Abstract
Objective
To describe the high-resolution CT (HRCT) findings of re-expansion pulmonary edema (REPE) following a thoracentesis for a spontaneous pneumothorax.
Materials and Methods
HRCT scans from 43 patients who developed REPE immediately after a thoracentesis for treatment of pneumothorax were retrospectively analyzed. The study group consisted of 41 men and two women with a mean age of 34 years. The average time interval between insertion of the drainage tube and HRCT was 8.5 hours (range, 1-24 hours). The patterns and distribution of the lung lesions were analyzed and were assigned one of the following classifications: consolidation, ground-glass opacity (GGO), intralobular interstitial thickening, interlobular septal thickening, thickening of bronchovascular bundles, and nodules. The presence of pleural effusion and contralateral lung involvement was also assessed.
Results
Patchy areas of GGO were observed in all 43 patients examined. Consolidation was noted in 22 patients (51%). The geographic distribution of GGO and consolidation was noted in 25 patients (58%). Interlobular septal thickening and intralobular interstitial thickening was noted in 28 patients (65%), respectively. Bronchovascular bundle thickening was seen in 13 patients (30%), whereas ill-defined centrilobular GGO nodules were observed in five patients (12%). The lesions were predominantly peripheral in 38 patients (88%). Of these lesions, gravity-dependent distribution was noted in 23 cases (53%). Bilateral lung involvement was noted in four patients (9%), and a small amount of pleural effusion was seen in seven patients (16%).
Conclusion
The HRCT findings of REPE were peripheral patchy areas of GGO that were frequently combined with consolidation as well as interlobular septal and intralobular interstitial thickening.
Keywords: Computed tomography (CT), high-resolution; Lung, pulmonary edema, re-expansion; Pneumothorax; Thoracentesis
Re-expansion pulmonary edema (REPE) is an uncommon iatrogenic complication that follows the re-expansion of the lung after performing a thoracentesis for large amounts of pneumothorax or pleural effusion (1-4). It usually occurs within one to two hours after a thoracentesis, but the onset time is variable and ranges from the period immediately following the procedure to 24 hours after. Though REPE may progress for one to two days, it usually resolves several days later (5, 6).
Although the diagnosis of REPE is usually based on characteristic serial radiographic features and sometimes a typical clinical course, the early recognition of the initial radiological manifestation, if present, is important since REPE is potentially fatal (1, 7). In addition, since REPE can mimic other diseases and there may be a possibility of underlying unexpected lung diseases which could be hidden in the collapsed lung, the radiologic differentiation of REPE from other lung lesions is also important. However, the initial chest radiographic findings, as such, are nonspecific, showing unilateral airspace opacification of the re-expanded lung (5-9). To the best of the authors' knowledge, a detailed analysis of HRCT imaging features of REPE has not yet been previously reported. Therefore, the aim of this study is to describe the HRCT findings in patients who developed REPE after chest tube drainage for spontaneous pneumothorax.
MATERIALS AND METHODS
This study was approved by our Institutional Review Board (IRB) and patient informed consent was waived for this retrospective study.
Patients
Over the past five years, we performed HRCT scans on 978 pneumothorax patients to facilitate the detection of bullous lung diseases caused by pneumothorax. Of these cases, 643 patients underwent a HRCT scan within 24 hours of a thoracentesis, and 43 patients (7%) were diagnosed as having REPE based on initial HRCT scans, serial radiographic findings, and clinical evidences, with an expected time course. Since REPE usually has been described as a radiologic diagnosis, with or without major clinical consequences in most cases in the literature (2, 5, 10), authors also primarily diagnose REPE by typical radiologic findings. These radiologic findings include ipsilateral airspace opacities in a previously collapsed lung after a thoracentesis on HRCT scans, and the rapid progression of radiologic findings within 1-2 days, followed by the rapid resolution of the opacities within several days. In addition, the patient series did not show any clinical evidence of other diseases such as infection, hemorrhage, or hydrostatic pulmonary edema. Although the serial radiographic findings could not be obtained in two patients showing only CT abnormalities, typical CT findings with no clinical evidence of other diseases could make the diagnosis of REPE possible.
The patient group was comprised of 41 men and two women, ranging in age from 15 to 72 years (mean age: 34 years). The time intervals between the thoracentesis and the HRCT examination ranged from 1 to 24 hours (mean interval: 8.5 hours).
