Abstract
Background
Bleeding is the most frequent complication of antithrombotic therapy for venous thromboembolism (VTE). However, little attention has been paid to the impact of bleeding after VTE in the community setting. The purpose of this investigation was to describe the incidence rate of bleeding after VTE, to characterize patients most at risk for bleeding, and to assess the impact of bleeding on rates of recurrent VTE and all-cause mortality.
Methods
The medical records of residents of the Worcester (MA) metropolitan area diagnosed with ICD-9 codes consistent with potential VTE during 1999, 2001, and 2003 were individually validated and reviewed by trained data abstracters. Clinical characteristics, acute treatment, and outcomes (including VTE recurrence rates, bleeding rates, and mortality) over follow-up (up to 3 years maximum) were evaluated.
Results
Bleeding occurred in 228 (12%) of 1,897 patients with VTE during our follow-up. Of these, 115 (58.8%) had evidence of early bleeding occurring within 30 days of VTE diagnosis. Patient characteristics associated with bleeding included impaired renal function and recent trauma. Other than a history of prior VTE, the occurrence of bleeding was the strongest predictor of recurrent VTE (HR 2.18; 95% CI 1.54-3.09) and was also a predictor of total mortality (HR 1.97 1.57-2.47).
Conclusions
The occurrence of bleeding following VTE is associated with an increased risk of recurrent VTE and mortality. Future study of antithrombotic strategies for VTE should be informed by this finding. Advances that result in decreased bleeding rates may paradoxically decrease the risk of VTE recurrence.
Keywords: venous thromboembolism, bleeding, hemorrhage, epidemiology
Introduction
Anticoagulant therapy has been established as the mainstay of treatment of venous thromboembolism (VTE) for the past several decades. Throughout this time, bleeding has been the most frequent as well as feared complication of this therapy. In order to be approved, new anticoagulants must demonstrate similar or improved efficacy for the prevention of recurrent VTE without any substantial increase in bleeding rates. Indeed, the rates of bleeding in recent clinical trials of VTE management have continued to improve and approximate 1% over a 3 to 6 month period of follow-up (1-3).
However, relatively little attention has been paid to the prevalence of bleeding after VTE in the broader community setting, the identification of patients most at risk for bleeding, or the impact of bleeding on subsequent long-term outcomes. This is in contrast to ongoing research in patients with acute myocardial infarction, which suggests that the occurrence of bleeding is associated with both an increase in recurrent ischemic events as well as in all-cause mortality (4-6).
The objectives of the Worcester Venous Thromboembolism study are to provide contemporary population-based data about the clinical epidemiology of VTE as well as its management and associated outcomes among residents of a large New England metropolitan area (7, 8). The purpose of the present investigation was to describe the incidence rates of bleeding episodes after a diagnosis of VTE, to characterize patients most at risk for bleeding, and to assess the impact of this complication on recurrent event rates of VTE and total mortality.
Methods
Patients
Computerized printouts of all metropolitan Worcester residents with healthcare system encounters in which any of 34 ICD-9 diagnosis codes possibly consistent with the occurrence of VTE had been listed in 1999, 2001, and 2003 were obtained from each of the 12 hospitals serving residents of the Worcester Standard Metropolitan Statistical Area (SMSA) (7, 8). These data queries were not limited to discharge diagnoses, but also encompassed all outpatient, emergency department, radiology, and laboratory encounters. For the first study cohort, namely, in- and out-patients diagnosed with possible VTE in 1999, the logs and/or computerized billing lists of patients evaluated in area ultrasound departments for potential DVT were also screened. This latter search was performed for purposes of identifying potential cases of VTE in greater Worcester residents that may have been missed due to coding errors and to identify patients referred directly from outside physicians' offices, rehabilitation facilities, and nursing homes for testing who then returned directly to these outside settings for treatment. Since this further screening process identified only a few additional cases of VTE, and in only 1 of 11 study hospitals, this search was not performed in the other 10 hospitals in our subsequent patient cohorts of 2001 and 2003. The Research Ethics boards of all participating greater Worcester hospitals approved this study.
Procedures
The medical records of all identified patients meeting the geographic inclusion criteria (residents of the Worcester SMSA, 2000 census = 477,800) were reviewed (7, 8). Trained physician and nurse abstractors using pre-specified criteria performed the validation of each potential case of VTE and characterization of each case of VTE as being definite, probable, possible, or absent. These criteria were based on a modification of a classification schema proposed by Silverstein et al (9). The study project coordinator (CE) also validated each case and its classification. If the classification of VTE was not immediately apparent using the diagnostic criteria specified, the study's principal investigator (FS) reviewed the medical record. Potential cases of recurrent VTE were classified using similar criteria as that employed for incident cases; the development of a definite or probable recurrence of VTE required the new occurrence of thrombosis in a previously uninvolved venous or pulmonary vessel by ultrasound or radiologic imaging.
