Abstract
Purpose
To modify the HRT-II Confocal Microscope with Rostock Corneal Module (HRT-RCM) to allow computerized control of the focal plane position (depth) within the cornea.
Methods
A threaded housing on the HRT-RCM microscope is normally rotated by hand to change the focal plane position within the cornea. This piece was removed to allow the front housing of the microscope to move freely. A linear actuator (Oriel Encoder Mike) was then attached to the side of the microscope, and coupled to a drive shaft which was connected to the front housing. The actuator was connected to an Oriel 18011 Encoder Mike controller, which was interfaced to a PC. Software was developed to allow control and display of the focal plane position using this PC, while image acquisition software was run on the HRT-RCM PC. The instrument was tested on one human volunteer.
Results
The modified instrument successfully allowed computer-controlled focusing throughout the entire cornea. Through-focus sequences could be collected on-line, and analyzed and reconstructed 3-dimensionally off-line using modified CMTF software.
Conclusions
Although this is only a prototype instrument, it significantly improves the examination proceedure by allowing completely “hands-free” operation of the HRT-RCM microscope. The data also demonstrates the feasibility of performing quantitative z-axis scans through the full thickness of the cornea with the HRT-RCM. Given the higher constrast images and improved optical sectioning of the HRT-RCM as compared to other instruments, these capabilities could have widespread application.
Keywords: Confocal Microscopy, Cornea, Imaging, 3-D Reconstruction
INTRODUCTION
In recent years, the clinical application of in vivo confocal microscopy has expanded rapidly. For example, confocal microscopy is often used to monitor the cellular events of epithelial and stromal wound healing following refractive surgical procedures (for reviews see 1-3). It can also be used for early detection and diagnosis of a number of infectious organisms (for review see 4, 5). In addition, temporal changes in the density of stromal cells6-10 and organization of subepithelial nerves in response to surgery or disease can be assessed.11-15 The effects of contact lens wear on the morphology and thickness of the corneal epithelium has also been quantified, and such studies have provided important insights into how lens type and wear pattern influence bacterial binding and corneal epithelial homeostasis.16-20 There are numerous other applications of this technology; many of which are discussed in several recent review articles.4, 5, 21
Three confocal imaging systems are currently used clinically: the tandem scanning confocal microscope (TSCM), the Confoscan 4 (a scanning slit system), and the HRT Rostock Cornea Module (a scanning laser system). The first scanning confocal microscope, developed by Petran et al,22, 23 used a modified Nipkow disk containing optically conjugate (source/detector) pinholes arranged in Archimedean spirals. In 1986 Lemp et al24 applied confocal imaging techniques to the study of the cornea ex vivo. This work led to the design of a TSCM with a horizontally oriented objective (Tandem Scanning Corp, Reston, VA), which was more suited to use in ophthalmology.25 Most TSCM systems use a specially designed surface contact objective (24x, 0.6 NA, 1.5 mm working distance). The position of the focal plane relative to the tip is varied by moving the lenses inside the objective casing. Thus, the depth of the focal plane within the cornea can be calibrated, making quantitative three-dimensional imaging possible with this system.26, 27 The TSCM has an axial (z-axis) resolution of approximately 9 μm.26
The Confoscan 4 is a variable-slit real-time scanning confocal microscope, available commercially from Nidek, Inc.. This design was originally described and applied to corneal imaging by Masters and Thaer.28 In this microscope, two independently adjustable slits are located in conjugate optical planes; a rapidly oscillating two-sided mirror is used to scan the image of the slit over the plane of the cornea to produce optical sectioning in real time.29 The system uses a 40x objective lens (0.75 NA) and digitized images are 460 × 345 μm in size. This is a user-friendly instrument that incorporates automated alignment and scanning software. In addition, the scanning slit design allows better light throughput and provides images with better contrast than the TSCM. However, this is achieved at the expense of axial resolution, which has been measured at approximately 26 μm.30
The HRT Rostock Corneal Module (Heidelberge Engineering, GmBH, Dossenheim, Germany) is a laser scanning confocal microscope.31 It operates by scanning a 670 nm laser beam (< 1 μm diameter) in a raster pattern over the field of view. This is accomplished using horizontally and vertically oriented scanning mirrors. The reflected light from the cornea is descanned using the same two mirrors, and directed to a photo detector using a beam splitter. The system typically uses a 63x objective lens (0.9 NA), and provides images that are 400μm × 400μm in size. The microscope produces images with excellent resolution and contrast, and has better axial resolution than the TSCM (7.6 μm), due to the higher NA objective.32 Unfortunately, automated scans of only 60μm can be generated at this time, and changing the focal plane over larger distances must be performed manually (by rotating the objective housing by hand).
