Abstract
An esophagorespiratory fistula (ERF) is an often fatal consequence of esophageal or bronchogenic carcinomas. The preferred treatment is placement of esophageal and/or airway stents. Stent placement must be performed as quickly as possible since patients with ERFs are at a high risk for aspiration pneumonia. In this review, choice of stents and stenting area, fistula reopening and its management, and the long-term outcome in the interventional management of malignant ERFs are considered. Lastly, a review of esophagopulmonary fistulas will also be provided.
Keywords: Interventional technique, Esophagorespiratory fistula, Esophagopleural fistula
An esophagorespiratory fistula (ERF) is an abnormal communication between the esophagus and the respiratory system; it is a devastating and life-threatening complication of esophageal and bronchogenic carcinomas, according to most etiologies (1). An ERF develops in approximately 1-22% of patients with an esophageal malignancy and in less than 1% of those with bronchogenic carcinoma (1-3). An ERF develops either because of direct tumor invasion and subsequent perforation or after radiation, laser therapy, chemotherapy or pre-existing stents (primarily, esophageal stents), or a combination of these (1, 4) (Fig. 1).
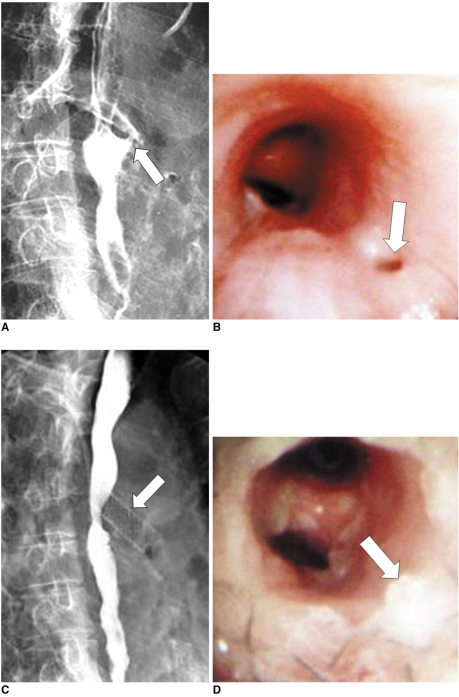
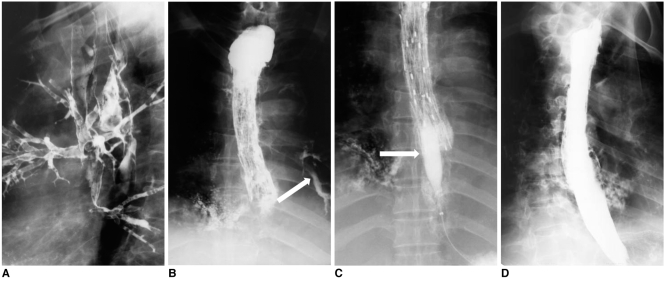
Fig. 1.
Esophagobronchial fistula due to pressure necrosis by esophageal stent.
Esophagogram (A) and bronchoscopy (B) after esophageal stent removal shows definite fistula (arrows) at proximal end of stent site.
Esophagogram (C) and bronchoscopy (D) after bronchial stent placement (arrows), shows successful closure of fistula.
The diagnosis of ERFs is usually not simple because of the dysphagia caused by the stenosis, which in turn occurs because of the cancer and the recurring aspiration resulting from the swallowing malfunction caused by paresis (3). Autopsy data indicate a higher incidence of fistulas, thus suggesting that fistulas are more common in patients than is usually diagnosed (3). ERFs can be confirmed based on clinical and endoscopic findings. A history of repeated coughing associated with eating, drinking, or both, with an increase in dysphagia and dyspnea are highly suggestive of an ERF. Endoscopic findings are sometimes inadequate in demonstrating a fistula, in which case an esophagogram is required. Esophagograms should be performed cautiously as aspiration could occur during swallowing of the contrast medium. CT is very important for assessing the extent of disease and pneumonia, as well as the relationship between the fistula and the surrounding tissue for planning the stent placement. For airway stenting, reconstructed CT images are very useful for measuring the distance between the fistula and a carina or vocal cord, in the determination optimal stent length.