The medical records of each patient's current, as well as previous, hospitalization(s) and/or outpatient visit(s) were reviewed to identify whether the index VTE event represented an incident (initial) or a recurrent case. Ambulatory patients presenting to greater Worcester hospitals with signs and symptoms consistent with VTE, or diagnosed with VTE within 24 hours of hospital presentation, were considered to have developed VTE in the outpatient setting. Information was collected about patients' demographic characteristics, medical history, clinical characteristics, diagnostic test results, and hospital management practices through review of the medical record. Only medical history variables documented in patients' medical records by a physician were abstracted. For variables in which several medical record entries were possible (e.g., cardiac procedures), data abstractors were instructed to record only entries matching those on a pre-specified list. Bleeding was defined as any episode of bleeding requiring transfusion of packed red blood cells or that required surgery or resulted in subsequent hospitalization, cerebrovascular accident, myocardial infarction, or death. The surgery variable included major operations where general or epidural anesthesia lasted 30 minutes or longer. Medical history variables defined as “recent” were those occurring or active in the 3 months prior to the diagnosis of VTE. Subjects were considered to have active cancer if they had malignancy (other than basal cell skin cancer) that was currently being treated or palliated. “Provoked” VTE was defined as VTE occurring within 3 months of a hospitalization, surgical procedure, pregnancy, trauma, or fracture. “Unprovoked” VTE was defined as VTE occurring in the absence of malignancy or any of the above “provoked” variables.
The development of a first recurrence of VTE or a bleeding episode was determined through the review of subsequent medical records at the same hospital site as the index event as well as through the screening of medical records from the other participating hospital sites. Information about deaths from all causes was obtained through hospital record reviews and review of death certificates at the Massachusetts Division of Vital Statistics. Follow-up data were available for a maximum of 3 years.
Statistical Analysis
Differences in the distribution of demographic and clinical characteristics as well as treatment practices in patients further stratified according to bleeding status were examined using chi-square tests of statistical significance for categorical variables and t-tests for continuous variables.
Cox proportional hazards regression analyses were used to examine the association between patient demographic, clinical, and treatment characteristics and occurrence of bleeding. All variables listed in Tables 1 were considered as potential covariates in this regression model. Candidate variables possibly associated with bleeding (p<0.25 after univariate analysis) were included in the model.
Table 1.
Demographic and Clinical Characteristics of Patients with Venous Thromboembolism According to Bleeding Status
| Bleeding (+) (n= 228) |
Bleeding (-) (n=1,669) |
P-value | |
|---|---|---|---|
| Demographic Characteristics | |||
| Age, mean (years) | 70.4 | 63.6 | <0.001 |
| Female (%) | 61.8 | 54.2 | 0.03 |
| Hospitalization history (%) | |||
| Admitted with non-VTE diagnosis | 35.1 | 27.1 | 0.04 |
| Other hospitalization in prior 3 months | 42.1 | 38.5 | 0.57 |
| Recent (< 3 mo) medical conditions (%) | |||
| Surgery | 28.6 | 27.7 | 0.93 |
| Malignancy | 36.8 | 28.9 | 0.01 |
| Severe infection | 31.6 | 25.2 | 0.04 |
| Central venous catheter | 22.4 | 17.9 | 0.11 |
| ICU discharge | 21.5 | 15.8 | 0.03 |
| Intubation | 18.0 | 15.5 | 0.33 |
| Fracture | 15.4 | 10.3 | 0.03 |
| Chemotherapy | 9.7 | 8.0 | 0.41 |
| Heart failure | 8.8 | 8.0 | 0.68 |
| Trauma | 20.2 | 13.8 | 0.01 |
| Cardiac procedures | 4.8 | 4.3 | 0.70 |
| Other medical history (%) | |||
| Prior VTE | 18.4 | 17.3 | 0.67 |
| Prior IVC filter | 9.7 | 7.8 | 0.34 |
| Prior CVA | 12.7 | 12.0 | 0.75 |
| Diabetes | 24.4 | 18.5 | 0.02 |
| Hypertension | 63.2 | 51.4 | <0.001 |
| Ischemic heart disease | 28.5 | 20.4 | 0.006 |
| Inflammatory bowel disease | 4.0 | 1.9 | 0.07 |
| Liver disease | 1.8 | 1.0 | 0.31 |
| Chronic renal insufficiency | 7.9 | 6.0 | 0.47 |
| Dialysis dependent | 4.0 | 2.0 | 0.09 |
| Laboratory data | |||
| Cr (mg/dl) | 1.43 (SD 1.21) |
1.21 (SD 1.10) |
0.008 |
| Hematocrit (%) | 34.2 (SD 5.3) |
36.1 (SD 5.8) |
<0.001 |
| Platelets | 245.7 (SD 107.5) |
250.2 (SD 110.4) |
0.58 |
| Type of VTE (%) | |||
| Isolated DVT | 66.2 | 72.0 | 0.08 |
| PE with or without DVT | 33.9 | 28.0 | |
| Unprovoked | 18.4 | 23.9 | 0.03 |
| Malignancy-related | 36.8 | 28.9 | 0.02 |
| Acute parenteral treatment (%) | |||
| UFH | 42.6 | 37.5 | 0.002 |
| LMWH | 29.3 | 30.7 | |
| Both UFH and LMWH | 18.5 | 13.5 | |
| Other parenteral therapy | 4.8 | 1.6 | 0.004 |
| Acute warfarin utilization | 71.1 | 73.0 | 0.54 |
Recent - occurring or active in the 3 months prior to index DVT or PE
Severe infection = infections were limited to those involving the blood, bone and joint, central nervous system, cardiovascular system, gastrointestinal system, respiratory system (pneumonia only), surgical sites, or the skin.
