Summary
Obesity is associated with systemic inflammation, immunological changes, increased risk of respiratory infections and chronic respiratory illness. Maternal obesity in pregnancy increases the risk of pregnancy complications, caesarean sections and adverse birth outcomes, which have in turn been associated with respiratory illness in children. To our knowledge, the possible influence of maternal obesity in pregnancy on respiratory illness in early childhood beyond the newborn period has not been explored. We examined the relationship between a high maternal body mass index (BMI) in pregnancy and lower respiratory tract infections and wheeze up to 18 months of age in the Norwegian Mother and Child study (MoBa), a population based cohort study that will include 100 000 pregnant women, conducted at the Norwegian Institute of Public Health. We analyzed data from the first 33 192 children, born between 1999 and 2005. In crude analyses maternal obesity in pregnancy was related to both respiratory infections and wheeze in the children. In multivariable analyses, only an effect on wheeze remained. The risk of wheeze increased linearly with maternal BMI in pregnancy, and was 3.3 % higher [95 % CI 1.2, 5.3]) for children with mothers who were obese during pregnancy, than for children of mothers with normal BMI. This effect was not mediated through obesity related pregnancy complications, low birthweight, preterm birth or caesarean sections.
Introduction
The intrauterine milieu exerts an important influence on the development of the foetal immune system and the foetal airways.1-3 A compromised foetal environment may impair the developing respiratory system and increase susceptibility to respiratory illness after birth. Complications and conditions in pregnancy, and neonatal characteristics such as preterm birth, mode of delivery, and birthweight have been associated with respiratory infections and wheeze in childhood.4-7 Obesity increases the risk of several obstetric complications, including preeclampsia, gestational diabetes, preterm birth, and caesarean sections.8-12 Obesity has also been associated to increased risk of respiratory infections, airway hyperresponsiveness, wheeze and immunological changes.13-22
The prevalence of respiratory diseases in childhood appears to have increased in industrialized countries 23,24 including Norway.25,26 The prevalence of overweight have increased concurrently with the respiratory diseases,27-34 and possible common aetiological pathways for obesity and inflammatory diseases should be investigated. As more women of child-bearing age become overweight and obese, possible effects of maternal obesity during pregnancy on children's health are important to identify.
Recently, a Danish study found maternal obesity in pregnancy to increase the risk of neonatal mortality, and suggested inflammation or infection related to obesity to be part of the causal pathway.35 Ramsay et al showed that maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways.36 Elevated levels of inflammatory substances in obese mothers during pregnancy could influence the in-utero environment and the foetal development either via inflammatory mediators or through impaired placental function. Studies on how maternal obesity in pregnancy may affect children's health have so far focused on metabolic and vascular consequences.36,37 However, the foetal immune system and respiratory system may also be influenced by maternal obesity during pregnancy.
To our knowledge, there are no published data on the potential role of maternal obesity during pregnancy in childhood respiratory health beyond the neonatal period. We used data on the first 33 192 children included in the Norwegian Mother and Child Study (MoBa) to evaluate the relationship between a high maternal body mass index (BMI) in pregnancy and lower respiratory tract infections and wheeze in children up to 18 months of age. We also explored if effects of a high maternal BMI were mediated through pregnancy complications or birth outcomes.
Methods
Population and study design
Data were collected at the Norwegian Institute of Public Health as part of the Norwegian Mother and Child Cohort Study (MoBa).38 Our subjects were the first 33 192 children in the study for whom questionnaires were obtained up to 18 months of age. MoBa is a pregnancy cohort started in 1999, with the aim of including 100 000 pregnant women by 2008. Pregnant women are recruited in week 13-17 of pregnancy, in connection with a routine ultrasound examination offered all pregnant women in Norway. The MoBa study includes all geographic areas of Norway, and both rural and urban areas are represented. The response rate is around 44 %.
Children in this analysis had questionnaire information from week 13 - 17 of pregnancy and age six and eighteen months. The questionnaires included items on maternal and child health, child development, socioeconomic status, nutrition, environmental exposures before, during, and after pregnancy. Mandatory birth records, filled in by midwives, were provided by the Medical Birth Registry of Norway (MBRN) 39 and included in the MoBa database. The birth records added information about complications and outcomes of pregnancy and the neonatal period, and selected maternal and neonatal characteristics.
Informed consent was obtained from each participant before inclusion in MoBa. The MoBa Study has been approved by the Regional Committee for Medical Research, the Norwegian Data Inspectorate and the Institution Review Board of the National Institute of Environment Health Sciences, USA. Our study population consisted of 33 192 children born between 1999 and 2005 who had reached 18 months of age and for whom all three questionnaires (the 17 week questionnaire in pregnancy, one at six months, and one at 18 months) had been processed.
Health outcomes
The health outcomes investigated were lower respiratory tract infections (LRTIs), hospitalization for LRTIs, and wheeze, reported in the questionnaires at six and 18 months after birth. The questionnaires can be viewed at the MoBa website.40 Lower respiratory tract infections were defined by maternal reports of respiratory syncytial-virus, bronchiolitis, bronchitis and pneumonia. We classified hospitalization for any of these conditions as “hospitalized for LRTI”. Wheeze was defined as any report of chest congestion or whistling/wheezing in the chest between six and 18 months of age. The questionnaires did not include questions on wheeze before six months of age. Outcomes were treated as dichotomous. LRTIs, with or without hospitalization, were compared to no episode of LRTI.
Main exposure
Maternal BMI was calculated from height and prepregnancy weight as reported on the first MoBa questionnaire. We excluded women with weight less than 35 kg (n = 9) and height less than 115 cm (n = 74). Depending on the analysis we treated maternal BMI as either a continuous variable or categorized it using the WHO scheme 41 of underweight (< 18.5), normal (18.5 - 24.9), overweight (25.0 - 29.9), and obese (BMI ≥ 30).
