Abstract
Many signaling pathways that contribute to tumorigenesis are also functional in pregnancy, although they are dysregulated in the former and tightly regulated in the latter. Transformation-related protein 53 (Trp53), which encodes p53, is a tumor suppressor gene whose mutation is strongly associated with cancer. However, its role in normal physiological processes, including female reproduction, is poorly understood. Mice that have a constitutive deletion of Trp53 exhibit widespread development of carcinogenesis at early reproductive ages, compromised spermatogenesis, and fetal exencephaly, rendering them less amenable to studying the role of p53 in reproduction. To overcome this obstacle, we generated mice that harbor a conditional deletion of uterine Trp53 and examined pregnancy outcome in females with this genotype. These mice had normal ovulation, fertilization, and implantation; however, postimplantation uterine decidual cells showed terminal differentiation and senescence-associated growth restriction with increased levels of phosphorylated Akt and p21, factors that are both known to participate in these processes in other systems. Strikingly, uterine deletion of Trp53 increased the incidence of preterm birth, a condition that was corrected by oral administration of the selective COX2 inhibitor celecoxib. We further generated evidence to suggest that deletion of uterine Trp53 induces preterm birth through a COX2/PGF synthase/PGF2α pathway. Taken together, our observations underscore what we believe to be a new critical role of uterine p53 in parturition.
Introduction
Pregnancy is a complex process. Ovulation, fertilization, preimplantation embryo development, oviductal embryo transport, embryo implantation, uterine decidualization, placentation, and parturition are all critical, and failure at any of these stages compromises pregnancy outcome. Implantation is the first encounter between embryo and mother, while parturition is the end of this encounter, although soluble factors from the uterus and/or embryo influence the embryo-uterine dialogue prior to implantation. Therefore, implantation and parturition are — conceptually, physiologically, and clinically — distinct conundrums with different molecular and genetic signatures. Although implantation failure is a significant cause of infertility and a clinical issue in in vitro fertilization programs, preterm birth and prematurity are problems with consequences that continue beyond birth, posing huge long-term social and economic liabilities.
Premature delivery accounts for 75% of early neonatal morbidity and mortality, making this disorder a global clinical, social, and economic burden (1). About 13 million premature births and more than 3 million stillbirths occur each year. Prematurity is a direct cause of nearly 30% of all neonatal deaths, totaling more than 1 million each year. In addition, many babies that survive premature birth suffer serious long-term disabilities (2). According to the 2006 report of the Institute of Medicine, National Academies of Sciences, the total cost of preterm birth is estimated to be at least $26 billion a year in the United States (3). Therefore, there is an urgent need to research new approaches for combating this public health concern. In the present study, we used a mouse model of conditional deletion of uterine Trp53 to demonstrate that p53 deficiency conferred premature uterine senescence and promoted preterm birth.
Several signaling pathways that function during pregnancy are also active during tumorigenesis, the difference being their tight regulation during pregnancy but dysregulation during tumorigenesis (4). Mutation of Trp53 is found in a variety of cancers (5); however, its roles in normal physiological processes, including female reproduction, remain poorly understood. A recent study reported that mice constitutively deleted of Trp53 (Trp53–/– mice) have implantation failure caused by downregulation of uterine leukemia inhibitory factor (LIF) on day 4 of pregnancy (with day 1 defined as the day the vaginal plug forms) (6). These systemic null mice, however, begin to die of cancer as early as 2 months of age (7), and a number of Trp53–/– fetuses, especially females, die prematurely due to the development of exencephaly (8). In addition, Trp53–/– males have compromised spermatogenesis (9). Such complications make these mice less than appropriate for studying female reproductive phenotypes devoid of other systemic and male effects. Therefore, we generated mice with conditional deletion of uterine Trp53 by crossing floxed Trp53 mice (Trp53loxP/loxP mice; ref. 10) with progesterone (P4) receptor–Cre (PR-Cre) transgenic mice (PgrCre/+ mice; ref. 11) and examined critical events during the course of pregnancy. Although all females with uterine deletion of Trp53 showed normal implantation, more than 50% had preterm birth with neonatal death associated with premature uterine senescence. Further studies to unravel the underlying mechanism revealed that p21 and phosphorylated Akt (pAkt) contributed to premature uterine senescence and that activation of the Akt/COX2 signaling pathway evoked premature birth. These results unravel what we believe to be a new role of p53 in parturition.