Unprovoked VTE = Any VTE in which there is NO recent fracture, recent trauma, recent surgery, recent hospitalization, current hospitalization, central venous catheter, pregnancy, hormonal therapy, or malignancy
Provoked VTE = Any VTE occurring within 3 months of fracture, trauma, surgery, hospitalization, central venous catheter placement, pregnancy, hormonal therapy. Patients with active malignancy at the time of VTE were not included in this category.
ICU = intensive care unit
VTE = venous thromboembolism
IVC = inferior vena cava
CVA = cerebrovascular accident
Cr = creatinine
DVT = deep vein thrombosis
UFH = unfractionated heparin
LMWH = low-molecular weight heparins (enoxaparin and dalteparin)
Cox proportional hazards regression analyses were also constructed to estimate hazards ratio for the occurrence of recurrent VTE or total mortality order adjusted for potential confounding covariates. For each outcome, we considered bleeding status as a discrete time varying covariate in the analyses. For analysis of all-cause mortality, we also considered recurrent VTE as a time varying covariate – similar to the manner in which we treated bleeding. Therefore, analyses of mortality had two discrete time varying covariates. Initial models included all variables listed in table 1. The final models were fit following combined forward and backwards stepwise regression.
We tested the assumption of proportional hazards using scaled Schoenfeld residuals and for those covariates that did not meet the assumption we graphically compared Kaplan-Meier estimates to Cox regression estimates to understand the varying proportional hazards. In both unadjusted and adjusted models proportional hazards assumptions were appropriate for the primary predictors of bleeding. In models for recurrent VTE, history of cerebrovascular disease, prior VTE, and malignancy did not meet proportional hazards assumptions. In models of mortality, prior trauma and history of diabetes did not meet proportional hazards assumption. Models adjusting for these factors adjust for average hazard even if non-time varying proportional hazards are used and are likely to provide appropriate adjustment for any major confounding. However we carried out additional models that allowed for time varying hazards ratios. Using two hazards ratios (< 400 and > 400 days from incident VTE diagnosis) provided sufficient estimates of the hazards ratio over time and proportional hazards assumptions were met. We illustrate survival curves (figures 1 and 2) based on the adjusted Cox regression models. Analyses were carried out in Stata version 10 (Survival Analysis and Epidemiologic Tables).
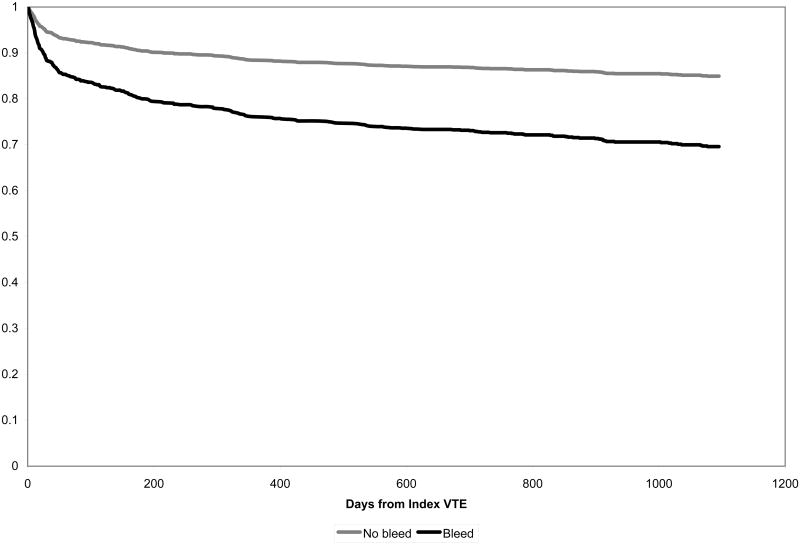
Figure 1.
