Abstract
Previous studies have demonstrated abnormal joint torque coupling and associated muscle coactivations of the upper extremity in individuals with unilateral stroke. We investigated the effect of upper limb configuration on the expression of the well-documented patterns of shoulder abduction/elbow flexion and shoulder adduction/elbow extension. Maximal isometric shoulder and elbow torques were measured in stroke subjects in four different arm configurations. Additionally, an isometric combined torque task was completed where subjects were required to maintain various levels of shoulder abduction/adduction torque while attempting to maximize elbow flexion or extension torque. The dominant abduction/elbow flexion pattern was insensitive to changes in limb configuration while the elbow extension component of the adduction/extension pattern changed to elbow flexion at smaller shoulder abduction angles. This effect was not present in control subjects without stroke. The reversal of the torque-coupling pattern could not be explained by mechanical factors such as muscle length changes or muscle strength imbalances across the elbow joint. Potential neural mechanisms underlying the sensitivity of the adduction/elbow extension pattern to different somatosensory input resultant from changes in limb configuration are discussed along with the implications for future research.
Keywords: Stroke, Arm, Posture, Torque, Electromyography
Introduction
Abnormal joint torque coupling and associated shoulder/elbow muscle coactivation is a persistent impairment in individuals with stroke who are commonly classified as being moderately to severely impaired (Dewald et al. 1995; Dewald and Beer 2001). Our group and others have previously quantified abnormal muscle coactivations and joint torque-coupling patterns in both static (Dewald et al. 1995; Beer et al. 1999; Dewald and Beer 2001; Lum et al. 2003) and dynamic (Beer et al. 2000, 2004) protocols reflecting constraints in the ability of individuals with stroke to generate certain joint torque combinations (Twitchell 1951; Brunnstrom 1970). The mutability of abnormal muscle coactivation and joint torque-coupling has been addressed recently in studies attempting to change or remediate the impairment through eight weeks of physical interventions (Lum et al. 2004; Ellis et al. 2005). In this report, we pose the question of whether these abnormal patterns are affected by altering afferent somatosensory feedback resulting from changes in static limb configuration. Motor output to distal upper extremity (Dominici et al. 2005; Ginanneschi et al. 2005; Ginanneschi et al. 2006) and lower extremity (Knikou and Rymer 2002a, b) muscles is known to be modulated by changes in static proximal joint postures in individuals without stroke. We designed a protocol where individuals with stroke and control subjects generated isometric single- and multi-joint torque combinations in four different arm configurations. We postulated that abnormal joint torque combinations would be affected by changes in static limb configuration and thus be modulated by afferent somatosensory input. If an altered limb configuration has an effect on abnormal joint torque-coupling we suggest that reorganization at the level of the spinal cord, specifically bulbospinal contributions, (Dewald et al. 1999) affects the integration of somatosensory input. Results of this study have significant implications for our understanding of sensory mechanisms underlying the expression of abnormal torque-coupling and for the development of associated measurement techniques.
Materials and methods
Subjects
Eleven individuals including 10 men and 1 woman, ages 31–66 years, ranging from 14 to 289 months after unilateral cortical and/or subcortical stroke, and four individuals without stroke, ages 45–69, participated in the study. All subjects were screened for the study by the primary author. Exclusion criteria were greater than mild impairment of upper extremity tactile sensation and proprioception, difficulty with sitting for long durations, recent changes in the medical management of hypertension, and any acute or chronic painful condition in the upper extremities or spine. The upper extremity portion of the Fugl-Meyer Motor Assessment (Fugl-Meyer et al. 1975) was administered to stroke subjects as an initial screening measure for the affected upper extremity to qualitatively determine the presence and extent of flexion synergy and categorize the level of impairment severity. The inclusion criteria for individuals with stroke required a broad range of impairment severity with exception of individuals without measurable impairment or individuals with near complete paralysis. Stroke subjects scored 18–49 out of 66 on the Fugl-Meyer Motor Assessment. All subjects were able to support the upper limb against gravity and generate at least minimal active elbow extension. All subjects had passive range of motion to at least 90° of shoulder flexion, abduction, and neutral internal/external rotation that was required to participate in the study. All subjects had intact upper extremity sensation and proprioception as measured by a tactile localization task and an awareness of movement task (O'Sullivan and Schmitz 2001). Overpressure at the end of the range of motion was used as a screening measure to verify the absence of inflammation at the shoulder, elbow, wrist and fingers (Hertling and Kessler 1996). Following screening, the subjects gave informed consent to participate in the study, which was approved by the Institutional Review Board of Northwestern University in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki for research involving human subjects.