Results
Trp53 is efficiently deleted in uteri of Trp53loxP/loxPPgrCre/+ females.
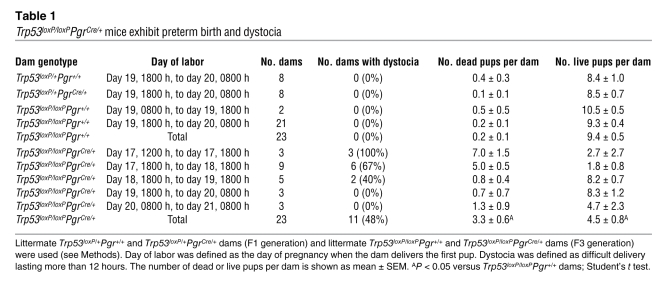
We generated Trp53loxP/loxPPgrCre/+ and Trp53loxP/loxPPgr+/+ mice by crossing Trp53loxP/loxP mice (FVB/129) with PgrCre/+ mice (C57BL6/129) to examine various events during pregnancy (see Methods). Throughout the present study, we used littermate females to compare reproductive functions between control and experimental groups to minimize the influence of genetic background on the observed phenotypes. PgrCre/+ females have been reported to have normal fertility (11). By breeding WT or PgrCre/+ females with PgrCre/+ males, we also confirmed that these females were fertile with normal gestational length (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI40051DS1). In addition, we found that heterozygous floxed Trp53 females with (Trp53loxP/+PgrCre/+) or without (Trp53loxP/+Pgr+/+) a PR-Cre allele on a mixed background had normal pregnancy outcome (Table 1). Therefore, PR haploinsufficiency or the presence of Cre did not show any adverse effect on pregnancy outcome. This is consistent with the findings of similar PR expression patterns in Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ pregnant uteri (Supplemental Figure 2). With these results in hand, we used 2-month-old littermate Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ dams on a mixed background to examine various reproductive events during pregnancy. We first confirmed that these dams had efficient deletion of uterine Trp53 (Figure 1, A–D), with no sign of tumorigenesis in the uterus or other organs as we previously reported (12), as opposed to that found in mice with global deletion of Trp53 (7).
Table 1 .
Trp53loxP/loxPPgrCre/+ mice exhibit preterm birth and dystocia
Figure 1. Efficient deletion of uterine Trp53.
(A and B) Trp53 mRNA was undetectable in 2-month-old Trp53loxP/loxPPgrCre/+ uteri analyzed by RT-PCR (A) and Northern hybridization (B). Actb and Rpl7 were used as internal controls. (C) p53 protein levels were very low to undetectable in irradiation-induced 2-month-old Trp53loxP/loxPPgrCre/+ uteri assessed by Western blotting. Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ dams were killed 6 hours after 9.5 Gy whole-body γ irradiation. Protein extracts from mouse lymphoma cell line WR19L were used as a positive control. (D) Immunostaining of p53 in Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ decidua on day 8 of pregnancy. Immunoreactive p53 was undetectable in Trp53loxP/loxPPgrCre/+ decidua. Fast Green solution was used to counterstain the cytoplasm. Dark green-black staining shows nuclear p53 localization. Scale bar: 200 μm.
Ovulation, fertilization, preimplantation development, and implantation are normal in Trp53loxP/loxPPgrCre/+ dams.
We examined ovulation and fertilization in Trp53loxP/loxPPgrCre/+ dams by flushing eggs and/or embryos on day 2 of pregnancy and found both processes to be normal (Supplemental Figure 3, A and B). We also confirmed normal development of preimplantation embryos and their timely transport from the oviduct into uterine lumens of Trp53loxP/loxPPgrCre/+ dams by recording the number of blastocysts retrieved in uterine flushings on day 4 of pregnancy (Supplemental Figure 3C).
