Abstract
We used a dual-task paradigm to investigate the spatial allocation of attention during smooth pursuit. Subjects tracked one character in a translating string of characters (block letter 8’s), and during maintained pursuit, one of the characters briefly changed to an E or 3. Based on the ability of subjects to correctly discriminate the probed character, we found that the primary focus of attention during smooth pursuit is centered on the tracked target with no appreciable lead or lag. Spatial cues were only partly effective in directing attention to other locations in our task, and these cueing effects were biased for locations ahead of the tracked target.
Keywords: attention, spatial, pursuit, eye movement, oculomotor
INTRODUCTION
Humans use a combination of saccadic and smooth pursuit eye movements to bring regions of interest in the visual field onto the fovea. Since attention can be defined as the selective enhancement of visual processing in a region of the visual field (Posner, 1980), eye movements constitute overt allocation of attention. Moreover, it is possible for humans to selectively enhance visual processing of a region of the visual field without an accompanying eye movement (Klein & Farrell, 1989; Posner, 1980); such an allocation of attention constitutes a covert allocation of attention. Unlike eye movements, which can be directly observed, covert shifts of attention must be indirectly measured as the spatially selective enhancement of performance on detection or discrimination tasks (Posner, 1980).
Despite the ability to covertly attend without moving the eyes, the direction of visual attention and the preparation of saccades are coupled both temporally and spatially. Performance on a discrimination task is highest at the saccade target immediately prior to a saccade, indicating that saccadic eye movements are preceded by shifts of attention to the saccade target (Deubel & Schneider, 1996; Hoffman & Subramaniam, 1995; Kowler, Anderson, Dosher, & Blaser, 1995). Although it is possible for subjects to attend to other areas of the visual field while making saccades, this decreases the accuracy and increases the latency of saccades (Deubel & Schneider, 1996; Kowler et al., 1995). These results are consistent with an obligatory link between saccade preparation and attention.
More recent studies suggest that the selection of targets for pursuit eye movements is likewise related to the allocation of visual attention. The latency of pursuit initiation is increased in the presence of distracters moving in the opposite direction of the pursuit target, and this effect is largely eliminated if the subject is also given a cue as to the location of the pursuit target (Krauzlis, Zivotofsky, & Miles, 1999). Similarly, initiation of pursuit of a moving region of a random dot configuration is shorter when attention is exogenously drawn to that region (Hashimoto, Suehiro, & Kawano, 2004). On the other hand, contrast sensitivity is not significantly reduced for background stimuli presented during the initiation of smooth pursuit, as might be expected if pursuit initiation required full attention to the tracked target (Schutz, Braun, & Gegenfurtner, 2007). Similarly, pre-cueing attention to one moving stimulus reduces, but does not eliminate, the tendency to average motion signals during the initial part of pursuit (Spering, Gegenfurtner, & Kerzel, 2006; Garbutt & Lisberger, 2006). These results indicate that pre-allocation of attention to a region of the visual field influences how pursuit is initiated, but they also suggest that the allocation of visual attention is malleable during this early phase.
The need for attention during the maintenance of pursuit is clearer and better established. The opposing motion of the background during smooth pursuit produces an overwhelming signal which would otherwise cancel pursuit were it not for the selection of the target and concurrent suppression of the background motion signal (Kowler, van der Steen, Tamminga, & Collewijn, 1984; Krauzlis, 2004). Allocation of attention to the pursuit target is one mechanism by which pursuit is maintained. During maintained pursuit, discrimination performance is better for the tracked stimulus than for non-tracked stimuli, even when retinal slip is comparable between the two (Khurana & Kowler, 1987). As might be expected if pursuit involves selectively attending the tracked stimulus, the contrast sensitivity for background stimuli is reduced during pursuit compared to fixation (Schutz Delipetkos, Braun, Kerzel & Gegenfurtner, 2007). Conversely, when subjects direct attention to stationary objects or to objects moving at velocities different from that of the pursuit target, pursuit gain is lower (Khurana & Kowler, 1987; Kerzel, Souto, & Ziegler, 2008). Similar effects on pursuit gain are also observed when the second task involves auditory, rather than visual stimuli (Hutton & Tegally, 2005). These results indicate that the maintenance of pursuit requires allocation of attention to the tracked stimulus.
Despite this evidence for a link between attention and smooth pursuit, the spatial allocation of attention during maintained pursuit has not been thoroughly investigated. The spatial scale of attention influences the gain of pursuit – it is higher when subjects attend a small part of a tracked object than when they attend a large part (Madelain, Krauzlis, and Wallman, 2005). However, the locus of spatial attention during maintained pursuit is not known. One possibility is that attention leads the eyes. Cognitive expectations about target trajectory control anticipatory smooth pursuit eye movements (Kowler, 1989), which might require attention to lead the target in order to allow accurate computation of the expected trajectory. This proposition is supported by the result that saccade latency and manual reaction time to targets appearing ahead of the pursuit target are lower than to targets appearing in the wake of pursuit (Tanaka, Yoshida, & Fukushima, 1998; van Donkelaar, 1999; van Donkelaar & Drew, 2002). However, the stimuli used in those studies included abrupt onsets in luminance and motion, which are known to capture attention and evoke involuntary eye movements (Abrams & Christ, 2003; Theeuwes, 1994; Yantis & Jonides, 1984, 1990). Therefore these experiments may have probed shifts of attention rather than the allocation of attention during pursuit.
In order to investigate the steady-state allocation of attention during maintained pursuit, we adopted a stimulus and task similar to that of Deubel and Schneider (1996) that minimizes abrupt visual onsets that might capture attention. We report that during maintained smooth pursuit, attention does not lead the target but is instead centered on the tracked stimulus. Using spatial cues, we find some evidence that attention may be voluntarily directed to locations ahead of, but not behind, the tracked target, but the primary locus of attention remains on the tracked stimulus.
METHODS
Subjects
Nine human subjects (four female and five male, aged 27–41 years, designated C, R, K, D, N, A, J, V, and T) participated in the experiment. One of the subjects (R) was an author of the study, whereas the other eight were naïve as to the experimental conditions and hypothesis. Four subjects (C, D, V, and T) had no previous experience of oculomotor testing. All subjects had normal or corrected to normal vision. All experimental procedures were reviewed and approved by the Institutional Review Board, and each subject gave informed consent. Subjects were paid a fixed amount of money for their participation. Individual sessions included approximately 400 trials and lasted for approximately 45 minutes; each subject participated in 8–20 sessions.
Stimuli and Experiments
Stimuli were generated on a Power Mac G4 using the Psychophysics Toolbox extensions for MATLAB (Brainard, 1997; Pelli, 1997), and displayed on a CRT video monitor (Eizo FX-E7, 75 Hz) at a viewing distance of 410 mm. The viewable dimensions of the screen were 45° horizontal by 35° vertical with a resolution of 0.055°/pixel. To minimize measurement errors, the subject’s head movements were restrained using a bite bar. Stimuli were presented in discrete trials that required the subject to fixate and pursue a target for 2 to 4 sec.