Treatment should be begun immediately after the diagnosis is confirmed since the usual cause of death in these patients is pulmonary sepsis resulting from chronic aspiration through the fistula. Unfortunately, at the time of diagnosis, the patient's performance and disease status usually precludes aggressive surgical therapy, thus leaving few therapeutic options (4). Esophageal intubation using conventional unexpandable plastic prostheses was the treatment of choice for ERFs until the early 1990s; however, complications such as perforation, hemorrhage, pressure necrosis, tube obstruction, tube dislodgment, and tube migration were reported in approximately 15-40% of patients (5). To overcome these limitations, several types of covered expandable metallic stents have been used in the treatment of ERFs (1, 3-12).
Traditionally, ERF means esophagotracheal or esophagobronchial fistulas. In the widest sense, an esophagopulmonary fistula can also be included. In this review, esophagotracheobronchial and esophagopulmonary fistulas will be considered, giving special emphasis to their interventional management.
ESOPHAGOTRACHEOBRONCHIAL FISTULA
Interventional Management and Follow-Up
Surgical management is very limited. Seto et al. (13) reported successful results in four patients that underwent an esophageal bypass using a gastric bypass and cardiostomy. However, they also cited the need for further clinical experience with more patients. This treatment is palliative and involves the restoration of the ability to ingest food and to prevent aspiration by inserting an esophageal or airway stent in most cases. Radiation therapy and chemotherapy are generally contraindicated due to the concern regarding fistula enlargement caused by tumor necrosis. A feeding gastrostomy or jejunostomy is only occasionally used for palliation since these procedures do not restore normal swallowing (11).
Covered expandable metallic stents were introduced in the mid-1990s and have shown a 67-100% stent closure rate (1, 3-12). We previously reported the long-term outcomes in 61 patients with malignant ERFs, each of whom had undergone the insertion of a covered expandable metallic stents. Stent placement was technically successful in all patients, and there were no immediate procedural complications (1). The stent completely sealed off the fistula in 80% (49 of 61) of the patients, and there were no further aspiration symptoms (initial clinical success) (Figs. 1, 2). Incomplete closure of the fistula caused by spillage of material through a gap between the proximal stent margin and the esophageal wall within seven days after stent placement (initial clinical failure), was seen in 12 (20%) of the 61 patients (1), and was due to the 'funnel phenomenon' which is difficult to manage despite the insertion of additional stents or glue injection to seal the gap (14-16). For esophageal stenosis with ERFs, 18-mm-diameter stents were used. Whereas, for esophageal stenosis without ERFs, 16-mm-diameter stents were generally used. In the study by Balazs et al. (12), which included 188 patients that underwent covered stent placement for malignant ERFs, improvement of swallowing and fistula closure were achieved in 77% of the patients (144 of 188).
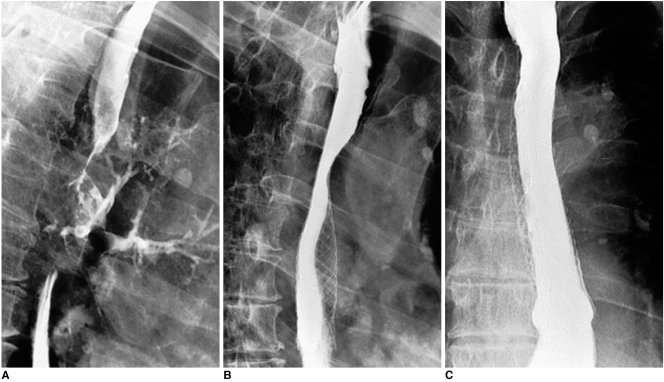
Fig. 2.
Esophageal cancer with development of esophagobronchial fistula. Ingested contrast medium is aspirated into left bronchi (A). Right anterior oblique (B) and anteroposterior (C) esophagograms obtained two days after placement of covered expandable metallic stent (18 mm in diameter), shows complete closure of fistula.
A close follow-up of patients with ERFs is important since non-sealing of a fistula after stent placement, and reopening of a fistula after initial sealing, might cause aspiration pneumonia which would certainly lead to the rapid demise of the patient. To prevent further aspiration pneumonia, an esophagogram obtained immediately after stent insertion is crucial in order to confirm the sealing of the fistula and subsequently allow a patient to eat a soft diet. If there is persistent leakage through the fistula, resulting from an incomplete stent expansion, a follow-up esophagogram should be obtained 2-3 days after stent placement in order to confirm stent expansion before food intake is resumed (1). The protocol of a repeat esophagogram at one week and then every one to two months after the procedure is suggested to evaluate fistula closure and stent patency or migration. This would also provide early detection of fistula reopening as well as long-term outcomes.