Covariates
Maternal characteristics obtained from the first pregnancy questionnaire included: asthma (any report of asthma before or in pregnancy), educational level (years of education completed: ≤12, >12 – <16, and ≥16, income in Nkr: <150 000, 150 000 – 299 000, and ≥300 000, age in years (< 25, 25 - 30, or > 30), parity (number of previous pregnancies of >20 weeks' duration: 0, 1, or >1), and smoking in pregnancy.
From the six month questionnaire, we dichotomized parental smoking after birth (any parental smoking 0 - 3 months after birth versus no smoking), and breast feeding at six months of age (yes or no). From the 18 month questionnaire, day care attendance was categorized as any attendance, cared for in a private home by a nanny, or at home by parent.
Covariates obtained from the MBRN included the child's sex, caesarean delivery, birthweight, gestational age, and maternal marital status (categorized as married, cohabiting (not married but living with partner), or single). We dichotomized variables for low birthweight (< 2500 grams versus ≥ 2500g), and preterm birth (born before 37 completed weeks of gestation versus born after 37 weeks). For pregnancy complications we considered conditions known to be related to maternal BMI, specifically diabetes, gestational diabetes, hypertension, and preeclampsia. Due to small numbers of these complications we combined them into a single variable for complications (any versus none).
Statistical methods
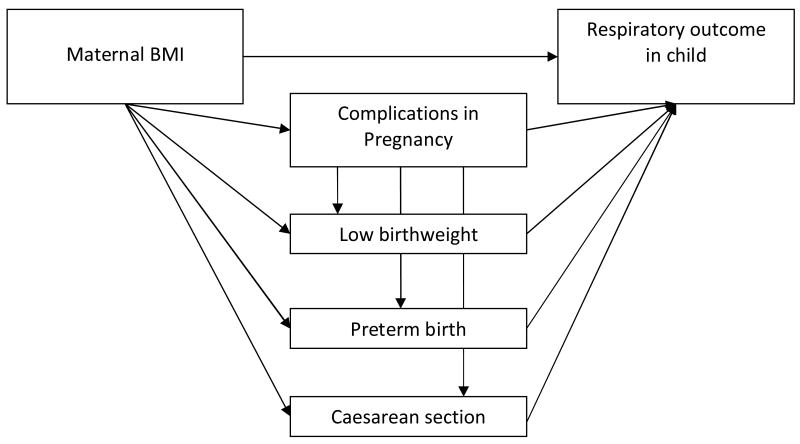
Our hypothesis was that a high maternal body mass index in pregnancy increased the risk of respiratory illness in early childhood. To explore direct and indirect effects of maternal BMI on childhood respiratory outcomes, we applied a path analyses,42 with one path directly from maternal BMI to childhood respiratory outcome, and other paths going via complications in pregnancy, low birthweight, preterm birth, and caesarean section. The path model is shown in figure 1. This allows estimation of the indirect effects of BMI that work through the pregnancy outcomes, in addition to the direct adjusted effect of BMI. All path equations included a BMI variable with four categories, maternal background variables (asthma, age, income, education, marital status, smoking in pregnancy, parity, plural births) and sex. The equations including the respiratory outcomes also contained postnatal variables, such as postnatal parental smoking, breast feeding and kindergarten attendance. The seven path equations were as follows:
Figure 1.
Path-model for investigating direct and indirect effects of maternal prepregnancy BMI on childhood respiratory health.
(outcome variable ∼ (regressed on) explanatory variables):
Complications ∼ BMI + background variables
Low birthweight ∼ BMI + Complications + Background variables
Preterm birth ∼ BMI + Complications + Background variables
Caesarean section ∼ BMI + Complications +Background variables
Wheeze ∼ BMI + Complications + Low birthweight + Preterm birth + Caesarean section + Background variables (including Breast feeding, Postnatal smoking and Kindergarten attendance)
LRTI ∼ BMI + Complications + Low birthweight + Preterm birth + Caesarean section + Background variables (including Breast feeding, Postnatal smoking and Kindergarten attendance)
Hospitalizations for LRTI ∼ BMI + Complications + Low birthweight + Preterm birth + Caesarean section + Background variables (including Breast feeding, Postnatal smoking and Kindergarten attendance)
We used an ordinary linear model with robust variance estimation. The robust option will give correct variance estimates in the presence of non-normal residuals and non-constant residual variance. From the models we reported the risk differences of the variables of interest. We also calculated the expected risk of the outcome when the variables of interest were at their reference values. Risk differences for one or more exposures can be added to the risk at reference to obtain total risk for combination of exposures. For example, to obtain the risk of wheeze for children born preterm of obese mothers, the risk differences for preterm birth and maternal obesity can be added to risk at reference for wheeze. This approach assumes that the additive model is appropriate.
The selections of background variables were based on presumptive associations with both respiratory outcomes and maternal BMI. To investigate how much background variables confounded the BMI effects, we adjusted for all the selected background variables (maternal age, parity, maternal education and income, maternal asthma, maternal smoking in pregnancy, parental smoking after birth, breast feeding and type of day care), and excluded pregnancy- and birth outcomes from the model. Then we added pregnancy- and birth outcomes (sex, complications in pregnancy, low birthweight, preterm birth, caesarean section, and plural births) in the model, and thereby obtained the direct effect of BMI not mediated through birth outcomes or pregnancy complications. The direct effects of BMI were estimated from equations 5-7. The indirect effects of BMI were obtained by multiplying the risk differences along each path of obstetric problems, and then adding the risk differences obtained for the indirect paths. The total effects were the sum of direct and indirect effects. The numbers presented are either proportions or risks (range 0 to 1), or the difference in risk between to groups (RD, range -1 to 1). To study the functional form of the direct effect of maternal BMI on wheeze, we modeled BMI as a continuous exposure in a generalized additive model (GAM) using S-Plus 6.2 software (Insightful, Seattle, WA, USA), and adjusted for obstetric outcomes and all pre- and postnatal background variables.