Probability of remaining free of recurrent VTE according to bleeding status
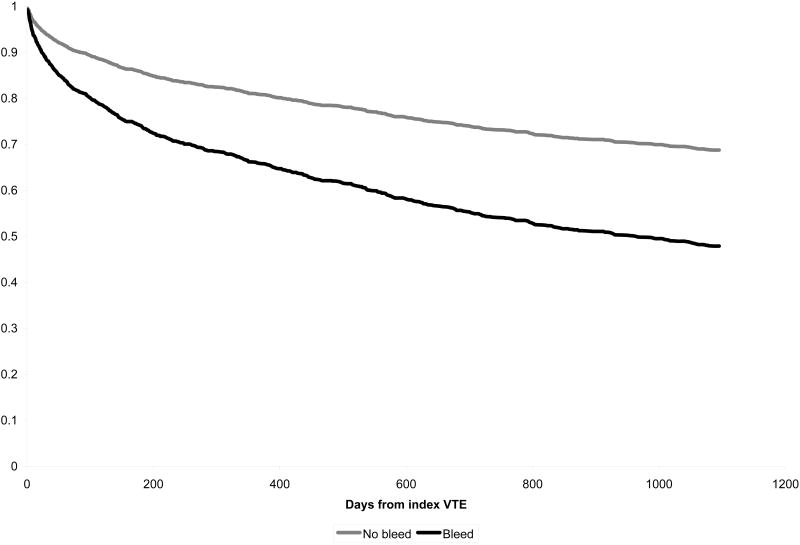
Figure 2.
Probability of survival according to bleeding status
Role of the funding source
The sponsors had no role in the design and conduct of this study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. The corresponding author had full access to the data and had final responsibility for the decision to submit for publication.
Results
Of 1,897 patients with validated VTE, 228 patients (12.0%) experienced a bleeding episode at some point over the course of our extended follow-up. Of these, 115 (58.8%) had early bleeding occurring within 30 days of the diagnosis of VTE; bleeding occurred after 30 days in the remaining 113 patients.
Demographic, clinical, and treatment characteristics of the study sample
The demographic and clinical characteristics of greater Worcester residents with independently confirmed VTE further stratified according to bleeding status are shown in Table 1. Patients who experienced bleeding after VTE were older, more likely to be female, and were more likely to have been hospitalized with a non-VTE diagnosis prior to developing VTE (as opposed to presenting from an outside setting with VTE). They were more likely to have a malignancy-related or other form of provoked VTE than patients were without bleeding. They were also more likely to have had diabetes, hypertension, or ischemic heart disease diagnosed in the past or a recent history of surgery, trauma, fracture, severe infection, or have been discharged from an intensive care unit. They were more likely to present with an elevated serum creatinine or a decreased hematocrit. They were more likely to be treated acutely with unfractionated heparin. Among patients with an estimated glomerular filtration rate (GFR) < 30 ml/min, 24% of those on LMWH (with or without UFH) and 21% of those receiving UFH alone suffered major bleeding. Among patients with an GFR 30-59 ml/min, 14 % of those on LMWH (with or without UFH) and 20% of those on unfractionated heparin experienced a major bleeding episode. Inferior vena cava filters were placed in 27 patients after major bleeding.
After Cox regression analysis, significant predictors of bleeding were recent trauma (HR 1.83; 1.24, 2.70), an estimated GFR < 30 ml/min (HR 1.85; 1.15, 3.00), and receipt of both unfractionated and low-molecular weight heparin (1.95; 1.12, 3.40) (See Table 2).
Table 2.
Factors Associated with Bleeding in Patients with Venous Thromboembolism
| Crude HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|
| Demographic Characteristics | ||
| Age (years) | ||
| <65 | 1.00 | 1.00 |
| 65-74 | 1.88(1.30,2.71) | 1.30 (0.86, 1.95) |
| 75-84 | 1.97(1.40,2.77) | 1.28 (0.87, 1.88) |
| ≥85 | 2.37(1.59,3.54) | 1.35 (0.85, 2.14) |
| Female | 1.35(1.03,1.78) | 1.26 (0.94, 1.70) |
| Hospitalization history | ||
| Admitted with non-VTE diagnosis | 1.67(1.27,2.21) | 1.12 (0.80, 1.57) |
| Recent (< 3 mo) medical conditions (%) | ||
| Surgery | 1.11(0.83,1.48) | 1.02 (0.73, 1.42) |
| Malignancy | 1.50(1.13,1.98) | 1.31 (0.94, 1.82) |
| Severe infection | 1.44(1.08,1.92) | 0.97 (0.69, 1.35) |
| Central venous catheter | 1.37(0.99,1.89) | 0.88 (0.57, 1.37) |
| ICU discharge | 1.67(1.22,2.31) | 1.43 (0.93, 2.20) |
| Fracture | 1.68(1.17,2.41) | 1.18 (0.77, 1.81) |
| Trauma | 1.60(1.16,2.22) | 1.83 (1.24, 2.70) |
| Other medical history (%) | ||
| Diabetes | 1.39(1.02,1.89) | 1.19 (0.85, 1.65) |
| Hypertension | 1.63(1.24,2.15) | 1.13 (0.82, 1.56) |
| Ischemic heart disease | 1.57(1.17,2.11) | 1.22 (0.88, 1.70) |
| Inflammatory bowel disease | 2.07(1.06,4.03) | 1.56 (0.75, 3.24) |
| Chronic renal insufficiency | ||
| GFR < 30 ml/min | 3.05(2.06,4.52) | 1.85 (1.15, 3.00) |