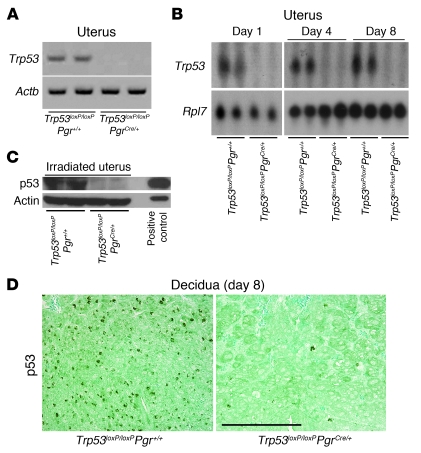
Successful implantation is the result of reciprocal interactions between the blastocyst and the receptive uterus. The receptive state of the uterus is transient and is defined as the time at which the uterine environment is conducive to blastocyst acceptance and implantation. In mice, generalized stromal edema late on the morning of day 4 leads to uterine luminal closure, placing the blastocyst in close apposition with the luminal epithelium. This is followed by an intimate adherence of the blastocyst trophectoderm with the luminal epithelium (attachment reaction), marking the first discernible sign of implantation on the night of day 4, between 2200 h and 2400 h. The attachment reaction coincides with increased stromal vascular permeability at the site of the blastocyst and can be demarcated by distinct blue bands along the uterus after intravenous injection of a blue dye solution (13). We found that Trp53loxP/loxPPgrCre/+ dams had numbers of distinct implantation sites comparable to those of Trp53loxP/loxPPgr+/+ dams on the night of day 4 (data not shown). The implantation process in progress was also normal in Trp53loxP/loxPPgrCre/+ dams on day 5 of pregnancy, as assessed by the blue dye method and histological analysis (Figure 2, A–C). COX2, encoded by Ptgs2, is an inducible rate-limiting enzyme required for the biosynthesis of prostaglandins (PGs). COX2 is expressed in the luminal epithelium and stroma exclusively at the site of blastocyst attachment and is critical for implantation (14). We found that expression patterns of Ptgs2 mRNA and COX2 protein were similar between Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ dams during implantation (Figure 2C and Supplemental Figure 4).
Figure 2. Mice with uterine deletion of Trp53 show normal implantation.
(A) Representative photographs of uteri with implantation sites (blue bands) on day 5 of pregnancy in Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ females, mated with fertile Trp53loxP/loxPPgr+/+ males. Arrows and arrowheads indicate implantation sites and ovaries, respectively. (B) Number of implantation sites on day 5 of pregnancy (mean ± SEM; P > 0.05; Student’s t test). (C) Representative photomicrographs of H&E-stained histology, and in situ hybridization of Ptgs2, in implantation sites on day 5 of pregnancy in Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ dams. Arrowheads indicate the location of implanting blastocysts. Scale bars: 200 μm. (D) In situ hybridization of Lif in Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ uterine sections on days 1 and 4 of pregnancy. Scale bars: 200 μm. (E and F) Lif mRNA levels in day 1 uteri of Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ dams (E) and in day 4, 0900 h, uteri of Trp53loxP/loxPPgr+/+, Trp53loxP/loxPPgrCre/+, WT, and Lif+/– dams (F), as determined by Northern hybridization. Values are mean ± SEM of 2–4 independent samples. *P < 0.05, Student’s t tests.