The tetrachoric correlation was used when calculating correlation coefficients. Missing data were not included in analyses. The rates of missing data for the health outcomes were: 2.1 % for wheeze, and 5.7 % for LRTIs. We checked if predicted risk were outside the 0 to 1 interval. We also looked for observations with high influence by plotting leverage versus squared residuals. Data were analyzed using Stata 9.2 (Stata Corporation, College Station, Texas).
Results
Having a BMI ≥ 25 was more prevalent among mothers with lower educational and income level, mothers who smoked, had asthma, higher parity, who did not breast-feed at six months, and who cared for their child at home (Table 1).
TABLE 1.
Percent of mothers in different BMI categories by characteristics, for 33 192 children in the Norwegian Mother and Child Study born 1999 – 2005 a
| Maternal prepregnancy BMI | |||||
|---|---|---|---|---|---|
| <18.5 | 18.5 - <25 | 25 - <30 | ≥30 | ||
| n | % | % | % | % | |
| Overall | 32 281 | 2.9 | 65.2 | 22.4 | 9.5 |
| Maternal marital status | |||||
| Married | 16 972 | 2.7 | 66.1 | 22.3 | 9.0 |
| Cohabiting | 14 294 | 3.0 | 64.2 | 22.7 | 10.2 |
| Single | 781 | 7.0 | 62.7 | 20.7 | 9.5 |
| Maternal age | |||||
| <25 | 4028 | 5.4 | 62.1 | 22.2 | 10.4 |
| 25-30 | 14 771 | 3.0 | 66.1 | 22.0 | 9.0 |
| >30 | 13 482 | 2.2 | 65.1 | 23.0 | 9.7 |
| Maternal education (years) | |||||
| Other | 590 | 4.2 | 62.5 | 22.7 | 10.5 |
| ≤12 | 12 595 | 3.5 | 57.8 | 25.2 | 13.5 |
| <12 - >16 | 13 254 | 2.3 | 68.3 | 21.8 | 7.6 |
| ≥16 | 5731 | 3.0 | 74.4 | 17.8 | 4.8 |
| Maternal income (in 1000 Nkr b) | |||||
| <150 | 6300 | 4.7 | 62.8 | 21.9 | 10.6 |
| 150 - <300 | 17 284 | 2.6 | 64.1 | 23.2 | 10.2 |
| ≥300 | 7599 | 2.1 | 70.1 | 20.8 | 7.0 |
| Maternal asthma | |||||
| No | 30 049 | 2.9 | 65.7 | 22.2 | 9.2 |
| Yes | 2232 | 3.1 | 57.9 | 25.8 | 13.2 |
| Parity | |||||
| 0 | 14 392 | 3.5 | 67.2 | 20.5 | 8.9 |
| 1 | 11 729 | 2.6 | 63.9 | 23.5 | 9.9 |
| >1 | 17 889 | 2.3 | 62.8 | 24.8 | 10.0 |
| Maternal smoking in pregnancy | |||||
| No | 28 968 | 2.7 | 65.9 | 22.4 | 9.1 |
| Yes | 3111 | 5.1 | 58.4 | 23.2 | 13.4 |
| Parental smoking after birth | |||||
| No | 22 034 | 2.7 | 66.8 | 22.0 | 8.6 |
| Yes | 8000 | 3.7 | 61.0 | 23.9 | 11.4 |
| Breast feeding (months) | |||||
| <6 | 5965 | 3.3 | 54.5 | 25.5 | 16.7 |
| ≥6 | 26 316 | 2.9 | 67.6 | 21.7 | 7.8 |
| Daytime care | |||||
| At home with parent | 10 100 | 3.6 | 61.9 | 23.2 | 11.3 |
| Nanny/private home | 10 195 | 2.5 | 66.9 | 21.6 | 8.9 |
| Kindergarten | 11 894 | 2.8 | 66.5 | 22.4 | 8.4 |
Number of children with information on maternal prepregnancy BMI = 32 281.
100 000 Nkr : ∼12 500€
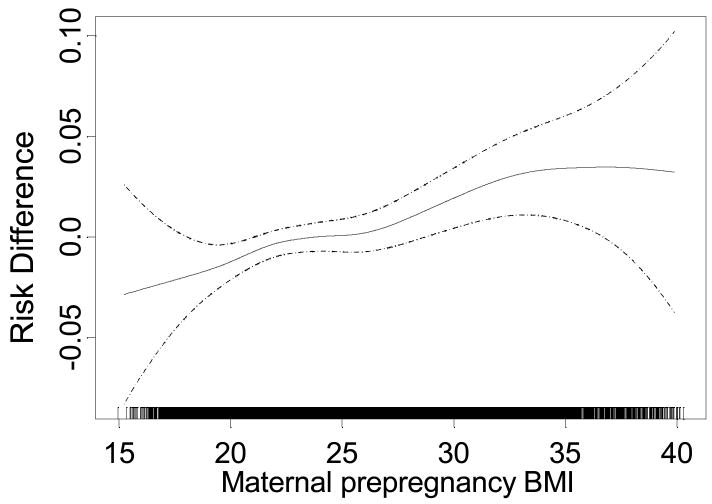
The incidence proportions of wheeze and LRTIs increased in the higher maternal BMI categories (table 2). For the respiratory infections, a weak significant increase of LRTI with maternal BMI was found in crude analyses, but when including both background variables and obstetric problems in analyses, neither LRTIs or hospitalizations for LRTIs were significantly associated with high maternal BMI. Adjustment for background variables also attenuated the crude positive association between wheeze and maternal BMI, and when we included pregnancy complications and birth outcomes in analyses, the BMI effects attenuated further. However, an increased risk of wheeze for children with mothers in the highest BMI category persisted, even after including both background variables and obstetric outcomes in the model, with an increased risk of 3.3 percentage points (risk difference: 0.033 [95% CI 0.012, 0.053]). Figure 2 displays the direct effect of maternal prepregnancy BMI on wheeze. There was a small, but significant linear increase of wheeze with increasing maternal BMI. The increased risk of wheeze per BMI unit was 0.0029 [95% CI 0.0015, 0.0043].