| GFR 30-60 ml/min | 1.77(1.31,2.39) | 1.23 (0.88, 1.73) |
| Dialysis dependent | 1.93(0.99,3.77) | 1.13 (0.50, 2.55) |
| Laboratory data | ||
| Hematocrit (per 1 % change) | 0.94(0.92,0.96) | 0.96 (0.93, 0.99) |
| Type of VTE (%) | ||
| PE with or without DVT | 1.33(1.01,1.76) | 1.20 (0.88,1.63) |
| Unprovoked | 0.68(0.48,0.95) | 1.53 (0.98, 2.58) |
| Acute parenteral treatment (%)* | ||
| UFH | 1.24(0.95,1.62) | 1.57 (0.95, 2.58) |
| LMWH | 0.87(0.65,1.16) | 1.59 (0.93, 2.72) |
| Both UFH and LMWH | 1.40 (1.00,1.96) | 1.95 (1.12, 3.40) |
Referent category: No parenteral treatment
VTE = venous thromboembolism
ICU = intensive care unit
GFR = glomerular filtration rate
PE = pulmonary embolism
DVT = deep vein thrombosis
UFH = unfractionated heparin
LMWH = low-molecular weight heparins (enoxaparin and dalteparin)
Outcomes
VTE recurrence
The occurrence rates of clinically recognized recurrent VTE were significantly increased in patients with bleeding compared to patients without bleeding at all time points examined. Almost half (43%) of recurrent VTE events after an episode of bleeding occurred within 30 days of hemorrhage. Survival free of recurrent VTE based on adjusted Cox regression is displayed in figure 1. After carrying out a Cox multivariable regression analysis controlling for a variety of long-term prognostic factors, occurrence of bleeding (HR 2.18, 95% CI 1.54, 3.09) was significantly associated with recurrence of VTE during our follow-up period (Table 3). Other variables associated with recurrent VTE include prior history of VTE, malignancy, and cerebrovascular disease. Increasing age was negatively associated with VTE recurrence after controlling for potentially confounding variables.
Table 3.
Factors Associated with Recurrent VTE in Patients with Venous Thromboembolism
| Recurrent VTE Hazard ratio (95% CI) (n=325) |
|
|---|---|
| Demographic Characteristics | |
| Age | |
| <65 | 1.0 |
| 65-74 | 0.79 (0.57, 1.10) |
| 75-84 | 0.68 (0.49, 0.93) |
| ≥85 | 0.57 (0.37, 0.88) |
| Recent (< 3 mo) medical conditions (%) | |
| Malignancy* | 1.38 (1.01-1.89) |
| Malignancy** | 0.76 (0.40, 1.42) |
| Other medical history (%) | |
| Prior VTE* | 1.06 (0.75, 1.50) |
| Prior VTE** | 2.50 (1.48, 4.23) |
| Cerebrovascular disease* | 1.70 (1.17, 2.48) |
| Cerebrovascular disease** | 0.95 (0.41, 2.23) |
| Complications | |
| Bleeding | 2.18 (1.54, 3.09) |
Unless otherwise specified, hazards ratios are for recurrent VTE occurring anytime following incident event. As malignancy, prior VTE, and cerebrovascular disease did not meet proportional hazards assumptions, hazard ratios are provided for recurrent VTE occurring < or > 400 days following incident VTE.
HR associated with development of VTE < 400 days from incident event
Hazards ration associated with development of VTE > 400 days from incident event.
All-cause Mortality
Mortality rates were significantly higher in patients who experienced a bleeding episode compared to those who did not develop bleeding at selected time points (Figure 2). Approximately one third (36%) of all deaths after a bleed occurred within 30 days of the bleeding episode. Survival free of recurrent VTE based on adjusted Cox regression is displayed in figure 2.
After Cox multivariable regression analysis, occurrence of bleeding (HR 1.97, 95% CI 1.57, 2.47) and occurrence of recurrent VTE (HR 2.37, 1.57, 2.47) were significant predictors of mortality. Other predictors of mortality included advancing age, female gender, admission to hospital with a non-VTE diagnosis or other hospitalization in the preceding 3 months, recent severe infection, recent intubation, recent chemotherapy, and diabetes (Table 3). Recent surgery was negatively associated with increased mortality after VTE.
