Abstract
Because sweetpotato [Ipomoea batatas (L.) Lam.] stem cuttings regenerate very easily and quickly, a study of their early growth and development in microgravity could be useful to an understanding of morphological changes that might occur under such conditions for crops that are propagated vegetatively. An experiment was conducted aboard a U.S. Space Shuttle to investigate the impact of microgravity on root growth, distribution of amyloplasts in the root cells, and on the concentration of soluble sugars and starch in the stems of sweetpotatoes. Twelve stem cuttings of ‘Whatley/Loretan’ sweetpotato (5 cm long) with three to four nodes were grown in each of two plant growth units filled with a nutrient agarose medium impregnated with a half-strength Hoagland solution. One plant growth unit was flown on Space Shuttle Colombia for 5 days, whereas the other remained on the ground as a control. The cuttings were received within 2 h postflight and, along with ground controls, processed in ≈45 min. Adventitious roots were counted, measured, and fixed for electron microscopy and stems frozen for starch and sugar assays. Air samples were collected from the headspace of each plant growth unit for postflight determination of carbon dioxide, oxygen, and ethylene levels. All stem cuttings produced adventitious roots and growth was quite vigorous in both ground-based and flight samples and, except for a slight browning of some root tips in the flight samples, all stem cuttings appeared normal. The roots on the flight cuttings tended to grow in random directions. Also, stem cuttings grown in microgravity had more roots and greater total root length than ground-based controls. Amyloplasts in root cap cells of ground-based controls were evenly sedimented toward one end compared with a more random distribution in the flight samples. The concentration of soluble sugars, glucose, fructose, and sucrose and total starch concentration were all substantially greater in the stems of flight samples than those found in the ground-based samples. Carbon dioxide levels were 50% greater and oxygen marginally lower in the flight plants, whereas ethylene levels were similar and averaged less than 10 nL·L −1. Despite the greater accumulation of carbohydrates in the stems, and greater root growth in the flight cuttings, overall results showed minimal differences in cell development between space flight and ground-based tissues. This suggests that the space flight environment did not adversely impact sweetpotato metabolism and that vegetative cuttings should be an acceptable approach for propagating sweetpotato plants for space applications.
Additional index words: hypoxia, Ipomea batata, Phytagel, carbohydrate metabolism, bioregenerative life support
Sweetpotato is being studied at Tuskegee University as a potential crop for use in the U.S. National Aeronautics and Space Administration’s Advanced Life Support program to provide food for long-term space exploration missions. Stem cuttings are commonly used in the propagation of sweetpotato. Although seeds of several crops have been grown in micro-gravity and their growth compared with ground-based controls (Cowles et al., 1994; Levine and Krikorian, 1991), plants that have been propagated vegetatively have not been studied under these conditions. Sweetpotato stem cuttings offer distinct advantages for space flight studies, especially those of short duration. Cuttings develop roots easier and quicker than seeds and the genetic makeup can be maintained from one initial planting.
Levine and Krikorian (1991) initiated roots of the monocot Hemerocallis (Baker.) M. Hotta and three populations of the dicot Haplopappus gracilis (Nutt.) Gray during a 5-d shuttle flight and reported greater overall root production compared with ground controls. Abrahamson et al. (1991) exposed eight sprouted seedlings [six alfalfa (Medicago sativa L.) and two white clover (Trifolium repens L.)] to microgravity for 6 d on a shuttle flight and found that root length:shoot length and root length:total length were greater compared with ground controls.
Plant regeneration from seeds during space flight studies has shown a decrease in the level of amyloplasts and a disorientation of root growth resulting from the absence of a strongly dominant gravity vector (Smith and Luttges, 1994). In addition, decreases in plant tissue starch from space flight have been one of the most consistent responses to microgravity (Brown et al., 1996). Musgrave et al. (2005) evaluated seed storage reserves and glucosinolates in Brassica rapa L. grown on the International Space Station and reported that deposition of storage reserves was more advanced in ground controls, whereas glucosinolate accumulation was enhanced by microgravity. They concluded that the spaceflight environment adversely influenced the overall flavor and nutritional quality of this crop by its direct impact on metabolite production. In contrast, Stutte et al. (2006) reported that there were no differences in the content of starch and soluble sugars in the leaves of flight and ground-based wheat (Triticum aestivum L.) plants grown for 21 d. They also found very little difference in cell development except that chloroplasts in the leaves of the plants grown in microgravity were more ovoid and the thylakoid membranes trended toward greater packing density. The researchers concluded that the space flight environment exerted minimal impact on wheat metabolism.
