Abstract
Objectives
To evaluate the association between plasma granulocyte colony-stimulating factor (G-CSF) levels and clinical outcomes including mortality in patients with acute lung injury (ALI) and to determine whether lower tidal volume ventilation was associated with a more rapid decrease in plasma G-CSF over time in patients with ALI.
Design
Retrospective measurement of G-CSF levels in plasma samples that were collected prospectively as part of a large multicenter clinical trial.
Setting
Intensive care units in ten university centers.
Patients
The study included 645 patients enrolled in the NHBLI ARDS Clinical Network trial of lower tidal volumes compared with traditional tidal volumes for ALI.
Measurements and Main Results
Baseline plasma levels of G-CSF were associated with an increased risk of death and a decrease in ventilator-free and organ failure-free days (VFD and OFD) in multivariate analyses controlling for ventilation strategy, age, and sex (OR death 1.2/log10 increment G-CSF, 95% CI 1.01 to 1.4). Stratification of G-CSF levels into quartiles revealed a strong association between the highest levels of G-CSF and increased risk of death and decreased VFD and OFD in multivariate analyses controlling for ventilation strategy, APACHE III score, PF ratio, creatinine, and platelet count (p<0.05). Subgroup multivariate analysis of patients with sepsis as their risk factor for ALI revealed a U-shaped association between mortality and G-CSF levels such that risk increased linearly from the second through fourth (highest) quartiles, yet also increased in the first (lowest) quartile. G-CSF levels decreased over time in both tidal volume groups and there was no statistical difference in the extent of decrease between ventilator strategies.
Conclusions
In patients with ALI, plasma G-CSF levels are associated with morbidity and mortality, yet these levels are not influenced by tidal volume strategy. In patients with sepsis-related ALI we find a bimodal association between baseline plasma G-CSF levels and subsequent morbidity and mortality from this disease.
Keywords: ALI, ARDS, G-CSF, low tidal volume ventilation, sepsis
Introduction
The potential role of granulocyte colony-stimulating factor (G-CSF) in the development of acute lung injury has been debated for the last 10 years (1–4). Several lines of evidence suggest a role for G-CSF in the pathogenesis of ALI/ARDS. G-CSF isa potent neutrophilic growth and release factor, and it has been associated with the development of an altered circulating neutrophil phenotype in animal models which is similar to that seen in ALI/ARDS patients (5–11). Furthermore, animal models (12–15) and clinical reports (16–18) suggest that the use of exogenous G-CSF may promote the development of ALI/ARDS. However, G-CSF, as a critical factor in innate immune function, is essential for the protective inflammatory response and possibly the balance of pro- and anti-inflammatory factors in the inflammatory milieu (4). Animals lacking G-CSF are more susceptible to bacterial infection and subsequent progression to sepsis and death (19), and several studies in normal mice, in fact, suggest that supplemental G-CSF may improve outcomes from ALI/ARDS due to pneumonia (14, 20, 21). Thus, G-CSF may serve as a ‘double-edged sword:’ Just as high levels of G-CSF might drive an over exuberant inflammatory response, low levels might leave the host susceptible to progression of the initiating insult (1, 22).
Clinical studies have demonstrated that high levels of G-CSF are present in the circulation and lungs of patients at risk for and with ALI/ARDS (23, 24). G-CSF is known to be released into the circulation by a wide range of cell types in response to many of the insults associated with the development of ALI/ARDS, particularly overwhelming infection and sepsis (25). In the lung, G-CSF is produced by both alveolar macrophages (26) and epithelial cells (27). Recent studies have shown that G-CSF is elevated in bronchoalveolar lavage (BAL) of both patients with ARDS and those at risk of developing ARDS (24), and that BAL levels of G-CSF may correlate with the severity of lung injury (23), and subsequent risk of death (28). Although it has been suggested that elevated plasma levels of G-CSF in the setting of ALI/ARDS correspond to worsened oxygenation and increased BAL neutrophilia (23), little else has been published examining circulating levels of this cytokine in ALI/ARDS. The association between circulating G-CSF and mortality, for example, is unknown.
We hypothesized that G-CSF is an important mediator in the pathogenesis of ALI/ARDS, and that systemic levels of this cytokine might be predictive of outcome from the disease. We further hypothesized, however, that unlike previously described ALI biomarkers, the relationship between G-CSF levels and outcomes might be nonlinear, particularly in patients with overwhelming infection and sepsis reflecting a dual role for G-CSF response in ALI pathogenesis. Lastly, we hypothesized that, similar to the effect seen with both IL-6 and IL-8 (29), a low tidal volume strategy would be associated with a more rapid decrease in levels of G-CSF over time. To examine these hypotheses, we measured baseline and day 3 G-CSF levels in previously banked plasma samples from the ARDS Clinical Trials Network’s ARMA trial, a randomized controlled trial of low tidal volume ventilation in patients with ALI/ARDS (30).
Methods
Patient selection
The 861 patients enrolled in the National Heart, Lung, and Blood Institute (NHLBI) ARDS Network multi-center randomized trial of 12ml/kg vs. 6ml/kg tidal volume ventilation served as the patient group for this study. This trial was conducted simultaneously with two other clinical trials in which ketoconazole or lisofylline were compared with placebo in a factorial design. Of the 861 patients enrolled, plasma was available at baseline (day 0) from 645 patients and from 556 patients both day 0 and day 3. As indicated in previous publications (30–32), the protocols were approved by a protocol review committee convened by the NHLBI and by the institutional review board at each of the participating institutions. Safety of the trials was monitored by an independent Safety Board.
