated cells (although signals for c and d were generally faint) and induced to varying degrees by IL-1 treatment. On the other hand, complex b was entirely induced by IL-1 treatment. The induction of NF-κB was transient, being apparent 5 min post-IL-1 treatment and decreased at 180 min. Further experiments focused on the 15 min time point where a marked IL-1 induced NF-κB activity was observed.
Fig. 6.
Time course action of IL-1 on NF-κB DNA binding. Hep3B cells were treated with 100 U/ml of IL-1 for the indicated period of time. Whole cell protein extracts were used for EMSA with a radiolabelled κB oligonucleotide probe. The different DNA–protein complexes are labelled as a–e (the free probe has migrated off from the gel). The results are representative of two independent experiments.
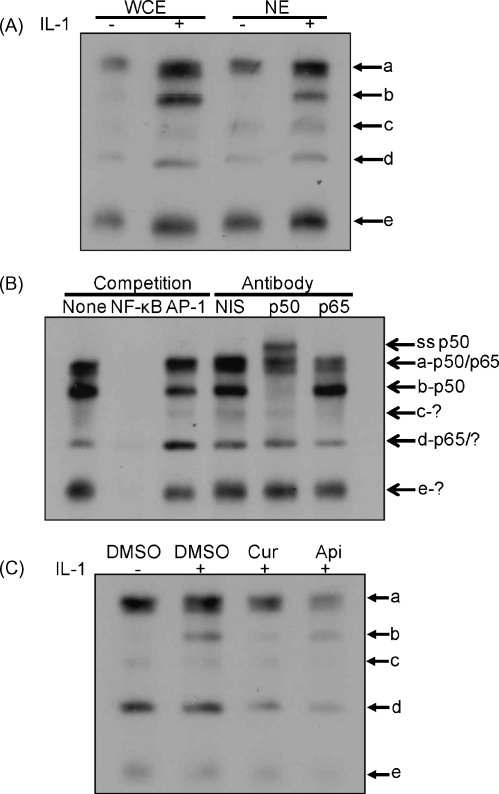
A similar profile of DNA–protein interactions was observed with both nuclear and whole cell extracts, thereby ruling out the possibility that the previously noted induction of NF-κB activity was peculiar to the use of whole cell extracts for EMSA (Fig. 7A). The specificity of DNA–protein complexes was analysed by competition EMSA and showed attenuation by pre-incubation of the extracts with a 250-fold molar excess of the unlabelled κB oligonucleotide but not by that containing the unrelated AP-1 binding site (Fig. 7B). The potential involvement of p50 and the p65 subunits, the major components of NF-κB in several modes of regulation (Pereira and Oakley, 2008), was investigated using antibody supershift/interference EMSA with non-immune serum serving as a control. Pre-incubation with the anti-p50 NF-κB antibody resulted in the formation of a slower migrating antibody-DNA-protein supershift complex, complete inhibition of complex b, and partial inhibition of complex a (Fig. 7B). On the other hand, pre-incubation with the anti-p65 antibody interfered with the formation of complexes a and d (Fig. 7B). These results suggest that, consistent with the data for siRNA-mediated knockdown (Fig. 4), both p50 and p65 were involved in DNA–protein interactions. Finally, the IL-1-induced NF-κB binding was attenuated by pre-treatment of the cells with apigenin or curcumin. Densitometric analysis showed that the induction of NF-κB binding by IL-1 was significant (P < 0.05) along with the attenuation of this by curcumin and apigenin (P < 0.01 and 0.05, respectively). Thus, similar to the previously noted effect on trans-activation (Fig. 5), apigenin and curcumin also attenuate the IL-1-induced NF-κB DNA binding.
Fig. 7.
Analysis of DNA binding to NF-κB binding site. EMSA was carried out using either whole cell extracts (WCE in panel A and all samples in panels B and C) or nuclear extracts (NE in panel A) from Hep3B cells that were either left untreated (−) or incubated with 100 U/ml of IL-1 for 15 min (+ and all samples in panel B). Competition assays in panel B included either no competitor (none) or a 250-molar excess of the κB binding site oligonucleotide (NF-κB) or that containing the AP-1 binding site (AP-1). Panel B also shows the outcome of antibody interference assays using non-immune serum (NIS) or antiserum against the p50 or p65 isoform of NF-κB as indicated. For panel C, curcumin (Cur; 50 μM) or apigenin (Api; 40 μM) were added to the cells 1 h before the addition of IL-1 and DMSO was used as a vehicle control. The different DNA–protein complexes are labelled a–e (the free probe has migrated off the gel). In panel B, ss p50 shows the antibody-DNA-protein supershift complex produced by the p50 antibody, and the potential proteins involved in the formation of each complex, as judged from antibody interference assays, are indicated on the right side (? indicates members other than p50 or p65). The data shown are representative of two to three independent experiments.