Selection of Stents and Stenting Area
Several kinds of covered esophageal stents are available in the United States (i.e., Ultraflex stent, Wallstent, and Z stent). In Korea, covered retrievable esophageal or airway stents made by S & G Biotech and Taewoong Medical are available; however, there are no randomized or controlled trials to compare the outcomes of any of these stents when used to treat malignant ERFs.
An understanding of the esophageal and airway anatomy of patients with ERFs is critical to determine stent placement into the esophagus, airway, or both. Airway stenting, with or without esophageal stenting, is useful in patients with Ivor Lewis esophagectomy, i.e., partial esophagectomy with bowel interposition, substantial airway stricture, previously placed esophageal stent, and an ERF secondary to pressure necrosis of the esophageal stent (1, 10, 11, 17) (Fig. 1).
The selection guide for determining the stenting area could be summarized as follows (Fig. 3): 1) esophageal stent placement if a patient has a stricture in the esophagus, but with no or only mild airway stricture since an esophageal stent can successfully treat both esophageal stricture and a fistula; 2) airway stent placement if a patient has no or only mild stricture in the esophagus, or has moderate to severe stricture in the airway, since an esophageal stent migrates well when esophageal stricture is absent or mild, and an airway stent can treat an airway stricture; or 3) both the airway and esophageal stent placement when a patient has moderate to severe stricture involving both the esophagus and the airway, since both the airway and esophageal stents are necessary to treat a stricture involving both the esophagus and the airway.
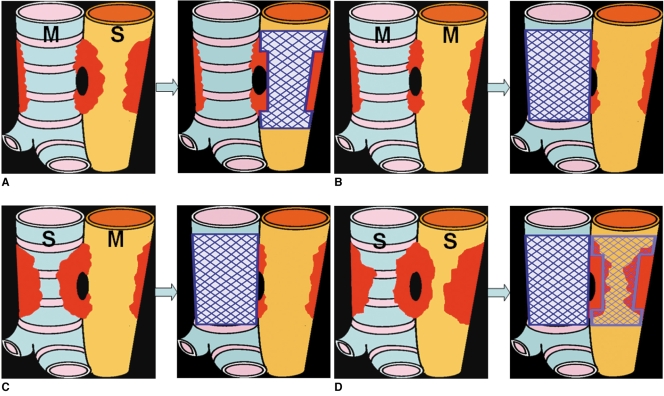
Fig. 3.
Determination of stenting area in various types of esophagorespiratory fistula.
A. Esophageal stenting is indicated when esophageal stricture is severe, but with no or only mild airway stricture.
B. Airway stenting is indicated when esophageal and airway strictures are non-existent or mild.
C. Airway stenting is indicated when airway stricture is severe, but without or with only mild esophageal stricture.
D. Both airway and esophageal stenting is indicated when both esophageal and airway stenosis is severe. M and S denotes mild or severe degree of stenosis, respectively.
In the selected patients, as the insertion of a single stent may be insufficient for palliation, placement of parallel stents may be indicated for patients with symptoms caused by malignant ERFs (11, 17) (Fig. 4). It is very important to carefully evaluate the airway stenosis with CT scans or bronchoscopes prior to esophageal stent placement, since it is possible to develop tracheal compression caused by expanding esophageal stents (17). Therefore, for insertion of both esophageal and airway stents during the same procedure, airway insertion should precede the esophageal stent insertion, since dyspnea could be aggravated immediately following the esophageal insertion.
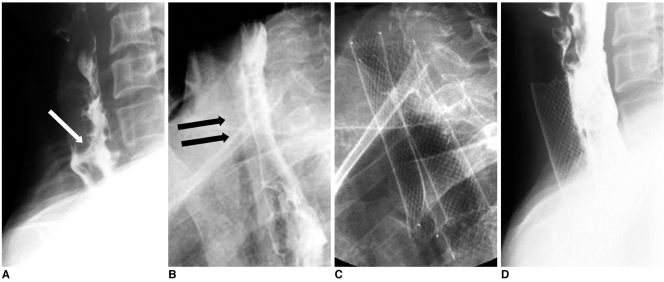
Fig. 4.
Esophageal cancer and esophagotracheal fistula.
A. Lateral esophagogram shows esophagotracheal fistula (arrow) and segmental luminal narrowing in cervical esophagus.
B. Radiograph obtained one week following esophageal stent placement shows diffuse tracheal narrowing (arrows).
C. Radiograph obtained following tracheal stent placement to relieve dyspnea.
D. Esophagogram obtained one week after tracheal stent placement shows good flow of contrast medium through esophageal stent without visualization of fistula and fully expanded tracheal stent.