The study design for the ventilator trial has been described extensively in previous publications (30–32). Briefly, patients with ALI/ARDS who required mechanical ventilation were randomized to either a 6ml/kg or a 12ml/kg tidal volume strategy. Ventilation and weaning from mechanical ventilation were defined by standardized protocols. Patients were followed for 180d or until discharge to home with unassisted breathing. At the time of enrollment APACHE III scores were calculated and the physician investigator identified the primary risk factor for the development of ALI as previously described (33). In the classification of sepsis, investigators were instructed to use the SCCM definition of clinical sepsis which includes a known or suspected source of systemic infection and at least two of the following: 1) temperature < 36°C or > 38°C; 2) Heat rate > 90 bpm; 3) Respiratory rate > 20 bpm or PaCO2 <32 mmHg; 4) White blood cell > 12,000/mm3, < 4,000/mm3, or > 10% bands. Known infection is a documented source of infection (e.g., positive blood cultures). Suspected infection is evidenced by one or more of the following: white cells in a normally sterile body fluid, perforated viscus, radiographic evidence of pneumonia plus purulent sputum, or a syndrome associated with a high risk of infection (e.g., ascending cholangitis) (34).
Cytokine measurements
Blood samples were obtained from the study participants at entry into the study (day 0) and again at day 3 as previously described. G-CSF levels were measured on samples from both days. The measurements were carried out by individuals blinded to all clinical data using Quantikine G-CSF immunoassay kits (R&D Systems, Minneapolis, MN). The lower limit of detection for G-CSF using this kit was 0.8pg/mL.
Study outcome variables
One of the primary outcomes for the ventilator trial was mortality before discharge home with unassisted breathing (30). This was used as the primary outcome for this study. Ventilator-free days (VFD) was also used as a primary outcome for the ventilator trial; it was used as a secondary outcome in the present analysis (35). It was calculated as the number of days of unassisted breathing from day 1–28 if unassisted breathing continued for >48h (35). As in previous studies (30–32), deaths prior to achieving unassisted breathing before day 28 were assigned zero VFD. Another secondary outcome was number of days alive without non-pulmonary organ failure (i.e. organ failure-free days [OFD]). Organ failure was determined as defined in the Brussels Organ Failure Table (36). As has been noted previously (30), differences in VFD or OFD could reflect a difference in mortality, ventilator days among survivors, or both.
Statistical analysis
Data analysis was conducted using SAS 9.1 (SAS Institute, Cary, NC). G-CSF levels were not normally distributed. Our theoretical model predicted that the relationship between G-CSF levels and adverse outcomes may be non-linear. Consequently, we divided baseline G-CSF levels into quartiles for analysis as we have done in previous studies examining biomarkers in the ARMA cohort (37). We also evaluated the impact of log10 transformed G-CSF levels and clinical outcomes.
We used multivariate logistic regression to analyze the longitudinal association between baseline G-CSF levels and the risk of mortality, controlling for age, sex, and ventilator group assignment. In analogous fashion, we used multivariate linear regression to study the association between baseline G-CSF levels and the two continuous study outcomes: VFD and OFD. Because there is no consensus on how to statistically analyze such physiologic and biochemical data, we present the results controlling for age, sex, and ventilator group and an additional multivariate analysis that controls for APACHE III score and other markers of acute illness severity (PaO2/FiO2 ratio, creatinine, and platelet count). Because lisofylline and ketoconazole did not change clinical outcomes (31, 32), they were not considered as confounding variables in any analysis.
We tested for a statistical interaction by sepsis as the primary clinical risk factor for ALI/ARDS. The likelihood ratio test was used to compare logistic regression models with and without interaction terms between G-CSF levels and sepsis as a risk factor for ALI/ARDS. Because we found a strong interaction by sepsis status, results are presented stratified by sepsis vs. other causes of ALI/ARDS. Because the power to detect statistical interactions is generally low, we used a cut-off alpha (2-tailed) of 0.10 to indicate a statistically significant interaction (38).
We observed a non-linear relationship between baseline G-CSF quartile and mortality in the subgroup with sepsis. To further evaluate the shape of the relationship between G-CSF and mortality, linear splines were generated with knots evenly spaced at quartiles of G-CSF.
To evaluate the second hypothesis, we studied the prospective impact of the 6 ml/kg tidal volume strategy on the change in G-CSF levels over time. We used analysis of covariance (ANCOVA) to examine the effect of mechanical ventilator treatment group on G-CSF levels measured at day 3, controlling for baseline level. To address the non-normal distribution of G-CSF levels, we performed ANCOVA on log10 transformed G-CSF levels.
Results
Baseline Characteristics
The baseline characteristics of the patients are shown in Table 1. There was no statistical difference in age, sex, ventilatory strategy assignment, APACHE III scores, creatinine, platelets, or PaO2/FIO2 between the patients included in the study (645) and those without baseline plasma samples available for analysis (257). A slightly lower proportion of patients included in the study had sepsis as a risk factor for ALI/ARDS compared to those without baseline plasma samples available for analysis. Because G-CSF levels were not significantly higher among septic vs. non-septic patients with ALI/ARDS (data not shown), it is unlikely that the differential sepsis representation introduced bias into the analysis of G-CSF and clinical outcomes. Moreover, stratification by sepsis status explicitly takes sepsis status into account, mitigating against any possible bias.
Table 1.
Baseline characteristics among those with and without available baseline G-CSF levels
| Variable | Included in study (n=645) |
No G-CSF levels (n=257) |
P value for comparison |
|---|---|---|---|
| Age (yrs) | 51 (17) | 53 (18) | 0.17 |
| Sex (% female) | 264 (41%) | 103 (40%) | 0.81 |
| Ventilator group | 303 (47%) | 126 (49%) | 0.58 |
| Sepsis as risk factor for ALI |
156 (24%) | 80 (31%) | 0.03 |
| APACHE III | 77 (28) | 76 (27) | 0.92 |
| Creatinine | 1.6 (1.5) | 1.7 (1.7) | 0.61 |
| Platelets | 155 (111) | 163 (114) | 0.33 |
| PaO2/FiO2 | 132 (65) | 129 (60) | 0.51 |
There were a total of 902 subjects who participated in the ARMA, LARMA, and KARMA studies.