4. Discussion
Circulating levels of several APP are elevated during inflammation and inflammatory disorders such as cardiovascular disease in response to increased transcription of these genes in hepatocytes by cytokines such as IL-1 (Ramji et al., 1993a,b; Baumann and Gauldie, 1994; Moshage, 1997; Ramji and Foka, 2002; Packard and Libby, 2008). Although the C/EBPs play crucial roles in such regulation, the mechanisms underlying the actions of cytokines on these transcription factors remain poorly understood (Ramji and Foka, 2002). We show here that IL-1 induces the expression of C/EBPδ mRNA and protein in Hep3B cells (Fig. 1) and this was attenuated by inhibitors of JNK, CK2 and NF-κB (Fig. 2). siRNA-mediated knockdown of the p50 and p65 NF-κB subunits attenuated the IL-1 induced expression of C/EBPδ (Fig. 4). IL-1 stimulated NF-κB DNA binding and trans-activation, which was attenuated by inhibitors of CK2 and JNK (Figs. 5–7). Although a role for JNK in IL-1-induced expression of C/EBPδ expression in hepatocytes, as judged by inhibition of the response in HepG2 cells by SP600125, has been reported (Dong et al., 2007), our studies also show important and novel roles for CK2 and NF-κB.
The association of the JNK pathway in the control of inflammation is well established and recent studies have also suggested an important role for CK2 (Singh and Ramji, 2008). In addition, these two pathways have also been implicated in IL-1 signalling (O’Neill and Greene, 1998; Singh and Ramji, 2008). For example, IL-1 has been shown to activate CK2 in intestinal epithelial cells and inhibition of this enzyme attenuates the expression of several genes induced by this cytokine (Parhar et al., 2007). Although inhibitors of JNK and CK2 attenuated the IL-1-induced expression of C/EBPδ (Fig. 2), no such effect was observed following siRNA-mediated knockdown of JNK-1/2 and CK2-α/α′ (e.g. Fig. II in supplementary data). The precise reasons for these results are currently unclear but could be due to functional redundancy between the pathways. Indeed, such functional redundancy is relatively common in signalling cascades involved in the regulation of gene expression as multiple pathways often regulate key transcription factors such as NF-κB (Mestre et al., 2001; Lowell et al., 1994). In addition, as siRNA-mediated depletion of a target protein only leads to a knockdown of expression, any remaining protein could function to compensate for its reduced expression.
Five NF-κB members exist in mammalian cells [RelA (p65), RelB, c-Rel, p50/p105 (NF-κB1) and p52/p100 (NF-κB2) (May and Ghosh, 1997; Pereira and Oakley, 2008). The p50 and p52 subunits are produced by proteolytic processing of precursor proteins (p105 and p100, respectively) (May and Ghosh, 1997; Pereira and Oakley, 2008). Transcriptional regulation by this family is complex in part due to extensive dimerization between the different proteins (May and Ghosh, 1997; Pereira and Oakley, 2008). RelA (p65), RelB and c-Rel contain a potent transcriptional activation domain at the carboxyl terminus (May and Ghosh, 1997). Although p50 and p52 lack these domains, activation by p50 homodimers has been demonstrated (May and Ghosh, 1997). Our studies show a crucial role for p50 and p65 in the IL-1-induced C/EBPδ expression. Both these subunits were involved in DNA–protein interactions, and IL-1 activated NF-κB DNA binding and trans-activation, which was attenuated by curcumin and apigenin (Figs. 5–7). Whether the action of these inhibitors on NF-κB is direct or a secondary response to inhibition of JNK and CK2 is currently unclear. However, cross-talk between JNK and NF-κB is relatively common (Wullaert et al., 2006). In addition, IL-1 has been found to increase the association of CK2 and NF-κB (Bird et al., 1997; Singh and Ramji, 2008). Furthermore, CK2 has been found to regulate the trans-activation potential of the p65 subunit along with other components of this pathway (Bird et al., 1997; Parhar et al., 2007; Singh and Ramji, 2008). For example, CK2-mediated phosphorylation of IκBα facilitates its degradation, thereby leading to stimulation of transcription of NF-κB gene targets (Singh and Ramji, 2008).