Discussion
In this population-based study of residents of a large New England metropolitan area, bleeding occurred in approximately 1 out of every 8 patients with VTE during our follow-up period. Approximately one-half of all bleeding episodes occurred within 1 month of the diagnosis of VTE. The bleeding rates observed in the present study are significantly higher than those observed in acute and chronic clinical trials of VTE treatment conducted during periods similar to those of our study enrollment (1-3). For example, in the Matisse trial, which randomized patients with acute VTE to either enoxaparin or fondaparinux followed by warfarin therapy, bleeding occurred in only 1.2% of patients in the standard treatment arm over a 3-month follow-up period (2). Even in the ELATE trial, which compared long-term low-intensity to conventional intensity warfarin for the secondary prophylaxis of VTE, bleeding was observed in only 2.5% of subjects over an approximate 2.5-year follow-up (3). The bleeding rates observed in our study are also higher than those reported by observational studies evaluating the efficacy of specialized anticoagulation services (approximately 1.5% major bleeding/year) (11, 12). Bleeding rates in our study more closely mirror those reported by other observational studies of patients receiving “usual care” long-term anticoagulation management (2.8% to 8.1%) (13-16).
The high rate of bleeding observed in our study likely reflects the advanced age, impaired renal function, concomitant malignancy, or history of recent trauma present in many patients diagnosed with VTE in the community (relative to patients enrolled in clinical trials). More than half of greater Worcester residents were 65 years of age or older and approximately one third had a concomitant malignancy. Numerous studies have identified these patient groups to be at increased risk for bleeding with unfractionated heparin or warfarin (17-20). Although the low-molecular-weight heparins offer a number of advantages over these agents, are generally associated with similar or lower bleeding rates (21, 22), and have been shown to be effective in elderly patients with malignancy (23), failure to properly adjust dosage in patients with impaired renal function can be dangerous (24). It must also be acknowledged that the higher bleeding rates observed in our study may reflect differences between ours and other studies' definitions of bleeding. However, in most studies, the need for blood transfusion is generally considered indicative of moderate to major bleeding.
Bleeding and Risk of Recurrent VTE and Mortality
Interestingly, the occurrence of bleeding after a confirmed diagnosis of VTE was a strong predictor of recurrent episodes of VTE as well as of subsequent mortality. As far as we are aware the former association has not been previously reported in the literature. One obvious explanation for these findings is that the occurrence of bleeding often leads to an interruption in the use of anticoagulation therapy, which may predispose to recurrent VTE. Indeed, the majority of bleeding episodes occurred within 30 days of the initial diagnosis of VTE. Moreover, among patients suffering recurrent VTE subsequent to bleeding, almost half of the recurrences occurred within 30 days of the bleeding event.
Other postulated mechanisms by which an episode of bleeding may lead to an increased risk of recurrent VTE might include the concomitant activation of coagulation, adverse effects of the resulting blood transfusions (25, 26), or perhaps even secondary to interventions performed for the management of the bleeding complications (e.g., IVC filter insertion, exploratory surgery). While we could not fully explore these issues using our database, we believe that a better understanding of the pathophysiologic relationship between bleeding and subsequent VTE is critical and deserves further study.
Our data suggest that prevention of early bleeding complications associated with VTE treatment may serve to decrease the risk of recurrent VTE as well as subsequent mortality. This concept mirrors that currently being studied in patients with acute coronary syndromes. For example, in a large randomized clinical trial comparing fondaparinux to enoxaparin in more than 20,000 patients with an acute coronary syndrome, patients randomized to fondaparinux showed a decreased likelihood for experiencing the combined endpoint of recurrent ischemia, myocardial infarction, or death and a significant reduction in death at 1 and 6 months follow-up (27). This occurred in the setting of a 50% reduction in major bleeding at 9 days in the fondaparinux arm suggesting that the differences in bleeding accounted for the differences observed in total mortality.
Based on these and other findings, further study of dosing strategies of currently utilized therapies and/or study of novel therapies for the acute treatment of VTE remain critical. These studies should include or even focus on the elderly, patients with significant renal disease, and patients with malignancy, who are at highest risk for bleeding. While further gains in the efficacy of antithrombotic therapy may be difficult, advances that result in decreased bleeding may paradoxically decrease the risk of recurrent thrombotic events and/or mortality.
Study limitations
The most significant limitation of our study is that we do not have information on patients' long-term treatment, and rates of adherence, with anticoagulation. The vast majority of patients were treated acutely with anticoagulation and our experience is that most will continue anticoagulation for at least 3 to 6 months barring a bleeding episode. Indeed, the occurrence of unprovoked VTE was not associated with recurrent VTE in our regression models - this is likely due to the use of extended duration anticoagulation in these patients. As such, we cannot comment on the timing, dosage, or type of therapy and its relationship to a bleeding event or subsequent outcomes. In addition, we cannot fully characterize bleeding episodes with respect to severity, as all bleeding events requiring transfusion were included. Since the thresholds for transfusion may vary between patients, physicians, and even hospitals this is a potential source of bias in interpreting our study findings.