Continuous variables are expressed as mean (sd); categorical variables as n (%).
Baseline G-CSF levels and clinical outcomes
G-CSF range at Day 0 was 0-555875 pg/mL (median 191pg/mL), demonstrating a marked elevation compared to those found in healthy human volunteers (typically <30pg/mL) (39, 40). Elevated baseline plasma levels of G-CSF were associated with significantly fewer ventilator-free days (VFD) and organ failure-free days (OFD) in both logistic regression analyses controlling for ventilation strategy (mean differences −0.9 per log10 increment, 95% CI −1.7 to −0.001, and −1.6, 95% CI −2.5 to −0.7, respectively), and multivariate analyses controlling for ventilation strategy, age, and sex (mean differences −1.1 per log10 increment, 95% CI −1.9 to −0.2 and −1.8, 95% CI −2.7 to −0.9, respectively). An increased risk of death was associated with baseline G-CSF levels when controlling for ventilation strategy, age, and sex (OR 1.2 per log10 increment, 95% CI 1.01 to 1.4), but this association did not reach significance when controlling for ventilation strategy alone. Associations between baseline G-CSF levels and VFD, OFD, and death were no longer statistically significant when additionally controlling for markers of disease severity (APACHE III score; data not shown). The lack of association after adjustment was, however, not unexpected given the possibility that the variables measuring disease severity might in fact be on the causal pathway between G-CSF elevation and morbidity and mortality, thereby potentially “over-adjusting” and minimizing any true underlying association.
To further investigate the association between baseline G-CSF levels and outcomes, we next examined the relationships by G-CSF quartiles (Table 2). This analysis showed a strong relationship between the highest quartile and subsequent organ dysfunction (decreased VFD and OFD) and death.
Table 2.
Quartile of baseline G-CSF level and patient outcomes
| Outcome | G-CSF range (pg/mL) |
Ventilator-free days |
Organ failure-free days |
Mortality OR (95% CI) |
|---|---|---|---|---|
| Mean difference (95% CI) |
Mean difference (95% CI) |
|||
| Quartile 1 | 0 – 77.9 | 0.6 (−1.7 to 2.8) | −0.7 (−3.0 to 1.5) | 1.28 (0.79 to 2.07) |
| Quartile 2(referent) | 78.0 – 190.2 | 0 | 0 | 1.0 |
| Quartile 3 | 190.8 – 595.6 | −0.2 (−2.5 to 2.0) | −1.4 (−3.6 to 0.9) | 0.99 (0.60 to 1.62) |
| Quartile 4 | 602.1 – 555874.5 | −2.8 (−5.0 to −0.5)* | −4.4 (−6.7 to −2.2)* | 1.70 (1.06 to 2.75)* |
All analyses control for age, sex, and ventilator group. Quartile 2 is used as referent to the nonlinear relationship between G-CSF levels and outcomes.
Ventilator-free days and organ failure-free days are mean days compared to referent group
Mortality is odds ratio compared to reference group.
p<0.05
Prognostic Value of Plasma G-CSF Levels in Patients with Sepsis
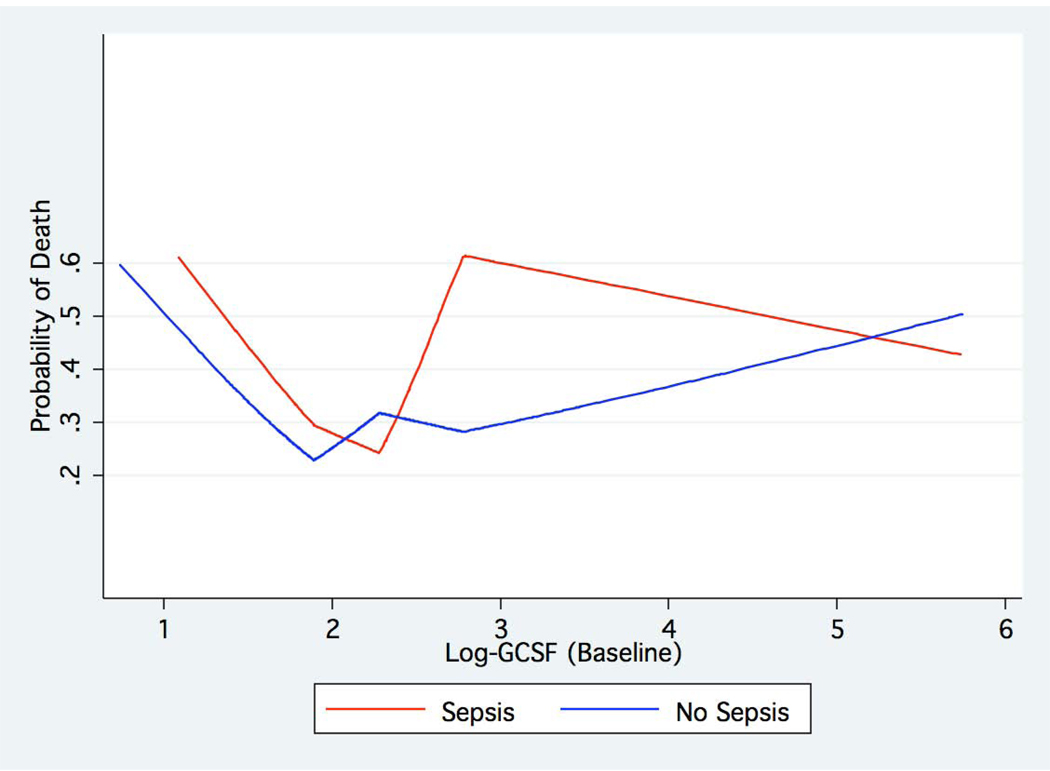
Given the known role of overwhelming infection in the stimulation of G-CSF release, we next tested for an interaction between the clinical diagnosis of sepsis in the cohort and baseline plasma G-CSF levels. Inspection of the data revealed that the association between G-CSF levels and adverse clinical outcomes in ALI/ARDS differed in patients with sepsis from those without sepsis, and the test for statistical interaction was positive by our pre-specified criteria (see Methods) (p=0.058). In particular, a strong association was found in patients whose primary clinical risk factor for ALI/ARDS was sepsis, whereas the results were much less strong among those without sepsis (Table 3). This analysis also identified the existence of a U-shaped distribution of morbidity and mortality in the sepsis subset, such that VFD, OFD, and risk of death appear to increase monotonically with increasing levels of G-CSF (quartiles 2, 3, and 4), yet were also higher in the lowest quartile. In the sepsis subset, these associations remain significant despite controlling for markers of acute and chronic disease severity (Table 4). An analysis using linear splines confirmed a U-shaped relationship between baseline G-CSF level and mortality among patients with sepsis (Figure 1).