METHODS
Hardware Modifications
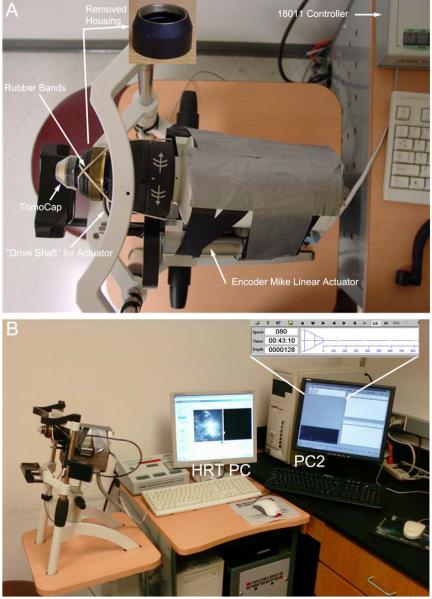
An HRT II with Rostock Corneal Module (HRT-RCM) was modified as shown in Figure 1A. A threaded housing on the HRT is normally rotated by hand to change the focal plane position within the cornea. This piece was removed (inset in Fig. 1A) to allow the front housing of the microscope to move freely. Two rubber bands were used to apply a constant rearward force to this front housing. A linear actuator (Oriel Encoder Mike) was then attached to the side of the microscope, and coupled to a stainless steel drive shaft which was connected to the back of the front housing. The force from the rubber bands ensured that the drive shaft remained in contact with the housing at all times, so that they moved in unison. The actuator was connected to an Oriel 18011 Encoder Mike controller.
Figure 1.
(A) The modified HRT-RCM prototype. A threaded housing on the HRT which is normally rotated by hand to change the focal plane position within the cornea was removed (inset) to allow the front housing of the microscope to move freely. Two rubber bands were used to apply a constant rearward force to this front housing. A linear actuator (Oriel Encoder Mike) was then attached to the side of the microscope, and coupled to a stainless steel “drive shaft” which was connected to the back of the front housing. The actuator was connected to an Oriel 18011 Encoder Mike controller. (B) In this prototype instrument, two PCs are used during scanning. Image acquisition is accomplished using the normal HRT software (HRT PC), and focusing is controlled using the arrow keys on a separate keyboard (PC2), using modified “CMTF” software. The software continuously displays the position of the focal plane with respect to the tip of the TomoCap (zoomed inset).
Software Modifications
Two PCs were used (Fig. 1B). The HRT PC with Heidelberg Eye Explorer software was used for image acquisition and storage. For control of the focal plane position, a second PC (PC2) was interfaced to the Oriel 18011 controller via a serial port. To control the 18011, we modified our previously described “CMTF” software which was originally designed for use with the TSCM system. This program allows the focal plane position and speed to be controlled using the arrow keys on the keyboard. Software modifications included changing the depth calibration from a non-linear curve to a line with a slope of 1, and reversing the direction of the actuator movement in response to the arrow keys. This was necessary since with the TSCM lenses inside the objective are moving, whereas with the HRT-RCM the front housing (with attached Tomocap) is being displaced. The software continuously displays the position of the focal plane with respect to the tip of the Tomocap (inset in Figure 1B).