Mechanical friction between the esophageal and airway stents may cause pressure necrosis of the interposed tissue between the two stents, thereby possibly resulting in a fatal hemorrhage (10, 17, 18). Thus, parallel stenting should only be performed after thoroughly reviewing a patient's clinical indications.
Fistula Reopening and Management
Reopening of a closed ERF is another life-threatening problem because reopening of the fistula indicates a return to the aspiration symptoms. In our large series report (1), the fistula reopened in 17 (35%) of 49 patients with initial clinical success at a mean follow-up time of 4.8 weeks. The reopening was caused primarily by complications associated with the placed stent, i.e., stent occlusion caused by tumor overgrowth or ingrowth, food impaction or granulation tissue formation, stent migration, and stent covering disruption (1, 6, 8, 14) (Fig. 5). Attention to dentition and adequate chewing could limit food-impaction-related problems. Furthermore, stent upgrade will be necessary in order to minimize tumor ingrowth or overgrowth as well as granulation tissue formation.
Fig. 5.
Reopening of esophagobronchial fistula caused by food impaction.
A. Initial lateral view shows fistula caused by esophageal cancer. Subsequently, placement of covered expandable stent was performed.
B. Esophagogram obtained one month after stent placement, shows reopening of fistula (arrow) due to food impaction, which is seen as filling defects within stent.
C. This patient underwent passage of inflated balloon catheter (arrow) up and down occluded stent to displace impacted food into stomach.
D. Esophagogram obtained after cleansing stent, shows stent patency and disappearance of fistula.
In a report by Shin et al. (1), eight of 17 patients underwent interventional treatment for reopened fistulas. Balloon irrigation was performed to displace the impacted food bolus into the stomach (Fig. 5). Balloon dilation and stent placement were performed for reopening caused by new granulation tissue formation; whereas, second stent placement was performed for incomplete covering of the fistula or stricture, or stent migration. Reopened fistulas can be treated successfully using interventional management if a patient's general condition is not poor.
Quality of Life and Long-Term Outcome
In a comparative study, health-related quality of life was remarkably improved in the stenting group, compared with the control group and the gastrostomy group in 35 patients with malignant ERFs (19).
In two, large series reports (1, 12), mean patient survival was reported to be 3.1-3.4 months. In one of these reports (12), the survival benefit was significant in patients in the stenting group (3.4 months) compared with the enterostomy group (1.1 months), and the supportive management group (1.3 months). In patients with stenting for malignant ERFs, survival depends on the successful sealing of the fistula (15.1 versus 6.2 weeks, p < 0.05) (1); therefore, suggesting that control of pulmonary contamination can provide the opportunity for both improved survival and quality of life.
ESOPHAGOPULMONARY FISTULA
Although the term 'esophagorespiratory fistula' is used to describe all fistulas located between the esophagus and airway tree, the fistula site is esophagotracheal in 52-57% of patients and esophagobronchial in 37-40% (2, 20, 21). In the remaining patients (3-11%), communication is established peripherally through the lung parenchyma, thus forming an esophagopulmonary fistula (2, 20, 21). However, previous reports regarding stent placement in patients with esophagopulmonary fistulas are very few (22).
In our recent study (22), we determined that 14 esophagopulmonary fistulas were caused by esophageal (n = 9) or bronchogenic (n = 5) carcinomas. Chemotherapy and radiation therapy appeared to be highly associated with fistula development, which occurred in 12 of our 14 study patients. At the time of stent placement, all patients had aspiration pneumonia and 11 had lung abscesses (79%), thus indicating lung contamination in all cases. Stent placement was technically successful in all cases, and clinical success, i.e., complete fistula sealing resulting in resolution of the aspiration symptoms, occurred in 12 patients (86%) (Fig. 6). During follow-up, the fistula reopened in two patients, who were subsequently treated with clinical success.
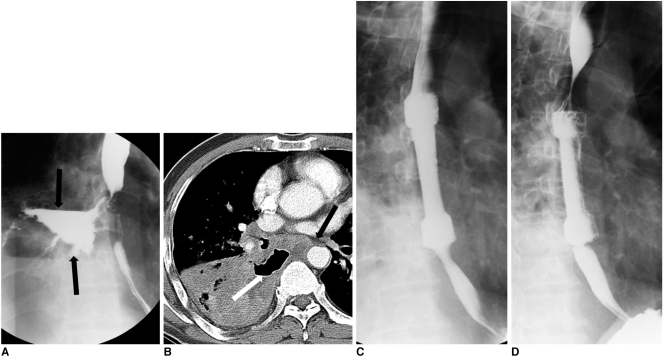
Fig. 6.