Table 3.
Quartile of baseline G-CSF level and patient outcomes by sepsis status
| Outcome | Ventilator-free days | Organ failure-free days | Mortality |
|---|---|---|---|
| Mean difference (95% CI) |
Mean difference (95% CI) |
OR (95% CI) | |
| Sepsis (n=156) | |||
| Quartile 1 | −4.7 (−9.5 to 0.1) | −6.2 (−10.5 to −1.8) | 3.20 (1.03 to 10.0) |
| Quartile 2 (referent) | 0 | 0 | 1.0 |
| Quartile 3 | −6.9 (−11.2 to −2.6)* | −6.7 (−10.6 to −2.7)* | 3.16 (1.13 to 8.80)* |
| Quartile 4 | −8.1 (−12.3 to −3.9)* | −8.3 (−12.1 to −4.5)* | 4.18 (1.52 to 11.52)* |
| No Sepsis (n=488) | |||
| Quartile 1 | 2.0 (−0.6 to 4.6) | 0.4 (−2.2 to 3.0) | 1.05 (.61 to 1.80) |
| Quartile 2 (referent) | 0 | 0 | 1.0 |
| Quartile 3 | 1.9 (−0.7 to 4.6) | 0.5 (−2.1 to 3.2) | 0.63 (0.35 to 1.14) |
| Quartile 4 | −0.9 (−3.5 to 1.8) | −2.9 (−5.6 to −0.2)* | 1.26 (0.72 to 2.18) |
All analyses control for age, sex, and ventilator group. Quartile 2 is used as referent due to the nonlinear relationship between G-CSF levels and outcomes.
Ventilator-free days and organ failure-free days are mean days compared to referent group
Mortality is odds ratio compared to reference group
p<0.05
Table 4.
Quartile of baseline G-CSF level and patient outcomes by sepsis status and controlling for disease severity
| Outcome | Ventilator-free days | Organ failure-free days | Mortality |
|---|---|---|---|
| Mean difference (95% CI) |
Mean difference (95% CI) |
OR (95% CI) | |
| Sepsis (n=156) | |||
| Quartile 1 | −5.45 (−10.26 to −0.64)* | −8.33 (−12.53 to −4.12)* | 4.31 (1.28 to 14.52)* |
| Quartile 2 (referent) | 0* | 0* | 1.0* |
| Quartile 3 | −6.80 (−11.09 to −2.51)* | −7.14 (−10.89 to −3.38)* | 3.29 (1.12 to 9.63)* |
| Quartile 4 | −6.12 (−10.48 to −1.77)* | −6.67 (−10.48 to −2.85)* | 3.18 (1.08 to 9.36)* |
| No Sepsis (n=488) | |||
| Quartile 1 | 1.27 (−1.23 to 3.78) | −0.03 (−2.45 to 2.39) | 1.17 (0.66 to 2.06) |
| Quartile 2 (referent) | 0 | 0 | 1.0 |
| Quartile 3 | 1.86 (−0.74 to 4.46) | 1.31 (−1.20 to 3.81) | 0.54 (0.29 to 1.02) |
| Quartile 4 | 0.96 (−1.69 to 3.61) | 0.10 (−2.45 to 2.66) | 0.79 (0.44 to 1.45) |
Analyses controls for age, sex, ventilator group, APACHE III score, PaO2/FIO2 ratio, creatinine, and platelet count. Quartile 2 is used as referent due to the nonlinear relationship between G-CSF levels and outcomes.
Ventilator-free days and organ failure-free days are mean days compared to referent group
Mortality is odds ratio compared to reference group
p<0.05
Figure 1. Relationship between baseline plasma G-CSF level and subsequent mortality.
Spline analysis of the relationship between baseline plasma G-CSF levels and subsequent mortality reveals the existence of a U-shaped curve with rising mortality at both the lowest and highest G-CSF levels.
Further subgroup analysis examining direct (bacterial/aspiration pneumonia) vs. indirect (all other) causes of lung injury was performed. This demonstrated a significant association between only the highest quartile G-CSF levels and worse outcomes in the indirect injury subgroup (Table 6), whereas no significant relationship was found between G-CSF levels and outcomes in the direct injury subgroup.
Table 6.