Scanning Procedure
The scanning procedure was generally similar to that normally used with the HRT system. The Heidelberg Eye Explorer software is used to initialize the scanning system and display images continuously on the monitor of the HRT PC. A Tomocap (Heidelberge Engineering, GmBH, Dossenheim, Germany) is placed on the front of the HRT, using GenTeal gel (Novartis Pharmaceuticals Corp, East Hanover, NJ) to couple it to the 63X objective. At this point, the CMTF software (on PC2) is used to move the front housing until the focal plane position corresponds the front surface of the Tomocap (identified by a bright reflection). The depth display on both the HRT and the CMTF software are set to zero at this point. The unit is then positioned so that the Tomocap is aligned perpendicular to the corneal surface and the cornea is then applanated, using GenTeal gel as an immersion medium. Scanning is then performed in a normal fashion, except that the focal plane position is controlled remotely using the CMTF software (arrow keys on PC2).
RESULTS
Routine Scanning
The new lens drive system was found to greatly improve the usability of the HRT system. The focal plane position could be changed smoothly and continuously without having to touch the microscope housing. The Heidelberg Eye Explorer Software is interfaced to an inductive displacement transducer which provides constant feedback on the focal plane position (based on movement of the front housing). This focal plane positional information was in good agreement with that displayed by the CMTF software.
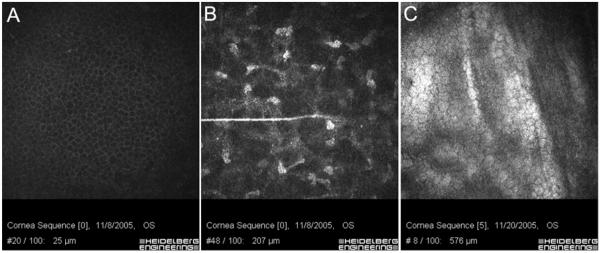
In vivo confocal data from one human is presented to demonstrate the general capabilities of the prototype system. As shown in Figure 2, high resolution images could be obtained from throughout the cornea. Since image acquisition was performed using the Heidelberg Eye Explorer software, the focal plane position, as detected by the inductive displacement transducer, is saved with each image.
Figure 2.
HRT-RCM images of the wing cells (A), stromal cells (B) and endothelium (C) obtained by focusing in and out of the cornea using the remote-controlled lens drive. Since image acquisition was performed using the Heidelberg Eye Explorer software, the focal plane position, as detected by the inductive displacement transducer, is saved with each image (shown on bottom of each image). Image width = 400 μm.
Confocal Microscopy Though-Focusing
To collect and quantify 3-D information from the cornea, a technique termed confocal microscopy through-focusing (CMTF) is typically used. Weigand et al. originally demonstrated that by rapidly focusing through the cornea at high speed, a z-axis intensity profile of the tissue can be obtained.33 CMTF scans can provide information about the depth and thickness of corneal cell layers, and also allow 3-D visualization of corneal tissue.34, 35 CMTF scans are obtained by scanning through the cornea from the epithelium to endothelium at a constant lens speed while continuously acquiring images.