TABLE 2.
Cumulative incidence, crude and adjusted risk differences (×100) for lower respiratory tract infections 0 - 18 months, hospitalizations for LRTIs 0 -18 months, and wheeze 6 -18 months, according to maternal prepregnancy BMI for 33 192 children born 1999 - 2005 in the Norwegian Mother and Child Cohort.
| Wheeze | LRTI | Hospitalization LRTI | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal BMI | n | % | cRD | aRDa | aRDb | 95%CI | % | cRD | aRDa | aRDb | 95%CI | % | cRD | aRDa | aRDb | 95%CI |
| 18.5 - <25 | 21 038 | 39.7 | 0 | 0 | 0 | 16.6 | 0 | 0 | 0 | 4.3 | 0 | 0 | 0 | |||
| 25 - <30 | 7237 | 41.1 | 1.4 | 0.6 | 0.4 | [-1.1, 1.8] | 17.4 | 0.8 | 0.3 | 0.3 | [-0.8, 1.3] | 4.7 | 0.5 | 0.1 | 0.0 | [-0.6, 0.7] |
| ≥ 30 | 3057 | 44.3 | 4.6 | 3.8 | 3.3 | [1.2, 5.3] | 18.3 | 1.7 | 0.8 | 0.6 | [-1.0, 2.2] | 4.6 | 0.5 | -0.3 | -0.5 | [-1.5, 0.5] |
| P-valuec | <0.001 | 0.001 | 0.008 | 0.042 | 0.613 | 0.724 | 0.229 | 0.785 | 0.570 | |||||||
Adjusted for maternal age, parity, maternal education and income, maternal marital status, maternal asthma, maternal smoking in pregnancy, parental smoking after birth, breast feeding, and type of daycare.
In addition adjusted for sex, birth weight, premature birth, caesarean section, complications in pregnancy and plural births.
Wald-test for effect of BMI ≥ 25.
Figure 2.
The adjusted relationship between maternal BMI in pregnancy and wheezing in children up to 18 months of age for 33 192 children in the Norwegian Mother and Child cohort study born 1999 - 2005. Adjusted for complications in pregnancy, low birthweight, preterm birth, caesarean section, sex, plural births, and maternal characteristics (asthma, age, income, education, marital status, smoking in pregnancy, parity), and breast feeding, postnatal smoking and type of day care. Dashed lines show the 95% confidence interval. The rug on the x-axis shows the individual data points. The y-scale shows risk difference for wheeze with value zero set arbitrary in the middle of the scale.
Table 3 shows the expected risk for each outcome for a reference group with value 0 for the factors listed in the same column as the outcome. Also displayed are the risk differences for each explanatory variable, adjusted for background variables. Overweight and obese mothers experienced increased risk of complications in pregnancy and caesarean sections. Obesity also increased the risk of preterm birth. We calculated the direct effects of BMI on the respiratory health outcomes by path equations 5, 6 and 7. The expected risk of wheeze (path equation 5) for children at reference value (in the reference categories for birthweight, maternal BMI, pregnancy complications, gestational age, and caesarean section), was 39.2 %. Risk differences can be added to the expected risk to find combined effects of different exposures. For example, to obtain the risk of wheeze for children born preterm of obese mothers, the contribution to wheeze from preterm birth (risk difference: 4.3 %) can be added to the increased risk of wheezing attributable to maternal obesity (risk difference: 3.3%), and both can be added to the risk at reference (39.2%), resulting in a risk of wheezing for this subgroup of children of 46.8%.
TABLE 3.
Expected risk at reference (%) and adjusted a risk differences (aRD) × 100, for wheeze 6 - 18 months, lower respiratory tract infections (LRTI) 0 - 18 months, and hospitalizations for LRTIs 0 - 18 months (LRTI hosp.), according to maternal BMI, complications in pregnancy, birthweight < 2500 grams (low birthweight), preterm birth and caesarean sections (C-section) for 33 192 children born 1999 - 2005 in the Norwegian Mother and Child cohort. Risk differences can be added to obtain total risk associated with combinations of different exposures b.
| Complications in pregnancy | Low birthweight | Preterm birth | C-section | Wheeze | LRTI | LRTI hosp. | ||
|---|---|---|---|---|---|---|---|---|
| Path Equation | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Risk at referencec | % | 3.7 | 3.4 | 5.2 | 11.4 | 39.2 | 16.0 | 4.4 |
| Maternal BMId | Pd | **** | * | **** | ** | |||
| normal (18.5 - <25) | aRD | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| overweight (25 - <30) | aRD | 3.9 | -0.3 | 0.3 | 3.6 | 0.3 | 0.3 | 0.1 |
| obese (≥ 30) | aRD | 9.2 | 0.5 | 1.5 | 8.8 | 3.3 | 0.6 | -0.4 |
| Complications in | ||||||||
| pregnancy | aRD | 9.1**** | 13.2**** | 15.1**** | 2.1 | 0.3 | 0.6 | |
| Low birthweight | aRD | 4.7* | 5.3* | 5.7*** | ||||
| Preterm birth | aRD | 4.3** | 3.5** | 3.1** | ||||
| C-section | aRD | 3.0*** | 0.7 | 0.7 |
p <0.05
p <0.01
p <0.001
p <0.0001
Adjusted for maternal marital status, maternal age, maternal income, maternal educational level, maternal smoking in pregnancy, parity, maternal asthma, sex and plural births. In path equation 5, 6 and 7 also adjusted for breast feeding, postnatal smoking and type of day care.
The risk difference for each exposure can be added to the risk at reference and to each other to obtain total risk when exposed to more than one exposure. For example, the risk of wheeze for preterm children born to obese mothers would be: 39.2% + 4.1% + 3.4% = 46.7 %.