Experimental arrangement
Each subject was seated in a Biodex chair with shoulder and waist strapping to restrain trunk and shoulder girdle movement during testing. Four different arm configurations were studied involving three positions with the shoulder in 75° of abduction and 40° of shoulder flexion (horizontal shoulder adduction) with the elbow at angles of 70° (position #1), 90° (position #2), and 130° (position #3) and one position with the shoulder in 20° of abduction and neutral flexion/extension with the elbow at 90° (position #4). The forearm, wrist, and hand were fixed to a 6-DOF load cell (JR3 Inc., Woodland, CA, USA; Model #45E15A) using fiberglass casting and a Delrin ring mounted at the wrist (Fig. 1). Prior to data collection in each arm position, the load cell was calibrated/zeroed with the subject fully at rest (quiescent EMG recordings). Orthogonal forces and moments measured by the load cell were filtered and converted on-line to torques at the elbow and shoulder via methods described by Beer et al. (1999). Real-time visual feedback was provided to the subject, via computer monitor, of the torque produced at the shoulder and/or elbow joint during both a single-task protocol and a dual-task protocol.
Fig. 1.
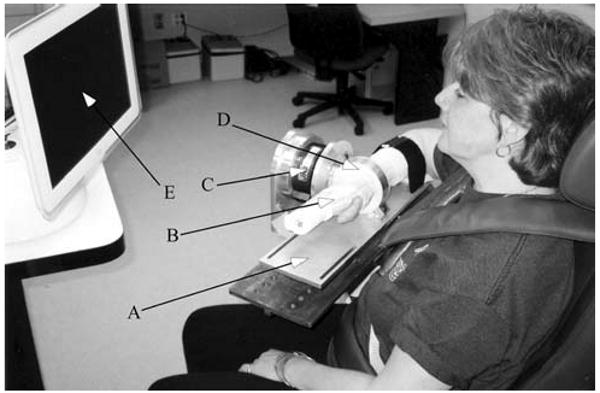
Image of the experimental setup in position 2. a Forearm interface plate mounted directly to load cell. b Fiberglass cast. c 6-DOF load cell. d Delrin cast interface ring. e Video feedback monitor
Electromyographic (EMG) signals were recorded during all trials from the brachioradialis; biceps brachii; lateral and long heads of triceps brachii; anterior, intermediate, and posterior deltoid; and vertical fibers of pectoralis major. Correct electrode placements were verified by examination of EMG activity. Active differential electrodes (Delsys, 16-channel Bagnoli EMG System, Boston, MA, USA) with 1 cm interelectrode distance were used to record surface EMG from the upper-limb muscles. The Delsys EMG system also provides pre-amplification (gain, 1,000) and single-pole high-pass filtering (cutoff frequency, 6 Hz). All EMG signals were filtered at 500 Hz (8-pole Butterworth; Frequency Devices Model 9016, Havelhill, MA, USA) to prevent aliasing and amplified in a second stage prior to data collection. The force/torque and EMG signals were collected at a sampling rate of 1,000 Hz via an analog/digital converter and stored on a computer for future analysis.
Single-task protocol
Maximum voluntary torques were measured at the shoulder and elbow in all limb positions. Three random blocks consisting of shoulder abduction/adduction, flexion/extension, and elbow flexion/extension were completed for position 1 mirroring methods reported previously (Dewald and Beer 2001) and in three additional positions (2, 3, and 4). Joint torques were concurrently measured at both the shoulder and elbow while the subject attempted to maximize the torque in the primary direction, which was shown in real-time on a computer monitor.
Dual-task protocol
Eight of the 11 subjects with stroke and all of the control subjects completed a dual-task protocol in positions 2 and 4. Subjects maintained various percentages of maximum shoulder abduction or adduction while attempting to maximize either elbow flexion or extension. Randomized blocks of 25, 50, and 75% of maximum abduction or adduction were completed for a total of 12 dual-task conditions. Each block consisted of three to ten repetitions such that the best representation of maximum elbow torque during abduction/adduction was achieved. Similar to the method of the single-task protocol, maximum elbow torque was ensured by verifying that three torque values were acquired and were within 10% in magnitude with the last torque not being the largest. Additional trials were taken until these criteria were met. Subjects had a 2 min rest break between each block and a 15–30 s rest break between each repetition. Each repetition was 10 s in duration and sampled at 1,000 Hz for all degrees of freedom (DOF).
During each block, the subject viewed a computer monitor that displayed, in real time, the magnitude of the isometric joint torques that they were generating for both the abduction/adduction DOF and the elbow flexion/extension DOF. Shoulder abduction was represented by counter-clockwise rotation of a speedometer-like needle within a circular cursor while vertical or horizontal movement of a circular cursor on the screen represented elbow flexion and/or extension. The vertical or horizontal assignment was dependent upon limb position and the side of the body being tested such that the cursor always moved on the screen in a direction intuitive to the subject as the direction they attempted to develop force in. A pie-piece shape within the circular cursor represented the target window for shoulder abduction torque, while a circular target at the side or top/bottom of the screen represented the subject's maximum level of elbow flexion/extension. The area of the pie-piece representing the required shoulder torque was set at the target torque with a tolerance of ±15%.
The goal for every repetition of each block was for the subject to develop and maintain the appropriate level of isometric shoulder torque and then generate their maximum elbow torque. Constant verbal encouragement was given to maintain appropriate shoulder torque while maximizing elbow torque.