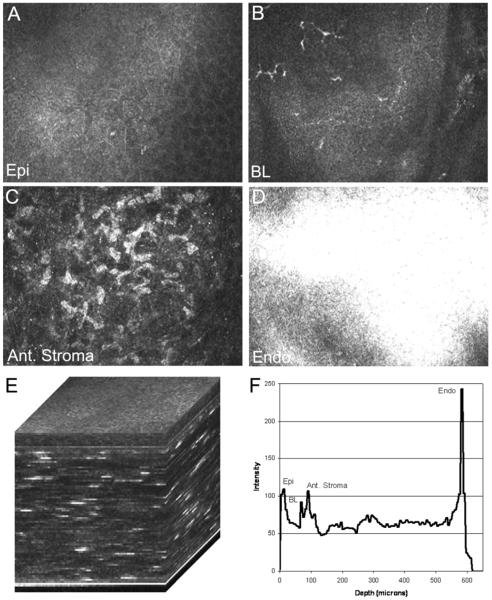
To test the feasibility of performing CMTF analysis using our prototype instrument, we first positioned the focal plane to the zero position corresponding to the Tomocap surface. We then set the speed of the lens drive to 200μm/sec on the CTMF software. We set the Heidelberg Eye Explorer to “sequence” mode, with an acquisition rate of 30 frames/second. To initiate the scan, we simultaneously pressed the foot pedal on the HRT (which started sequential image acquisition) and pressed the right arrow key on the CMTF software (which started forward scanning of the lens drive). With these settings the HRT acquires 100 frames in 3.52 seconds, which corresponds to a step size between images of 7 μm. After the HRT acquisition was complete, we pressed the arrow key again to stop the lens movement. The sequence of images was exported as a series of “jpeg” files, which could be read into Photoshop off-line. Selected images from the CMTF stack are shown in Fig. 3A-D, whereas the entire sequence is shown in Supplemental Movie 1. Note that all cell layers of the cornea were obtained in a single scan.
Figure 3.
(A-D) Selected images from a CMTF stack collected using the modified HRT-RCM. Note that images from all cell layers of the cornea were obtained in a single scan. (E) A 3-D reconstruction (surface rendering) generated from the CMTF software. (F) The corresponding intensity profile. Note that peaks corresponding to the epithelial surface (Epi), basal epithelial nerve plexus (BL), anterior stroma (Ant. Stroma) and endothelium (Endo) were clearly identified. Image width for A-D = 384 μm.
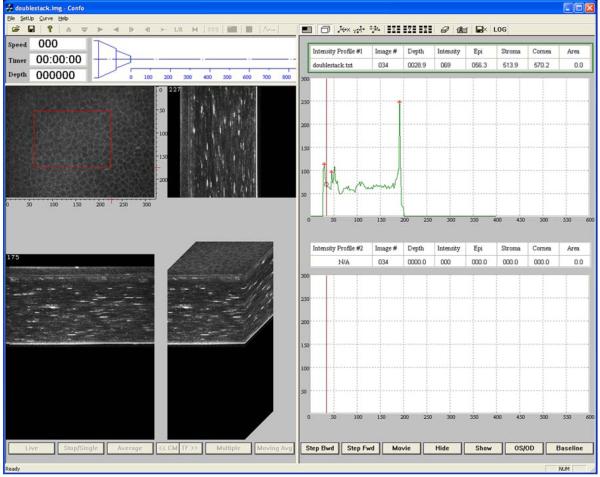
In order to further analyze the CMTF data, we modified the image stack so that it could be loaded into the CMTF software, which is set-up to read a stack of 400, 320×240 pixel images. First, the image sequence was read into MetaMorph using the “build stack” feature. Next, the images in the stack were all cropped to 320×240 pixels. We then used the “add plane” feature to create a new stack in which each image was shown twice (200 planes total). We then padded the stack with 200 black images (pixel intensity of 0). To create the intensity profile, we calculated the average intensity of each image in this stack using the “region measurements” tool, and exported the data to excel. A text file with the curve data in a format compatible with the CMTF software was then generated. A generic header file for the image stack was also created. The header, image and text file were successfully read into the CMTF software. Figure 4 shows a screen shot of the CMTF software after loading the image sequence data. As the user moves a cursor along the intensity curve, the corresponding images are displayed. In this way, the user can identify images of interest and record their exact z-axis positions. In addition, side views and 3-D surface renderings of the CMTF stack can be viewed interactively. Figures 3E and 3F show higher magnification views of a 3-D reconstruction (surface rendering) generated from the CMTF software, and the corresponding intensity profile. Note that peaks corresponding to the epithelial surface, basal epithelial nerve plexus, anterior stroma and endothelium were clearly identified.
Figure 4.