Esophagopulmonary fistula caused by lung cancer. Esophagogram (A) and CT scan (B) show large lung abscess (arrows) connected to esophagus. Immediate (C) and one-month (D) follow-up esophagograms show successful closure of fistula.
In our recent report, the mean patient survival time was 101 days (22), and the cause of death was usually aspiration pneumonia (13 of 14 patients). The survival period seems to be similar to that of esophagotracheobronchial fistulas, which range between 3.1-3.4 months (1, 12). Lung abscesses decreased in size, but persisted even after stent placement, probably because the natural drainage of lung abscesses into the esophagus would be closed after stent placement (22). Concomitant abscess drainage procedures should thus be considered.
To increase the length of patient survival, the diagnosis of esophagopulmonary fistulas must be established early, ideally before the onset of pneumonia or the development of lung abscesses. High-risk patients with esophageal or bronchogenic carcinomas and who undergo chemotherapy or radiation therapy should be closely followed in order to detect symptoms associated with fistula formation.
SUMMARY
Placement of covered expandable metallic stents is a safe and effective palliative treatment for patients with ERFs. Furthermore, although the initial clinical success rate was poor and the rate of reopening was high, interventional management may be effective for sealing off reopened ERFs.
Placement of covered expandable metallic esophageal stents can be a successful alternative for palliative treatment of malignant esophagopulmonary fistulas. However, persistence of lung abscesses and the worsening of a patient's clinical condition are frequently observed even following stent placement.
Acknowledgement
The authors thank Bonnie Hami, MA, of the Department of Radiology, University Hospitals Health System, Cleveland, OH, for her editorial assistance in the preparation of this manuscript.
Footnotes
This study was supported by a grant of the Korea Health 21 R & D Project, Ministry of Health & Welfare, Republic of Korea (A060603).
References
- 1.Shin JH, Song HY, Ko GY, Lim JO, Yoon HK, Sung KB. Esophagorespiratory fistula: long-term results of palliative treatment with covered expandable metallic stents in 61 patients. Radiology. 2004;232:252–259. doi: 10.1148/radiol.2321030733. [DOI] [PubMed] [Google Scholar]
- 2.Martini N, Goodner JT, D'Angio GJ, Beattie EJ., Jr Tracheoesophageal fistula due to cancer. J Thorac Cardiovasc Surg. 1970;59:319–324. [PubMed] [Google Scholar]
- 3.Balazs A, Galambos Z, Kupcsulik PK. Characteristics of esophagorespiratory fistulas resulting from esophageal cancers: a single-center study on 243 cases in a 20-year period. World J Surg. 2009;33:994–1001. doi: 10.1007/s00268-009-9988-3. [DOI] [PubMed] [Google Scholar]
- 4.Murthy S, Gonzalez-Stawinski GV, Rozas MS, Gildea TR, Dumot JA. Palliation of malignant aerodigestive fistulae with self-expanding metallic stents. Dis Esophagus. 2007;20:386–389. doi: 10.1111/j.1442-2050.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 5.Weigert N, Neuhaus H, Rosch T, Hoffmann W, Dittler HJ, Classen M. Treatment of esophagorespiratory fistulas with silicone-coated self-expanding metal stents. Gastrointest Endosc. 1995;41:490–496. doi: 10.1016/s0016-5107(05)80009-4. [DOI] [PubMed] [Google Scholar]
- 6.Abadal JM, Echenagusia A, Simo G, Camunez F. Treatment of malignant esophagorespiratory fistulas with covered stents. Abdom Imaging. 2001;26:565–569. doi: 10.1007/s002610000193. [DOI] [PubMed] [Google Scholar]
- 7.Tomaselli F, Maier A, Sankin O, Woltsche M, Pinter H, Smolle-Juttner FM. Successful endoscopical sealing of malignant esophageotracheal fistulae by using a covered self-expandable stenting system. Eur J Cardiothorac Surg. 2001;20:734–738. doi: 10.1016/s1010-7940(01)00867-3. [DOI] [PubMed] [Google Scholar]