Imaging Technique
High-resolution CT scans were obtained with a Tomoscan AV E1 (Philips, Best, Netherlands), Somatom Sensation 16 (Siemens Medical Imaging, Forchheim, Germany), Somatom Sensation 64 (Siemens Medical Imaging, Forchheim, Germany), and LightSpeed VCT (GE Medical Systems, Milwaukee, WI). The scanning parameters were 120 kVp and 50-190 mA. The section thicknesses were 1.0-2.0 mm, and the reconstruction intervals were 2.0 mm from the lung apex to the carina and 5.0-10.0 mm from the carina to the lung base. All images were reconstructed using high-spatial-frequency or bone algorithms and were displayed with a lung window setting (window level: -700 to -800 HU; width: 1500 HU).
Data Analysis
High-resolution CT images were retrospectively reviewed by two thoracic radiologists, and decisions regarding each finding were reached by consensus. The patterns, extent, and distributions of the lung lesions were analyzed. The different patterns were classified as consolidation, ground-glass opacity (GGO), interlobular septal thickening, bronchovascular bundle thickening, intralobular interstitial thickening, and nodules. The distributions were also categorized as upper, middle, or lower lung zones; central or peripheral zones; and dependent or nondependent portions. The presence of contralateral lung involvement and pleural effusion was also assessed. The time interval between the initial radiologic presentation of the lesions and complete resolution of the lesions on follow-up chest radiographs was also evaluated.
The presence of symptoms during the course of REPE, and the number of fatal cases or death related to REPE were also investigated.
RESULTS
On HRCT scans, patchy areas of GGO were seen in all 43 patients (Fig. 1). Consolidation was noted in 22 patients (51%; Fig. 2). In 25 patients (58%), geographic distribution of GGO and consolidation was noted (Fig. 2). Interlobular septal thickening was found in 28 patients (65%), and this was predominantly located in the anterior portion of the lung (Figs. 1, 3). Intralobular interstitial thickening, which was superimposed on GGO, was also frequently observed with 28 identified cases (65%) (Figs. 1, 3). Bronchovascular bundle thickening was noted in 13 patients (30%). Ill-defined centrilobular GGO nodules were noted in five patients (12%).
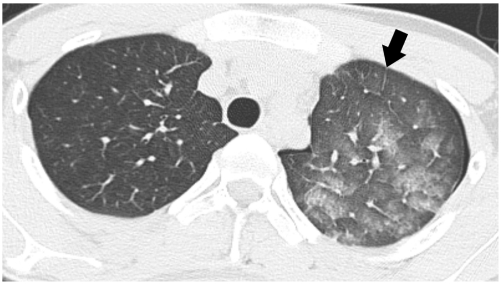
Fig. 1.
Re-expansion pulmonary edema predominantly showing ground-glass opacity pattern. High-resolution CT scan of 19-year-old man taken two hours after insertion of chest tube for left pneumothorax demonstrates patchy ground-glass opacity in left upper lobe with intralobular septal thickening. Note lobular distribution of ground-glass opacity and mild interlobular septal thickening, anteriorly (arrow).
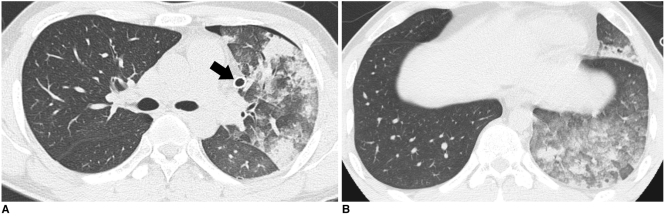
Fig. 2.
Re-expansion pulmonary edema with geographic distribution of consolidation and ground-glass opacity.
A. High-resolution CT scan obtained at five hours after insertion of chest tube, in 26-year-old man, shows geographic patchy areas of consolidation and ground-glass opacity in left lung. Chest tube is located in medial portion of pleura (arrow).
B. High-resolution CT scan of 36-year-old man that underwent chest tube insertion 14 hours before, demonstrates geographic distribution of ground-glass opacity and consolidation. Note gravity-dependent distribution of opacities.