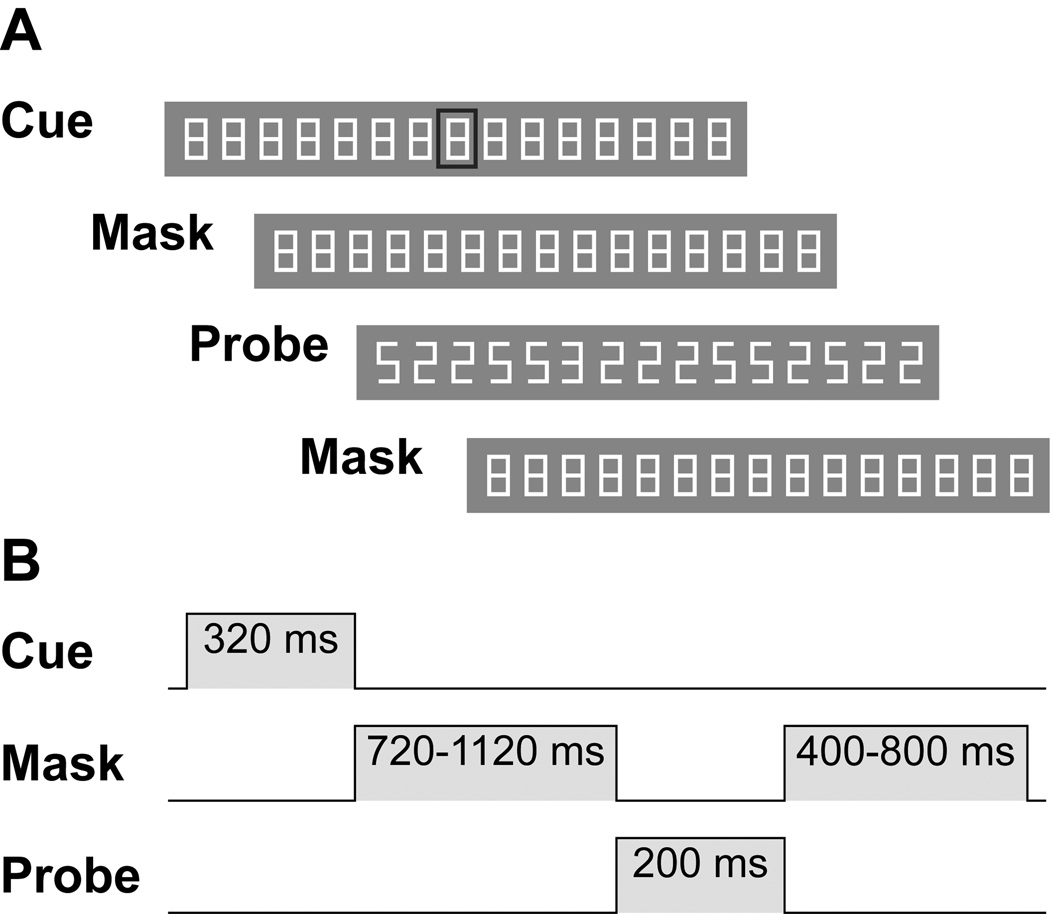
We used a stimulus consisting of an array of fifteen equally spaced characters (Figure 1), subtending an angle of 9° horizontally. We refer to the characters by numbering them from left to right; the eighth character position was at the center of the array. At the center of the screen, each character was 0.28° in width by 0.5° in height with a stroke width of 0.06° and a center-to-center spacing of 0.6°. At the beginning of a trial, the pursuit or fixation target was marked by a rectangle surrounding one of the characters. The rectangle was 0.61° wide by 0.83° tall with a stroke width of 0.12°. The background had a luminance of 53 cd/m2, the characters 86 cd/m2, and the tracking cue 40 cd/m2.
Figure 1.
The task used to test spatial attention during pursuit. (A) Illustration of the stimuli. Segment is actual size if viewed from 410 mm. Probe frame illustrates a 3 in position 5. (B) Schematic diagram of the temporal sequence of an individual trial used in all experiments, in which stimulus transitions from cue to mask to probe and back to mask.
The time course of the trial is depicted in Figure 1. At the outset of a trial, the stimulus appeared at either the far left or far right of the screen in the case of pursuit trials, or, in the case of fixation trials, at a randomly selected position uniformly distributed within the central 10° of the screen. The stimulus array then remained stationary for a randomly selected interval (500–1000 ms) during which a tracking cue indicated the pursuit or fixation target (Figure 1A, “Cue”). Following this period, the character array moved either to the right or to the left at absolute stimulus velocities of approximately 8, 12, or 16°/s. After 320 ms, the target cue disappeared and the stimulus continued to move (“Mask”). After an additional interval of time sufficient to place the stimulus at a randomly selected position within the central 10° of the screen, the stimulus changed from its mask configuration to its probe configuration for 200 ms (“Probe”). The probe involved a change in all characters from block letter 8’s to either 2’s or 5’s except for one character, which changed either to a block letter E or 3. At the end of the probe interval, the mask reappeared and the stimulus continued to translate across the screen until it reached the opposite edge. At the end of each trial, subjects reported by button press whether they had detected an E or a 3. Subjects received no feedback as to whether they had correctly identified the character.
We conducted several variants of this basic task in separate experiments to address different questions about the allocation of attention during pursuit. Experiment 1 examined how discrimination performance varied as a function of position with respect to the tracked character. In this experiment, the discrimination character could appear at any one of the central positions on any given trial, and the identity of the character was chosen at random. The array either moved at a constant velocity (pursuit trials: 8, 12, or 16 °/s, rightward or leftward) or remained stationary (fixation trials); there were equal numbers of pursuit trials at each velocity, and twice as many fixation trials as for any given velocity. Subjects performed either 416 or 520 trials per session and performed enough sessions to achieve sufficient statistical significance in our analysis of performance. This goal was met with at least 3,500 trials collected in at least eight sessions for each subject. All nine subjects participated in this experiment.
Experiment 2 tested whether the results for discrimination ability were due to limitations of visual acuity during the task. In this experiment, we interleaved two classes of trials. The first class of trials served to corroborate our main effect and involved conditions like those described above: the array of characters moved either at 0 or ± 16°/s and the central character served as the tracking target. For the second class of trials, subjects were presented with a simple spot stimulus, rather than the array of fifteen characters. After an initial fixation period, the spot moved at constant velocity (pursuit trials: 16 °/s, rightward or leftward) or remained stationary (fixation trials). Once the spot reached a randomly selected position within the central 10° of the screen, the stimulus was briefly joined by a single probe character (either a block letter E or 3), matched to the spot’s speed, and placed at a position offset randomly selected from 10 possible positions spanning ±3° from the tracked spot. The probe character was presented for 200 ms, after which it disappeared, and the moving spot stimulus continued to translate by itself across the screen (or remain stationary), until the end of each trial. As in the other experiments, subjects reported by button press whether they had detected an E or a 3, and received no feedback as to whether they had correctly identified the character. Thus, this experiment tested the same letter discrimination ability as in the other experiments, but the probe stimulus was newly appearing and there were no distracters. Four subjects (T, J, R, and V) participated in this experiment.
Experiment 3 tested whether the results depended on the position of the tracked character in the array. The fixation or pursuit target could be either at the center of the array (character 8), as in experiment 1, or offset by 1.75° to the left (character 5) or right (character 11). Unlike in experiment 1, the stimulus moved either at 0 or ± 16°/s. There were equal numbers of trials for each velocity and tracking position. Two subjects (K and N) served in this experiment.
Experiment 4 tested how spatial cues about the likely location of the probe affected discrimination performance. The design of this experiment was similar to that for experiments 1–3 above: subjects tracked or fixated the array of characters moving at either ±16 or 0°/s and the central character served as the tracking target. However, during the fixation period at the beginning of the trial, subjects were usually (16/22 trial types or 73%) given a visual cue about the likely location of the probe character; on the remaining trials (6/22, 27%), no cue was provided. The visual cues were partially valid (75%) and consisted of a small dot above the character that was presented for 320 ms at the position mostly likely to contain the probe character presented later in the trial. The position of the visual cue was randomly selected from 2 possible positions: +1.75° or −1.75° from the tracked character (i.e., at character positions 11 or 5). On invalidly cued trials, the probe was equally likely to be either at the tracked position at the center of the array, or at the uncued eccentric position (±1.75°) on the side opposite the visual cue. On trials without a cue, the probe was equally likely to be at the array center or at one of the two eccentric positions (±1.75°). Subjects again reported their answer (E or 3) by button press, with no feedback. Four subjects (T, J, R, and V) participated in this experiment.
Data Acquisition and Analysis
Presentation of stimuli, and the acquisition, display, and storage of data were controlled by a personal computer using the Tempo software package (Reflective Computing). The visual display computer provided feedback signals to the Tempo computer at the onset of each new frame, allowing us to synchronize data collection to stimulus presentation with 1-msec resolution. Eye movements were measured with an infrared video-based eye tracking system (RK-726, ISCAN Inc.) that reported the horizontal and vertical positions of the pupil with 12-bit resolution using a proprietary algorithm that computes the centroid of the pupil at 240 Hz. Before each block, we calibrated the output from the eye tracker by recording the raw digital values as subjects fixated 19 known locations three times in a pseudorandom sequence. The mean values during 500-msec fixation intervals at each location were used to generate a smooth function (using cubic spline interpolation) for converting raw eye tracker values to horizontal eye position. To minimize measurement errors, we focused our analysis on the horizontal component of eye movements because the stimuli were moving exclusively along the horizontal meridian. In addition, we used the calibration data to compute the precision of our eye movement recordings. Typical values ranged from 0.07° to 0.14° depending on the subject and the session.
All eye movement data and events related to the onset of stimuli were stored on disk during the experiment and later transferred to a Linux-based system for subsequent offline analysis. An interactive analysis program was used to filter, display, and make measurements from the data. To generate smooth traces free of high frequency noise, we applied a low-pass filter (−3dB at 50 Hz) to the calibrated horizontal eye position signals. Horizontal eye velocities were obtained by applying a finite impulse response (FIR) filter (−3dB at 54 Hz) to the filtered eye position signals. Signals encoding eye acceleration were then obtained by applying the same FIR filter to the signals encoding velocity. We detected the occurrence of saccades by applying a set of amplitude criteria to the eye velocity and eye acceleration signals, as described previously (Krauzlis & Miles, 1996). With the eye tracker data, this algorithm permitted us to detect saccades with amplitudes as small as ~0.3 deg. To prevent any contamination of our measurements of smooth eye movements by saccades, we excluded from analysis any trial in which a saccade occurred within 100 msec of the probe interval. This resulted in exclusion of a relatively small proportion of trials per subject (C: 10%, R: 5%, A: 3%, K: 5%, N: 2%, D: 10%). Measurements on eye position and velocity were then exported to MATLAB for further analysis.