There is evidence that indicates plant responses when grown in space may be influenced by the gaseous environment. For example, Musgrave et al. (1998, 2000) obtained smaller seeds and variable weight in space experiments and have hypothesized that the composition of the gaseous environment changed as a result of the lack of buoyancy-driven convection in microgravity. Ground-based studies by Blasiak et al. (2006) reported that accretionary seed growth in pepper (Capsicum annum L.) was limited by the availability of oxygen and suggested that the variation in seed quality could be attributed to localized limitations in oxygen supply. Shoots of B. rapa grown in microgravity had greater sucrose and total soluble carbohydrates compared with ground control shoots, and it was suggested that this response was the result of root zone hypoxia caused by microgravity-induced changes in fluid and gas distribution (Stout et al., 2001).
Successful root growth is the all-important first step in the establishment of a sweetpotato crop in a closed environment. Of particular importance to this crop is the rapid growth of adventitious roots because these will influence the eventual development of storage roots. If sweetpotato is to be used successfully in future bioregenerative systems that recycle wastes into food, water, and oxygen, reliable plant propagation and growth in microgravity must be demonstrated necessitating a comprehensive understanding of the effects of gravity on both the plant’s physiology and environment (Stout et al., 2001).
The primary objective of this experiment was to demonstrate the feasibility of the use of stem cuttings for plant propagation in microgravity. The root growth, distribution of amyloplasts in the root cells, and carbohydrates in the stems were examined and compared with their ground-based counterparts.
Materials and Methods
Planting material and growth conditions
Sweetpotato stem cuttings were obtained from ‘TU-82-155’ (now ‘Whatley/Loretan’) and developed at Tuskegee University that has produced high storage root yields (comparable to field-grown) in controlled environments (Mortley et al., 1994, 1996, 2001). Stem cuttings were grown in two plant growth units (PGU) as part of Bioserve Space Technologies (University of Colorado, Boulder) Commercial Generic Bioprocessing Apparatus (CGBA) payload within the Isothermal Containment Module (ICM) locker. Each PGU (16 ×10 cm) contained four substrate containers each 10 cm wide and 4.5 cm deep.
Four grams of Phytagel (Sigma-Aldrich, St. Louis, MO), a nutrient agarose media used in tissue culture, were autoclaved for 15 min at 121 °C in 500 mL of half-strength Hoagland nutrient solution and cooled for 10 min. After cooling, 100 mL were poured into each of the four substrate containers of the PGU and allowed to solidify overnight. Twelve 5-cm long stem cuttings were planted into each PGU (flight and ground-based control) 24 h before launch. One PGU was placed in the CGBA-ICM, which was then stowed in a shuttle middeck locker and flown aboard Space Shuttle Colombia for 5 d. During the flight, the ICM locker was actively ventilated with cabin air and the temperature averaged 23 °C; fluorescent lamps provided continuous lighting. A ground-based unit was placed in a chamber with similar environmental conditions at Kennedy Space Center, FL.
Postflight procedures and plant growth unit air analysis
The plant growth unit was retrieved within 2 h of landing. After air samples (see air analysis subsequently), root length, and numbers were measured, stem cutting samples were stored in dry ice for subsequent shoot soluble sugars analysis. Fibrous root samples were transferred to vials containing 10 mL of primary fixative (3% glutaraldehyde, 1% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH = 7.2) at room temperature (modified from Karnovsky, 1968) and transported back to Tuskegee University for electron microscopy evaluations.
Air samples were collected from the headspace separating the two containers holding the cuttings of both ground-based and space flight plant growth units. Two syringe samples were taken from the flight and ground-based growth units. The samples were screened for ethylene and carbon dioxide with a gas chromatograph (model 6890; Hewlett-Packard, Houston, TX) with a thermal conductivity detector for permanent gas analysis in tandem with a flame ionization detector for ethylene analysis. Oxygen was quantified using a gas chromatograph (model 5880; Hewlett-Packard) coupled with a thermal conductivity detector.
Carbohydrate analysis
Approximately 20 mg of freeze-dried and ground stem tissue was extracted with 3 mL of 80% ethanol at 80 °C for 5 min. This procedure was repeated three times and the extract was concentrated and used for soluble sugar determination. The residue was boiled in 0.4 mL of 0.2 N KOH and neutralized with 80 μL of 1 M acetic acid. Starch was hydrolyzed to liberate glucose by adding 3 mL of 10-unit/mL amyloglucosidase enzyme in 50 mM citrate buffer (pH 4.5) and incubated at 55 °C for 1 h. The hydrolysate-containing glucose released from starch was centrifuged and quantitatively transferred to a clear 10-mL volumetric flask. Carbohydrates in 80% ethanol extract and enzyme hydrolysate were determined by ion chromatography coupled with pulsed amperometric detection using a chromatograph system [DX-500; Dionex, Sunnyvale, CA (Dionex Corp., 1989)].