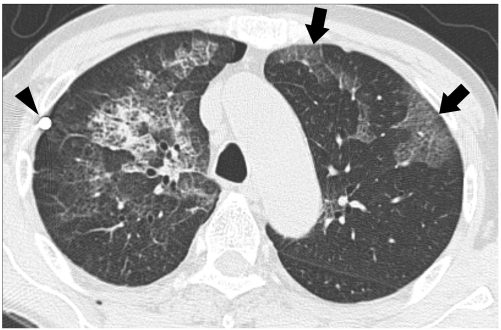
Fig. 3.
72-year-old man with bilateral re-expansion pulmonary edema. High-resolution CT taken seven hours after thoracentesis for right pneumothorax reveals mixed ground-glass opacity and minimal consolidation combined with intralobular reticulations and interlobular septal thickening; thus, suggesting ipsilateral reexpansion pulmonary edema. Like-natured opacities are also seen in contralateral lung (arrows), which consequently suggests contralateral re-expansion pulmonary edema. Note beam hardening artifact from tip of chest tube (arrowhead).
The lesions were predominantly peripheral in 38 patients (88%). Of these patients, gravity-dependent distribution was noted in 23 cases (53%, Fig. 2B). In the remaining five patients (12%), the lesions were distributed randomly in the peripheral and central portions. There was no upper, middle, or lower zonal predominance.
Additional contralateral lung involvement was noted in four patients (9%), and this manifested as relatively small patchy areas of GGO with intralobular interstitial thickening, which were similar to the CT findings of the ipsilateral REPE, but to a lesser extent (Fig. 3). A small amount of pleural effusion was seen in seven patients (16%).
Three patients experienced severe cough (n = 3), agitation (n = 1), tachycardia (n = 2), or tachypnea (n = 2), immediately after a thoracentesis, and there were relatively large areas of opacities on their HRCT scans. No fatality cases were found to be related to REPE. REPE were resolved within 0.8 to 9.8 days (mean, 4.1 days), after initial presentation on serial chest radiographs in 41 patients, except for two patients which only showed CT abnormalities.
DISCUSSION
The risk factors of REPE include a longer duration of collapse, rapid drainage of pleural air or fluid, application of negative intrapleural pressure, the degree of lung collapse, and younger age (1-3). However, it can also arise if the lung has been collapsed for a short period of time or if it is re-expanded without high negative pressure (1, 4). The clinical features are variable and may range from patients that are asymptomatic, but have radiographic findings to those that experience severe cardiorespiratory insufficiency. If symptoms occur, they are dramatic and their onset usually occurs immediately after reexpansion; typically 64% of patients complain of symptoms within one hour (5, 6). In our series, only a few patients experienced severe cough, agitation, tachycardia, and tachypnea, with relatively large areas of disease involvement on HRCT scans. Also no fatality cases were caused by REPE. Due to a small number of cases causing clinically significant symptoms, an analysis by correlation between the symptoms and CT findings could not be performed.
The incidence of REPE was 7% among the 643 patients in our study who underwent HRCT scans within 24 hours after a thoracentesis, which is between the two previously reported incidences, i.e., 1% and 14%, respectively (2, 10). Recently, one study demonstrated a considerably high incidence (30%) of REPE (11), which was probably due in part to a CT-based diagnosis of REPE, whereas most previous studies was diagnosed based on chest radiography results. In addition, they also treated the all patients with a tube thoracotomy using negative pressure, which could be an additional cause of the high incidence of REPE in their study. In our cases, we also performed HRCT scans in all enrolled patients, and most REPE patients showed a subclinical course, which lead to the consideration that the actual incidence of REPE may be more common than is reported (5, 12).
Although the exact mechanism of REPE has not been identified, it appears to be caused by multiple mechanisms. Increased capillary permeability due to hypoxic injury, reperfusion injury with release of toxic oxygen free radicals, and surfactant depletion, are all thought to play a major role. Furthermore, a delay in lymphatic return by stasis during prolonged collapse and bronchial occlusion may also partly account for the development of REPE (5, 9, 13-15). As hydrostatic forces are also likely to play a role, as suggested by increasing venous return and the pressure gradient across pulmonary capillaries (6, 14, 16), the pathogenesis of REPE can ultimately be explained by mixed permeability and hydrostatic edema.