Statistical Analysis
We recorded the proportion of correct responses at each combination of position and velocity for each experiment. Error bars for proportion data are 95% confidence intervals calculated using the MATLAB© function “binofit”. To assess the statistical difference between proportions of correct responses across conditions, we used a Chi-square test. Depending on the particulars of each experiment, we used multiple ANOVA to assess the impact of various factors including pursuit eye position offset, pursuit eye velocity gain, stimulus velocity, probe position, and cue position on task performance (correct versus incorrect judgment).
RESULTS
The four experiments in this study involved a dual-task paradigm in which subjects pursued or fixated a moving or stationary array of characters and performed a character discrimination task at different positions in the array. In the first experiment, the central character of the array was the pursuit or fixation target, and average performance on the discrimination task provided an assessment of the allocation of spatial attention during maintained pursuit. In the second experiment, we tested whether the results for discrimination performance were due to acuity limitations with our stimuli. In the third experiment, to test whether the results depended on the position of the tracked character in the array, one of three different characters could be the pursuit or fixation target. Finally, in a fourth experiment, we tested whether discrimination performance in our task could also be affected by explicit spatial cues.
Experiment 1
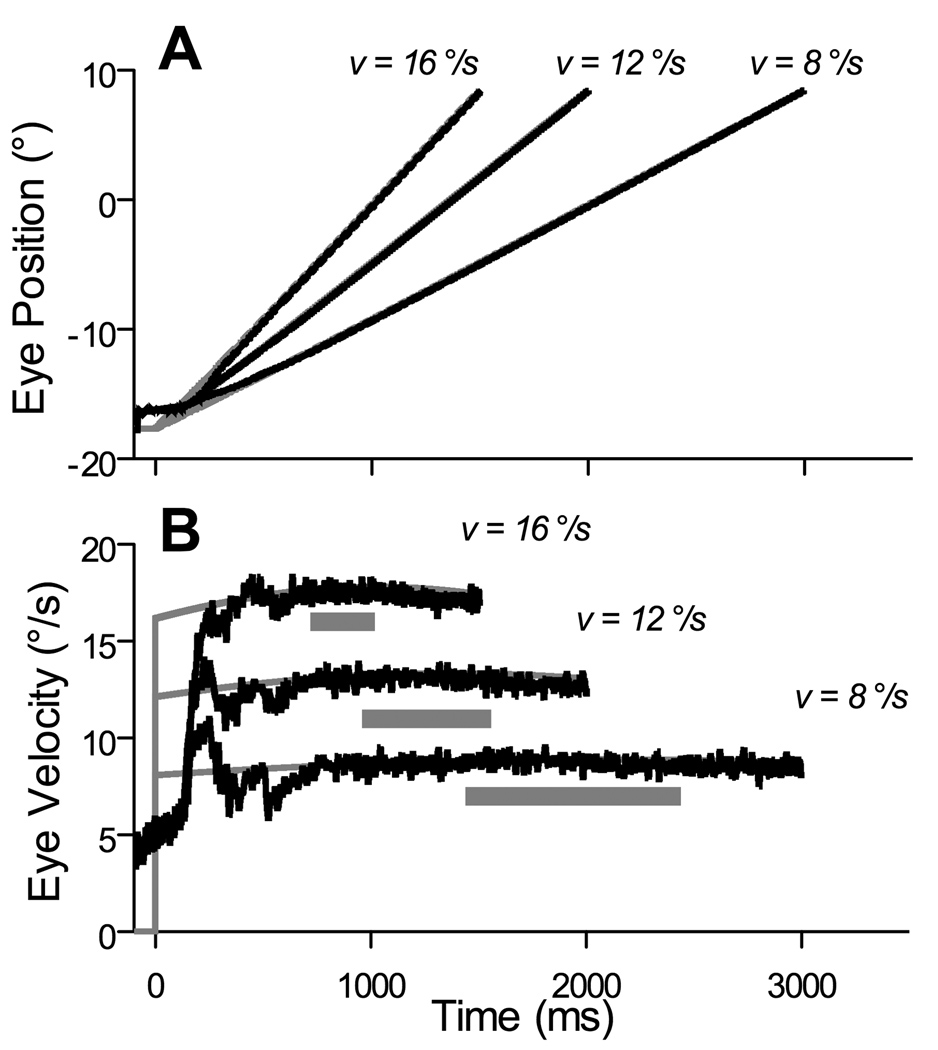
As intended from the experimental design, subjects were in maintained smooth pursuit during the probe interval, and the appearance of the probe did not disrupt pursuit. Figure 2 shows the horizontal displacement and velocity of the stimulus as a function of time for three stimulus velocities, 16, 12, and 8 °/s rightward (gray lines), along with mean eye position and velocity tracings for one subject (black lines). The horizontal bars in panel B indicate the interval of time during which the 200-ms probe could appear. All subjects showed some amount of anticipation of stimulus motion prior to its onset, and they all accurately reached stimulus velocity before the earliest possible onset of the probe interval. Mean gain during the probe interval was 0.97 to 1.0 for all stimulus velocities and subjects. Due to tangent error, stimuli moved slightly more slowly on the periphery than in the center of the screen. Nevertheless, the tangent function was approximately linear in the region of the screen in which the probe appeared, and thus the stimulus velocity never varied by more than 0.4% during a probe interval and by no more than 0.74% between probe intervals of a given velocity. Mean position and velocity for leftward pursuit, although not shown, was similar to that for rightward pursuit. Additionally, the mean velocity does not include saccades; saccades have been excised from the trace and the mean velocity at any given time includes at least 95% of all trials from a single session.
Figure 2.
Sample pursuit eye movements during the task. (A) Mean horizontal eye position for Subject C on rightward pursuit trials. Gray lines indicate actual stimulus trajectory; black lines indicate eye position. (B) Mean horizontal eye velocity for Subject C on rightward pursuit trials. Gray lines indicate actual stimulus velocity; black lines indicate eye velocity. Horizontal bars indicate interval of time in which the probe could first appear. Note that pursuit velocity has already matched stimulus velocity before these intervals.