A screen shot of the CMTF software taken after loading the image sequence data from the modified HRT-RCM prototype instrument. With this interactive software, as the user moves a cursor along the intensity curve (right), the corresponding images are displayed. In this way, the user can identify images of interest and record their exact z-axis positions. In addition, side views and 3-D surface renderings of the CMTF stack can be viewed interactively (left).
DISCUSSION
The HRT Rostock Corneal Module provides images with superior resolution and contrast than other commerically available confocal systems. However, in order to change the focal plane position, a cylindrical housing must be rotated by hand. This housing is within inches of the patient cornea, and can sometimes interfere with the examination process. Overall, changing the focal plane position is somewhat cumbersome, and this has been an important limitation of the instrument. In this study, we modified the HRT so that the focal plane position could be controlled using our previously described “CMTF” software.35 In this prototype instrument, image acquisition is accomplished using the normal HRT software, while focusing is controlled using the arrow keys on a separate PC. This modification significantly improved the examination procedure by allowing completely “hands-free” operation of the HRT-II microscope.
The HRT uses a Tomocap which applanates the corneal surface and stabilizes the cornea, thus quantitative z-scanning is possible with this system. The HRT software incorporates a feature which allows automated volume scans to be acquired using an internal focusing mechanism; unfortunately, automated scans of only 60μm can be generated, and changing the focal plane over larger distances must be performed manually (by rotating the objective housing by hand). Recntly, Guthoff and coworkers used a novel PMMA cap to reduce compression artifacts and improve visualization of the superficial epithelium with the HRT.32 They then used automated volume scanning to collect z-series of images through the entire epithelium. Various 3-D reconstruction techniques were applied which allowed assessment and visualiazation of the corneal surface. In the same study, they also collected sequences of images through the entire cornea while manually changing the focal plane position, and performed 3-D reconstructions off-line. Our prototype instrument allows image sequences through the cornea to be obtained using an automated lens drive system, which ensures a constant velocity and even spacing between images. These image stacks can be used to generate intensity profile curve through the cornea, and loaded into our previously described CMTF software for interactive 3-D visualization and analysis.35
Unlike other more widely used methods for measuring corneal and epithelial thickness, confocal microscopy provides a series of high resolution microscopic images which directly correspond to peaks in the intensive curve. Thus, the origin of the intensity peaks can be confirmed if necessary. CMTF analysis of TSCM images has had numerous clinical and research applications, and has been particularly useful for studying the effects of refractive surgical procedures such as PRK and LASIK.6, 36-40 Given the higher contrast images and improved optical sectioning of the HRT-II as compared to other instruments, the ability to perform CMTF scans on this instrument could have widespread application.
There are still important limitations to the capabilities of our prototype instrument. First, the reflection from the Tomocap often intereferes with visualization of the epithelial surface. As mentioned above, a novel PMMA cap has recently been developed which appears to minimize this reflection.32 A second limitation is that the HRT software only collects 100 images during a sequential acquire. Thus the step size between images in a CMTF stack is 7 microns. This is signifcantly higher than that used for the TSCM system (2.12 microns), and limits the resolution of epithelial and corneal thickness measurements. To overcome this limitation, the HRT software could potentially be modified to increase the number of images collected in a sequence, or the video signal from the HRT laser camera could be fed directly into the CMTF software. Both of these options are currently under investigation. Finally, the depth calibration of the modified instrument has yet to be experimentally verified.
It should be noted that CMTF curves can also be generated using the Confoscan 4 clinical confocal microscope. However, the Confoscan system uses a non-contact objective lens (for patient comfort), and movement of the entire objective lens is necessary to change the focal plane position within the cornea and generate CMTF scans. A trade-off with this design is that the cornea can move randomly with respect to the lens tip during a CMTF scan. A “Z-Ring” which touches the corneal surface can be used to allow accurate calculation of z-axis position within the cornea during scanning,41 but the distance between images in CMTF scans is generally not as uniform as that obtained using applanating objectives. There are clearly multiple trade-offs in all designs that must be considered when evaluating an instrument.