Electron microscopy analysis
After removal from the primary fixative, excised samples were rinsed twice for 10 min each in 0.1 M sodium cacodylate buffer, pH 6.9, and postfixed in 1% osmium tetroxide in the same buffer for 2 h (modified from Palade, 1952). Samples were rinsed once in buffer followed by five successive deionized water rinses for 10 min each. Tissues were then stained en bloc with 0.5% aqueous uranyl acetate for 2 h (Terzakis, 1968), after which samples were dehydrated in a graded series of ethanol and then transferred to propylene oxide. Tissues were infiltrated and embedded with 50% Spurr’s low-viscosity medium (Spurr, 1969). The distal most section [≈3 mm (i.e., root tip)] of samples were also infiltrated with 50% Spurr’s low-viscosity medium and then embedded in fresh 100% Spurr’s medium. Resin was cured at 60 °C for 48 h. Sections were cut using a Dupont Sorvall MT2B ultramicrotone (Dupont-Sorvall, Wilmington, DE) and a diamond knife (Microstar Technologies, Huntsville, TX). Sections were collected on 75 ×300-mesh copper grids and stained with uranyl acetate (Watson, 1958) and lead citrate (Reynolds, 1963). Stained sections were viewed at 60 kV with a transmission electron microscope (model 201; Phillips Electronics Instruments, Mahwah, NJ). All micrographic images were recorded on electron microscopy film (Kodak 4489; Eastman Kodak, Rochester, NY).
Experimental design and statistical analysis
Sweet-potato stem cuttings were arranged in a completely randomized design with repeated measurements. Data were analyzed by one-way analysis of variance according to the general linear model procedures of SAS (SAS Institute, Cary, NC). Significant effects were determined by differences in least squares means at the 0.05 significance level.
Results and Discussion
Root growth
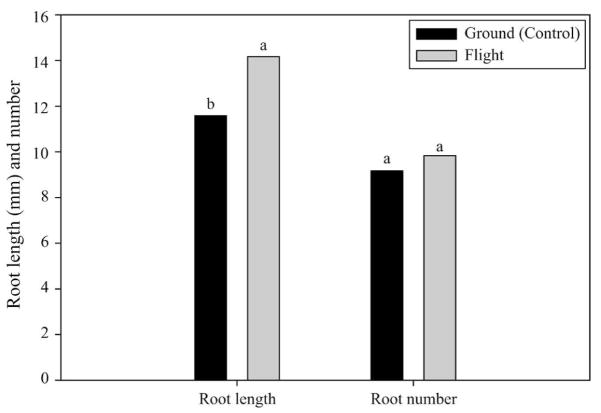
Adventitious root development of sweet-potato stem cuttings grown in microgravity for 5 d was compared with ground-based controls. Stem cuttings appeared to have regenerated fibrous roots normally in spaceflight and ground-based controls, but roots tended to grow perpendicular to the stem cuttings in the space flight samples. Statistical analysis indicated that total root length was significantly (≈ 12%) greater for roots developed in microgravity versus ground-based controls (Fig. 1). The total number of roots produced by the stem cuttings grown in microgravity was almost identical to those produced by the stems of ground-based controls.
Fig. 1.
Number and length of adventitious roots from flight and ground-based sweetpotato stem cuttings. Mean values with different letters are significantly different. Significant effects were determined by differences in least squares means at P ≤ 0.05.
Carbohydrate
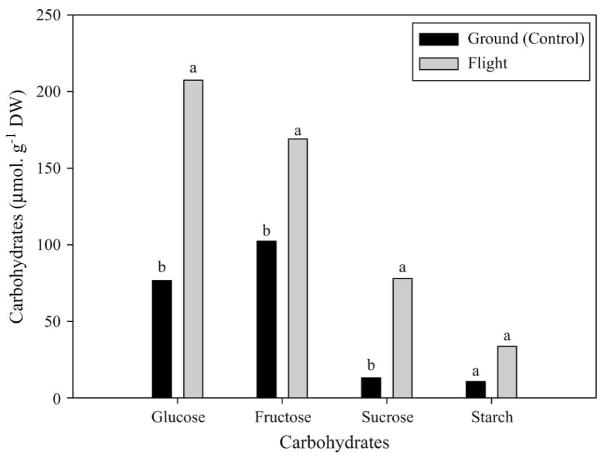
Statistical analysis of soluble sugars in space flight and corresponding ground control stem cuttings showed a significant accumulation of total soluble sugars in the stem cuttings grown in microgravity (Fig. 2). Glucose (P ≤ 0.02) and fructose (P ≤ 0.01) concentrations in the flight stems significantly exceeded those in the ground-based stems by 91% and 61%, respectively. Similarly, the concentration of sucrose (P ≤0.04) was four times greater in flight than in ground-based controls, respectively. Glucose was the most abundant soluble carbohydrate in both the spaceflight and ground-based control samples. Starch concentration in the spaceflight stem cutting samples (although not significant) was approximately three times greater than in ground-based stem cuttings.