Reference: Normal BMI and value 0 for the exposures listed in the same column.
Wald-test for effect of BMI ≥ 25.
We found preterm birth, low birthweight and complications in pregnancy to be independent risk factors for wheeze and lower respiratory tract infections. The correlation between low birthweight and preterm birth was 0.91. When including low birthweight and preterm birth simultaneously in analyses, the effects of these correlated exposures weakened substantially, but each still had an independent effect on childhood respiratory health. Caesarean sections were significantly associated to both respiratory infections and wheeze in crude analyses (data not shown). However, in adjusted analyses, caesarean sections did not influence the risk for respiratory infections, but remained significant for wheeze, increasing the risk by 3.0 percentage points.
Table 4 presents the direct and indirect effects of a high maternal BMI in pregnancy on the respiratory outcomes. Maternal overweight (BMI 25 - < 30) had no direct effect on respiratory infections or wheeze, and the indirect effects were negligible. Maternal obesity (BMI ≥ 30) did not affect the risk for respiratory infections substantially, but slightly increased the risk of wheeze. This effect was mainly a direct effect of obesity. Through obesity-related obstetric problems the risk of wheeze only increased by 0.9 percentage points.
TABLE 4.
Direct effect of maternal BMI, indirect effects of BMI via obstetric problems (complications in pregnancy, preterm birth and caesarean sections) and total effects of BMI and obstetric problems given as adjusteda risk differences (RD) × 100 for lower respiratory tract infections (LRTI) 0 - 18 months, hospitalizations for LRTIs 0 -18 months, and wheeze 6 - 18 months for 33 291 children in the Norwegian Mother and Child cohort study born 1999 - 2005.
| Maternal BMI | Wheeze | LRTI | Hospitalization for LRTI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Direct effect RD |
Indirect effect RD |
Total effect RD |
Direct Effect RD |
Indirect effect RD |
Total effect RD |
Direct effect RD |
Indirect effect RD |
Total effect RD |
|
| 18.5 - <25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 - <30 | 0.3 | 0.2 | 0.5 | 0.3 | 0.1 | 0.4 | 0.0 | 0.1 | 0.1 |
| ≥ 30 | 3.3 | 0.9 | 4.2 | 0.6 | 0.3 | 0.9 | -0.5 | 0.3 | -0.2 |
Adjusted for maternal marital status, maternal age, maternal income, maternal educational level, maternal smoking in pregnancy, parity, maternal asthma, sex, plural births, low birthweight, breast feeding, postnatal smoking and type of day care.
The models predicted no risks above 1, and only a low percentage below zero in a few models, thus we deemed them acceptable. No observations with high influence (leverage) were found, thus the results were robust against outliers.
Discussion
Maternal obesity during pregnancy was related to respiratory disease in children up to 18 months in crude analyses. However, lifestyle and maternal characteristics associated with high maternal BMI attenuated the associations. For wheeze, a modest effect of high BMI persisted after adjustment for background variables and after removing indirect effects of BMI via adverse pregnancy outcomes. Although low birthweight and preterm birth were significant risk factors for wheeze and respiratory infections, the effect attributable a high maternal BMI mediated through these birth outcomes was small.
Studying obesity as a cause of respiratory symptoms is a challenge because social and behavioural characteristics of the mother, including age, parity, educational level, marital status, income, smoking, and breast feeding practice may be associated with both maternal obesity and respiratory disease in children. We found that a high maternal BMI was related to several lifestyle factors associated with an increased risk of childhood respiratory disease, such as smoking and shorter duration of breast feeding. Controlling for a variety of covariates reduced the possibility of confounding, however, lifestyle factors not accounted for may represent unmeasured confounding.
The participation rate in MoBa was around 44%. When comparing MoBa with the MBRN which includes all births in Norway, there were indications of some differences between MoBa participants and the general population. For example, women in MoBa were slightly older, smoked less, and had fewer caesarean sections (13.9% in MoBa compared to 15.4% in MBRN). Thus, prevalence estimates based on MoBa may not be representative for the Norwegian population in general. The distribution of prepregnancy BMI among women in the MBRN is not reported. However, over- or under-representation of different BMI categories within the study populations would not necessarily bias associations.
The respiratory outcomes were based on maternal reports, and some misclassification is therefore possible. Reports of wheeze are dependent on maternal awareness and interpretation, which may be related to educational level or maternal asthma.43 Attempts to validate parental reports have given contrasting results. Some studies have found discrepancy between parental and clinicians' report of wheeze,44 while other studies on infant wheeze find acceptable agreement between parental reports in questionnaires and clinical findings.45,46
Misclassification problems might be less severe for LRTIs and LRTI hospitalizations, since theses outcomes are less dependent on maternal interpretation and usually a result of a doctor diagnosis. Because we obtained information on complications, birth outcomes and several of the maternal and neonatal characteristics from the MBRN, we do not expect differential misclassification or substantial information bias in these covariates, and misreporting would most likely lead to an underestimation of a true effect.
Obesity has been associated to obstetric complications and medical conditions in pregnancy, including preeclampsia, hypertension, and caesarean sections.8,11,12 Some have found associations between caesarean section and wheezing in childhood,5 but others have not.47,48 Complications in pregnancy, preterm delivery, low birthweight and mode of delivery have been associated with wheezing and respiratory infections in childhood in other studies.4-7,49 We found that a high maternal BMI in pregnancy was associated with complications during pregnancy, caesarean sections, and preterm birth. Preterm birth and low birthweight were independent risk factors for wheeze and lower respiratory tract infections. The pregnancy complications we considered (hypertension, diabetes, and preeclampsia) were not significantly associated to the respiratory outcomes. We found that caesarean sections were significantly associated to all three respiratory outcomes in crude analyses, but after adjusting for covariates, only an effect on wheeze remained. In several studies the associations between mode of delivery and childhood respiratory disease have disappeared when adjusting for known confounders,50-52 and associations have been suggested to be caused by confounding.51 We believe that the association between caesarean section and respiratory outcomes in our data might not be causal, but attributed unknown confounding.