Data analysis
Analysis of single-task torque data
MVTs for each of the six torque directions were determined using custom software written within the Matlab environment (Mathworks, Inc.). For each 5 s trial, and in each torque direction, the peak torque was determined by identifying the 250 ms window with the largest average torque magnitude. The MVT for each direction was taken as the maximum peak torque across all trials. Secondary shoulder and elbow torques generated concurrently during the 250 ms window of peak shoulder abduction and shoulder adduction torque were measured to quantify the abnormal joint torque-coupling pattern (Dewald and Beer 2001). All joint torques were normalized to the MVT occurring within the testing session for comparison of abnormal torque-coupling between subjects. The normality of the data was confirmed using the Shapiro–Wilkes test. A one-factor analysis of variance (ANOVA) was completed to test the effect of limb position on normalized secondary joint torque during shoulder abduction or adduction. Post hoc comparisons were based on Scheffe's test.
Absolute maximal joint torques were used in a one-factor ANOVA to test the effect of limb position on MVT for each of the tested DOFs. The normality of the data was confirmed using the Shapiro–Wilkes test. Multiple comparisons were accounted for using Scheffe's test.
Analysis of dual-task protocol data
Torque data were obtained for all repetitions of each of the 12 dual-task blocks in both limb positions. Maximum elbow flexion/extension torque during various percentages of shoulder abduction/adduction was determined using custom software written within the Matlab environment (Mathworks, Inc.). For each 10 s trial, criteria were established to identify the maximum elbow torque generated for each percent abduction/adduction level. For trials in which subjects were attempting to maximize elbow flexion, whether it was during abduction or adduction, the custom software searched for the peak elbow flexion (250 ms window) occurring during the appropriate level of shoulder torque or, in cases where subjects generated elbow extension instead of elbow flexion, the custom software searched for the lowest elbow extension torque. The opposite was the case for when subjects were attempting to maximize elbow extension during various percentages of shoulder abduction/adduction. If elbow extension did not occur then the software would search for the lowest elbow flexion. Subjects were required to sustain the appropriate shoulder torque for at least 250 ms in order to identify the peak elbow torque. These criteria allowed us to identify the best performance of generating the required elbow torques during a controlled amount of shoulder abduction/adduction. Peak elbow torques were normalized to maximum elbow flexion and extension MVTs. Normalized elbow torques were averaged across subjects within each group for each of the 12 dual-task combinations of both limb positions. Separate two-factor ANOVAs were completed for each group to test the effect of limb position and percent shoulder torque on best elbow torque performance for both elbow flexion and extension trials. Three-factor ANOVAs with interactions were completed to test the effect of group, limb position and percent shoulder torque on best elbow torque performance for each of the four shoulder/elbow dual-task combinations. The normality of the data was confirmed using the Shapiro–Wilkes test. Post hoc comparisons were based on Scheffe's test. A significant effect or difference was defined as a P value of less than 0.05.
Analysis of EMG data
Individual muscle activation or EMG was determined during both single- and dual-task trials for all subjects. EMG signals were rectified and averaged during a 250 ms window that was temporally offset by 30 ms to the corresponding peak torque time window to deal with the electromechanical delay of the human skeletal muscle (Cavanagh and Komi 1979; Bober et al. 1982; Kornecki and Zschorlich 1994). Averaged EMG was normalized by the maximum EMG measured within the same testing session to facilitate comparison between limb positions. All EMG data were confirmed to be normally distributed by the Shapiro–Wilkes test. A single-factor ANOVA was completed to test the effect of limb position on muscle activation for each muscle during both single- and dual-task conditions. Post hoc comparisons were based on Scheffe's test.
Results
Single-task abnormal torque-coupling
There was a significant effect (P < 0.05) of limb position on secondary elbow torque and associated muscle electromyography for both single-task abduction and adduction MVTs. During abduction MVTs, normalized secondary elbow flexion (44 ± 11%) was less in position 4 as compared to positions 1–3 which ranged from 67 ± 7 to 83 ± 5%. Concurrent normalized elbow flexor activity corresponded with joint torques in that it was less in position 4 (BRD, 36 ± 8%; BIC, 45 ± 9%) than in positions 1–3 (BRD, 56 ± 7 to 67 ± 5%; BIC, 65 ± 6 to 70 ± 3%) while elbow extensor activity (TRILA, 21 ± 6 to 28 ± 8%; TRILH, 13 ± 2 to 23 ± 7%) remained unchanged in all positions. During adduction MVTs, normalized secondary elbow torque was reversed from extension in the first three positions (64 ± 9 to 0 ± 17%) to flexion (54 ± 8%) in position 4. Concurrent elbow flexor activity corresponded with the torque reversal in that it was greater in position 4 (BRD, 45 ± 5%; BIC, 57 ± 7%) than in positions 1–3 (BRD, 18 ± 4 to 39 ± 6%; BIC, 19 ± 5 to 37 ± 6%) while elbow extensor activity was less in position 4 (TRILA, 22 ± 3%; TRILH, 15 ± 2%) than in positions 1–3 (TRILA, 40 ± 6 to 61 ± 6%; TRILH, 56 ± 5 to 61 ± 5%).