Fig. 2.
Glucose, fructose, sucrose, and starch content in stem cuttings of flight and ground-based sweetpotato stem cuttings. Mean values with different letters are significantly different. Significant effects were determined by differences in least squares means at P ≤ 0.05.
Microscopic observation
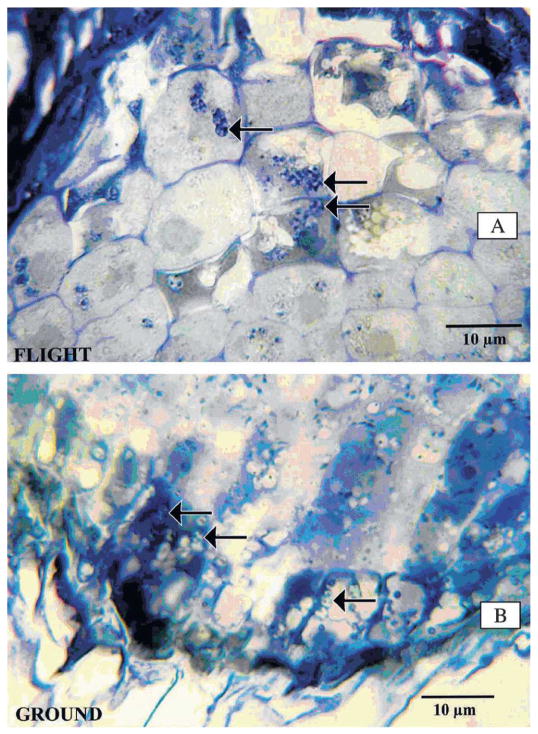
Light micrographs indicated sedimentation of amyloplasts at the base of columella cells in the root tip sections of ground-based controls and a more random distribution in cells of the flight samples (Fig. 3A–B).
Fig. 3.
Light micrographs of root tip sections of (A) flight and (B) ground-based sweetpotato. Arrows indicate a random distribution of amyloplasts at the base of columella cells in (A) flight samples and sedimentation in (B) ground-based sweetpotato.
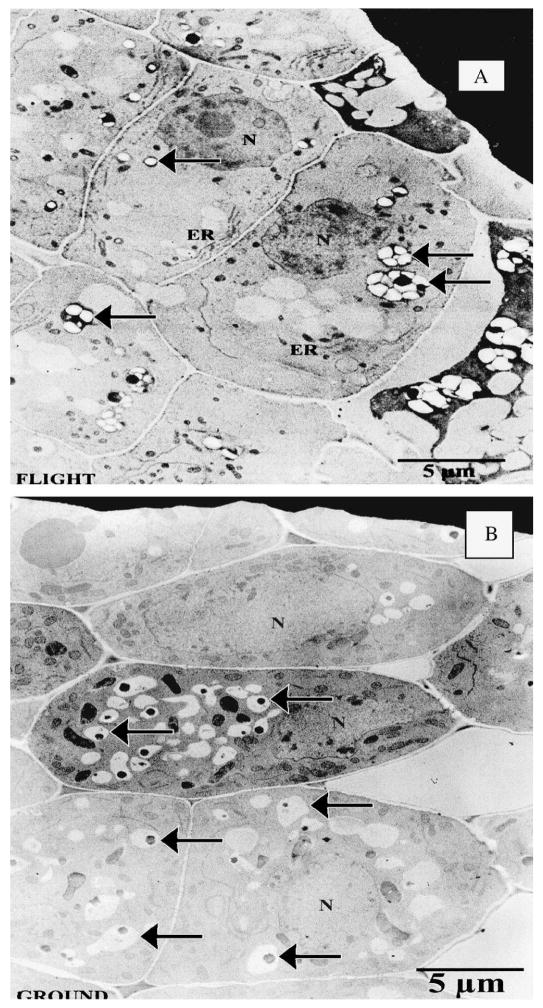
Root ultrastructure analysis indicated that cell development was similar between ground-based and space flight samples (Fig. 4A–B). It was observed that starch grains were distributed throughout the cytoplasm in flight samples (Fig. 4A). In addition, starch grains appeared to be smaller in the space flight samples compared with ground-based samples and tended to be in clusters of two to three versus ground-based samples that appeared singly in a section of a cell.