Supplementary Material
Acknowledgments
This study was supported in part by NIH R01 EY 013322, NIH R24 EY016664, and an unrestricted grant and Senior Scientific Investigator Award (WMP) from Research to Prevent Blindness, Inc., NY, NY.
REFERENCES
- 1.Petroll WM, Jester JV, Cavanagh HD. Clinical Confocal Microscopy. Curr Opinion Ophthalmol. 1998;9:59–65. doi: 10.1097/00055735-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Tervo T, Moilanen J. In vivo confocal microscopy for evaluation of wound healing following corneal refractive surgery. Progress in Retinal & Eye Research. 2003;22:339–358. doi: 10.1016/s1350-9462(02)00064-2. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman SC, Kaufman HE. How has confocal microscopy helped us in refractive surgery? Curr Opin Ophthalmol. 2006;17:380–388. doi: 10.1097/01.icu.0000233959.73262.99. [DOI] [PubMed] [Google Scholar]
- 4.Labbe A, Khammari C, Dupas B, Gabison E, Brasnu E, Labetoulle M, Baudouin C. Contribution of in vivo confocal microscopy to the diagnosis and management of infectious keratitis. The Ocular Surface. 2009;7:41–52. doi: 10.1016/s1542-0124(12)70291-4. [DOI] [PubMed] [Google Scholar]
- 5.Efron N. Contact lens-induced changes in the anterior eye as observed in vivo with the confocal microscope. Prog Retin Eye Res. 2007;26:398–436. doi: 10.1016/j.preteyeres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Erie JC, Patel SV, McLaren JW, Hodge DO, Bourne WM. Corneal keratocyte deficits after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol. 2006;141:799–809. doi: 10.1016/j.ajo.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Patel SV, McLaren JW, Hodge DO, Bourne WM. Normal human keratocyte density and corneal thickness measurement by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 2001;42:333–339. [PubMed] [Google Scholar]
- 8.Erie JC, Nau CB, McLaren JW, Hodge DO, Bourne WM. Long-term Keratocyte Deficits in the Corneal Stroma after LASIK. Ophthalmology. 2004;111:1356–1361. doi: 10.1016/j.ophtha.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Ku JYF, Niederer RL, Patel DV, Sherwin T, McGhee CNJ. Laser Scanning In Vivo Confocal Analysis of Keratocyte Density in Keratoconus. Ophthalmology. 2008:115. doi: 10.1016/j.ophtha.2007.04.067. [DOI] [PubMed] [Google Scholar]
- 10.Niederer RL, Perumal D, Sherwin T, McGhee CNJ. Laser Scanning In Vivo Confocal Microscopy Reveals Reduced Innervation and Reduction in Cell Density in All Layers of the Keratoconic Cornea. Invest Ophthalmol Vis Sci. 2008:49. doi: 10.1167/iovs.07-0968. [DOI] [PubMed] [Google Scholar]
- 11.Auran JD, Koester CJ, Kleiman NJ, Rapaport R, Bomann JS, Wirotsko BM, Florakis GJ, Koniarek JP. Scanning slit confocal microscopic obervation of cell morphology and movement within the normal human anterior cornea. Ophthalmology. 1995;102:33–41. doi: 10.1016/s0161-6420(95)31057-3. [DOI] [PubMed] [Google Scholar]
- 12.Masters BR, Thaer AA. In vivo human corneal confocal microscopy of identical fields of subepithelial nerve plexus, basal epithelial, and wing cells at different times. Microsc Res Tech. 1994;29:350–356. doi: 10.1002/jemt.1070290505. [DOI] [PubMed] [Google Scholar]