Absolute torques were compared between limb positions to rule out changes in muscle length and moment arms as an explanation for the effect of limb position, specifically at position 4, on secondary torque-coupling. There was no significant difference (P > 0.05) in absolute MVTs between position 4 and positions 1–3 for abduction (position 4, 24.4 ± 4.1 Nm; positions 1–3, 22.2 ± 2 to 24.3 ± 2.2 Nm), elbow flexion (position 4, 32.6 ± 3.1 Nm; positions 1–3, 27.6 ± 2.1 to 36.3 ± 3.6 Nm), and elbow extension (position 4, 20.5 ± 3.5 Nm; positions 1–3, 14.6 ± 3.0 to 23.9 ± 2.3 Nm). Adduction was only slightly less but significantly different (P < 0.05) in position 4 (19.7 ± 2.1 Nm) than in positions 1–3 (28.1 ± 3.7 to 31.4 ± 3.0 Nm).
Dual-task torque pattern generation
There was a significant effect of abduction level but not limb position on best elbow torque performance during the abduction dual-task in stroke subjects. The effect of abduction level was an expected result and is consistent with previous reports (Beer et al. 1999). Individuals with stroke generated higher levels of elbow flexion torque and lower levels of elbow extension torque as the percent of required shoulder abduction increased from 25 to 50 to 75% maximum for both positions 2 and 4. In position 2, elbow flexion torque increased from 50 ± 8 to 76 ± 7% while elbow extension torque decreased from 32 ± 13 to below 0% into a flexion moment of 7 ± 15% at higher levels of abduction consistent with previous reports (Beer et al. 1999). In position 4, elbow flexion torque increased from 59 ± 10 to 75 ± 18% while elbow extension torque decreased from 14 ± 20% to below 0% into a flexion moment of 8 ± 18% at higher levels of abduction. In both positions, stroke subjects were able to generate a net extension torque only at the lowest abduction level (25%).
There was also a significant effect of group (stroke vs. control) and no interaction effects on best elbow torque performance in that control subjects were able to generate near maximal levels of elbow flexion (71 ± 8 to 84 ± 8%) and elbow extension torque (64 ± 9 to 76 ± 7%) regardless of abduction level while stroke subjects only generated similar elbow flexion torques at the 50% (position 2, 67 ± 9%; position 4, 77 ± 12%) and 75% (position 2, 76 ± 7%; position 4, 75 ± 18%) abduction levels. Therefore, stroke subjects were only able to perform at the level of controls when operating maximally within the abnormal coupling pattern of abduction/elbow flexion despite limb position.
There was a significant effect of limb position and adduction level on best elbow torque performance during the adduction dual-task. Notably, the effect of limb position in the stroke group reflected the results found in the single-task adduction MVTs. Stroke subjects generated decreasing levels of elbow flexion in position 2 and increasing levels of elbow flexion in position 4 as the required level of adduction increased (Fig. 2a). Similarly, stroke subjects generated greater levels of elbow extension in position 2 than in position 4 (Fig. 2b) at all levels of adduction.
Fig. 2.
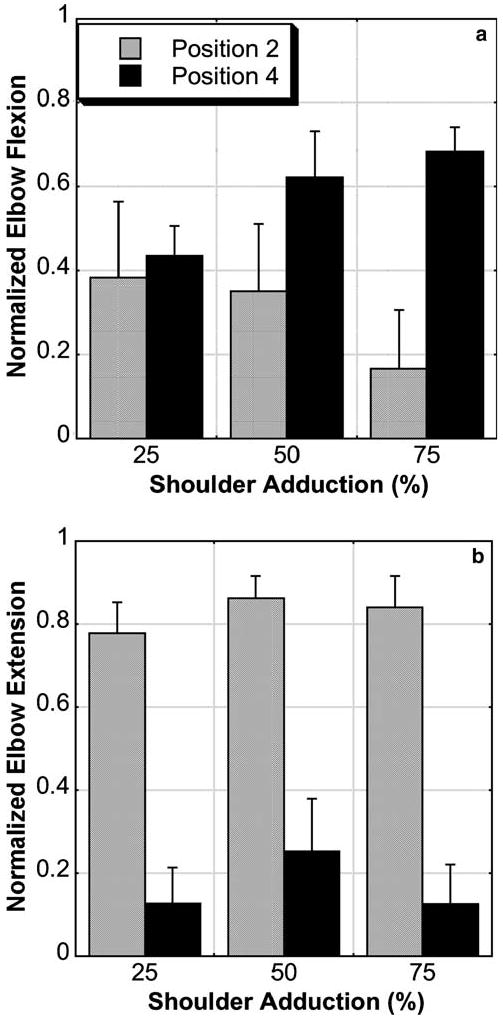
Mean with standard error bars of normalized elbow flexion torque (a) and extension torque (b) during various percentages of maximum shoulder adduction in positions 2 and 4. Subjects were able to generate more elbow flexion and less elbow extension during adduction in position 4
Concurrent elbow flexor and extensor EMG was consistent with the measured torques in that there was a significant effect of both adduction level and limb position on normalized EMG. Elbow extensor activity was consistently less in position 4 than in position 2 during adduction/elbow extension (Fig. 3d) and elbow flexor activity increased more with greater levels of adduction during adduction/elbow flexion (Fig. 3a) with the arm in position 4.