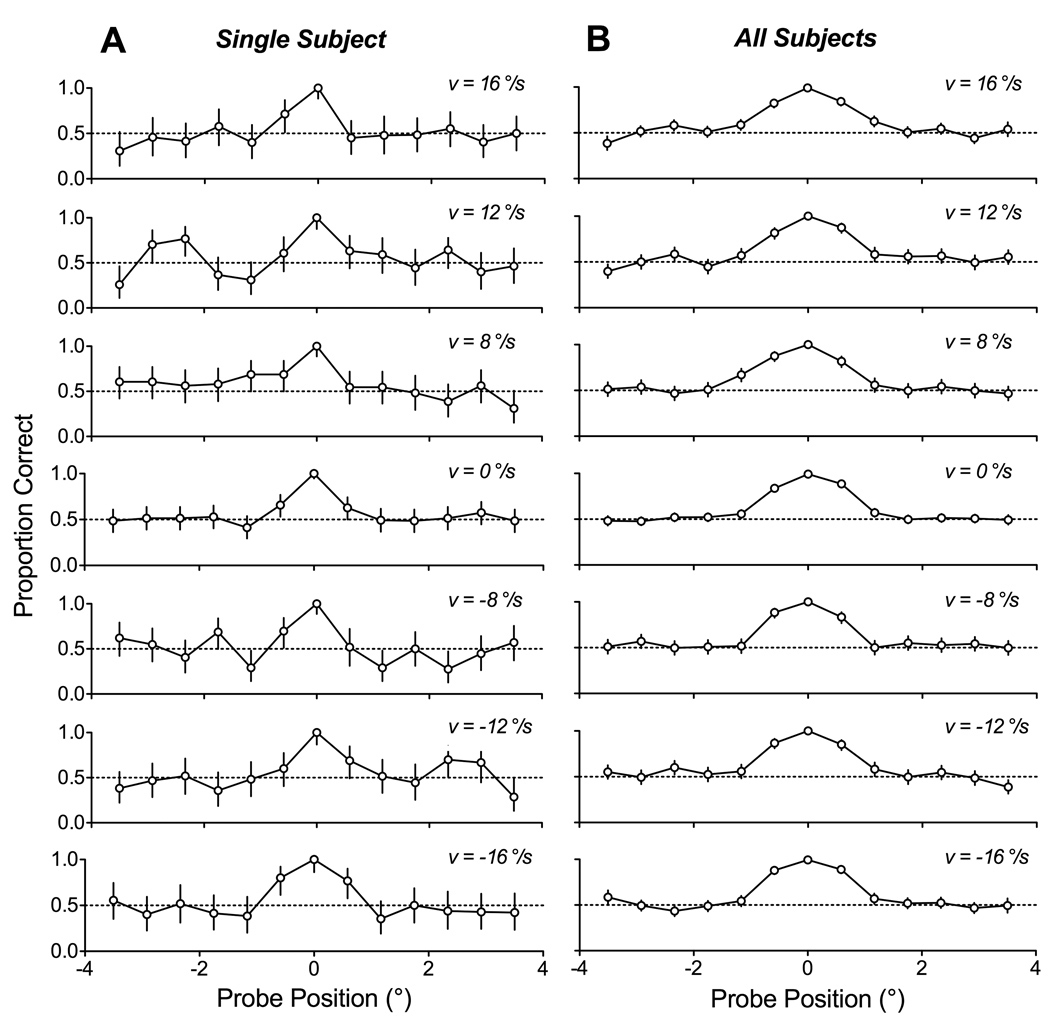
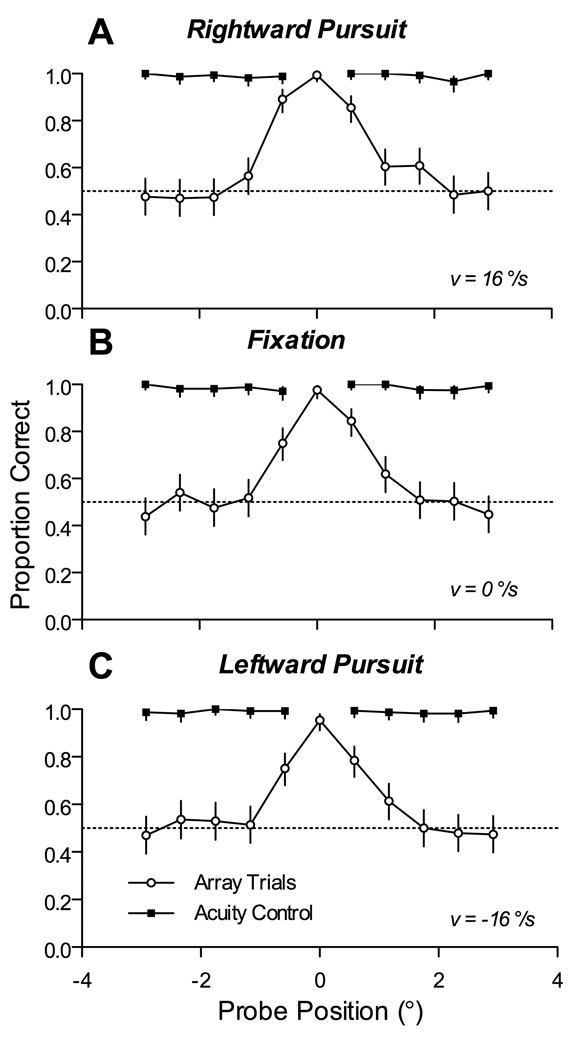
Best performance was at the central character of the array and did not systematically change as a function of stimulus velocity for any of the characters. Figure 3 shows data from a single subject (Subject C) as well as the pooled data from all subjects in experiment 1. The plots give the proportion of correct responses observed for each position of the probed character, and the error-bars indicate the 95% confidence intervals.
Figure 3.
The spatial profile of discrimination performance during pursuit. (A) Sample data from Subject C. Individual panels show plots of performance as a function of probe position (0° represents the tracked character), for a particular direction and speed of pursuit. Top panel represents fastest rightward pursuit while bottom panel represents fastest leftward pursuit. Error bars indicate 95% confidence intervals on estimate of proportion correct pooled across sessions. (B) Pooled data across all 6 subjects that participated in this experiment. All conventions are the same as in (A).
The data from the single subject illustrated in Figure 3 show that performance was perfect, or nearly so, when the probe appeared at the middle character. Proportion correct across all conditions was 100% when the probe was at 0°. However, performance fell off rapidly for eccentric positions. On average, proportion correct was 64% for probes at 0.6°, but only 45% at 1.2°, a proportion that was not significantly different from chance. As evident from the similar appearance across the stack of plots in Figure 3, this same pattern was observed across the full range of stimulus velocities and directions. In particular, performance did not depend on whether the probed characters were ahead or behind the tracked position. During pursuit, proportion correct was 62% for probes appearing 0.6° ahead of the tracked character and 67% for probes appearing 0.6° behind the tracked character; these proportions were significantly greater than chance but not significantly different from each other.
The same pattern of performance was found across all subjects (right column of Figure 3). Although there were idiosyncratic differences between subjects in the profile of performance, all showed based performance within a spatial window that spanned approximately three characters, or about 2° of visual angle. The proportion of correct response, averaged across all subjects was 99.6% when the probe was at 0°, 86% for probes placed at 0.6°, and only 57% for probes at 1.2°. Again, performance did not depend on pursuit speed, or on whether the probe appeared ahead or behind the tracked position: on average, proportion correct was 86% for probes 0.6° ahead, and 84% for probes at 0.6° behind, the tracked character.
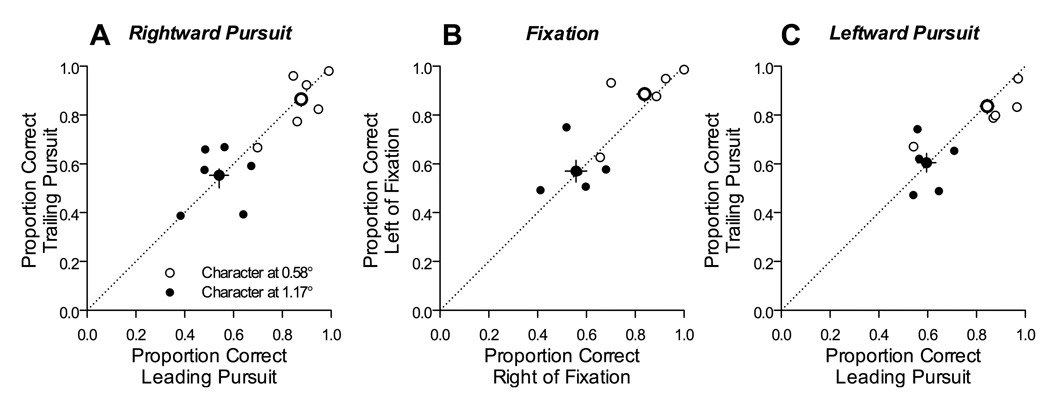
To confirm that performance did not depend on the direction of pursuit, we directly compared performance for probes placed just ahead and behind the pursuit target. The graphs in Figure 4 plot proportion correct for probe positions trailing the pursuit target by 0.6 or 1.2° (open and filled symbols, respectively) against proportion correct for probe positions leading the pursuit target by the same amount. The smaller symbols show individual subject performance and the larger symbols show the group means. If pursuit direction influenced performance, then the data should be systematically biased away from the line of unity slope. In particular, if spatial attention were allocated ahead of the tracked target, the data should lie below the line. Although there were some individual experimental conditions in which this was true, there were also counter-examples, and similar idiosyncratic asymmetries could also be found during fixation (middle plot in Figure 4). Overall, the group means (large symbols with 95% confidence intervals) showed no significant effect of probe location: performance was not significantly different for probes presented just ahead or behind the pursuit target.
Figure 4.
Comparison of performance at the characters directly leading and trailing the tracked character. Each panel plots the proportion of correct responses at the characters just behind the tracked character, as a function of the proportion of correct responses at the characters just ahead of the tracked character. Individual panels show data obtained during rightward pursuit (A), fixation (B), and leftward pursuit (C). The smaller symbols in each panel show the data from individual experimental conditions from the sample subject C, for probes placed one character away from the tracked character (open symbols) and two characters away from the tracked character (filled symbols). Larger symbols in each panel show the group means across all 6 subjects, with confidence intervals.
Performance also did not depend on the small offsets in eye position that occurred during pursuit. As might be expected, subjects tended to lag slightly behind the exact center of the tracked character during pursuit. For example, as illustrated by Figure 5, during pursuit of the 16°/s stimulus, subjects trailed the center of the array during the probe interval by an average of 0.24°. However, there was no evidence that these offsets influenced performance in the discrimination task. Specifically, there was no significant difference in the magnitude of the offset on correct versus incorrect trials (MANOVA), as might be expected if performance were sensitive to the size or direction of the offset. Also, reiterating the point made by Figure 4, even though the offsets during pursuit placed the lagging character slightly closer to the center of gaze than the leading character, overall there was no significant difference between the proportions of correct responses at these two character positions.