- 13.Darwish T, Brahma A, O’Donnell C, Efron N. Subbasal nerve fiber regeneration after LASIK and LASEK assessed by noncontact esthesiometry and in vivo confocal microscopy: Prospective study. J Cataract Refract Surg. 2007;33:1515–1521. doi: 10.1016/j.jcrs.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Benítez-del-Castillo JM, Acosta MC, Wassfi MA, Díaz-Valle D, Gegúndez JA, Fernandez C, García-Sánchez J. Relation between Corneal Innervation with Confocal Microscopy and Corneal Sensitivity with Noncontact Esthesiometry in Patients with Dry Eye. Invest Ophthalmol Vis Sci. 2007:48. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- 15.Tuisku IS, Konttinen YT, Konttinen LM, Tervo TM. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjö gren’s syndrome. Experimental eye Research. 2008;86:879–885. doi: 10.1016/j.exer.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Imayasu M, Petroll WM, Jester JV, Patel SK, Cavanagh HD. The relationship between contact lens oxygen transmissibility and binding of pseudomonas aeruginosa to the cornea after overnight wear. Ophthalmol. 1994;101:371–386. doi: 10.1016/s0161-6420(94)31326-1. [DOI] [PubMed] [Google Scholar]
- 17.Ren DH, Yamamoto K, Ladage PM, Molai M, Li L, Petroll WM, Jester JV, Cavanagh HD. Adaptive effects of 30-night wear of hyper-O2 transmissible contact lenses on bacterial binding and corneal epithelium: A 1 year clinical trial. Ophthalmology. 2002;109:27–39. doi: 10.1016/s0161-6420(01)00867-3. [DOI] [PubMed] [Google Scholar]
- 18.Cavanagh HD, Ladage PM, Li SL, Yamamoto K, Molai M, Ren DH, Petroll WM, Jester JV. Effects of daily and overnight wear of a novel hyper oxygen-transmissible soft contact lens on bacterial binding and corneal epithelium. Ophthalmology. 2002;109:1957–1969. doi: 10.1016/s0161-6420(02)01278-2. [DOI] [PubMed] [Google Scholar]
- 19.Ladage PM, Yamamoto K, Ren DH, Li L, Jester JV, Petroll WM, Cavanagh HD. Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated cells. Ophthalmology. 2001;108:1279–1288. doi: 10.1016/s0161-6420(01)00639-x. [DOI] [PubMed] [Google Scholar]
- 20.Robertson DM, Petroll WM, Cavanagh HD. The effect of nonpreserved care solutions on 12 months of daily and extended silicone hydrogel contact lens wear. Ophthalmology. 2008;49:7–15. doi: 10.1167/iovs.07-0940. [DOI] [PubMed] [Google Scholar]
- 21.Dhaliwal JS, Kaufman SC, Chiou AGY. Current applications of clinical confocal microscopy. Curr Opin Ophthalmol. 2007;18:300–307. doi: 10.1097/ICU.0b013e3281b11665. [DOI] [PubMed] [Google Scholar]
- 22.Petran M, Hadravsky M, Egger MD, Galambos R. Tandem scanning reflected light microscope. J Opt Soc Am. 1968;58:661–664. [Google Scholar]
- 23.Petran M, Hadravsky M, Benes J, Kucera R, Boyde A. The tandem scanning reflected light microscope: Part I: the principle, and its design. Proc R Microsc Soc. 1985;20:125–129. [Google Scholar]
- 24.Lemp MA, Dilly PN, Boyde A. Tandem scanning (confocal) microscopy of the full thickness cornea. Cornea. 1986;4:205–209. [PubMed] [Google Scholar]
- 25.Cavanagh HD, Shields W, Jester JV, Lemp MA, Essepian J. Confocal microscopy of the living eye. CLAO J. 1990;16:65–73. [PubMed] [Google Scholar]
- 26.Petroll WM, Cavanagh HD, Jester JV. 3-Dimensional reconstruction of corneal cells using in vivo confocal microscopy. J Microsc. 1993;170:213–219. doi: 10.1111/j.1365-2818.1993.tb03344.x. [DOI] [PubMed] [Google Scholar]