Lif, an estrogen response gene, is required for uterine receptivity and implantation (15). It is first expressed in uterine glands on the morning of day 4, then in the stroma surrounding the blastocyst at the time of the attachment reaction the night of day 4, persisting through the morning of day 5 (16). Because reduced uterine Lif expression was previously reported to be the cause of implantation failure in Trp53–/– dams (6), we compared uterine Lif expression between Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ dams on days 1 and 4 of pregnancy and found their expression patterns to be similar (Figure 2D). We also found comparable levels of uterine Lif expression in Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ dams on day 1 (Figure 2E), when the uterus is under the influence of preovulatory estrogen surge, as well as in ovariectomized uteri after estrogen treatment (Supplemental Figure 5). However, similar to the previous report (6), we found about 60% reduction in Lif expression levels in Trp53loxP/loxPPgrCre/+ uteri on the morning of day 4, although these levels were comparable to those in Lif+/– dams (Figure 2F), which showed normal implantation and pregnancy (Supplemental Figure 6 and refs. 16, 17). Because reduced uterine Lif expression sustained normal pregnancy in Lif+/– mice, we believe that a similar reduction in uterine Lif expression in Trp53loxP/loxPPgrCre/+ mice would fail to prevent implantation. Furthermore, the stromal Lif expression pattern surrounding the blastocyst at the time of attachment on the night of day 4, also considered important for implantation (16, 17), was normal in Trp53loxP/loxPPgrCre/+ dams (Figure 2D). These findings show that reduced Lif expression levels in Trp53-deleted uteri on the morning of day 4 were not a limiting factor for implantation to occur, although this reduction could be detrimental to implantation in other conditions, as reported by Hu et al. (6). Because the expression of Trp53 is primarily restricted to the stroma prior to and during implantation (18), and because the stroma can influence epithelial phenotypes (19), it is possible that some reduction in Lif expression levels on day 4 is secondary to stromal deficiency of p53 in Trp53loxP/loxPPgrCre/+ mice.
Deletion of uterine Trp53 leads to decidual growth restriction with polyploidy, terminal differentiation, and senescence.
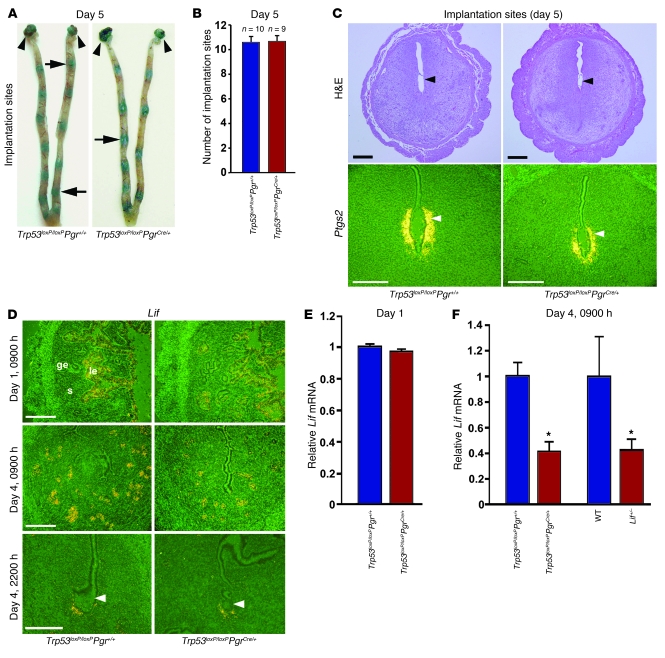
With the implantation process in progress, stromal cells surrounding the implanting embryo undergo decidualization, which is essential for sustaining pregnancy. To determine whether this process is normal in Trp53loxP/loxPPgrCre/+ dams, we first recorded weights of implantation sites in Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ dams on day 8 of pregnancy, the day of maximal decidual growth. The number of sites remained comparable between the 2 groups (Figure 3, A and B), but weights of implantation sites were lower in Trp53loxP/loxPPgrCre/+ dams (Figure 3, A and C), suggestive of alteration in decidual cell growth. To circumvent any effects of embryo genotype on implantation or decidualization, we performed reciprocal blastocyst transfer experiments. Day 4 Trp53loxP/loxPPgr+/+ blastocysts were transferred into uteri of Trp53loxP/loxPPgr+/+ or Trp53loxP/loxPPgrCre/+ recipients on day 4 of pseudopregnancy. Again, implantation rates were comparable between Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ recipient dams (Figure 3D), although weights of implantation sites were lower in Trp53loxP/loxPPgrCre/+ recipients (Figure 3E), on day 8 of pregnancy. Hoxa10 and Bmp2 are highly expressed in decidual cells and critical for decidualization (20–22). Expression patterns of Hoxa10 and Bmp2 in decidual cells surrounding the implanting blastocysts in Trp53loxP/loxPPgrCre/+ dams were similar to those in Trp53loxP/loxPPgr+/+ dams (Figure 3F), although their expression levels were about 40% lower in Trp53loxP/loxPPgrCre/+ mice on day 8 (Figure 3G). Together, these findings suggested a role for uterine p53 in normal decidual growth and led us to explore the mechanism by which uterine p53 deficiency limits decidual growth.