Fig. 3.
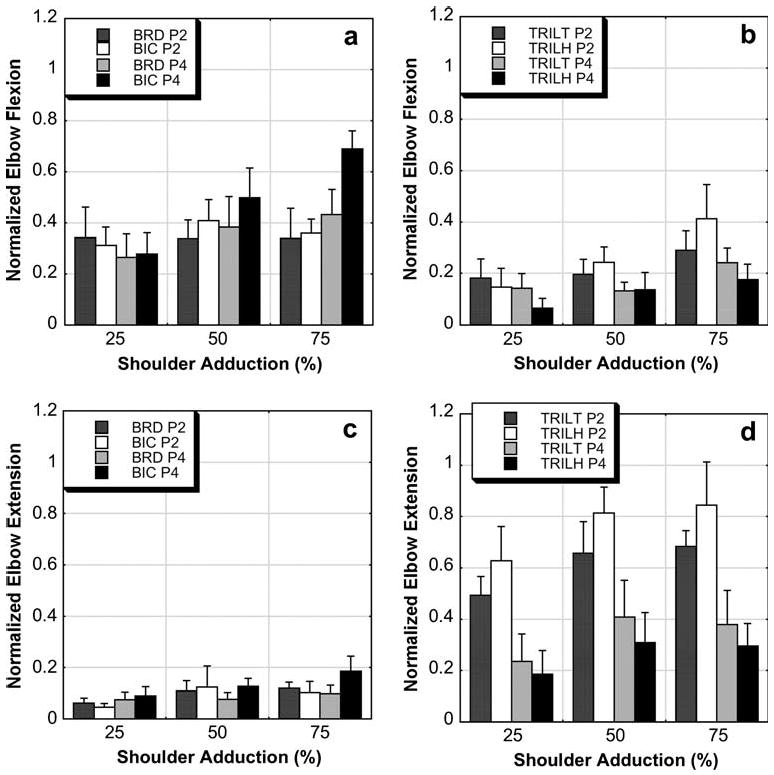
Mean with standard error bars of normalized elbow flexor and extensor EMG occurring during the adduction/elbow extension task and adduction/elbow flexion task. The graphs are organized by muscle group (flexors, BRD and BIC; extensors, TRILT and TRILH) and task such that the effects of position (P2 position 2, P4 position 4) and adduction level (25, 50, and 75% of maximum adduction) can be visually appreciated. During the adduction/elbow flexion task, there was an effect of position and adduction level for the BIC (a) and an effect of level and position on the TRILH (b). During the adduction/elbow extension task, there was an effect of position for both elbow extensors (d) but not for the flexors (c). These EMG data reflect the torque data
There was a significant effect of group on best elbow torque performance during the adduction dual-task in that control subjects were able to generate near maximal levels of elbow torque regardless of adduction level (Fig. 4). Furthermore, there was an interaction effect between group and position for both adduction tasks (data from Figs. 2, 4). Most notably, in the adduction/elbow flexion task, elbow flexion in position 4 for the stroke group was significantly greater than in position 2 but still significantly less than either position in the control group. Similarly, for the adduction/elbow extension task, elbow extension in position 4 was significantly less than in position 2 and either position in the control group. There was no effect of adduction level or subsequent interaction effect involving adduction level. Therefore, in stroke subjects, the abnormal coupling pattern of adduction/elbow extension was present only while in position 2. While in position 4, the abnormal coupling pattern of adduction/elbow extension was no longer evident. Instead, elbow flexion was strongly coupled to both abduction and adduction in position 4, which was inconsistent with previous reports (Beer et al. 1999) where the limb was only tested in the horizontal plane (similar to position 2).
Fig. 4.