Several studies have found a high BMI to be related to increased risk of lower respiratory tract infections in children and adults.13,14 Obesity has also been related to increased airway hyper-responsiveness.15,21,22 For most children, wheezing represents transient episodes caused by acute respiratory infections.53 However, wheezing and episodes of lower respiratory tract infections in early childhood has been associated to persistent wheezing and later occurrence of asthma.54-56
Inflammation plays a major role in respiratory diseases.57 Systemic inflammation, with elevated levels of inflammatory factors similar to those seen in infection, may be involved in, or caused by obesity.19,58-60 The systemic inflammation related to obesity has also been found in children.59 One substance linking obesity and inflammation is leptin, a hormone secreted by adipocytes. Leptin levels correlate with BMI,61 and leptin receptors are involved in growth regulation in human lung tissue.62 Leptin stimulates the production of proinflammatory cytokines and is able of modulating the immune response.63,64
Associations found between maternal obesity and respiratory outcomes attenuated when controlling for characteristics and obstetric problems. This was especially true for the respiratory infections. For wheeze, an effect remained, indicating a possible direct effect of maternal obesity. Altered immunological mechanisms influencing the in utero environment and, thus, the growing fetus, may be a possible explanation for these associations, but unmeasured and residual confounding possibly influencing the associations can not be excluded.
To our knowledge this is the first published evidence linking maternal obesity in pregnancy to the development of respiratory illness beyond the neonatal period in children. Given the morbidity from early wheezing and its impact on the development of asthma in later childhood, this finding could be of potential public health significance. Follow-up of children to older ages when asthma can be reliably diagnosed will be important.
Acknowledgments
The Norwegian Association of Heart and Lung patients with EXTRA funds from the Norwegian Foundation for Health and Rehabilitation; Division of Intramural Research, National Institute of Environmental Health Sciences, National Institute of Health, USA. We gratefully acknowledge the participation of the MoBa parents. The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health, Division of Intramural Research, National Institute of Environmental Health Sciences, National Institute of Health, USA. Contract no. HHSN291200775558C and Project no. Z01-ES-85433), NIH/NINDS (grant no.1 UO1 NS 047537-01), and the Norwegian Research Council/FUGE (grant no. 151918/S10).
References
- 1.Jones CA, Holloway JA, Warner JO. Does atopic disease start in foetal life? Allergy. 2000;55:2–10. doi: 10.1034/j.1398-9995.2000.00109.x. [DOI] [PubMed] [Google Scholar]
- 2.Prescott SL. Maternal allergen exposure as a risk factor for childhood asthma. Current Allergy and Asthma Reports. 2006;6:75–80. doi: 10.1007/s11882-006-0014-7. [DOI] [PubMed] [Google Scholar]
- 3.Warner JO. The early life origins of asthma and related allergic disorders. Archives of Disease in Childhood. 2004;89:97–102. doi: 10.1136/adc.2002.013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrington LJ, Langley-Evans SC. Wheezing and eczema in relation to infant anthropometry: evidence of developmental programming of disease in childhood. Maternal & Child Nutrition. 2006;2:51–61. doi: 10.1111/j.1740-8709.2006.00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Negele K, Heinrich J, Borte M, von Berg A, Schaaf B, Lehmann I, et al. Mode of delivery and development of atopic disease during the first 2 years of life. Pediatric Allergy and Immunology. 2004;15:48–54. doi: 10.1046/j.0905-6157.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 6.Riccetto AG, Ribeiro JD, Silva MT, Almeida RS, Arns CW, Baracat EC. Respiratory syncytial virus (RSV) in infants hospitalized for acute lower respiratory tract disease: incidence and associated risks. The Brazilian Journal of Infectious Diseases. 2006;10:357–61. doi: 10.1590/s1413-86702006000500011. [DOI] [PubMed] [Google Scholar]
- 7.Rusconi F, Galassi C, Forastiere F, Bellasio M, De Sario M, Ciccone G, et al. Maternal complications and procedures in pregnancy and at birth and wheezing phenotypes in children. American Journal of Respiratory and Critical Care Medicine. 2007;175:16–21. doi: 10.1164/rccm.200512-1978OC. [DOI] [PubMed] [Google Scholar]
- 8.Catov JM, Ness RB, Kip KE, Olsen J. Risk of early or severe preeclampsia related to pre-existing conditions. International Journal of Epidemiology. 2007;36:412–9. doi: 10.1093/ije/dyl271. [DOI] [PubMed] [Google Scholar]
- 9.Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. The Medical Journal of Australia. 2006;184:56–9. doi: 10.5694/j.1326-5377.2006.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 10.Kumari AS. Pregnancy outcome in women with morbid obesity. International Journal of Gynaecology and Obstetrics. 2001;73:101–7. doi: 10.1016/s0020-7292(00)00391-x. [DOI] [PubMed] [Google Scholar]
- 11.Raatikainen K, Heiskanen N, Heinonen S. Transition from overweight to obesity worsens pregnancy outcome in a BMI-dependent manner. Obesity (Silver Spring) 2006;14:165–71. doi: 10.1038/oby.2006.20. [DOI] [PubMed] [Google Scholar]
- 12.Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. International Journal of Obesity and Related Metabolic Disorders. 2001;25:1175–82. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- 13.Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Archives of Internal Medicine. 2000;160:3082–8. doi: 10.1001/archinte.160.20.3082. [DOI] [PubMed] [Google Scholar]