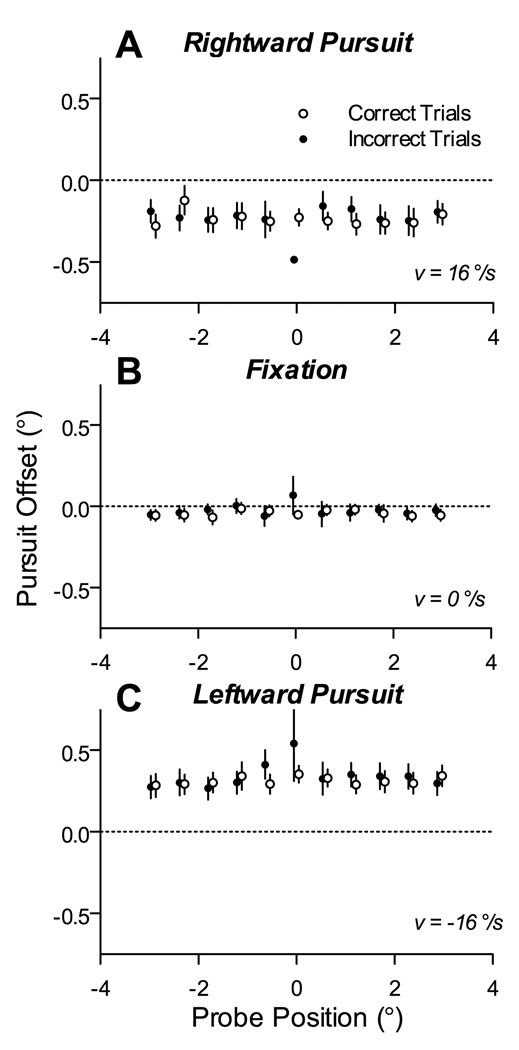
Figure 5.
Eye position offsets during correctly and incorrectly performed trials. Each panel plots the average pursuit offset during the probe interval as a function of the probe position. Individual panels show data obtained during rightward pursuit (A), fixation (B), and leftward pursuit (C). Symbols show group means, with 95% confidence intervals, of the eye position offsets measured from trials in which the letter discrimination was correct (open) and incorrect (filled). None of the pairwise comparisons between correct and incorrect trials showed a significant difference (3-way ANOVA).
We conclude from these data that discrimination performance during pursuit was best at the tracked stimulus and decreased symmetrically with eccentricity from this position, regardless of pursuit direction and independent of the small position offsets that accompany pursuit.
Experiment 2
Because our stimulus array subtended 9° of visual angle, it is possible that the performance of our subjects in Experiment 1 was limited by visual acuity. Because acuity falls off with distance from the center of the fovea (Bex, Dakin, & Simmers, 2003; Millodot, 1966), the narrow and symmetric spatial profiles of performance in our task might simply reflect the limitations of early visual processing, rather than the allocation of spatial attention. To investigate this possibility, we tested the ability of 4 subjects to discriminate characters presented singly and briefly at the same range of spatial locations occupied by our standard array.
For this experiment, we presented probe characters (either a block letter E or 3) at the same spatial locations and with the same timing as in the previous experiments, but without any accompanying distracter characters (block letter 8’s). Rather than tracking the center of the character array, subjects tracked a simple spot stimulus; when the spot reached a randomly selected position within the central 10 ° of the display, a probe character moving at the same speed as the spot was briefly (200 ms) presented at a randomly selected eccentricity (±3 °)from the tracked spot. As a positive control, on interleaved trials subjects performed the discrimination task while tracking the full array of characters as in the previous experiments.
Discrimination of probe characters presented singly was nearly perfect across the full range of eccentricities tested, in contrast to the results found when probes were presented amidst distracter characters. The results were nearly identical across all 4 subjects, and are summarized by the plots of pooled data in Figure 6. When a single probe was presented, the proportion of correct responses remained near 1 for all ten probe positions ranging from ±3° from the tracked spot, regardless of pursuit speed. These results show that visual acuity for our stimuli is excellent across the range of eccentricities used in our experiments. In contrast, when the probe was accompanied by the full array of distracter characters, discrimination performance was best at the tracked character and fell off rapidly with eccentricity. The spatial profile of discrimination performance was therefore dependent on the presence of distracter characters and was not a limitation of visual acuity in our task.
Figure 6.
Discrimination performance in the presence and absence of distracter characters in the array. The three plots show proportion of correct responses during rightward pursuit (A), fixation (B), and leftward pursuit (C). Solid squares indicate performance on the single character acuity task, whereas open circles represent performance on the discrimination task in the presence of the entire character array. Error bars represent 95% confidence intervals on the proportion correct pooled across all 4 subjects that participated in this experiment.
Experiment 3
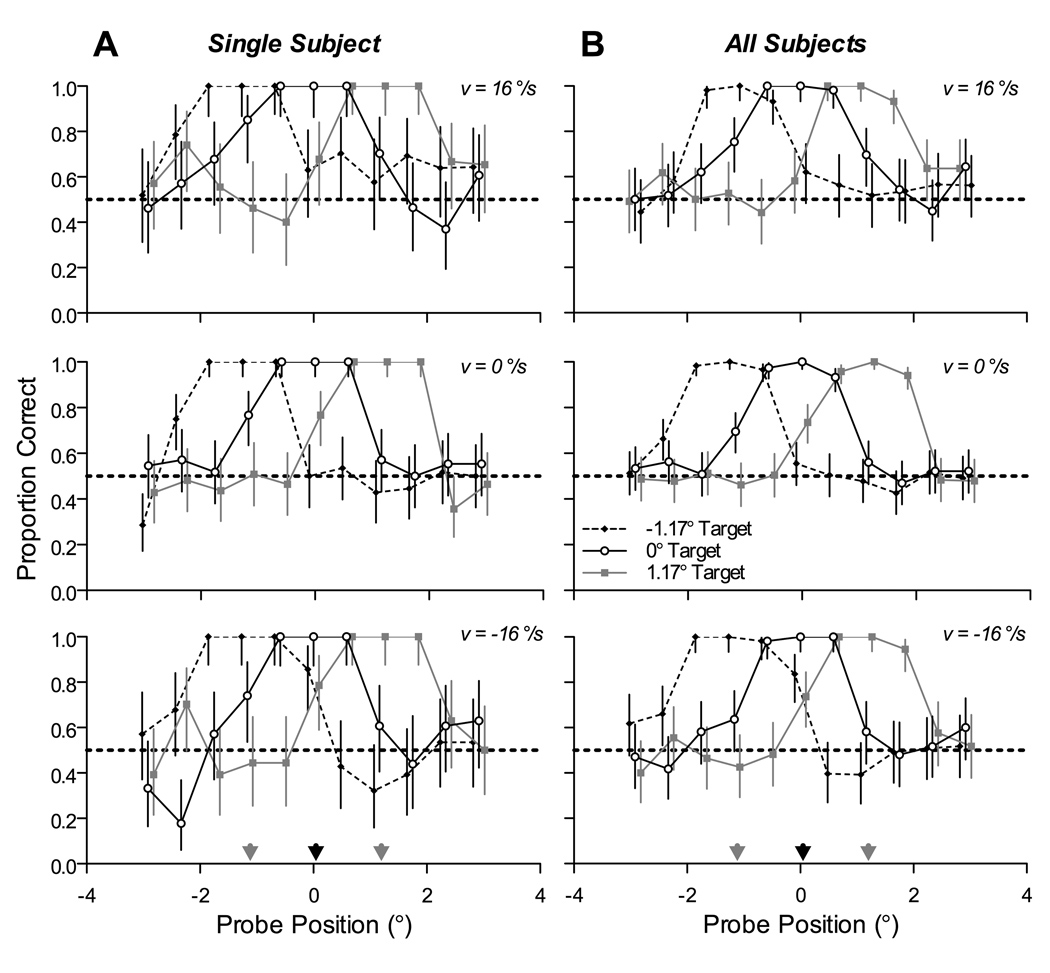
Although performance on the discrimination task was best at the central target in Experiment 1, it is possible that attention was drawn to the center of mass of the array rather than being directed to the pursuit target. To distinguish between these explanations, we performed a third experiment in which two of the subjects from Experiment 1 tracked characters other than the central character.
Best performance was again centered on the pursuit target, even when the target was not centered in the array. The left column of Figure 7 shows performance for a single subject (K) for each possible probe character position, stimulus velocity, and tracked character position (positions 5 and 11 in the 15-character array, as well as 8). In each case, peak performance shifted to the position of the intended pursuit target. The right column of Figure 7 shows the results pooled across both subjects that participated in this experiment. Again, performance was nearly perfect at the tracked character position, fell off slightly for the immediately adjacent positions, and fell to chance levels within 2–3 characters from the tracked position.