Fig. 4.
Electron micrographs of root tip sections of (A) flight and (B) ground-based control sweetpotato. N = nucleus; ER = endoplasmic reticulum. Arrows indicate starch granules. Note that the starch granules are distributed throughout the cytoplasm in the flight samples.
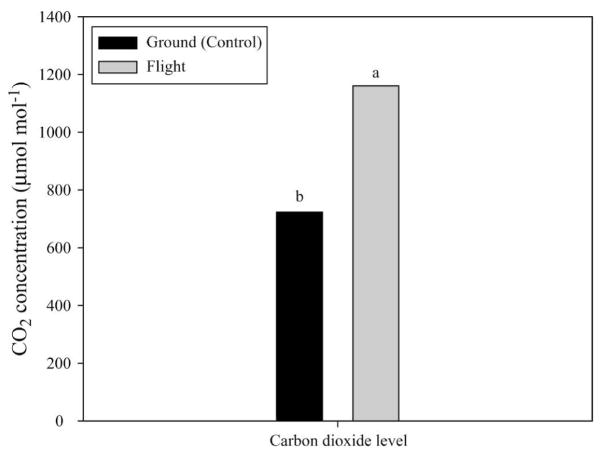
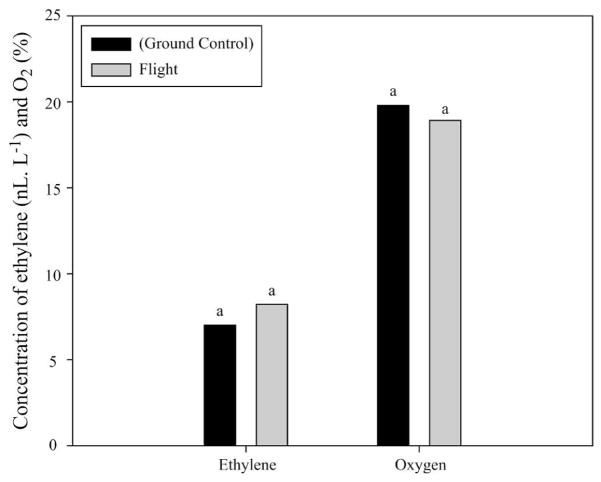
Plant growth unit air analysis
Carbon dioxide concentration was ≈50% greater (P ≤ 0.05) for the flight growth unit compared with that of the ground control (Fig. 5). In contrast, both ethylene and oxygen concentration were similar for flight and ground-based growth units (Fig. 6).
Fig. 5.
Carbon dioxide concentration in growth units of flight and ground-sweetpotato stem cuttings. Mean values with different letters are different. Significant effects were determined by differences in least squares means at P ≤ 0.05.
Fig. 6.
Ethylene and oxygen levels in growth units of flight and ground-based sweetpotato stem cuttings. Mean values with different letters are significantly different. Significant effects were determined by differences in least squares means at P ≤ 0.05.
These results show that sweetpotato stem cuttings successfully regenerated adventitious roots in microgravity (after being transplanted in standard gravity conditions) during a 5 d Space Shuttle flight experiment. Although there was a significant increase in the length of adventitious roots obtained among the flight cuttings compared with that of ground-based controls, there were no differences in root number. This suggests that the space flight environment had no negative effect on the ability of vegetative cuttings to form roots and that use of cuttings should be an acceptable means for propagating sweetpotato for future space applications.