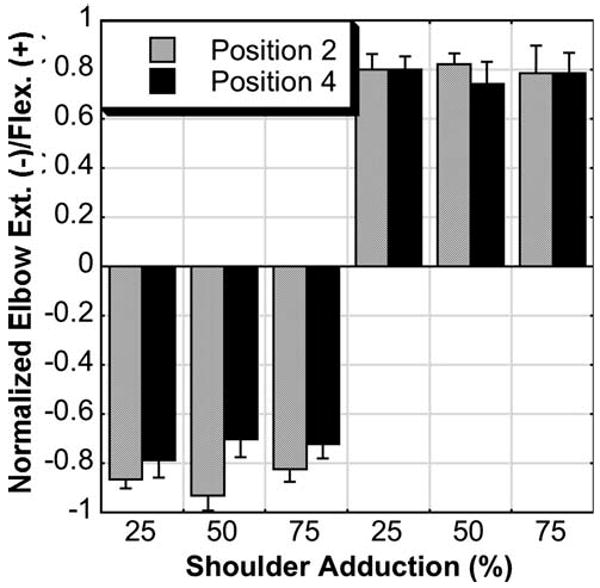
Mean with standard error bars of normalized elbow extension (−) and flexion (+) torque during various percentages of required adduction in positions 2 and 4 for control subjects. Individuals without stroke were able to generate near maximum elbow torques regardless of required adduction level
Discussion
This study demonstrated abnormal torque-coupling similar to what Beer et al. (1999) reported and expanded on the inquiry by measuring different limb positions in an attempt to better understand whether the integration of somatosensory information is altered following stroke. A significant effect of shoulder abduction angle (position) on torque coupling was identified when stroke subjects were required to generate maximal adduction torque (see single-task subsection). When the arm abduction angle was reduced such that the arm was near to the side of the body, there was a reversal in the torque-coupling pattern during adduction that was not seen in other arm positions. Subjects with stroke were also no longer able to generate adduction/elbow extension torque patterns in the 20° abduction angle position (position 4) as they were in the 75° abduction angle position (position 2) and as the control subjects were (see Figs. 2, 4). Conversely, subjects were able to generate abnormal abduction/elbow flexion in all limb positions. This suggests that the expression of the abnormal joint torque-coupling pattern of abduction/elbow flexion is robust. We expected this result considering other investigators (Lum et al. 2003) have reported the presence of the abduction/elbow flexion pattern in shoulder positions more adducted than used in our previous studies (Beer et al. 1999; Dewald and Beer 2001). The pattern involving adduction was not robust reflecting a clear effect of limb position. However, in control subjects, there was no effect of position (see Fig. 4) during the dual task illustrating their ability to centrally alter the changing somatosensory input resultant from different shoulder angles. Stroke subjects showed no difference in absolute maximum elbow torques in various positions ruling out muscle length changes as a contributing factor to the effect of position (see single-task subsection). Similarly, there was no difference between elbow flexion and elbow extension torques indicating that elbow strength imbalances in our stroke subjects did not contribute to the torque-coupling patterns as reported elsewhere (Lum et al. 2003). The lack of significant mechanical effects suggests that following stroke the central nervous system reorganizes affecting how somatosensory input is integrated within the central nervous system.
Somatosensory input from the shoulder and arm is processed at the level of the spinal cord and conveyed to supraspinal centers through both monosynaptic and oligosynaptic pathways (Diederichsen et al. 2002) although its exact role in relation to the control of human movement remains controversial (Prochazka et al. 2000). Central nervous system reorganization following stroke (Chen et al. 2002) is associated with changes at the level of the spinal cord due to a loss of descending corticospinal input and a subsequent greater influence of bulbospinal input (Dewald et al. 1999). Increased monoaminergic input to motoneurons originating from the metabotropic outputs of the raphe nucleus (Alvarez et al. 1998) and locus coeruleus nucleus (Giroux et al. 1999) is known to result in increased spinal motoneuronal excitability (Heckman et al. 2003). In addition to the neuromodulatory influences on motoneurons, primary descending input for volitional movement is likely through an ionotropic mechanism (Powers and Binder 2001). Descending pontomedullary reticular formation output to the limbs is shown primarily to be from shoulder abductors and elbow flexors (Davidson and Buford 2004) and contribute to the bilateral control of reaching movements in the cat (Schepens and Drew 2004) and monkey (Davidson and Buford 2006). These reports were consistent with prior work documenting the prevalent response of ipsilateral projections from the pontomedullary reticular formation to limb flexors (Drew and Rossignol 1990a, b). Considering these linkages, we attribute the robust abduction/elbow flexion pattern to be a result of increased influence from bulbospinal pathways, specifically ipsilateral reticulospinal pathways, with the coactivation of abductors and flexors explained by the multisegmental collateralization of these pathways (Matsuyama et al. 2004). Such influence would explain the persistent abduction/flexion pattern despite changing somatosensory inputs generated by different limb configurations and therefore the dominant clinical presentation of abduction/elbow flexion coupling following stroke (Foerster 1936; Twitchell 1951; Brunnstrom 1970).
The position dependence of the adductor pattern in stroke subjects may still be explained by enhanced bulbospinal influence following stroke, but more specifically, may indicate a shift in balance between two constituent brainstem motor systems, namely the vestibulospinal and reticulospinal pathways. The vestibular system contributes to arm-trunk coordination during reaching (Adamovich et al. 2001; Mars et al. 2003) and is sensitive to the biomechanical constraints of the task (Tunik et al. 2003). In the absence of visual and proprioceptive feedback, vestibulospinal contribution was primarily evident during reaching toward an ipsilateral target as opposed to a contralateral target (Tunik et al. 2003). Activation of the vestibular pathway through galvanic stimulation has been shown to elicit triceps brachii activity when the arm is used in an isometric postural manner (Baldissera et al. 1990; Britton et al. 1993). In our stroke subjects, significant triceps activity resulting in net elbow extension torque during adduction was only possible when the arm was positioned in the horizontal plane out away from the body. Outward positions of the arm changed the biomechanical constraints of the volitional efforts and may have resulted in an increased contribution from the vestibulospinal system similar to that reported by Tunik and colleagues (Tunik et al. 2003) explaining the increased activation of elbow extensors during adduction. Conversely, changes in sensory feedback resulting from placing the upper arm in a more adducted position may have reduced the contribution of the vestibulospinal system and shifted the balance toward reticulospinal activity thus resulting in an elbow flexion bias during the generation of adduction torques.