Figure 3. Uterine deletion of Trp53 restricts normal decidual growth.
(A) Representative photographs of day 8 pregnant implantation sites in Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ mice. (B and C) Number and weight of implantation sites in Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ mice on day 8 of pregnancy (mean ± SEM; *P < 0.05). (D and E) Trp53loxP/loxPPgr+/+ blastocysts were transferred into uteri of Trp53loxP/loxPPgr+/+ or Trp53loxP/loxPPgrCre/+ recipients on day 4 of pseudopregnancy. The number and weight of implantation sites were evaluated on day 8, 4 days after embryo transfer (mean ± SEM; *P < 0.05). Numbers in parentheses denote the ratio of implantation sites/total blastocysts transferred. (F) In situ hybridization of Hoxa10 and Bmp2 in Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ uteri on day 8 of pregnancy. Em, embryo; M, mesometrial pole; AM, antimesometrial pole. Scale bar: 200 μm. (G) Northern hybridization of Hoxa10 and Bmp2 in day 8 uteri of Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ dams. In each group, 2–3 independent samples were examined (mean ± SEM; *P < 0.05). (H and I) Loss of p53 upregulated pAkt, p110α, and p21 levels in day 8 implantation sites. As assessed by Western blotting (H), quantitatively analyzed band intensities of pAkt were normalized against total Akt, and those of p110α and p21 were normalized against actin (I). In each group, 3 independent samples from different mice were examined (mean ± SEM; *P < 0.05).
Heightened stromal cell proliferation, differentiation, and polyploidy are hallmarks of normal decidualization in mice; alteration in any of these processes may influence decidual growth. One major role of the deciduum is to accommodate and support the developing embryo. It was previously shown using an in vitro model of human decidualization that withdrawal of decidualization stimuli downregulates p53, but induces Akt activation (23, 24). We also found increased levels of pAkt in implantation sites of Trp53loxP/loxPPgrCre/+ mice on day 8 of pregnancy (Figure 3, H and I). These increased levels were consistent with immunostained pAkt-positive decidual cells in Trp53loxP/loxPPgrCre/+ dams (data not shown). We also found increased levels of p110α, a catalytic subunit of PI3K, in the Trp53loxP/loxPPgrCre/+ mice (Figure 3, H and I). A recent study shows that PIK3CA, which encodes p110α, has 2 alternate promoters, and p53 binds directly to one of them to inhibit its transcription (25). In contrast, inactivation of p53 upregulates PIK3CA, resulting in enhanced PI3K activity with increased levels of pAkt (25). Therefore, our observations of increased levels of p110α and pAkt provide evidence that conditional deletion of Trp53 in the uterus stimulates PI3K activity, which in turn elevates pAkt levels.