In conclusion, we show here for the first time that NF-κB plays a crucial role in the IL-1 induced expression of C/EBPδ, a key transcription factor in the activation of APP gene transcription. JNK and CK2 were also involved in the response and modulated the IL-1-induced trans-activation and DNA binding by NF-κB. The studies therefore provide novel insights into the molecular mechanisms underlying the IL-1-mediated induction of APP gene transcription in hepatocytes.
Acknowledgement
We thank the British Heart Foundation for financial support (grant FS/03/015/15141).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.biocel.2009.09.018.
Appendix A. Supplementary data
References
- Alam T., An M.R., Papaconstantinou J. Differential expression of three C/EBP isoforms in multiple tissues during the acute phase response. J Biol Chem. 1992;267:5021–5024. [PubMed] [Google Scholar]
- Bannach F.G., Gutierrez-Fernandez A., Parmer R.J., Miles L.A. Interleukin-6-induced plasminogen gene expression in murine hepatocytes is mediated by transcription factor CCAAT/enhancer binding protein β (C/EBPβ) J Throb Haemost. 2004;2:2205–2212. doi: 10.1111/j.1538-7836.2004.01022.x. [DOI] [PubMed] [Google Scholar]
- Baumann H., Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Bird T.A., Schooley K., Dower S.K., Hagen H., Virca G.D. Activation of nuclear transcription factor NF-κB by interleukin-1 is accompanied by casein kinase II-mediated phosphorylation of the p65 subunit. J Biol Chem. 1997;272:32606–32612. doi: 10.1074/jbc.272.51.32606. [DOI] [PubMed] [Google Scholar]
- Cantwell C.A., Sterneck E., Johnson P.F. Interleukin-6-specific activation of the C/EBPδ gene in hepatocytes is mediated by Stat3 and Sp1. Mol Cell Biol. 1998;18:2108–2117. doi: 10.1128/mcb.18.4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.M., Liao W.S. Differential acute-phase response of rat kininogen genes involves type I and type II interleukin-6 response elements. J Biol Chem. 1993;268:25311–25319. [PubMed] [Google Scholar]
- Coulouarn C., Lefebvre G., Derambure C., Lequerre T., Scotte M., Francois A. Altered gene expression in acute systemic inflammation detected by complete coverage of the human transcriptome. Hepatology. 2004;39:353–364. doi: 10.1002/hep.20052. [DOI] [PubMed] [Google Scholar]
- Coulouarn C., Lefebvre G., Daveau R., Letellier F., Hiron M., Drouot L. Genome-wide response of the human Hep3B hepatoma cell to proinflammatory cytokines, from transcription to translation. Hepatology. 2005;42:946–955. doi: 10.1002/hep.20848. [DOI] [PubMed] [Google Scholar]
- Dong J., Fujii S., Li H., Nakabayshi H., Sakai M., Nishi S. Interleukin-6 and mevastatin regulate plasminogen activator inhibitor-1 through CCAAT/enhancer-binding protein-δ. Arterioscler Thromb Vasc Biol. 2005;25:1078–1084. doi: 10.1161/01.ATV.0000159701.24372.49. [DOI] [PubMed] [Google Scholar]
- Dong J., Fujii S., Li H., Nakabayashi H., Sakai M., Nishi S. IL-1 and IL-6 induce hepatocyte plasminogen activator inhibitor-1 expression through independent signaling pathways converging on C/EBPδ. Am J Physiol Cell Physiol. 2007;292:C209–C215. doi: 10.1152/ajpcell.00157.2006. [DOI] [PubMed] [Google Scholar]
- Dunne A., O’Neill L.A. The interleukin-1 receptor/toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2003;171:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- Foka P., Singh N.N., Salter R.C., Ramji D.P. The tumour necrosis factor-α-mediated suppression of the CCAAT/enhancer binding protein-α gene transcription in hepatocytes involves inhibition of autoregulation. Int J Biochem Cell Biol. 2009;41:1189–1197. doi: 10.1016/j.biocel.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Granger R.L., Hughes T.R., Ramji D.P. Stimulus- and cell-type-specific regulation of CCAAT-enhancer binding protein isoforms in glomerular mesangial cells by lipopolysaccharide and cytokines. Biochim Biophys Acta. 2000;1501:171–179. doi: 10.1016/s0925-4439(00)00016-8. [DOI] [PubMed] [Google Scholar]