Quartile of baseline G-CSF level and patient outcomes by direct vs. indirect lung injury
| Type of lung injury | Ventilator-free days | Organ failure-free days | Mortality |
|---|---|---|---|
| Mean difference (95% CI) | Mean difference (95% CI) | OR (95% CI) | |
| Direct (n=301) | |||
| Quartile 1 | 1.27 (−2.03 to 4.57) | −0.46 (−3.73 to 2.81) | 1.01 (0.52 to 1.93) |
| Quartile 2 (referent) | 0 | 0 | 1.0 |
| Quartile 3 | 1.92 (−1.76 to 5.89) | −0.59 (−4.23 to 3.05) | 0.62 (0.29 to 1.34) |
| Quartile 4 | 0.016 (−3.55 to 3.59) | −2.05 (−5.59 to 1.48) | 0.93 (0.46 to 1.90) |
| Indirect (n=344) | |||
| Quartile 1 | 0.22 (−3.06 to 3.50) | −1.35 (−4.56 to 1.86) | 1.48 (0.70 to 3.12) |
| Quartile 2 (referent) | 0 | 0 | 1.0 |
| Quartile 3 | −1.79 (−4.69 to 1.11) | −2.07 (−4.91 to 0.77) | 1.45 (0.74 to 2.85) |
| Quartile 4 | −4.92 (−7.85 to −1.99)* | −6.40 (−9.27 to −3.54)* | 2.87 (1.48 to 5367)* |
Direct lung injury = bacterial/aspiration pneumonia.
All analyses control for age, sex, and ventilator group. Quartile 2 is used as referent due to the nonlinear relationship between G-CSF levels and outcomes.
Ventilator-free days and organ failure-free days are mean days compared to referent group
Mortality is odds ratio compared to reference group
p<0.05
Change in G-CSF levels over time and the impact of tidal volume strategy
G-CSF levels decreased over time (Day 0 to Day 3) in both tidal volume groups (Table 5). However, unlike the previously reported association between low tidal volume strategy and more rapid decrease in IL-6 and IL-8 (29), there was no statistical difference in the extent of decrease in G-CSF between ventilator strategies (p=0.16). Furthermore, there was no evidence that sepsis modified the impact of ventilator strategy on G-CSF levels over time (p=0.86).
Table 5.
G-CSF levels over time and mortality
| Alive | Dead | |||||
|---|---|---|---|---|---|---|
| Time | N | G-CSF level (pg/mL) (median) |
25th–75th IQR |
N | GCS-F level (pg/mL) (median) |
25th–75th IQR |
| OVERALL | ||||||
| Entire group | ||||||
| Baseline | 423 | 188 | 80–540 | 222 | 207 | 74–887 |
| Day 3 | 373 | 50 | 24–94 | 183 | 69 | 35–196 |
| 6 ml / kg group | ||||||
| Baseline | 233 | 179 | 78–464 | 109 | 214 | 78–835 |
| Day 3 | 212 | 48 | 24–96 | 91 | 78 | 38–232 |
| 12 ml / kg group | ||||||
| Baseline | 190 | 200 | 83–616 | 113 | 202 | 74–847 |
| Day 3 | 161 | 51 | 26–92 | 92 | 63 | 29–154 |
| SEPSIS | ||||||
| Baseline | 92 | 181 | 95–616 | 65 | 371 | 106–923 |
| Day 3 | 83 | 54 | 24–119 | 52 | 82 | 43–213 |
| NON-SEPSIS | ||||||
| Baseline | 331 | 188 | 77–539 | 157 | 165 | 62–782 |
| Day 3 | 290 | 49 | 23–89 | 131 | 67 | 33–180 |
G-CSF range at Day 0 was 0 – 555875 pg/mL (median 191 pg/mL); G-CSF range at Day 3 was 0 – 45451 pg/mL (median 55 pg/mL). IQR = interquartile range.
Discussion
In this study we demonstrate a significant, though complex, relationship between baseline plasma G-CSF levels in ALI/ARDS and subsequent morbidity and mortality from this disease. Our initial analysis, examining a large heterogenous cohort of ALI/ARDS patients (Table 2), suggested an association between elevated plasma levels of G-CSF and subsequent organ failure and death, as has been seen for other inflammatory cytokines in this disease (29, 41–44). Given the established relationship between infection (particularly sepsis) and G-CSF release, we examined the impact of sepsis on the association between G-CSF and clinical outcomes in ALI/ARDS by stratification. Previous studies of ALI/ARDS have shown that individual biomarkers may demonstrate specificity within only certain risk factor groups (29).
The analysis demonstrated a strong relationship between G-CSF levels and adverse clinical outcomes in the sepsis group (Table 3), with essentially no association in the non-sepsis group. A similar strengthening of relation between cytokine levels and outcomes in sepsis patients has been seen in other analyses (29). No previous study has examined G-CSF in ALI/ARDS due to sepsis; however, three studies have examined this cytokine in patients with sepsis alone. Although two small studies have suggested correlations between either high (45) or low (46) levels of circulating G-CSF and increased mortality, the largest study (n=82) showed no independent association (47), leaving the relationship unclear. Our analysis included a relatively large number of patients with sepsis and ALI/ARDS and demonstrates a U-shaped relationship between mortality and plasma G-CSF levels: risk increases linearly from the second through fourth (highest) quartiles, yet also increases in the first (lowest) quartile. Similar associations are seen for measures of organ dysfunction (VFD and OFD) in our analysis (Table 3). Of the numerous biomarkers investigated in ALI/ARDS, G-CSF appears to be unique in displaying this relationship. Furthermore, these results are maintained despite controlling for markers of disease severity (Table 4), and the association between the lowest quartile and worse outcome is strengthened in this analysis. Subgroup analysis examining indirect causes of lung injury failed to demonstrate a similar U-shaped relationship between G-CSF and outcomes in this subgroup (Table 6), suggesting that this bimodal relationship is unique to the sepsis cohort and not related to indirect mechanisms of injury in general.
The finding of a bimodal distribution of G-CSF-associated morbidity and mortality may be explained by the role of this cytokine as a “double-edged sword” in the immune response (1, 22). Failure of the innate immune response to sepsis, as is seen in neutropenic infection, may lead to rapid deterioration and death; yet, at the other extreme, an over-exuberant inflammatory response is postulated to drive ALI/ARDS. This may explain why covariate analysis with disease severity markers weakens the association between baseline G-CSF and subsequent outcomes in the highest quartile, but strengthens this association in the lowest quartile: G-CSF release may in part be driven by the severity of illness (as is more apparent in the analysis of the entire cohort), but failure to produce an adequate G-CSF response (the lowest quartile) predicts progression of sepsis and subsequent morbidity and mortality. The finding of a bimodal population may also shed light on the equivocal results of recent clinical trials of exogenous G-CSF in nonneutropenic sepsis and pneumonia (48–51) in which, in theory, patients with extremely low G-CSF levels may have benefited from the intervention, while those with extremely high levels may have worsened.