- 8.Han YM, Song HY, Lee JM, Cho SI, Chung GH, Kim CS, et al. Esophagorespiratory fistulae due to esophageal carcinoma: palliation with a covered Gianturco stent. Radiology. 1996;199:65–70. doi: 10.1148/radiology.199.1.8633174. [DOI] [PubMed] [Google Scholar]
- 9.Saxon RR, Barton RE, Katon RM, Petersen BD, Lakin PC, Timmermans H, et al. Treatment of malignant esophageal obstructions with covered metallic Z stents: long-term results in 52 patients. J Vasc Interv Radiol. 1995;6:747–754. doi: 10.1016/s1051-0443(95)71180-0. [DOI] [PubMed] [Google Scholar]
- 10.Kishi K, Nakao T, Goto H, Kimura M, Sonomura T, Yamanaka N, et al. A fast placement technique for covered tracheobronchial stents in patients with complicated esophagorespiratory fistulas. Cardiovasc Intervent Radiol. 2005;28:485–489. doi: 10.1007/s00270-003-0203-x. [DOI] [PubMed] [Google Scholar]
- 11.van den Bongard HJ, Boot H, Baas P, Taal BG. The role of parallel stent insertion in patients with esophagorespiratory fistulas. Gastrointest Endosc. 2002;55:110–115. doi: 10.1067/mge.2002.119731. [DOI] [PubMed] [Google Scholar]
- 12.Balazs A, Kupcsulik PK, Galambos Z. Esophagorespiratory fistulas of tumorous origin. Non-operative management of 264 cases in a 20-year period. Eur J Cardiothorac Surg. 2008;34:1103–1107. doi: 10.1016/j.ejcts.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Seto Y, Yamada K, Fukuda T, Hosoi N, Takebayashi R, Chin K, et al. Esophageal bypass using a gastric tube and a cardiostomy for malignant esophagorespiratory fistula. Am J Surg. 2007;193:792–793. doi: 10.1016/j.amjsurg.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Wang MQ, Sze DY, Wang ZP, Wang ZQ, Gao YA, Dake MD. Delayed complications after esophageal stent placement for treatment of malignant esophageal obstructions and esophagorespiratory fistulas. J Vasc Interv Radiol. 2001;12:465–474. doi: 10.1016/s1051-0443(07)61886-7. [DOI] [PubMed] [Google Scholar]
- 15.Deviere J, Quarre JP, Love J, Cremer M. Self-expandable stent and injection of tissue adhesive for malignant bronchoesophageal fistula. Gastrointest Endosc. 1994;40:508–510. doi: 10.1016/s0016-5107(94)70226-8. [DOI] [PubMed] [Google Scholar]
- 16.Saxon RR, Barton RE, Katon RM, Lakin PC, Timmermans HA, Uchida BT, et al. Treatment of malignant esophagorespiratory fistulas with silicone-covered metallic Z stents. J Vasc Interv Radiol. 1995;6:237–242. doi: 10.1016/s1051-0443(95)71104-6. [DOI] [PubMed] [Google Scholar]
- 17.Nam DH, Shin JH, Song HY, Jung GS, Han YM. Malignant esophageal-tracheobronchial strictures: parallel placement of covered retrievable expandable nitinol stents. Acta Radiol. 2006;47:3–9. doi: 10.1080/02841850500334989. [DOI] [PubMed] [Google Scholar]
- 18.Binkert CA, Petersen BD. Two fatal complications after parallel tracheal-esophageal stenting. Cardiovasc Intervent Radiol. 2002;25:144–147. doi: 10.1007/s00270-001-0088-5. [DOI] [PubMed] [Google Scholar]
- 19.Hu Y, Zhao YF, Chen LQ, Zhu ZJ, Liu LX, Wang Y, et al. Comparative study of different treatments for malignant tracheoesophageal/bronchoesophageal fistulae. Dis Esophagus. 2009;22:526–531. doi: 10.1111/j.1442-2050.2009.00950.x. [DOI] [PubMed] [Google Scholar]
- 20.Duranceau A, Jamieson GG. Malignant tracheoesophageal fistula. Ann Thorac Surg. 1984;37:346–354. doi: 10.1016/s0003-4975(10)60745-x. [DOI] [PubMed] [Google Scholar]
- 21.Angorn IB. Intubation in the treatment of carcinoma of the esophagus. World J Surg. 1981;5:535–541. doi: 10.1007/BF01655006. [DOI] [PubMed] [Google Scholar]
- 22.Kim KR, Shin JH, Song HY, Ko GY, Kim JH, Yoon HK, et al. Palliative treatment of malignant esophagopulmonary fistulas with covered expandable metallic stents. AJR Am J Roentgenol. 2009;193:W278–W282. doi: 10.2214/AJR.08.2176. [DOI] [PubMed] [Google Scholar]