Although we conducted a broad screening for all possible cases of VTE occurring in the greater Worcester population, we cannot claim complete case ascertainment of index VTE events, episodes of VTE recurrence, or episodes of bleeding. To the extent that collection of these data may have varied according to patient's bleeding status, some bias may have been introduced into our study. Failure to include patients seeking care for events after their index VTE at hospitals outside of the Worcester metropolitan area would result in an underestimation of the magnitude of recurrent VTE and/or episodes of major bleeding. Finally, due to the extremely low autopsy rates during the period under study, we are unable to estimate the rates of fatal episodes of VTE.
Conclusions
Approximately 12% of patients diagnosed with VTE in this central New England community suffered bleeding over our follow-up period - more than half of these bleeding episodes occurred within 30 days of the diagnosis of VTE. Patients with recent trauma or with severe renal dysfunction were at highest risk for bleeding. The occurrence of bleeding was the second most important predictor of a recurrent episode of VTE and a strong predictor of subsequent mortality. Efforts aimed at decreasing the rates of bleeding following the diagnosis and treatment of VTE in the community remain critical to improving the long-term prognosis of patients with this increasingly prevalent clinical syndrome.
Table 4.
Factors Associated with Mortality in Patients with Venous Thromboembolism
| Mortality Hazard ratio (95% CI) (n=639) |
|
|---|---|
| Demographic Characteristics | |
| Age | |
| <65 | 1.0 |
| 65-74 | 2.10 (1.66, 2.65) |
| 75-84 | 2.87 (2.32, 3.55) |
| ≥85 | 4.13 (3.23, 5.27) |
| Female | 1.27 (1.08, 1.49) |
| Hospitalization history | |
| Admitted with non-VTE diagnosis | 1.51 (1.26, 1.80) |
| Other hospitalization in prior 3 months | 1.40 (1.18, 1.67) |
| Recent (< 3 mo) medical conditions (%) | |
| Surgery | 0.70 (0.57, 0.86) |
| Severe infection | 1.33 (1.11, 1.59) |
| Intubation | 1.29 (1.01, 1.64) |
| Chemotherapy | 3.04 (2.44, 3.79) |
| Heart failure | 1.20 (0.94, 1.55) |
| Trauma* | 0.91 (0.69, 1.21) |
| Trauma** | 0.50 (0.32, 0.78) |
| Other medical history (%) | |
| Prior VTE | 0.80 (0.64, 1.02) |
| Diabetes* | 1.14 (0.91, 1.43) |
| Diabetes** | 1.42 (1.05, 1.93) |
| Ischemic heart disease | 0.87 (0.72, 1.05) |
| Complications | |
| Recurrent VTE | 2.37 (1.93, 2.92) |
| Bleeding | 1.97 (1.57, 2.47) |
Unless otherwise specified, hazards ratios are for recurrent VTE occurring anytime following incident event. As malignancy, prior VTE, and cerebrovascular disease did not meet proportional hazards assumptions, hazard ratios are provided for recurrent VTE occurring < or > 400 days following incident VTE.
HR associated with development of VTE < 400 days from incident event
Hazards ration associated with development of VTE > 400 days from incident event.
Acknowledgments
This study was made possible by the cooperation of administrators, physicians, and medical records personnel in 12 central Massachusetts hospitals.
Supported by a grant from the National Heart, Lung, and Blood Institute (R01-HL70283) Drs. Spencer and Crowther hold Career Investigator Awards from the Heart and Stroke Foundation of Canada. Drs. Spencer and Crowther are also supported by Team Grant #154190, Studies in Venous Thromboembolism, Canadian Institutes of Health Research.