Why such a wide distribution of G-CSF response in septic patients with lung injury should occur is less clear, but may reflect several variables in the septic cohort, including genetic polymorphism and host factors. G-CSF response has recently been shown to vary in the general population such that individuals may be categorized as either ‘high’ or ‘low’ responders based on the response of their leukocytes to lipopolysaccharide stimulation (52). In that study, expression of csf3 (the gene encoding G-CSF) was found to be more than 4-fold greater in ‘high’ compared to ‘low’ responders, and was the gene showing the greatest differential regulation between the two populations. Polymorphisms in the promoter region of csf3 have recently been described that appear to be associated with this differential response (M. Wurfel, personal communication). Among possible host factors that may account for differences in G-CSF expression, alcoholism has been shown to suppress the G-CSF response in a rat model of sepsis (53), and the association between alcoholism and leukopenic sepsis has been well-described (54). Unfortunately, none of these variables were captured in the ARMA study and thus cannot be examined in this cohort.
In this study no association was found between tidal volume strategy and the rate of decline in plasma G-CSF levels over time. This is contrast to the previously reported effects of tidal volume on biomarkers such as IL-6, IL-8, and soluble TNF receptor I (29,55). Our failure to find a similar association between tidal volume and rate of decrease in G-CSF levels suggests that G-CSF may not participate in the pathogenesis of ventilator-induced lung injury in the setting of ALI, and further emphasizes the unique biology of G-CSF in this disease compared to previously studied cytokines. Given the small numbers of patients in the tidal volume cohorts examined, caution should be exercised in interpreting these results.
A limitation of this study is the conceptual difficulty in formulating a multivariate analysis. As we have previously discussed (29), it is uncertain how best to statistically control for markers of disease severity, such as APACHE III score, in studies that relate biomarkers to clinical outcomes. Our a priori causal model was that G-CSF might be a pathophysiologic mediator in the disease. Therefore, controlling for variables that measure acute illness severity and are on the causal pathway between G-CSF elevation and death, may result in “over adjusted” effect estimates that are biased toward the null value. The results adjusted only for age, sex, and ventilator group may actually be better estimates of the “true” relation between cytokines and outcomes. Because there is no consensus on how to statistically analyze such physiologic and biochemical data, we present both multivariate analysis and analysis adjusted only for ventilator group. The observation that the adjusted, but not unadjusted, confidence intervals do not exclude “no effect” could reflect this overadjustment phenomenon. Furthermore, caution must observed in interpreting any study employing post-hoc analysis with multiple comparisons as we have performed.
An additional potential limitation of the study is the method used for ARDS risk factor assignment in the ARMA trial, which was based on clinical judgment, rather than on strictly specified criteria. Although this method of classification may not allow direct comparison to other studies in which, for instance, sepsis has been defined by different methods, the observed higher mortality in patients classified with sepsis in the ARMA study is similar to that reported in previous studies, suggesting that the classification of causes used in ARMA is comparable (33). Finally, it must be noted that a slightly lower proportion of patients included in the study had sepsis as a risk factor for ALI/ARDS compared to those without baseline plasma samples available for analysis. Because the proportion of septic patients was only slightly lower, we do not believe that it had a substantive impact on our results. Moreover, we would expect the inclusion of slightly fewer septic patients to lead to more conservative estimates of the impact of G-CSF and clinical outcomes and could in fact slightly underestimate the impact of G-CSF on clinical outcomes.
Conclusions
Our study identifies G-CSF as a biomarker with unique characteristics in ALI/ARDS compared to those previously described (56). We find a bimodal association between baseline plasma G-CSF levels and subsequent morbidity and mortality from this disease. This finding suggests a complex role for G-CSF in the innate immune response and the delicate balance between critical host defense and catastrophic inflammatory injury. Furthermore these findings may shed some light on heretofore ambiguous results of multiple clinical trials of G-CSF therapy in critical illness, and suggest the possibility that, following confirmation our findings, a more targeted approach in the use of this cytokine might be more efficacious in the future. Further investigation of the complex role of G-CSF in critical illness is warranted.
Acknowledgments
Financial Support: Supported by NIH R01 HL084200, K08 HL0049, NO1HR46064, KL2RR024130, and UVM New Research Initiative.