While contributions from bulbospinal systems were not directly measured in the present study, their role in abnormal joint torque-coupling should be demonstrated in future work to elucidate the contribution of reticulo- and/or vestibulospinal pathways to the generation of stroke-induced impairments. This may be realized in the stroke population through the application of direct probes of brainstem centers such as galvanic vestibular stimulation (Cauquil and Day 1998), auditory stimulation (Troiani et al. 2004), startle reflex elicitation (Valls-Sole et al. 1999), and pharmacologic monoaminergic manipulations.
Conclusion
In the presence of changing somatosensory input, upper extremity motor outflow following stroke is subservient to abnormal torque-coupling mostly in the abduction/elbow flexion pattern and less so in the adduction/elbow extension pattern. This relationship should be considered in future work attempting to quantify this impairment. Identification of specific brainstem contributions to the torque-coupling impairment is also a critical avenue of future research. This and our past work have identified techniques to quantify abnormal torque-coupling (Dewald et al. 1995; Beer et al. 1999, 2004; Dewald and Beer 2001); however, the definitive physiological mechanism responsible remains unknown. Our current and future work will seek to identify and discriminate between contributions from the cortex, brainstem, and spinal cord to the expression of abnormal joint torque-coupling patterns.
Acknowledgments
The authors express our thanks to Dr. Charles Heckman for his suggestions relating to the contributions of the monoaminergic system. The authors would also like to express our thanks to the manuscript reviewers for their constructive and thorough review. A National Institutes of Health RO1 Grant (HD39343) supported this work.
References
- Adamovich SV, Archambault PS, Ghafouri M, Levin MF, Poizner H, Feldman AG. Hand trajectory invariance in reaching movements involving the trunk. Exp Brain Res. 2001;138:288–303. doi: 10.1007/s002210100694. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Pearson JC, Harrington D, Dewey D, Torbeck L, Fyffe RE. Distribution of 5-hydroxytryptamine-immunoreactive boutons on alpha-motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol. 1998;393:69–83. [PubMed] [Google Scholar]
- Baldissera F, Cavallari P, Tassone G. Effects of transmastoid electrical stimulation on the triceps brachii EMG in man. Neuroreport. 1990;1:191–193. doi: 10.1097/00001756-199011000-00003. [DOI] [PubMed] [Google Scholar]
- Beer RF, Given JD, Dewald JP. Task-dependent weakness at the elbow in patients with hemiparesis. Arch Phys Med Rehabil. 1999;80:766–772. doi: 10.1016/s0003-9993(99)90225-3. [DOI] [PubMed] [Google Scholar]
- Beer RF, Dewald JP, Rymer WZ. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: evidence for disturbed control of limb dynamics. Exp Brain Res. 2000;131:305–319. doi: 10.1007/s002219900275. [DOI] [PubMed] [Google Scholar]
- Beer RF, Dewald JP, Dawson ML, Rymer WZ. Target-dependent differences between free and constrained arm movements in chronic hemiparesis. Exp Brain Res. 2004;156:458–470. doi: 10.1007/s00221-003-1807-8. [DOI] [PubMed] [Google Scholar]
- Bober T, Kornecki S, Lehr RP, Jr, Zawadzki J. Biomechanical analysis of human arm stabilization during force production. J Biomech. 1982;15:825–830. doi: 10.1016/0021-9290(82)90047-1. [DOI] [PubMed] [Google Scholar]
- Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res. 1993;94:143–151. doi: 10.1007/BF00230477. [DOI] [PubMed] [Google Scholar]
- Brunnstrom S. Movement therapy in hemiplegia: a neurophysiological approach. Harper and Row; New York: 1970. [Google Scholar]
- Cauquil AS, Day BL. Galvanic vestibular stimulation modulates voluntary movement of the human upper body. J Physiol. 1998;513(Pt 2):611–619. doi: 10.1111/j.1469-7793.1998.611bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh PR, Komi PV. Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1979;42:159–163. doi: 10.1007/BF00431022. [DOI] [PubMed] [Google Scholar]
- Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus-triggered averaging. J Neurophysiol. 2004;92:83–95. doi: 10.1152/jn.00083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res. 2006 doi: 10.1007/s00221-006-0374-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001;24:273–283. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118(Pt 2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Beer RF, Given JD, McGuire JR, Rymer WZ. Reorganization of flexion reflexes in the upper extremity of hemiparetic subjects. Muscle Nerve. 1999;22:1209–1221. doi: 10.1002/(sici)1097-4598(199909)22:9<1209::aid-mus7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Diederichsen L, Krogsgaard M, Voigt M, Dyhre-Poulsen P. Shoulder reflexes. J Electromyogr Kinesiol. 2002;12:183–191. doi: 10.1016/s1050-6411(02)00019-6. [DOI] [PubMed] [Google Scholar]