There was a substantial accumulation of glucose, fructose, sucrose, and starch in the flight stems. Similar results have been reported for rapid-cycling B. rapa in microgravity (Stout et al., 2001), for other species under water-logging conditions (Castonguay et al., 1993; Daugherty and Musgrave, 1994; Setter et al., 1987), or in source-sink experiments (Hall and Milthorpe, 1977; Plaut et al., 1987; Setter et al., 1980). When Daugherty and Musgrave (1994) exposed rapid-cycling B. rapa to water-logging conditions, they found that as available oxygen decreased within the root zone, there was an increase in soluble carbohydrates and starch in the leaves. This increase was observed as early as 12 h after the treatments were initiated. Although root zone O2 levels were not measured directly, the levels within the plant growth unit averaged 18.9%. Because this aerial O2 level was lower in the space environment, it is reasonable to infer that concentration in the root zone might have been lower in space as well. Although alcohol dehydrogenase activity, a sensitive biochemical indicator of hypoxic conditions (Daugherty and Musgrave, 1994, Porterfield et al., 2000), was not measured in this study, it is probable that the level of its activity may also have been high in the adventitious roots of these stem cuttings. Although there was a substantial difference in starch concentration between the flight and ground-based stem cuttings, the difference was not significant (P ≤ 0.08). This may be attributable in part to the fact that inferences based on P values are not as sensitive for the detection of significance as are likelihood ratios (Edwards, 1972). Based on likelihood ratio analyses (data not shown), there is strong evidence of a difference in glucose, fructose, sucrose, and starch concentration between treatments. The possibly ambiguous conclusion from the nearly significant P value for starch (P ≤ 0.08) is resolved by the strong support for a significant difference in the likelihood ratio of 38.6: 1 for the nonzero difference of 22.9 for starch concentration of the flight minus the ground-based control stem cuttings.
The concentration of carbon dioxide within the flight plant growth unit was ≈50% greater than that found in the corresponding ground-based unit. In previous studies evaluating the impact of CO2 enrichment (ambient to 1000 μmol·mol−1) on content of starch, sucrose, glucose, and fructose (Center for Food and Environmental Systems for Human Exploration of Space, 1997), we found that starch increased an average of 12%, whereas sucrose, glucose, and fructose increased with CO2 up to 750 μmol·mol−1. However, as CO2 was increased further to 1000 μmol·mol−1, starch and soluble sugars decreased somewhat. Thus, it is unlikely that the higher CO2 (1200 μmol·mol−1 in space versus 700 μmol·mol−1 on the ground) can explain the differences in carbohydrate levels.
Although atmospheric ethylene accumulation showed no difference between flight and ground cuttings, it is possible that flight tissue might have produced more ethylene internally and that chamber leakage prevented any buildup. Several studies suggest that when plants are gravitationally disoriented such as when turned horizontally or placed on clinostats, ethylene production increases (Leather et al., 1972; Wheeler et al., 1986). In addition, spaceflight plants can show ethylene-like symptoms (Brown et al., 1974; Johnson and Tibbitts, 1968). This could explain the greater adventitious root growth in flight (Mattoo and Suttle, 1991) or might be related to the elevated carbohydrate levels in the space cuttings. However, because there were no direct assays for ethylene activities in the tissue, this is only speculation.
The apparent reduction in the size of starch grains in root samples of space flight stem cuttings as well as the tendency to be in clusters of two to three versus ground-based samples are consistent with that documented by others (Croxdale et al., 1997; Kordyum et al., 1997). These researchers showed that amyloplast size, starch volume, or both could decrease in root cap statocytes of higher plants in microgravity. Kordyum et al. (1997) suggested that this response could be the result of an increase in hydrolysis of amylose or a decrease in starch synthesis by ADP-glucose pyrophosphorylase. This response could also be related to mission length (5 d in this case) and environmental changes that could have affected cell maturity (Croxdale et al., 1997).
These results document that sweetpotato stem cuttings successfully regenerated adventitious roots in microgravity and that overall development was generally comparable to ground-based control plants. These findings suggest that vegetative stem cuttings should be a viable means for propagating sweetpotato plants in space, although longer-duration tests are needed to demonstrate actual shoot regeneration from the rooted cuttings.
Acknowledgments
This research was supported by funds from the U.S. National Aeronautics and Space Administration (Grant No. NCC9-158 and the U.S. Department of Agriculture (Grant No. ALX-SP-1).
We thank Dr. Darwin Poritz of Lyndon B. Johnson Space Center, Houston, for assistance with statistical analysis; and Carla Goulart and James Classen (Bioserve Space Technologies), University of Colorado, Boulder, for their technical assistance. Electron microscopy services were provided by the Tuskegee University Center for Biomedical Research/RCMI/NH Grant #5G12RR03059.
Footnotes
Contribution No. 356 of the George Washington Carver Agricultural Experiment Station, Tuskegee University.
Contributor Information
Desmond G. Mortley, Center for Food and Environmental Systems for Human Exploration of Space and G.W. Carver Agricultural Experiment Station, Tuskegee University, 214 Milbank Hall, Tuskegee, AL 36088.