References
- 1.Quezado ZMN, Eichacker PQ. Prophylactic granulocyte colony-stimulating factor in the critically ill: carefully balancing the benefits and risks. Crit Care Med. 2002;30:2162–2164. doi: 10.1097/00003246-200209000-00045. [DOI] [PubMed] [Google Scholar]
- 2.Azoulay E, Attalah H, Harf A, et al. Granulocyte colony-stimulating factor or neutrophil-induced pulmonary toxicity: myth or reality? Systematic review of clinical case reports and experimental data. Chest. 2001;120:1695–1701. doi: 10.1378/chest.120.5.1695. [DOI] [PubMed] [Google Scholar]
- 3.Bauer M, Reinhart K. From mice and MOF: rodent models, immune modulation, and outcome in the critically ill. Crit Care Med. 2006;34:921–923. doi: 10.1097/01.CCM.0000202439.45624.E8. [DOI] [PubMed] [Google Scholar]
- 4.Weiss M, Moldawer LL, Schneider EM. Granulocyte colony-stimulating factor to prevent the progression of systemic nonresponsiveness in systemic inflammatory response syndrome and sepsis. Blood. 1999;93:425–439. [PubMed] [Google Scholar]
- 5.Price TH, Chatta GS, Dale DC. Effect of recombinant granulocyte colony-stimulating factor on neutrophil kinetics in normal young and elderly humans. Blood. 1996;88:335–340. [PubMed] [Google Scholar]
- 6.Spiekermann K, Roesler J, Emmendoerffer A, et al. Functional features of neutrophils induced by G-CSF and GM-CSF treatment: differential effects and clinical implications. Leukemia. 1997;11:466–478. doi: 10.1038/sj.leu.2400607. [DOI] [PubMed] [Google Scholar]
- 7.Carulli G. Effects of recombinant human granulocyte colony-stimulating factor administration on neutrophil phenotype and functions. Haematologica. 1997;82:606–616. [PubMed] [Google Scholar]
- 8.Fowler AA, Fisher BJ, Centor RM, et al. Development of the adult respiratory distress syndrome: progressive alteration of neutrophil chemotactic and secretory processes. Am J Pathol. 1984;116:427–435. [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher MP, Vassar MJ, Holcroft JW. Patients with adult respiratory distress syndrome (ARDS) demonstrate in vivo neutrophil activation associated with diminished binding of neutrophil-specific monoclonal antibody 31D8. Inflammation. 1988;12:455–473. doi: 10.1007/BF00919439. [DOI] [PubMed] [Google Scholar]
- 10.Chollet-Martin S, Montravers P, Gibert C, et al. Subpopulation of hyperresponsive polymorphonuclear neutrophils in patients with adult respiratory distress syndrome. Role of cytokine production. Am Rev Respir Dis. 1992;146:990–996. doi: 10.1164/ajrccm/146.4.990. [DOI] [PubMed] [Google Scholar]
- 11.Van Eeden SF, Kitagawa Y, Klut ME, et al. Polymorphonuclear leukocytes released from the bone marrow preferentially sequester in lung microvessels. Microcirculation. 1997;4:369–380. doi: 10.3109/10739689709146801. [DOI] [PubMed] [Google Scholar]
- 12.King J, Deboisblanc BP, Mason CM, et al. Effect of granulocyte colony-stimulating factor on acute lung injury in the rat. Am J Respir Crit Care Med. 1995;151:302–309. doi: 10.1164/ajrccm.151.2.7531097. [DOI] [PubMed] [Google Scholar]
- 13.Freeman BD, Correa R, Karzai W, et al. Controlled trials of rG-CSF and CD11b-directed MAb during hyperoxia and E. coli pneumonia in rats. J Appl Physiol. 1996;80:2066–2076. doi: 10.1152/jappl.1996.80.6.2066. [DOI] [PubMed] [Google Scholar]
- 14.Karzai W, von Specht BU, Parent C, et al. G-CSF during Escherichia coli versus Staphylococcus aureus pneumonia in rats has fundamentally different and opposite effects. Am J Respir Crit Care Med. 1999;159:1377–1382. doi: 10.1164/ajrccm.159.5.9806082. [DOI] [PubMed] [Google Scholar]
- 15.Azoulay E, Attalah H, Yang K, et al. Exacerbation with granulocyte colony-stimulating factor of prior acute lung injury during neutropenia recovery in rats. Crit Care Med. 2003;31:157–165. doi: 10.1097/00003246-200301000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi Y, Kobayashi Y, Chikayama S, et al. Effect of granulocyte/colony-stimulating factor on the onset of the adult respiratory distress syndrome. Acta Haematol. 1999;101:124–129. doi: 10.1159/000040937. [DOI] [PubMed] [Google Scholar]
- 17.Takatsuka H, Takemoto Y, Mori A, et al. Common features in the onset of ARDS after administration of granulocyte colony-stimulating factor. Chest. 2002;121:1716–1720. doi: 10.1378/chest.121.5.1716. [DOI] [PubMed] [Google Scholar]
- 18.Karlin L, Darmon M, Thiery G, et al. Respiratory status deterioration during G-CSF-induced neutropenia recovery. Bone Marrow Transplant. 2005;36:245–250. doi: 10.1038/sj.bmt.1705037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieschke GJ, Grail D, Hodgson G, et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–1746. [PubMed] [Google Scholar]
- 20.Futenma M, Kawakami K, Saito A. Production of tumor necrosis factor-alpha in granulocytopenic mice with pulmonary candidiasis and its modification with granulocyte colony-stimulating factor. Microbiol Immunol. 1995;39:411–417. doi: 10.1111/j.1348-0421.1995.tb02221.x. [DOI] [PubMed] [Google Scholar]
- 21.Shirai R, Kadota J, Tomono K, et al. Protective effect of granulocyte colony-stimulating factor (G-CSF) in a granulocytopenic mouse model of Pseudomonas aeruginosa lung infection through enhanced phagocytosis and killing by alveolar macrophages through priming tumour necrosis factor-alpha (TNF-alpha) production. Clin Exp Immunol. 1997;109:73–79. doi: 10.1046/j.1365-2249.1997.4211317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niederman MS. Granulocyte colony-stimulating factor for severe pneumonia: what do we do when the best laid plans for men (and mice and rats...) fail? Crit Care Med. 2003;31:635–637. doi: 10.1097/01.CCM.0000048630.38193.72. [DOI] [PubMed] [Google Scholar]
- 23.Wiedermann FJ, Mayr AJ, Kaneider NC, et al. Alveolar granulocyte colony-stimulating factor and alpha-chemokines in relation to serum levels, pulmonary neutrophilia, and severity of lung injury in ARDS. Chest. 2004;125:212–219. doi: 10.1378/chest.125.1.212. [DOI] [PubMed] [Google Scholar]