Figure 7.
Spatial profile of discrimination performance when the pursuit target is at different positions in the array of characters. (A) Sample data from Subject K. Individual panels show plots of performance as a function of probe position, for rightward pursuit (top), fixation (middle), and leftward pursuit (bottom). Within each panel, the three curves show the spatial profile of discrimination performance when the tracked character was 1.8° to left (black diamonds), 1.8° to the right (gray squares), or centered in the array (white circles) Gray and black arrows in bottom plot indicate position of tracked character. Error bars indicate 95% confidence intervals. (B) Pooled data from both subjects that participated in this experiment. All conventions are the same as in (A).
Just as there was no change in performance on the task other than shifting its peak to the intended pursuit target, there was also no change in the quality of pursuit when subjects pursued characters other than the central character. There was no systematic change in pursuit gain, and the distribution of offsets in eye positions was identical for each velocity when corrected for the displacement of the tracked character. We performed ANOVA comparisons between the three distributions at each velocity and failed to reject the null hypothesis that the distributions were identical (p-values near 1.0 for all comparisons and both subjects).
Experiment 4
The results from the preceding experiments indicate that performance remained best at the tracked character because of how spatial attention was deployed during the pursuit task in the presence of distracters, rather than because of acuity limitations or technical aspects of the experimental design. If so, then one might expect that classic manipulations of visual attention might alter the spatial profile of performance in our task. To test this idea, we conducted a fourth experiment in which we provided spatial cues to the subjects that indicated the likely location of the upcoming probe character.
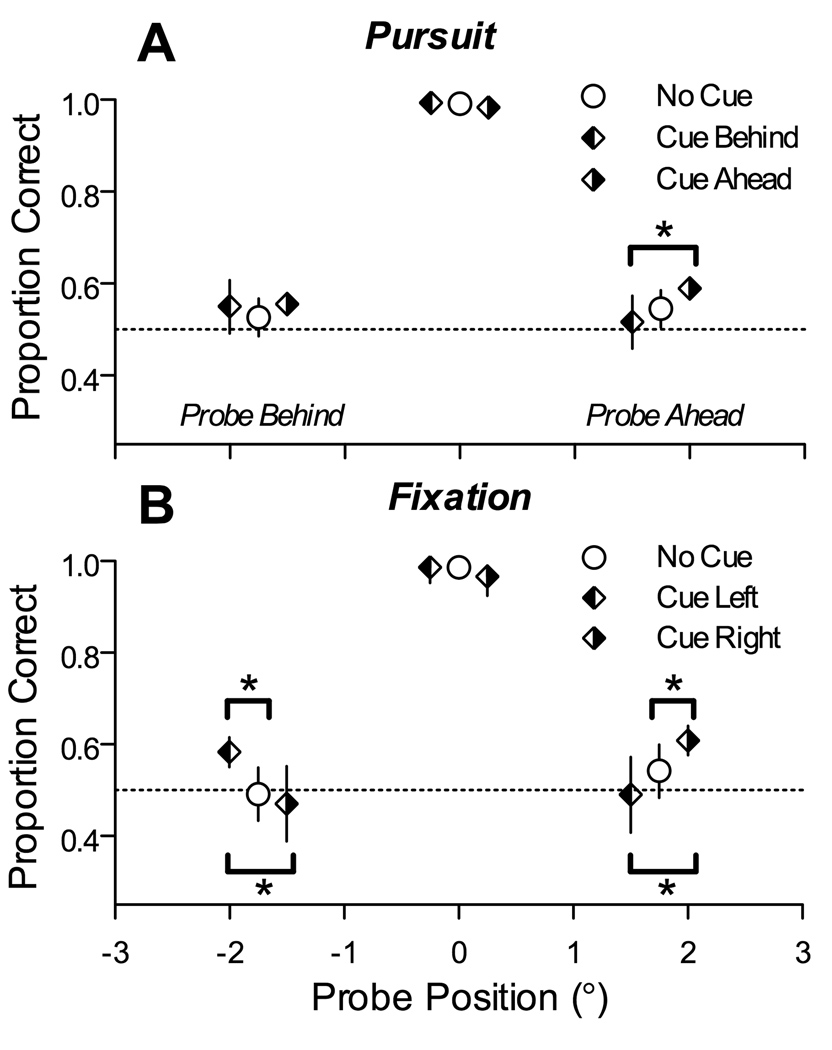
As in experiments 1–3, subjects tracked or fixated an array of characters and were required to discriminate the briefly presented probe character. In this experiment, however, on most trials (16/22 trial types, 73%) subjects were given a visual cue about the likely location of the probe character during the fixation period at the start of the trial. The visual cue was partially valid – it correctly indicated the location of the upcoming probe character on 75% of the trials. To economize on the number of conditions, we used two cued locations, falling on either tail of the normal spatial profile of performance in the task (two characters away from the tracked character on either the left or right side). On invalidly cued trials, the probe character was equally likely to be at the uncued position on the opposite side of the array, or at the tracked position at the center. On the remainder of the trials (6/22 trial types, 27%) subjects were given no visual cue, and the probe character was equally likely to be at the array center, or at either eccentric position (two characters away from the center).
We found small but significant effects of spatial cues in this task. As summarized by the plots in Figure 8, which pools the data from 4 subjects, spatial cues produced small changes in performance, and these effects depended on the locations of the probe and the cue with respect to the direction of pursuit. As shown in Figure 8A, during pursuit when the probe was located ahead of the tracked character, performance was significantly better on validly cued trials (“Cue Ahead”) than on invalidly cued trials (“Cue Behind”) (p=0.019, Chi-square test); the difference between performance on validly cued trials and no-cue trials did not reach significance (p=0.06). In contrast, during pursuit when the probe was located behind the tracked character, performance was not significantly different between any of the three cue conditions. During the fixation condition (Figure 8B), valid cues produced significantly better performance than invalid cues (and no cue) regardless of where the probe appeared. In addition to these cueing effects for slightly eccentric locations, overall performance was again best when the probe appeared at the tracked character, during both pursuit and fixation. Thus, at least under the conditions of this experiment, spatial cues caused small but significant changes in discrimination performance, and these effects were observed during pursuit only when valid cues were presented at positions ahead of the tracked character.
Figure 8.
Effects of spatial cues on discrimination performance. In this experiment, probes appeared either at the tracked, central character, or at the character 1.75° either to the left or right of the tracked character, during either pursuit or fixation. (A) Proportion of correct responses during pursuit, pooled across rightward and leftward directions. As indicated by the three symbol types, responses on each trial were preceded by no cue (open circles), by a cue located 1.75° behind the tracked character (left-filled diamonds), or by a cue located 1.75° ahead of the tracked character (right-filled diamonds). (B) Proportion of correct responses during fixation. The three symbol types indicate trials with no cue (open circles), a cue located 1.75° left of center (left-filled diamonds), or a cue located 1.75° right of center (right-filled diamonds). The data points for the three cueing conditions are slightly offset horizontally for clarity. Error bars represent 95% confidence intervals on the proportion correct pooled across all 4 subjects that participated in this experiment. Brackets with asterisks highlight comparisons that showed a significant difference (p<0.05, Chi-square test).
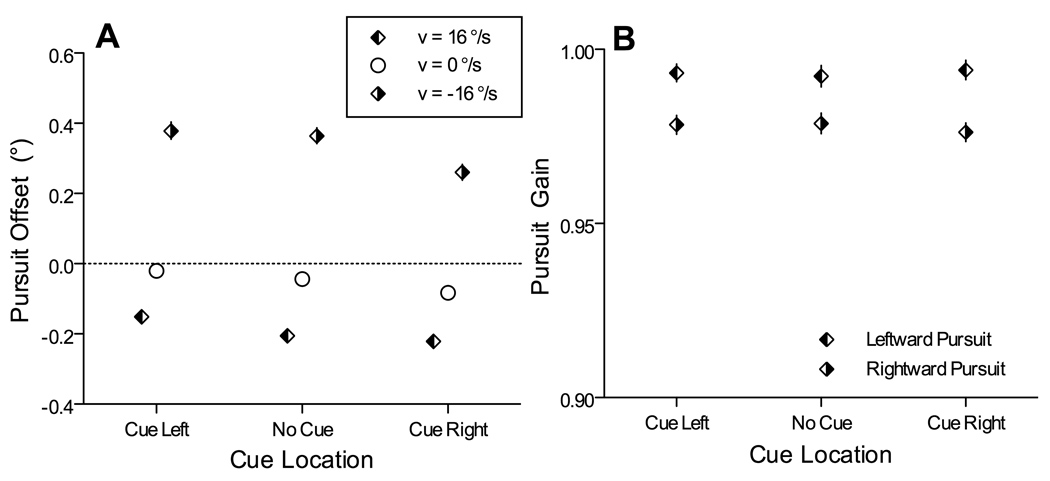
Spatial cues also caused minor changes in the metrics of pursuit, and these primarily affected the position offsets during pursuit rather than pursuit eye velocity. During the probe interval of this experiment, subjects again tended to lag slightly behind the center of the tracked character during pursuit, and this was sometimes altered by the spatial cue. As illustrated in Figure 9A, during rightward pursuit subjects trailed the center by about 0.2°, and this offset was significantly smaller on trials that included a left cue. During leftward pursuit subjects trailed the center by slightly less than 0.4°, and this offset was significantly smaller on trials with a right cue. These offsets were in the opposite direction from what one might expect if eye position were simply biased toward the cue location. In contrast, spatial cues had no effect on the gain of pursuit velocity during the probe interval. As shown in Figure 9B, pursuit gain during leftwards pursuit was significantly higher than pursuit gain during rightwards pursuit, but there were no significant differences in pursuit gain across the different cue conditions.