Conrad K. Bonsi, Center for Food and Environmental Systems for Human Exploration of Space and G.W. Carver Agricultural Experiment Station, Tuskegee University, 214 Milbank Hall, Tuskegee, AL 36088
Walter A. Hill, Center for Food and Environmental Systems for Human Exploration of Space and G.W. Carver Agricultural Experiment Station, Tuskegee University, 214 Milbank Hall, Tuskegee, AL 36088
Carlton E. Morris, Center for Food and Environmental Systems for Human Exploration of Space and G.W. Carver Agricultural Experiment Station, Tuskegee University, 214 Milbank Hall, Tuskegee, AL 36088
Carol S. Williams, Center for Biomedical Research/RCMI, Tuskegee University, Tuskegee, AL 36088
Ceyla F. Davis, Center for Biomedical Research/RCMI, Tuskegee University, Tuskegee, AL 36088
John W. Williams, Center for Biomedical Research/RCMI, Tuskegee University, Tuskegee, AL 36088
Lanfang H. Levine, Dynamac Corporation, Mail Code DYN-3, Kennedy Space Center, FL 32899
Barbara V. Petersen, ASRC Aerospace, Mail Code KT-F, Kennedy Space Center, FL 32899
Raymond M. Wheeler, NASA Biological Sciences Office, Mail Code KT-B-1, Kennedy Space Center, FL 32899
Literature Cited
- Abrahamson KS, Lisec JT, Derby JA, Simske SJ, Luttges MW. Effect of weightlessness on leguminous sprouts. Amer Soc Gravitational Space Biol Bul. 1991;5:59. [Google Scholar]
- Blasiak J, Kuang A, Farhangi CS, Musgrave ME. Roles of intra-fruit oxygen and carbon dioxide in controlling pepper (Capsium annum L.) seed development and storage reserve deposition. J Amer Soc Hort Sci. 2006;131:164–173. [Google Scholar]
- Brown AH, Chapman DK, Liu SWW. A comparison of leaf epinasty induced by weightlessness and by clinostat rotation. Bioscience. 1974;24:518–520. [Google Scholar]
- Brown CC, Tripathy BC, Stutte GW. Photosynthesis and carbohydrate metabolism in microgravity. In: Sige H, editor. Plants in space biology. Tohuku University Press; Tokyo: 1996. pp. 127–134. [Google Scholar]
- Castonguay Y, Nadeau P, Simard RR. Effects of flooding on carbohydrate and ABA levels in roots and shots of alfalfa. Plant Cell Environ. 1993;16:695–702. [Google Scholar]
- Center for Food and Environmental Systems for Human Exploration of Space. Tuskegee University; Tuskegee, AL: 1997. Annual report. [Google Scholar]
- Cowles J, Lemay R, Jahns G. Seedling growth and development on space shuttle. Adv Space Res. 1994;14:3–12. doi: 10.1016/0273-1177(94)90273-9. [DOI] [PubMed] [Google Scholar]
- Croxdale J, Cook M, Tibbitts TW, Brown CS, Wheeler RM. Structure of potato tubers formed during space flight. J Expt Bot. 1997;48:2037–2043. doi: 10.1093/jexbot/48.317.2037. [DOI] [PubMed] [Google Scholar]
- Daugherty CJ, Musgrave ME. Characterization of populations of rapid- cycling Brassica rapa L. selected for differential waterlogging tolerance. J Expt Bot. 1994;45:385–392. [Google Scholar]
- Dionex Corp. Tech. Dionex Corp; Sunnyvale, CA: 1989. Analysis of carbohydrates and anion exchange chromatography with pulsed amperometric detection. Note 20. [Google Scholar]
- Edwards AW. Likelihood. Cambridge University Press; London, UK: 1972. [Google Scholar]
- Hall AJ, Milthorpe FL. Assimilate source-sink relationships in Capsicum annum L. III. The effects of fruit excision on photosynthesis and leaf and stem carbohydrates. Aust J Plant Physiol. 1977;4:771–783. [Google Scholar]
- Johnson SP, Tibbits TW. The liminal angle of a plagiotropic organ under weightlessness. BioScience. 1968;18:655–661. [Google Scholar]
- Karnovsky MJ. A formaldehyde-glutaraldehyde fixation at high osmolality for use in electron microscopy. J Cell Biol. 1968;27:137A–138A. [Google Scholar]
- Kordyum E, Baranenko V, Nedukha E, Samoilov V. Development of potato minitubers in microgravity. Cell Physiol. 1997;38:1111–1117. doi: 10.1093/oxfordjournals.pcp.a029095. [DOI] [PubMed] [Google Scholar]