- Han Z., Boyle D.L., Chang L., Bennett B., Karin M., Yang L. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S.M., Harvey E.J., Hughes T.R., Ramji D.P. The interferon-γ-mediated inhibition of lipoprotein lipase gene transcription in macrophages involves casein kinase 2- and phosphoinositide-3-kinase-mediated regulation of transcription factors Sp1 and Sp3. Cell Signal. 2008;20:2296–2301. doi: 10.1016/j.cellsig.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey E.J., Li N., Ramji D.P. Critical role for casein kinase 2 and phosphoinositide-3-kinase in the interferon-γ-induced expression of monocyte chemoattractant protein-1 and other key genes implicated in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:806–812. doi: 10.1161/01.ATV.0000258867.79411.96. [DOI] [PubMed] [Google Scholar]
- Heissmeyer V., Krappmann D., Wulczyn F.G., Scheidereit C. NF-κB p105 is a target of IκB kinases and controls signal induction of Bcl-3-p50 complexes. EMBO J. 1999;18:4766–4778. doi: 10.1093/emboj/18.17.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiron M., Daveau M., Arnaud P., Bauer J., Lebreton J.P. The human hepatoma Hep3B cell line as an experimental model in the study of the long-term regulation of acute-phase proteins by cytokines. Biochem J. 1992;287:255–259. doi: 10.1042/bj2870255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt J.A., O’Rourke J.P., DeWille J. Signal transducer and activator of transcription 3 activates CCAAT enhancer binding protein δ gene transcription in G0 growth-arrested mouse mammary epithelial cells and in involuting mouse mammary gland. J Biol Chem. 2000;275:29123–29131. doi: 10.1074/jbc.M004476200. [DOI] [PubMed] [Google Scholar]
- Li X., Shi X., Liang D.Y., Clark J.D. Spinal CK2 regulates nociceptive signaling in models of inflammatory pain. Pain. 2005;115:182–190. doi: 10.1016/j.pain.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Lowell C.A., Soriano P., Varmus H.E. Functional overlap in the src gene family: inactivation of hck and fgr impairs natural immunity. Genes Dev. 1994;8:387–398. doi: 10.1101/gad.8.4.387. [DOI] [PubMed] [Google Scholar]
- Magalini A., Savoldi G., Ferrari F., Garnier M., Ghezzi P., Albertini A. Role of IL-1β and corticosteroids in the regulation of the C/EBP-α, β and δ genes in vivo. Cytokine. 1995;7:753–758. doi: 10.1006/cyto.1995.0090. [DOI] [PubMed] [Google Scholar]
- May M.J., Ghosh S. Rel/NF-κB and IκB proteins: an overview. Semin Cancer Biol. 1997;8:63–73. doi: 10.1006/scbi.1997.0057. [DOI] [PubMed] [Google Scholar]
- Mead J.R., Hughes T.R., Irvine S.A., Singh N.N., Ramji D.P. Interferon-γ stimulates the expression of the inducible cAMP early repressor in macrophages through the activation of casein kinase 2. A potentially novel pathway for interferon-γ-mediated inhibition of gene transcription. J Biol Chem. 2003;278:17741–17751. doi: 10.1074/jbc.M301602200. [DOI] [PubMed] [Google Scholar]
- Mestre J.R., Mackrell P.J., Rivadeneira D.E., Stapleton P.P., Tanabe T., Daly J.M. Redundancy in the signaling pathways and promoter elements regulating cyclooxygenase-2 gene expression in endotoxin-treated macrophage/monocytic cells. J Biol Chem. 2001;276:3977–3982. doi: 10.1074/jbc.M005077200. [DOI] [PubMed] [Google Scholar]
- Moshage H. Cytokines and the hepatic acute phase response. J Pathol. 1997;181:257–266. doi: 10.1002/(SICI)1096-9896(199703)181:3<257::AID-PATH756>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- O’Neill L.A., Greene C. Signal transduction pathways activated by the IL-1 receptor family: ancient signalling machinery in mammals, insects, and plants. J Leukoc Biol. 1998;63:650–657. [PubMed] [Google Scholar]