- Dominici F, Popa T, Ginanneschi F, Mazzocchio R, Rossi A. Cortico-motoneuronal output to intrinsic hand muscles is differentially influenced by static changes in shoulder positions. Exp Brain Res. 2005;164:500–504. doi: 10.1007/s00221-005-2270-5. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat. I. Movements evoked by microstimulation. J Neurophysiol. 1990a;64:767–781. doi: 10.1152/jn.1990.64.3.767. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat. II. Electromyographic activity evoked by microstimulation. J Neurophysiol. 1990b;64:782–795. doi: 10.1152/jn.1990.64.3.782. [DOI] [PubMed] [Google Scholar]
- Ellis MD, Holubar BG, Acosta AM, Beer RF, Dewald JP. Modifiability of abnormal isometric elbow and shoulder joint torque coupling after stroke. Muscle Nerve. 2005;32:170–178. doi: 10.1002/mus.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster O. Motorische Felder und Bahnen. In: Foerster BaO., editor. Handbuch der Neurologie. Vol. 6. Springer; Berlin Heidelberg New York: 1936. pp. 1–357. [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Ginanneschi F, Del Santo F, Dominici F, Gelli F, Mazzocchio R, Rossi A. Changes in corticomotor excitability of hand muscles in relation to static shoulder positions. Exp Brain Res. 2005;161:374–382. doi: 10.1007/s00221-004-2084-x. [DOI] [PubMed] [Google Scholar]
- Ginanneschi F, Dominici F, Biasella A, Gelli F, Rossi A. Changes in corticomotor excitability of forearm muscles in relation to static shoulder positions. Brain Res. 2006;1073–1074:332–338. doi: 10.1016/j.brainres.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Giroux N, Rossignol S, Reader TA. Autoradiographic study of alpha1- and alpha2-noradrenergic and serotonin1A receptors in the spinal cord of normal and chronically transected cats. J Comp Neurol. 1999;406:402–414. [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hertling D, Kessler RM. Management of common musculoskeletal disorders: physical therapy principles and methods. Lippincott; Philadelphia: 1996. [Google Scholar]
- Knikou M, Rymer WZ. Hip angle induced modulation of H reflex amplitude, latency and duration in spinal cord injured humans. Clin Neurophysiol. 2002a;113:1698–1708. doi: 10.1016/s1388-2457(02)00285-7. [DOI] [PubMed] [Google Scholar]
- Knikou M, Rymer Z. Effects of changes in hip joint angle on H-reflex excitability in humans. Exp Brain Res. 2002b;143:149–159. doi: 10.1007/s00221-001-0978-4. [DOI] [PubMed] [Google Scholar]
- Kornecki S, Zschorlich V. The nature of the stabilizing functions of skeletal muscles. J Biomech. 1994;27:215–225. doi: 10.1016/0021-9290(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Lum PS, Burgar CG, Shor PC. Evidence for strength imbalances as a significant contributor to abnormal synergies in hemiparetic subjects. Muscle Nerve. 2003;27:211–221. doi: 10.1002/mus.10305. [DOI] [PubMed] [Google Scholar]
- Lum PS, Burgar CG, Shor PC. Evidence for improved muscle activation patterns after retraining of reaching movements with the MIME robotic system in subjects with post-stroke hemiparesis. IEEE Trans Neural Syst Rehabil Eng. 2004;12:186–194. doi: 10.1109/TNSRE.2004.827225. [DOI] [PubMed] [Google Scholar]
- Mars F, Archambault PS, Feldman AG. Vestibular contribution to combined arm and trunk motion. Exp Brain Res. 2003;150:515–519. doi: 10.1007/s00221-003-1485-6. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Nakajima K, Drew T, Aoki M, Mori S. Locomotor role of the corticoreticular-reticulospinalspinal interneuronal system. Prog Brain Res. 2004;143:239–249. doi: 10.1016/S0079-6123(03)43024-0. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SB, Schmitz TJ. Physical rehabilitation: assessment and treatment. Davis; Philadelphia: 2001. [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol. 2001;143:137–263. doi: 10.1007/BFb0115594. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Clarac F, Loeb GE, Rothwell JC, Wolpaw JR. What do reflex and voluntary mean? Modern views on an ancient debate. Exp Brain Res. 2000;130:417–432. doi: 10.1007/s002219900250. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol. 2004;92:2217–2238. doi: 10.1152/jn.01189.2003. [DOI] [PubMed] [Google Scholar]
- Troiani D, Ferraresi A, Zei U, Manni E. Low-frequency loud acoustic stimulation and goal-directed arm movements. Acta Otolaryngol. 2004;124:395–399. doi: 10.1080/00016480410016414. [DOI] [PubMed] [Google Scholar]
- Tunik E, Poizner H, Levin MF, Adamovich SV, Messier J, Lamarre Y, Feldman AG. Arm-trunk coordination in the absence of proprioception. Exp Brain Res. 2003;153:343–355. doi: 10.1007/s00221-003-1576-4. [DOI] [PubMed] [Google Scholar]
- Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Rothwell JC, Goulart F, Cossu G, Munoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol. 1999;516(Pt 3):931–938. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