References
- 1.Fiessinger JN, Huisman MV, Davidson BL, et al. Ximelagatran vs low-molecular-weight heparin and warfarin for the treatment of deep vein thrombosis. A randomized trial. JAMA. 2005;293:681–89. doi: 10.1001/jama.293.6.681. [DOI] [PubMed] [Google Scholar]
- 2.Buller HR, Davidson BL, Decousus H, et al. Matisse Investigators. Fondaparinox or enoxaparin for the initial treatment of symptomatic deep vein thrombosis. A randomized trial. Ann Intern Med. 2004;140:867–73. doi: 10.7326/0003-4819-140-11-200406010-00007. [DOI] [PubMed] [Google Scholar]
- 3.Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349:631–39. doi: 10.1056/NEJMoa035422. [DOI] [PubMed] [Google Scholar]
- 4.Eikelboom JW, Mehta SR, Anand SS, et al. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–82. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 5.Rao SV, O'Grady K, Pieper KS, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96:1200–06. doi: 10.1016/j.amjcard.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 6.Spencer FA, Moscucci M, Granger CB, et al. GRACE Investigators. Does comorbidity account for the excess mortality in patients with major bleeding in acute myocardial infarction. Circulation. 2007;116:2793–01. doi: 10.1161/CIRCULATIONAHA.107.694273. [DOI] [PubMed] [Google Scholar]
- 7.Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism Study: A population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006;21:722–27. doi: 10.1111/j.1525-1497.2006.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer FA, Gore JM, Lessard D, et al. Outcomes after deep vein thrombosis and pulmonary embolism in the community: The Worcester Venous Thromboembolism Study. Arch Intern Med. 2008;168:425–430. doi: 10.1001/archinternmed.2007.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverstein MD, Heit JA, Mohr DN, et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism. A 25-year population-based study. Arch Intern Med. 1998;158:585–93. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 10.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 81:515–526. [Google Scholar]
- 11.Palaretti G, Manotti C, DAngelo A, et al. Thrombotic events during oral anticoagulant treatement : results of the inception-cohort, prospective, collaborative ISCOAT study : iSCOAT study group (Italian Study on Complications of Oral Anticoagulant Therapy) Thrombo Haemost. 1997;78:1438–1443. [PubMed] [Google Scholar]
- 12.Abdelhafiz AH, Wheeldon NM. Results of an open-label prospective study of anticoagulant therapy for atrial fibrillation in an outpatient anticoagulation clinic. Clin Ther. 2004;26:1470–1478. doi: 10.1016/j.clinthera.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105:91–9. doi: 10.1016/s0002-9343(98)00198-3. [DOI] [PubMed] [Google Scholar]
- 14.Gitter MJ, Jaeger TM, Petterson TM, et al. Bleeding and thromboembolism during anticoagulant therapy: a population based study in Rochester, Minnesota. Mayo Clin Proc. 1995;70:725–733. doi: 10.4065/70.8.725. [DOI] [PubMed] [Google Scholar]
- 15.Steffensen FH, Kristensen K, Ejlersen E, et al. Major haemorrhagic complications during oral anticoagulant therapy in a Danish population-based cohort. J Intern Med. 1997;242:497–503. doi: 10.1111/j.1365-2796.1997.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 16.Willey VJ, Bullano MF, Hauch O, et al. Management patterns and outcomes of patients with venous thromboembolism in the usual community practice setting. Clin Ther. 2004;26:1149–1159. doi: 10.1016/s0149-2918(04)90187-7. [DOI] [PubMed] [Google Scholar]
- 17.Pengo V, Legnani C, Noventa F, et al. ISCOAT Study Group. (Italian Study on Complications of Oral Anticoagulant Therapy) Oral anticoagulant therapy in patients with nonrheumatic atrial fibrillation and risk of bleeding: a multicenter inception cohort study. Thromb Haemost. 2001;85:418–22. [PubMed] [Google Scholar]
- 18.Hylek E, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120:897–02. doi: 10.7326/0003-4819-120-11-199406010-00001. [DOI] [PubMed] [Google Scholar]
- 19.White RH, Beyth RJ, Zhou H, et al. Major bleeding after hospitalization for deep vein thrombosis. Am J Med. 1999;107:414–24. doi: 10.1016/s0002-9343(99)00267-3. [DOI] [PubMed] [Google Scholar]
- 20.Prandoni P, Lensing AWA, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–88. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 21.Iorio A, Guercini F, Pini M. Low-molecular-weight heparin for the long-term treatment of symptomatic venous thromboembolism: meta-analysis of the randomized comparisons with oral anticoagulants. J Thromb Haemost. 2003;1:1906–13. doi: 10.1046/j.1538-7836.2003.00364.x. [DOI] [PubMed] [Google Scholar]
- 22.van Dongen CJJ, van den Belt AGM, Prins MH, et al. Cochrane Peripheral Vascular Diseases Group. Fixed dose subcutaneous low-molecular-weight heparin versus adjusted dose unfractionated heparin for venous thromboembolism (update in Cochrane Database Syst Rev. 2008) Cochrane Database of Systematic Reviews. 2008 1. [Google Scholar]
- 23.Lee AYY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–53. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 24.LaPointe NM, Chen AY, Alexander KP, et al. Enoxaparin dosing and associated risk of in-hospital bleeding and death in patients with non-ST-segment elevation acute coronary syndromes. Arch Intern Med. 2007;167:1539–44. doi: 10.1001/archinte.167.14.1539. [DOI] [PubMed] [Google Scholar]
- 25.Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:555–62. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 26.Sabatine MS, Morrow DA, Guigliano RP, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042–49. doi: 10.1161/01.CIR.0000162477.70955.5F. [DOI] [PubMed] [Google Scholar]
- 27.Yusuf S, Mehta SR, Chrolavicius S, et al. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med. 2006;354:1464–76. doi: 10.1056/NEJMoa055443. [DOI] [PubMed] [Google Scholar]