Re-expansion pulmonary edema may lead to severe hypoxemia, which will increase lung damage and may result in extensive adult respiratory distress syndrome (ARDS) and multiorgan failure (1, 5, 7, 17). Also, it can mimic other diseases which could be hidden in the collapsed lung or could be developed during the course of REPE. Therefore, it is evident that the early recognition and differential diagnosis of REPE are important. There were no specific diagnostic criteria for REPE, and it usually has been described as a radiologic diagnosis with or without major clinical consequences in most cases (2, 5, 10). Although chest radiographic findings, as such, are nonspecific and show a variable degree of unilateral airspace opacities immediately after a thoracentesis, there appears to be a typical course with time; progression of opacities for 1-2 days, and resolution within 3-7 days (5-8).
Pneumonia, hemorrhage, and cryptogenic organizing pneumonia could be included in the differential diagnosis of REPE on HRCT scans. The diagnostic possibility of pneumonia or hemorrhage could be excluded based on clinical findings with no symptoms or signs such as fever, hemoptysis, or leukocytosis, and no underlying diseases causing pulmonary hemorrhage. In addition, typical rapid progression of radiologic findings within 1-2 days, followed by the rapid resolution of the opacities within several days, could exclude the diagnosis of organizing pneumonia or other underlying lung diseases from the differential diagnosis.
Re-expansion pulmonary edema can mimic other diseases, such as aspiration, pneumonia, or even pleural effusion on chest radiographs, and can occur unexpectedly in patients that are given HRCT for another purpose, i.e. to detect bullae as a possible cause of spontaneous pneumothorax. Therefore, it is important to recognize the appearance of REPE on HRCT. To the best of the authors' knowledge, two studies on REPE have described CT images, which included one radiologic case report (9) and one recent clinical study (11). However, a systematic and detailed review of the HRCT findings of REPE has not previously been reported.
Very rarely, bilateral or contralateral REPE have been reported (16, 18). In our series, four cases (9%) of additional contralateral REPE developed, and each case presented as a relatively small area of GGO with intralobular interstitial thickening, which were similar to the CT findings of the ipsilateral REPE, to a lesser degree. As a small GGO might not be definitely identified on a chest radiograph, the actual incidence of contralateral REPE could be higher than the reported numbers. The mechanisms underlying contralateral REPE are even less clear. However, a similar pathogenesis to that which leads to ipsilateral REPE can also affect the contralateral lung; this includes the presence of various tissue factors from the ipsilateral injured lung that results in both local and systemic alteration of capillary permeability, spillage of edema fluid from the reexpanded ipsilateral lung, and acute reexpansion of both ipsilateral and contralateral lungs (16, 19).
There are a few limitations to our study. Firstly, all cases were from spontaneous pneumothorax. Although the authors cannot definitively determine whether or not the HRCT findings of REPE from other preceding diseases, such as pleural effusion, are identical, it can be assumed that the same HRCT findings might be present in cases of REPE that occur after the drainage of pleural effusion. This can be explained by the fact that the mechanisms of REPE after a thoracentesis, for both pneumothorax and pleural effusion, are identical. Secondly, since most of our patients were asymptomatic and no fatal cases were included in this study, the CT findings of fatal REPE could not be analyzed, which presumably show more extensive findings than those of the clinically modest REPE.
In conclusion, the HRCT findings of patchy areas of GGO, combined with the consolidation and intra- and interlobular interstitial thickening, are seen in the majority of patients with REPE, which suggests mixed permeability and hydrostatic edema. The familiarity with the HRCT features of REPE, acquired from this study, will enable a more prompt diagnosis.