- 24.Matute-Bello G, Liles WC, Radella F, 2nd, et al. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Crit Care Med. 2000;28:1–7. doi: 10.1097/00003246-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78:2791–2808. [PubMed] [Google Scholar]
- 26.Hierholzer C, Kelly E, Tsukada K, et al. Hemorrhagic shock induces G-CSF expression in bronchial epithelium. Am J Physiol. 1997;273:L1058–L1064. doi: 10.1152/ajplung.1997.273.5.L1058. [DOI] [PubMed] [Google Scholar]
- 27.Koyama S, Sato E, Masubuchi T, et al. Alveolar type II-like cells release G-CSF as neutrophil chemotactic activity. Am J Physiol. 1998;275:L687–L693. doi: 10.1152/ajplung.1998.275.4.L687. [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal A, Baker CS, Evans TW, et al. G-CSF and IL-8 but not GM-CSF correlate with severity of pulmonary neutrophilia in acute respiratory distress syndrome. Eur Respir J. 2000;15:895–901. doi: 10.1034/j.1399-3003.2000.15e14.x. [DOI] [PubMed] [Google Scholar]
- 29.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;31:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230–232. [DOI] [PubMed] [Google Scholar]
- 30.ARDS Clinical Trials Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 31.ARDS Clinical Trials Network. Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2000;283:1995–2002. doi: 10.1001/jama.283.15.1995. [DOI] [PubMed] [Google Scholar]
- 32.ARDS Clinical Trials Network. Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30:1–6. doi: 10.1097/00003246-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Eisner MD, Thompson T, Hudson LD, et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:231–236. doi: 10.1164/ajrccm.164.2.2011093. [DOI] [PubMed] [Google Scholar]
- 34.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 35.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30:1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Bernard G. The Brussels Score. Sepsis. 1997;1:43–44. [Google Scholar]
- 37.Ware LB, Matthay MA, Parsons PE, et al. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007;35:1821–1828. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenland S. Basic problems in interaction assessment. Environ Health Perspect. 1993;101 Suppl 4:59–66. doi: 10.1289/ehp.93101s459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watari K, Asano S, Shirafuji N, et al. Serum granulocyte colony-stimulating factor levels in healthy volunteers and patients with various disorders as estimated by enzyme immunoassay. Blood. 1989;73:117–122. [PubMed] [Google Scholar]
- 40.Kawakami M, Tsutsumi H, Kumakawa T, et al. Levels of serum granulocyte colony-stimulating factor in patients with infections. Blood. 1993;76:1962–1964. [PubMed] [Google Scholar]
- 41.Hack CE, Hart M, van Schijndel RJ, et al. Interleukin-8 in sepsis: relation to shock and inflammatory mediators. Infect Immun. 1992;60:2835–2842. doi: 10.1128/iai.60.7.2835-2842.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 43.Pinsky MR, Vincent JL, Deviere J, et al. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993;103:565–575. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 44.Meduri GU, Headley S, Kohler G, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 45.Bozza FA, Salluh JI, Japiassu AM, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11:R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen YM, Whang-Peng J, Chern CH, et al. The prognostic value of serum cytokine levels in patients with acute infections. Chinese Med J (Taipei) 1995;56:75–79. [PubMed] [Google Scholar]
- 47.Presneill JJ, Waring PM, Layton JE, et al. Plasma granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor levels in critical illness including sepsis and septic shock: relation to disease severity, multiple organ dysfunction, and mortality. Crit Care Med. 2000;28:2344–2354. doi: 10.1097/00003246-200007000-00028. [DOI] [PubMed] [Google Scholar]
- 48.Wunderink R, Leeper K, Jr, Schein R, et al. Filgrastim in patients with pneumonia and severe sepsis or septic shock. Chest. 2001;119:523–529. doi: 10.1378/chest.119.2.523. [DOI] [PubMed] [Google Scholar]
- 49.Root RK, Lodato RF, Patrick W, et al. Multicenter, double-blind, placebo-controlled study of the use of filgrastim in patients hospitalized with pneumonia and severe sepsis. Crit Care Med. 2003;31:367–373. doi: 10.1097/01.CCM.0000048629.32625.5D. [DOI] [PubMed] [Google Scholar]
- 50.Pettila V, Takkunen O, Varpula T, et al. Safety of granulocyte colony-stimulating factor (filgrastim) in intubated patients in the intensive care unit: interim analysis of a prospective, placebo-controlled, double-blind study. Crit Care Med. 2000;28:3620–3625. doi: 10.1097/00003246-200011000-00011. [DOI] [PubMed] [Google Scholar]
- 51.Nelson S, Summer W, Bagby G, et al. Granulocyte colony-stimulating factor enhances pulmonary host defenses in normal and ethanol-treated rats. J Infect Dis. 1991;164:901–906. doi: 10.1093/infdis/164.5.901. [DOI] [PubMed] [Google Scholar]
- 52.Wurfel MM, Park WY, Radella F, et al. Identification of high and low responders to lipopolysaccharide in normal subjects: an unbiased approach to identify modulators of innate immunity. J Immunol. 2005;175:2570–2578. doi: 10.4049/jimmunol.175.4.2570. [DOI] [PubMed] [Google Scholar]
- 53.Bagby GJ, Zhang P, Stoltz DA, et al. Suppression of the granulocyte colony-stimulating factor response to Escherichia coli challenge by alcohol intoxication. Alcohol Clin Exp Res. 1998;22:1740–1745. [PubMed] [Google Scholar]
- 54.Perlino CA, Rimland D. Alcoholism, leukopenia, and pneumococcal sepsis. Am Rev Respir Dis. 1985;132:757–760. doi: 10.1164/arrd.1985.132.4.757. [DOI] [PubMed] [Google Scholar]
- 55.Parsons PE, Matthay MA, Ware LB, et al. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L426–L431. doi: 10.1152/ajplung.00302.2004. [DOI] [PubMed] [Google Scholar]
- 56.Ware LB. Prognostic determinants of acute respiratory distress syndrome in adults: impact on clinical trial design. Crit Care Med. 2005;33:S217–S222. doi: 10.1097/01.ccm.0000155788.39101.7e. [DOI] [PubMed] [Google Scholar]