- 14.Jedrychowski W, Maugeri U, Flak E, Mroz E, Bianchi I. Predisposition to acute respiratory infections among overweight preadolescent children: an epidemiologic study in Poland. Public Health. 1998;112:189–95. doi: 10.1038/sj.ph.1900438. [DOI] [PubMed] [Google Scholar]
- 15.Johnston RA, Zhu M, Rivera-Sanchez YM, Lu FL, Theman TA, Flynt L, et al. Allergic airway responses in obese mice. American Journal of Respiratory and Critical Care Medicine. 2007;176:650–8. doi: 10.1164/rccm.200702-323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nystad W, Meyer HE, Nafstad P, Tverdal A, Engeland A. Body mass index in relation to adult asthma among 135,000 Norwegian men and women. American Journal of Epidemiology. 2004;160:969–76. doi: 10.1093/aje/kwh303. [DOI] [PubMed] [Google Scholar]
- 17.Huang SL, Shiao G, Chou P. Association between body mass index and allergy in teenage girls in Taiwan. Clinical and Experimental Allergy. 1999;29:323–9. doi: 10.1046/j.1365-2222.1999.00455.x. [DOI] [PubMed] [Google Scholar]
- 18.Luder E, Ehrlich RI, Lou WY, Melnik TA, Kattan M. Body mass index and the risk of asthma in adults. Respiratory Medicine. 2004;98:29–37. doi: 10.1016/j.rmed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 19.von Mutius E, Schwartz J, Neas LM, Dockery D, Weiss ST. Relation of body mass index to asthma and atopy in children: the National Health and Nutrition Examination Study III. Thorax. 2001;56:835–8. doi: 10.1136/thorax.56.11.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Visser M. Higher levels of inflammation in obese children. Nutrition. 2001;17:480–1. doi: 10.1016/s0899-9007(01)00509-3. [DOI] [PubMed] [Google Scholar]
- 21.Shore SA. Obesity and asthma: lessons from animal models. Journal of Applied Physiology. 2007;102:516–28. doi: 10.1152/japplphysiol.00847.2006. [DOI] [PubMed] [Google Scholar]
- 22.Ulger Z, Demir E, Tanac R, Goksen D, Gulen F, Darcan S, et al. The effect of childhood obesity on respiratory function tests and airway hyperresponsiveness. The Turkish Journal of Pediatrics. 2006;48:43–50. [PubMed] [Google Scholar]
- 23.Beasley R. The burden of asthma with specific reference to the United States. The Journal of Allergy and Clinical Immunology. 2002;109:S482–S489. doi: 10.1067/mai.2002.122716. [DOI] [PubMed] [Google Scholar]
- 24.Burr ML, Butland BK, King S, Vaughan-Williams E. Changes in asthma prevalence: two surveys 15 years apart. Archives of Disease in Childhood. 1989;64:1452–6. doi: 10.1136/adc.64.10.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lodrup Carlsen KC, Haland G, Devulapalli CS, Munthe-Kaas M, Pettersen M, Granum B, et al. Asthma in every fifth child in Oslo, Norway: a 10-year follow up of a birth cohort study. Allergy. 2006;61:454–60. doi: 10.1111/j.1398-9995.2005.00938.x. [DOI] [PubMed] [Google Scholar]
- 26.Selnes A, Nystad W, Bolle R, Lund E. Diverging prevalence trends of atopic disorders in Norwegian children. Results from three cross-sectional studies. Allergy. 2005;60:894–9. doi: 10.1111/j.1398-9995.2005.00797.x. [DOI] [PubMed] [Google Scholar]
- 27.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. International Journal of Obesity and Related Metabolic Disorders. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 28.Heslehurst N, Ells LJ, Simpson H, Batterham A, Wilkinson J, Summerbell CD. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15-year period. British Journal of Obstetrics and Gynaecology. 2007;114:187–94. doi: 10.1111/j.1471-0528.2006.01180.x. [DOI] [PubMed] [Google Scholar]
- 29.Government Statistical Service, NHS Health and Social Care Information Centre. Health Survey for England. 2005 Available at: http://www.ic.nhs.uk/webfiles/publications/hlthsvyeng2004upd/HealthSurveyForEnglandTrend161205_PDF.pdf.
- 30.Norwegian Institute of Public Health (NIPH) Obesity development in Norway. 2004 February 3; NIPH Web site. Available at: http://www.fhi.no/eway/default.aspx?pid=233&trg=MainLeft_5631&MainArea_5661=5631:0:15,2689:1:0:0:∷0:0&MainLeft_5631=5544:44492∷1:5641:1:∷0:0.
- 31.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. The Journal of the American Medical Association. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 32.Ulset E, Undheim R, Malterud K. Has the obesity epidemic reached Norway? [Norwegian] Tidsskrift for den Norske Laegeforening. 2007;127(1):34–37. [PubMed] [Google Scholar]
- 33.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of Overweight and Obesity Among US Children, Adolescents, and Adults, 1999-2002. The Journal of the American Medical Association. 2004;291:2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 34.WHO. WHO Fact sheet N°311. 2006 September; WHO website. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 35.Nohr EA, Vaeth M, Bech BH, Henriksen TB, Cnattingius S, Olsen J. Maternal Obesity and Neonatal Mortality According to Subtypes of Preterm Birth. Obstetrics and Gynecology. 2007;110:1083–90. doi: 10.1097/01.AOG.0000286760.46679.f8. [DOI] [PubMed] [Google Scholar]
- 36.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. The Journal of Clinical Endocrinology and Metabolism. 2002;87:4231–7. doi: 10.1210/jc.2002-020311. [DOI] [PubMed] [Google Scholar]
- 37.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 38.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) International Journal of Epidemiology. 2006;35:1146–50. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 39.Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstetricia et Gynecologica Scandinavica. 2000;79:435–9. [PubMed] [Google Scholar]
- 40.The Norwegian Institute of Public Health. The Mother and Child Cohort Study: Questionnaires. 2008 Feb 25; MoBa Website. Available at: http://www.fhi.no/eway/default.aspx?pid=238&trg=MainArea_5811&MainArea_5811=5903:0:15,3138:1:0:0:∷0:0.
- 41.WHO. BMI classification. 2007 November 19; WHO website. Avaialable at: http://www.who.int/bmi/index.jsp?introPage=intro_3.html.