- Packard R.R.S., Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk production. Clin Chem. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- Parhar K., Morse J., Salh B. The role of protein kinase CK2 in intestinal epithelial cell inflammatory signaling. Int J Colorectal Dis. 2007;22:601–609. doi: 10.1007/s00384-006-0193-7. [DOI] [PubMed] [Google Scholar]
- Pereira S.G., Oakley F. Nuclear factor-kappaB1: regulation and function. Int J Biochem Cell Biol. 2008;40:1425–1430. doi: 10.1016/j.biocel.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Poli V. The role of C/EBP isoforms in the control of inflammatory and native immune functions. J Biol Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- Ramji D.P., Cortese R., Ciliberto G. Regulation of C-reactive protein, haptoglobin and hemopexin gene expression. In: Mackiewicz A., Kushner I., Baumann H., editors. Acute phase proteins: molecular biology, biochemistry and clinical applications. CRC–Taylor and Francis; 1993. pp. 365–394. [Google Scholar]
- Ramji D.P., Vitelli A., Tronche F., Cortese R., Ciliberto G. The two C/EBP isoforms, IL-6DBP/NF-IL6 and C/EBPδ/NF-IL6β, are induced by IL-6 to promote acute phase gene transcription via different mechanisms. Nucleic Acids Res. 1993;21:289–294. doi: 10.1093/nar/21.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramji D.P., Foka P. CCAAT/enhancer binding proteins: structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Ray B.K. Serum amyloid A gene expression under acute-phase conditions involves participation of inducible C/EBP-β and C/EBP-δ and their activation by phosphorylation. Mol Cell Biol. 1994;14:4324–4332. doi: 10.1128/mcb.14.6.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford D.C., DeWille J.W. C/EBPδ is a downstream mediator of IL-6 induced growth inhibition of prostate cancer cells. The Prostate. 2005;63:143–154. doi: 10.1002/pros.20159. [DOI] [PubMed] [Google Scholar]
- Singh N.N., Ramji D.P. Protein kinase CK2, an important regulator of the inflammatory response? J Mol Med. 2008;86:887–897. doi: 10.1007/s00109-008-0352-0. [DOI] [PubMed] [Google Scholar]
- Singh N.N., Ramji D.P. Transforming growth factor-β-induced expression of the apolipoprotein E gene requires c-Jun N-terminal kinase, p38 kinase, and casein kinase 2. Arterioscler Thromb Vasc Biol. 2006;26:1323–1329. doi: 10.1161/01.ATV.0000220383.19192.55. [DOI] [PubMed] [Google Scholar]
- Tengku-Muhammad T.S., Hughes T.R., Ranki H., Cryer A., Ramji D.P. Differential regulation of macrophage CCAAT-enhancer binding protein isoforms by lipopolysaccharide and cytokines. Cytokine. 2000;12:1430–1436. doi: 10.1006/cyto.2000.0711. [DOI] [PubMed] [Google Scholar]
- Tobe M., Isobe Y., Tomizawa H., Nagasaki T., Takahashi H., Fukazawa T. Discovery of quinazolines as a novel structural class of potent inhibitors of NF-κB activation. Bioorg Med Chem. 2003;11:383–391. doi: 10.1016/s0968-0896(02)00440-6. [DOI] [PubMed] [Google Scholar]
- Wullaert A., Heyninck K., Beyaert R. Mechanisms of crosstalk between TNF-induced NF-κB and JNK activation in hepatocytes. Biochem Pharmacol. 2006;72:1090–1101. doi: 10.1016/j.bcp.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Yamada M., Katsuma S., Adachi T., Hirosawa A., Shiojima S., Kadowaki T. Inhibition of protein kinase CK2 prevents the progression of glomerulonephritis. Proc Natl Acad Sci USA. 2005;102:7736–7741. doi: 10.1073/pnas.0409818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Tobita K., Osada S., Nishihara T., Imagawa M. CCAAT/enhancer-binding protein-δ gene expression is mediated by APRF/STAT3. J Biochem. 1997;121:731–738. doi: 10.1093/oxfordjournals.jbchem.a021647. [DOI] [PubMed] [Google Scholar]
- Yao J., Kim T.W., Qin J., Jiang Z., Qian Y., Xiao H. Interleukin-1 (IL-1)-induced TAK1-dependent versus MEKK3-dependent NFκB activation pathways bifurcate at IL-1-receptor associated kinase modification. J Biol Chem. 2007;282:6075–6089. doi: 10.1074/jbc.M609039200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.