References
- 1.Mahfood S, Hix WR, Aaron BL, Blaes P, Watson DC. Reexpansion pulmonary edema. Ann Thorac Surg. 1988;45:340–345. doi: 10.1016/s0003-4975(10)62480-0. [DOI] [PubMed] [Google Scholar]
- 2.Matsuura Y, Nomimura T, Murakami H, Matsushima T, Kakehashi M, Kajihara H. Clinical analysis of reexpansion pulmonary edema. Chest. 1991;100:1562–1566. doi: 10.1378/chest.100.6.1562. [DOI] [PubMed] [Google Scholar]
- 3.Miller WC, Toon R, Palat H, Lacroix J. Experimental pulmonary edema following re-expansion of pneumothorax. Am Rev Respir Dis. 1973;108:654–656. [PubMed] [Google Scholar]
- 4.Sherman SC. Reexpansion pulmonary edema: a case report and review of the current literature. J Emerg Med. 2003;24:23–27. doi: 10.1016/s0736-4679(02)00663-7. [DOI] [PubMed] [Google Scholar]
- 5.Tarver RD, Broderick LS, Conces DJ., Jr Reexpansion pulmonary edema. J Thorac Imaging. 1996;11:198–209. [PubMed] [Google Scholar]
- 6.Gluecker T, Capasso P, Schnyder P, Gudinchet F, Schaller MD, Revelly JP, et al. Clinical and radiologic features of pulmonary edema. Radiographics. 1999;19:1507–1531. doi: 10.1148/radiographics.19.6.g99no211507. [DOI] [PubMed] [Google Scholar]
- 7.Smolle-Juettner FM, Prause G, Ratzenhofer B, Pongratz M, Friehs G, List WF. The importance of early detection and therapy of reexpansion pulmonary edema. Thorac Cardiovasc Surg. 1991;39:162–166. doi: 10.1055/s-2007-1013955. [DOI] [PubMed] [Google Scholar]
- 8.Humphreys RL, Berne AS. Rapid re-expansion of pneumothorax. A cause of unilateral pulmonary edema. Radiology. 1970;96:509–512. doi: 10.1148/96.3.509. [DOI] [PubMed] [Google Scholar]
- 9.Murat A, Arslan A, Balci AE. Re-expansion pulmonary edema. Acta Radiol. 2004;45:431–433. doi: 10.1080/02841850410005624. [DOI] [PubMed] [Google Scholar]
- 10.Rozenman J, Yellin A, Simansky DA, Shiner RJ. Re-expansion pulmonary oedema following spontaneous pneumothorax. Respir Med. 1996;90:235–238. doi: 10.1016/s0954-6111(96)90293-0. [DOI] [PubMed] [Google Scholar]
- 11.Kim YK, Kim H, Lee CC, Choi HJ, Lee KH, Hwang SO, et al. New classification and clinical characteristics of reexpansion pulmonary edema after treatment of spontaneous pneumothorax. Am J Emerg Med. 2009;27:961–967. doi: 10.1016/j.ajem.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 12.Waqaruddin M, Bernstein A. Re-expansion pulmonary oedema. Thorax. 1975;30:54–60. doi: 10.1136/thx.30.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodring JH. Focal reexpansion pulmonary edema after drainage of large pleural effusions: clinical evidence suggesting hypoxic injury to the lung as the cause of edema. South Med J. 1997;90:1176–1182. doi: 10.1097/00007611-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Maniwa K, Tanaka E, Inoue T, Sakuramoto M, Minakuchi M, Maeda Y, et al. Interstitial pulmonary edema revealed by high-resolution CT after relief of acute upper airway obstruction. Radiat Med. 2005;23:139–141. [PubMed] [Google Scholar]
- 15.Pavlin DJ, Nessly ML, Cheney FW. Increased pulmonary vascular permeability as a cause of re-expansion edema in rabbits. Am Rev Respir Dis. 1981;124:422–427. doi: 10.1164/arrd.1981.124.4.422. [DOI] [PubMed] [Google Scholar]
- 16.Ketai LH, Godwin JD. A new view of pulmonary edema and acute respiratory distress syndrome. J Thorac Imaging. 1998;13:147–171. [PubMed] [Google Scholar]
- 17.Peatfield RC, Edwards PR, Johnson NM. Two unexpected deaths from pneumothorax. Lancet. 1979;1:356–358. doi: 10.1016/s0140-6736(79)92893-9. [DOI] [PubMed] [Google Scholar]
- 18.Heller BJ, Grathwohl MK. Contralateral reexpansion pulmonary edema. South Med J. 2000;93:828–831. [PubMed] [Google Scholar]
- 19.Ragozzino MW, Greene R. Bilateral reexpansion pulmonary edema following unilateral pleurocentesis. Chest. 1991;99:506–508. doi: 10.1378/chest.99.2.506. [DOI] [PubMed] [Google Scholar]