- 42.Schumacker RE, Lomax RG. Path Models. In: Mahwah Riegert D., editor. A Beginners Guide to Structural Equation Modelling. Lawrence Erlbaum Associates, Inc.; 2004. pp. 149–166. [Google Scholar]
- 43.Michel G, Silverman M, Strippoli MP, Zwahlen M, Brooke AM, Grigg J, Kuehni CE. Parental understanding of wheeze and its impact on asthma prevalence estimates. The European Respiratory Journal. 2006;28:1124–30. doi: 10.1183/09031936.06.00008406. [DOI] [PubMed] [Google Scholar]
- 44.Levy ML, Godfrey S, Irving CS, Sheikh A, Hanekom W, Bush A, Lachman P. Wheeze detection: recordings vs. assessment of physician and parent. The Journal of Asthma. 2004;41:845–53. doi: 10.1081/jas-200038451. [DOI] [PubMed] [Google Scholar]
- 45.Chong Neto HJ, Rosario N, Dela Bianca AC, Sole D, Mallol J. Validation of a questionnaire for epidemiologic studies of wheezing in infants. Pediatric Allergy and Immunology. 2007;18:86–7. doi: 10.1111/j.1399-3038.2006.00488.x. [DOI] [PubMed] [Google Scholar]
- 46.Mallol J, Garcia-Marcos L, Aguirre V, Martinez-Torres A, Perez-Fernandez V, Gallardo A, Calvo M, Rosario FN, Rocha W, Fischer G, Baeza-Bacab M, Chiarella P, Pinto R, Barria C. The International Study of Wheezing in Infants: questionnaire validation. International Archives of Allergy and Immunology. 2007;144:44–50. doi: 10.1159/000102613. [DOI] [PubMed] [Google Scholar]
- 47.Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB. Elective caesarean section and respiratory morbidity in the term and near-term neonate. Acta Obstetricia et Gynecologica Scandinavica. 2007;86:389–94. doi: 10.1080/00016340601159256. [DOI] [PubMed] [Google Scholar]
- 48.Maitra A, Sherriff A, Strachan D, Henderson J. Mode of delivery is not associated with asthma or atopy in childhood. Clinical and Experimental Allergy. 2004;34:1349–55. doi: 10.1111/j.1365-2222.2004.02048.x. [DOI] [PubMed] [Google Scholar]
- 49.Nafstad P, Magnus P, Jaakkola JJ. Risk of childhood asthma and allergic rhinitis in relation to pregnancy complications. The Journal of Allergy and Clinical Immunology. 2000;106:867–73. doi: 10.1067/mai.2000.110558. [DOI] [PubMed] [Google Scholar]
- 50.Annesi-Maesano I, Moreau D, Strachan D. In utero and perinatal complications preceding asthma. Allergy. 2001;56:491–7. doi: 10.1034/j.1398-9995.2001.056006491.x. [DOI] [PubMed] [Google Scholar]
- 51.Bernsen RM, de Jongste JC, Koes BW, Aardoom HA, van der Wouden JC. Perinatal characteristics and obstetric complications as risk factors for asthma, allergy and eczema at the age of 6 years. Clinical and Experimental Allergy. 2005;35:1135–40. doi: 10.1111/j.1365-2222.2005.2155.x. [DOI] [PubMed] [Google Scholar]
- 52.McKeever TM, Lewis SA, Smith C, Hubbard R. Mode of delivery and risk of developing allergic disease. The Journal of Allergy and Clinical Immunology. 2002;109:800–2. doi: 10.1067/mai.2002.124046. [DOI] [PubMed] [Google Scholar]
- 53.Wright AL. Epidemiology of asthma and recurrent wheeze in childhood. Clinical Reviews in Allergy & Immunology. 2002;22:33–44. doi: 10.1007/s12016-002-0004-z. [DOI] [PubMed] [Google Scholar]
- 54.Nafstad P, Brunekreef B, Skrondal A, Nystad W. Early respiratory infections, asthma, and allergy: 10-year follow-up of the Oslo Birth Cohort. Pediatrics. 2005;116:e255–e262. doi: 10.1542/peds.2004-2785. [DOI] [PubMed] [Google Scholar]
- 55.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. New England Journal of Medicine. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 56.Sznajder M, Stheneur C, Albonico V, Dib S, Cau D, Chevallier B. Respiratory development of 5- to 6- year-old children experiencing a first bronchiolitis episode before age one. European Annals of Allergy and Clinical Immunology. 2005;37:392–6. [PubMed] [Google Scholar]
- 57.Busse WW, Lemanske RF., Jr Asthma. New England Journal of Medicine. 2001;344:350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 58.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. C-reactive protein and gestational diabetes: the central role of maternal obesity. The Journal of Clinical Endocrinology and Metabolism. 2003;88:3507–12. doi: 10.1210/jc.2003-030186. [DOI] [PubMed] [Google Scholar]
- 59.Schwarzenberg SJ, Sinaiko AR. Obesity and inflammation in children. Paediatric Respiratory Reviews. 2006;7:239–46. doi: 10.1016/j.prrv.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–3. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 61.Considine RV. Human leptin: an adipocyte hormone with weight-regulatory and endocrine functions. Seminar in Vascular Medicine. 2005;5:15–24. doi: 10.1055/s-2005-871738. [DOI] [PubMed] [Google Scholar]
- 62.Tsuchiya T, Shimizu H, Horie T, Mori M. Expression of leptin receptor in lung: leptin as a growth factor. European Journal of Pharmacology. 1999;365:273–9. doi: 10.1016/s0014-2999(98)00884-x. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez-Margalet V, Martin-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clinical and Experimental Immunology. 2003;133:11–9. doi: 10.1046/j.1365-2249.2003.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? The Journal of Allergy and Clinical Immunology. 2004;114:254–9. doi: 10.1016/j.jaci.2004.03.053. [DOI] [PubMed] [Google Scholar]