- 27.Jester JV, Petroll WM, Feng W, Essepian J, Cavanagh HD. Radial keratotomy: I. The wound healing process and measurement of incisional gape in two animal models using in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 1992;3:3255–3270. [PubMed] [Google Scholar]
- 28.Masters BR, Thaer AA. Real-time scanning slit confocal microscopy of the in vivo human cornea. Appl Optics. 1994;33:695–701. doi: 10.1364/AO.33.000695. [DOI] [PubMed] [Google Scholar]
- 29.Brakenhoff GJ, Visscher K. Confocal imaging with bilateral scanning and array detectors. J Microsc. 1992;165:139–146. [Google Scholar]
- 30.Erie EA, McLaren JW, Kittleson KM, Patel SV, Erie JC, Bourne WM. Corneal Subbasal Nerve Density: A Comparison of Two Confocal Microscopes. Eye & Contact Lens. 2008;34:322–325. doi: 10.1097/ICL.0b013e31818b74f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guthoff RF, Baudouin C, Stave J. Atlas of confocal laser scanning in vivo microscopy in ophthalmology. Heidelberg/Springer; Berlin: 2006. [Google Scholar]
- 32.Zhivov A, Stachs O, Stave J, Guthoff RF. In vivo three-dimensional confocal laser scanning microscopy of corneal surface and epithelium. Br J Ophthalmol. 2009;93:667–672. doi: 10.1136/bjo.2008.137430. [DOI] [PubMed] [Google Scholar]
- 33.Wiegand W, Thaer AA, Kroll P, Geyer OC, Garcia AJ. Optical sectioning of the cornea with a new confocal in vivo slit-scanning video-microscope. Ophthalmology. 1993;100(9A):128. doi: 10.1016/s0161-6420(95)30981-5. [DOI] [PubMed] [Google Scholar]
- 34.Li HF, Petroll WM, Moller-Pedersen T, Maurer JK, Cavanagh HD, Jester JV. Epithelial and corneal thickness measurements by in vivo confocal microscopy through focusing (CMTF) Current Eye Research. 1997;16:214–221. doi: 10.1076/ceyr.16.3.214.15412. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Jester JV, Cavanagh HD, Black TD, Petroll WM. On-line 3-dimensional confocal imaging in vivo. Invest Ophthalmol Vis Sci. 2000;41:2945–2953. [PubMed] [Google Scholar]
- 36.Moller-Pedersen T, Li H, Petroll WM, Cavanagh HD, Jester JV. Confocal microscopic characterization of wound repair after photorefractive keratectomy using in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 1998;39:487–501. [PubMed] [Google Scholar]
- 37.Møller-Pedersen T, Vogel M, Li HF, Petroll WM, Cavanagh HD, Jester JV. Quantification of stromal thinning, epithelial thickness, and corneal haze after photorefractive keratectomy using in vivo confocal microscopy. Ophthalmology. 1997;104:360–368. doi: 10.1016/s0161-6420(97)30307-8. [DOI] [PubMed] [Google Scholar]
- 38.McCulley JP, Petroll WM. Quantitative assessment of corneal wound healing following IntraLASIK using in vivo confocal microscopy. Trans Am Ophthalmol Soc. 2008;106:84–92. [PMC free article] [PubMed] [Google Scholar]
- 39.Erie JC, Patel SV, McLaren JW, et al. Effect of myopic laser in situ keratomileusis on epithelial and stromal thickness. Ophthalmology. 2002;109:1447–1452. doi: 10.1016/s0161-6420(02)01106-5. [DOI] [PubMed] [Google Scholar]
- 40.Vesaluoma M, Perez-Santonja J, Petroll WM, Linna T, Alio J, Tervo T. Corneal stromal changes induced by myopic LASIK. Invest Ophthalmol Vis Sci. 2000;41:369–376. [PubMed] [Google Scholar]
- 41.McLaren JW, Nau CB, Patel SV, Bourne WM. Measuring corneal thickness with the confoscan 4 and Z-ring adapter. Eye Contact Lens. 2007;33:185–190. doi: 10.1097/ICL.0b013e31802b3114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.