Figure 9.
Effects of spatial cues on eye position offsets and eye velocity gain. (A) Eye position offsets during the probe interval measured during leftward pursuit (left-filled diamonds), fixation (open cicles), and rightward pursuit (right-filled diamonds). Data from these trials were sorted according to the cue condition, as indicated along the x-axis. Symbols show group means, with 95% confidence intervals, which in each case are smaller than the height of the symbol. (B) Gain of eye velocity during the probe interval during leftward and rightward pursuit, sorted according to cue condition. Conventions as in (B).
DISCUSSION
We conclude that during smooth pursuit eye movements spatial attention is centered on the tracked target. Using a challenging letter discrimination task, we found that performance was always best at the character position corresponding to the pursuit target, and fell off steeply (within 1–2°) and relatively symmetrically for positions ahead or behind of the tracked target. Moreover, these spatial properties of discrimination performance during pursuit were invariant across a range of pursuit speeds (8, 12, 16 °/s). We also considered the possibility that attention was allocated based on the center-of-mass of the array rather than the position of the tracked target. To test this possibility, we performed a control experiment in which the position of the pursuit target varied within the array, and found that the best performance shifted to whichever character position corresponded to the intended pursuit target. These results confirm that spatial attention is centered on the pursuit target rather than shifted ahead or behind it.
The exact spatial profile of performance we observed was certainly influenced by the details of our stimulus – for example, the spacing of the characters in our array. In the following, we briefly consider the factors that likely contributed to this spatial profile, before discussing the significance of our finding that the best performance stays fixed at the tracked character.
Factors that contributed to the narrow spatial profile of performance in our task
A key feature of our results is the fact that discrimination performance fell off steeply for positions adjacent to the tracked character – the sharpness of this tuning allowed us to make sensitive measurements of where attention was allocated during pursuit. Of course, a prerequisite for this approach is that visual acuity is not a limiting factor in the task. In all subjects, the fall off in performance was much more spatially restricted than we would have expected based on visual acuity alone; at the stroke width we used in our stimuli (0.06° or 3.3 minutes of visual arc), visual acuity within the central 3–4° of the stimulus should not vary (Bex, Dakin, & Simmers, 2003; Millodot, 1966). Nonetheless, to directly address this issue, we tested discrimination performance using single characters presented at the same range of spatial locations as in the main experiment, and found that performance was essentially perfect. Thus, our finding that spatial attention was centered on the tracked target is not due to a simple fall-off in visual acuity with eccentricity. The narrow spatial profile in our task was probably due to visual crowding or lateral masking (for a review, see Levi, 2008). The “ordinary” masks in our task (i.e., the block letter 8 that preceded and followed the probe letter at the same position) made it necessary for subjects to commit sustained attention to the stimulus. However, the presence of these masks still allowed nearly perfect performance in the single-character task, implying that ordinary masking was not responsible for the narrow spatial profile.
On the other hand, visual crowding (or lateral masking) could be responsible, because the narrow profile was only observed when the probes were flanked by other characters in the array. Visual crowding is known to play a major role in limiting the readability of text, especially for peripheral (or at least extrafoveal) visual stimuli (Chung, Levi, & Legge, 2001; Pelli, Palomares, & Majaj, 2004). The fall off in performance that we found (best performance within 1–2° of the tracked character) is in reasonably good agreement with the classic rule that crowding occurs when competing features lie within 0.5 the eccentricity of the target object (Bouma, 1970; Toet and Levi, 1992). Given the 0.6° spacing of our characters, this rule predicts crowding effects to occur at about 1.2° degrees, which matches what we observed.
The explanation for crowding remains unsettled (Levi, 2008), but some form of the widely accepted “pooling hypothesis” could explain the pattern of results in our task (Parkes et al., 2001; Levi, Klein & Hariharan, 2002; Pelli et al., 2004). According to this view, subjects recognized letters in our task by first detecting individual features (e.g., edges) and then pooling these features within a window small enough to avoid mixing together parts that do not belong together (e.g., edges from different characters). In principle, this narrow integration window could be centered on arbitrary locations in the array of characters. In practice, we found that it remained centered on the tracked character in our task, regardless of pursuit direction or speed.
Spatial attention is not allocated ahead of the pursuit target
Previous findings demonstrated that the allocation of attention during smooth pursuit is ahead of the pursuit target (van Donkelaar, 1999; van Donkelaar & Drew, 2002). Such a model is appealing because it is consistent with the view that smooth pursuit movements anticipate the motion of the target along an expected trajectory (Kowler, 1989), and the forward projection of attention would provide an opportunity for accurate computation of such expectations. Our results contradict this model. We suggest that the discrepancies between our results and previous reports can be resolved by recognizing that those studies involved attention capture, while ours did not.
The initial support for the hypothesis that the locus of attention was allocated ahead of the pursuit target came from observation of directional asymmetry in saccade latency to targets appearing on either side of a pursuit stimulus (Tanaka et al., 1998). Tanaka et al. (1998) offered several competing hypotheses to explain their results. Two of the relevant explanations were that a shift of attention into the wake of a pursuit movement was inhibited, or that attention was asymmetrically allocated along the pursuit path. The second hypotheses inspired a manual reaction time version of the experiment (van Donkelaar, 1999) that found that manual reaction time was lower to targets ahead of the pursuit target. A subsequent character discrimination task, in which only one character underwent a substantial change in form, indicated that the degree to which the best performance led the pursuit target depended upon pursuit velocity (van Donkelaar & Drew, 2002). Another investigation lent credence to the hypothesis that saccade initiation was inhibited in the wake of pursuit (Kanai, van der Geest, & Frens, 2003). That study abolished the asymmetry in saccade latency by cuing the direction of the upcoming saccade.
While those experiments have lent support to both of the hypotheses put forth by Tanaka et al. (1998), all involved abrupt onsets that captured attention. The stimuli involved abrupt onsets in luminance (Kanai et al., 2003; Tanaka et al., 1998; van Donkelaar, 1999), significant shape changes (van Donkelaar & Drew, 2002), or abrupt onsets in motion (Kanai et al., 2003; Tanaka et al., 1998). Abrupt changes in luminance and shape have long been known to capture attention and invoke involuntary eye movements (Theeuwes, 1994; Theeuwes, Kramer, Hahn, & Irwin, 1998; Yantis & Jonides, 1984, 1990), and recently abrupt motion onsets have also been shown to capture attention (Abrams & Christ, 2003). Thus the directional asymmetry in saccade latencies and manual reaction occurred in the context of attention capture and may be more reflective of an asymmetry in the capture of attention rather than in the steady state allocation of attention during maintained pursuit.
Attention capture is not an important factor in our conclusions because our main experiments did not include a spatially localized abrupt onset. We used a stimulus that was specifically designed to avoid these effects. Although the stimulus has been described previously (Deubel & Schneider, 1996), it is worthwhile to briefly highlight some of its important aspects. First, the stimulus presentation time was short, which allowed us to probe the momentary allocation of attention. Second, while the change from mask to probe and back created small local abrupt onsets in a pre-structured field of objects, the onsets were not correlated in any way to the position of the probe character. In addition, the difference between the probe and the distracters was solely the configuration of the same number of line segments. Thus we did not induce automatic allocation of attention to the probe character.
Nevertheless, we determined in our fifth experiment that subjects were marginally able to voluntarily allocate attention ahead of the pursuit target in response to spatially specific cues. This result is reminiscent of the finding that response times are decreased to onsets ahead of the pursuit target. Therefore we propose that our results and those of van Donkelaar and Drew are best reconciled by the proposition that attention has a greater capacity to be allocated ahead of the pursuit target than behind, either voluntarily or in response to abrupt onsets.