- Leather GR, Forrence LE, Abeles FB. Increased ethylene production during clinostat experiments may cause leaf epinasty. Plant Physiol. 1972;49:183–186. doi: 10.1104/pp.49.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine HG, Krikorian AD. Shoot growth, root formation and chromosome damage results from the chromax I Experiment (Shuttle Mission STS-29) Amer Soc Gravitational Space Biol Bul. 1991;5:28. [Google Scholar]
- Mattoo AK, Suttle JC. The plant hormone ethylene. CRC Press; Boca Raton, FL: 1991. [Google Scholar]
- Mortley DG, Bonsi CK, Hill JH, Hill WA. Nutrient management of sweetpotato grown in nutrient film technique. Acta Hort. 2001;548:567–574. [Google Scholar]
- Mortley DG, Bonsi CK, Loretan PA, Hill WA, Morris CE. Relative humidity influences yield, edible biomass and linear growth rate of sweetpotato. HortScience. 1994;29:609–610. [Google Scholar]
- Mortley DG, Hill WA, Loretan PA, Bonsi CK, Morris CE. Growth responses of hydroponically grown sweetpotato tolerant and intolerant of a continuous daily light period. HortScience. 1996;31:209–212. [Google Scholar]
- Musgrave ME, Kuang A, Brown CS, Matthews SW. Changes in Arabidopsis leaf ultrastructure, chlorophyll and carbohydrate content during spaceflight depend on ventilation. Ann Bot (Lond) 1998;81:503–512. doi: 10.1006/anbo.1998.0585. [DOI] [PubMed] [Google Scholar]
- Musgrave ME, Kuang A, Tuominen LK, Levine LH, Morrow RC. Seed storage reserves and glucosinolates in Brassica rapa grown on the International Space Station. J Amer Soc Hort Sci. 2005;130:848–856. [Google Scholar]
- Musgrave ME, Kuang A, Xiao Y, Stout SC, Bingham GE, Briarty LG, Levinskikh MA, Sychev VN, Podolski IG. Gravity independence of seed-to-seed cycling in Brassica rapa. Planta. 2000;210:400–406. doi: 10.1007/pl00008148. [DOI] [PubMed] [Google Scholar]
- Palade GE. A study of fixation for electron microscopy. J Exp Med. 1952;95:285–298. doi: 10.1084/jem.95.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield DM, Barta DJ, Ming DW, Morrow RC, Musgrave ME. Astroculture root metabolism and cytochemical analysis. Adv Space Res. 2000;26:315–318. doi: 10.1016/s0273-1177(99)00578-5. [DOI] [PubMed] [Google Scholar]
- Plaut Z, Mayoral ML, Reinhold L. Effect of altered sink: Source ratio on photosynthetic metabolism of source leaves. Plant Physiol. 1987;85:786–791. doi: 10.1104/pp.85.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter TL, Brun WA, Brenner ML. Effect of obstructed translocation on leaf abscisic acid associated stomatal closure and photosynthesis decline. Plant Physiol. 1980;65:1111–1115. doi: 10.1104/pp.65.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter TL, Water I, Greenway H, Atwell BJ, Kupkanchanakul T. Carbohydrate status of terrestrial plants during flooding. In: Crawford RMM, editor. Plant life in aquatic and amphibious habitats. Blackwell Scientific; Oxford, UK: 1987. pp. 411–433. [Google Scholar]
- Smith JD, Luttges MW. White clover growth and root tip cell morphology under three gravity conditions: Spaceflight, clinorotation, and nominal. Amer Soc Gravitational Space Biol Bul. 1994;8:15. [Google Scholar]
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stout SC, Porterfield DM, Briarty LG, Kuang A, Musgrave ME. Evidence of root zone hypoxia in Brassica rapa L. grown in microgravity. Int J Plant Sci. 2001;162:240–255. doi: 10.1086/319585. [DOI] [PubMed] [Google Scholar]
- Stutte GW, Monje O, Hatfield RD, Paul AL, Ferl RJ, Simone CG. Microgravity effects on leaf morphology, cell structure, carbon metabolism and mRNA expression of dwarf wheat. Planta. 2006;224:1038–1049. doi: 10.1007/s00425-006-0290-4. [DOI] [PubMed] [Google Scholar]
- Terzakis JA. Uranyl acetate, a stain and fixative. J Ultrastruct Res. 1968;22:168–184. doi: 10.1016/s0022-5320(68)90055-5. [DOI] [PubMed] [Google Scholar]
- Watson ML. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958;4:475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RM, White RG, Salisbury FB. Gravitropism in higher plant shoots. IV. Further studies on participation of ethylene. Plant Physiol. 1986;82:534–542. doi: 10.1104/pp.82.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]