Putting our current results together with those of previous studies, it appears that during maintained pursuit, attention is allocated to the intended pursuit target in an approximately symmetrical fashion. In addition, subjects are better able to allocate attention ahead of a pursuit target than behind it. Objects or other obstacles that might influence the trajectory of the pursuit target will capture attention if they appear abruptly; if they do not appear abruptly, they will first appear on the periphery and, as they approach the fovea, there will be sufficient time for computation of an expected trajectory. Furthermore, if the pursuit target can change direction of its own accord, then attention would optimally be allocated to the target rather than in its expected path. Overall, there is little heuristic justification for a model in which attention is normally allocated ahead of the pursuit target rather than to the pursuit target itself.
CONCLUSIONS
Overall, our results demonstrate that performance on a discrimination task is best at the tracked target during pursuit eye movements, and falls off symmetrically for positions ahead or behind the target, regardless of speed. This indicates that the focus of spatial attention during smooth pursuit eye movements is centered on the pursuit target. In addition, subjects were marginally able to allocate attention ahead of the pursuit target but not behind it.
ACKNOWLEDGEMENTS
This work was supported by Grant EY12212 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lee P. Lovejoy, Email: lovejoy@salk.edu.
Garth A. Fowler, Email: g-fowler@northwestern.edu.
REFERENCES
- Abrams RA, Christ SE. Motion onset captures attention. Psychol Sci. 2003;14(5):427–432. doi: 10.1111/1467-9280.01458. [DOI] [PubMed] [Google Scholar]
- Bouma H. Interaction effects in parafoveal letter recognition. Nature. 1970;226(5241):177–178. doi: 10.1038/226177a0. [DOI] [PubMed] [Google Scholar]
- Bex PJ, Dakin SC, Simmers AJ. The shape and size of crowding for moving targets. Vision Res. 2003;43(27):2895–2904. doi: 10.1016/s0042-6989(03)00460-7. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Brown L, Li X. Confidence intervals for two sample binomial distribution. Journal of Statistical Planning and Inference. 2005;130:359–375. [Google Scholar]
- Chung ST, Levi DM, Legge GE. Spatial-frequency and contrast properties of crowding. Vision Res. 2001;41(14):1833–1850. doi: 10.1016/s0042-6989(01)00071-2. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 1996;36(12):1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Garbutt S, Lisberger SG. Directional cuing of target choice in human smooth pursuit eye movements. J Neurosci. 2006;26(48):12479–12486. doi: 10.1523/JNEUROSCI.4071-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Suehiro K, Kawano K. Temporospatial properties of the effects of bottom-up attention on smooth pursuit initiation in humans. Exp Brain Res. 2004;156(1):88–93. doi: 10.1007/s00221-003-1758-0. [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Percept Psychophys. 1995;57(6):787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Tegally D. The effects of dividing attention on smooth pursuit eye tracking. Exp Brain Res. 2005;163(3):306–313. doi: 10.1007/s00221-004-2171-z. [DOI] [PubMed] [Google Scholar]
- Johnson NL, Kotz S, Kemp AW. Univariate discrete distributions. 2nd ed. New York: Wiley; 1992. [Google Scholar]
- Kanai R, van der Geest JN, Frens MA. Inhibition of saccade initiation by preceding smooth pursuit. Exp Brain Res. 2003;148(3):300–307. doi: 10.1007/s00221-002-1281-8. [DOI] [PubMed] [Google Scholar]
- Kerzel D, Souto D, Ziegler NE. Effects of attention shifts to stationary objects during steady-state smooth pursuit eye movements. Vision Res. 2008;48(7):958–969. doi: 10.1016/j.visres.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Khurana B, Kowler E. Shared attentional control of smooth eye movement and perception. Vision Res. 1987;27(9):1603–1618. doi: 10.1016/0042-6989(87)90168-4. [DOI] [PubMed] [Google Scholar]
- Klein R, Farrell M. Search performance without eye movements. Percept Psychophys. 1989;46(5):476–482. doi: 10.3758/bf03210863. [DOI] [PubMed] [Google Scholar]
- Kowler E. Cognitive expectations, not habits, control anticipatory smooth oculomotor pursuit. Vision Res. 1989;29(9):1049–1057. doi: 10.1016/0042-6989(89)90052-7. [DOI] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res. 1995;35(13):1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Kowler E, van der Steen J, Tamminga EP, Collewijn H. Voluntary selection of the target for smooth eye movement in the presence of superimposed, full-field stationary and moving stimuli. Vision Res. 1984;24(12):1789–1798. doi: 10.1016/0042-6989(84)90010-5. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. Recasting the smooth pursuit eye movement system. J Neurophysiol. 2004;91(2):591–603. doi: 10.1152/jn.00801.2003. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Miles FA. Initiation of saccades during fixation or pursuit: evidence in humans for a single mechanism. J Neurophysiol. 1996;76(6):4175–4179. doi: 10.1152/jn.1996.76.6.4175. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Zivotofsky AZ, Miles FA. Target selection for pursuit and saccadic eye movements in humans. J Cogn Neurosci. 1999;11(6):641–649. doi: 10.1162/089892999563706. [DOI] [PubMed] [Google Scholar]
- Levi DM. Crowding--an essential bottleneck for object recognition: a mini-review. Vision Res. 2008;48(5):635–654. doi: 10.1016/j.visres.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Klein SA, Hariharan S. Suppressive and facilitatory spatial interactions in foveal vision: foveal crowding is simple contrast masking. J Vis. 2002;2(2):140–166. doi: 10.1167/2.2.2. [DOI] [PubMed] [Google Scholar]
- Liu L, Arditi A. Apparent string shortening concomitant with letter crowding. Vision Res. 2000;40(9):1059–1067. doi: 10.1016/s0042-6989(99)00247-3. [DOI] [PubMed] [Google Scholar]
- Madelain L, Krauzlis RJ, Wallman J. Spatial deployment of attention influences both saccadic and pursuit tracking. Vision Res. 2005;45(20):2685–2703. doi: 10.1016/j.visres.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Millodot M. Foveal and extra-foveal acuity with and without stabilized retinal images. Br J Physiol Opt. 1966;23(2):75–106. [PubMed] [Google Scholar]
- Parkes L, Lund J, Angelucci A, Solomon JA, Morgan M. Compulsory averaging of crowded orientation signals in human vision. Nat Neurosci. 2001;4(7):739–744. doi: 10.1038/89532. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Pelli DG, Palomares M, Majaj NJ. Crowding is unlike ordinary masking: distinguishing feature integration from detection. J Vis. 2004;4(12):1136–1169. doi: 10.1167/4.12.12. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Schutz AC, Braun DI, Gegenfurtner KR. Contrast sensitivity during the initiation of smooth pursuit eye movements. Vision Res. 2007;47(21):2767–2777. doi: 10.1016/j.visres.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Schutz AC, Delipetkos E, Braun DI, Kerzel D, Gegenfurtner KR. Temporal contrast sensitivity during smooth pursuit eye movements. J Vis. 2007;7(13):1–15. doi: 10.1167/7.13.3. 3. [DOI] [PubMed] [Google Scholar]
- Spering M, Gegenfurtner KR, Kerzel D. Distractor interference during smooth pursuit eye movements. J Exp Psychol Hum Percept Perform. 2006;32(5):1136–1154. doi: 10.1037/0096-1523.32.5.1136. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Yoshida T, Fukushima K. Latency of saccades during smooth-pursuit eye movement in man. Directional asymmetries. Exp Brain Res. 1998;121(1):92–98. doi: 10.1007/s002210050440. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Endogenous and exogenous control of visual selection. Perception. 1994;23(4):429–440. doi: 10.1068/p230429. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Kramer AF, Hahn S, Irwin DE. Our eyes do not always go where we want them to go - capture of the eyes by new objects. Psychological Science. 1998;9(5):379–385. [Google Scholar]
- Toet A, Levi DM. The two-dimensional shape of spatial interaction zones in the parafovea. Vision Res. 1992;32(7):1349–1357. doi: 10.1016/0042-6989(92)90227-a. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P. Spatiotemporal modulation of attention during smooth pursuit eye movements. Neuroreport. 1999;10(12):2523–2526. doi: 10.1097/00001756-199908200-00016. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P, Drew AS. The allocation of attention during smooth pursuit eye movements. Prog Brain Res. 2002;140:267–277. doi: 10.1016/S0079-6123(02)40056-8. [DOI] [PubMed] [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: evidence from visual search. J Exp Psychol Hum Percept Perform. 1984;10(5):601–621. doi: 10.1037//0096-1523.10.5.601. [DOI] [PubMed] [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: voluntary versus automatic allocation. J Exp Psychol Hum Percept Perform. 1990;16(1):121–134. doi: 10.1037//0096-1523.16.1.121. [DOI] [PubMed] [Google Scholar]