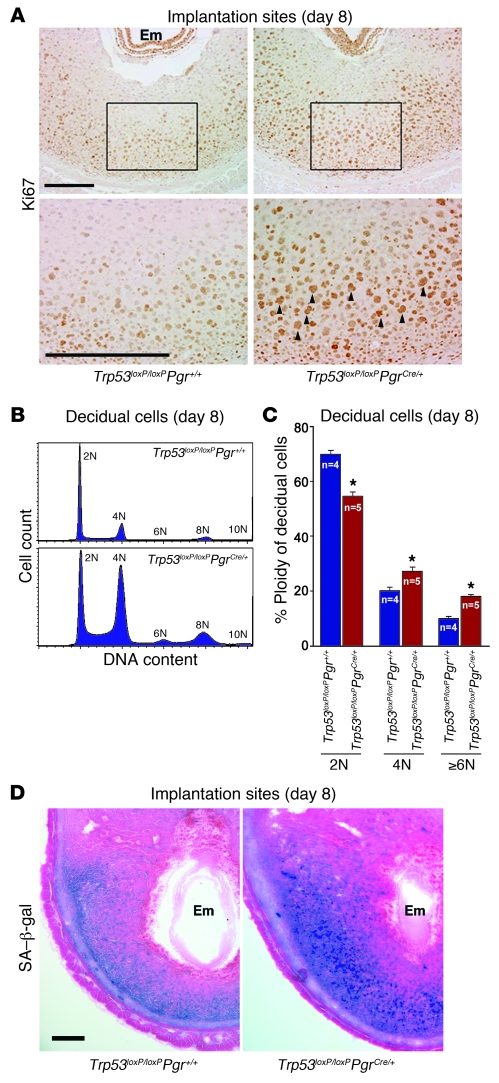
We also found heightened expression of p21, a cyclin-dependent kinase inhibitor (Figure 3, H and I). Normally, p21 is transcriptionally upregulated by p53 after DNA damage. However, p21 is also induced independently of p53 in multiple cell lineages with terminal differentiation during tissue development (26, 27). In this respect, the function of p53 in developing tissues is different from its role in adult tissues. Since the growing deciduum is a maternal counterpart during embryo development, the roles of p53 and its targets in decidua may be similar to those in developing embryos. Stromal cells often enter the endoreplication cycle and become polyploid with p21 expression during normal decidual differentiation (28); therefore, the upregulation of p21 in Trp53loxP/loxPPgrCre/+ uteri suggests that p53 deficiency stimulates decidual polyploidy, favoring terminal cell differentiation. In fact, we found numerous Ki67-positive decidual cells with large, irregularly shaped nuclei in Trp53loxP/loxPPgrCre/+ dams (Figure 4A), and flow cytometry analysis confirmed substantial increases in decidual cells with a DNA complement higher than 2N in Trp53loxP/loxPPgrCre/+ dams on day 8 of pregnancy (Figure 4, B and C). Taken together, these results provide evidence that uterine loss of p53 enhances the endoreplication cycle, ultimately pushing decidual cells toward terminal differentiation. Our findings are consistent with previous findings of increased numbers of polyploid cells in pancreases of Trp53–/– pups (29), increased p21 expression in Trp53–/– megakaryocytes during polyploidization (30), and decreased Trp53 expression with terminal differentiation of developing tissues (31). More interestingly, we found that enhanced terminal differentiation of decidua in Trp53loxP/loxPPgrCre/+ dams was associated with increased senescence-associated β-galactosidase (SA–β-gal) activity (Figure 4D), suggestive of senescence-induced decidual growth restriction, in Trp53loxP/loxPPgrCre/+ uteri. p21 is known to be a major contributor to senescence (32), and Akt activation also induces senescence (33). In addition, pAkt can enhance the stability of p21 protein through phosphorylation (34). Thus, upregulated p21 and pAkt levels are likely causes of senescence-induced decidual growth restriction in mice with uterine deletion of Trp53.
Figure 4. Uterine deletion of Trp53 confers decidual polyploidy, terminal differentiation, and senescence.
(A) Uterine loss of p53 increased Ki67-positive decidual cells with large nuclei. Higher-magnification views of the boxed regions are shown below. Arrowheads indicate Ki67-positive cells with large, irregularly shaped nuclei. Scale bars: 200 μm. (B) Representative histogram of flow cytometry analyses showing DNA content (propidium iodide). Ploidy is denoted by -N (i.e., 2N, diploid; 4N–10N, polyploid). (C) In Trp53loxP/loxPPgrCre/+ decidua, the number of diploid cells decreased, whereas the number of polyploid cells increased (mean ± SEM; *P < 0.05, Student’s t tests). (D) Uterine Trp53 deletion enhanced SA–β-gal activity in decidua. Scale bar: 200 μm.
Deletion of uterine Trp53 promotes preterm delivery.
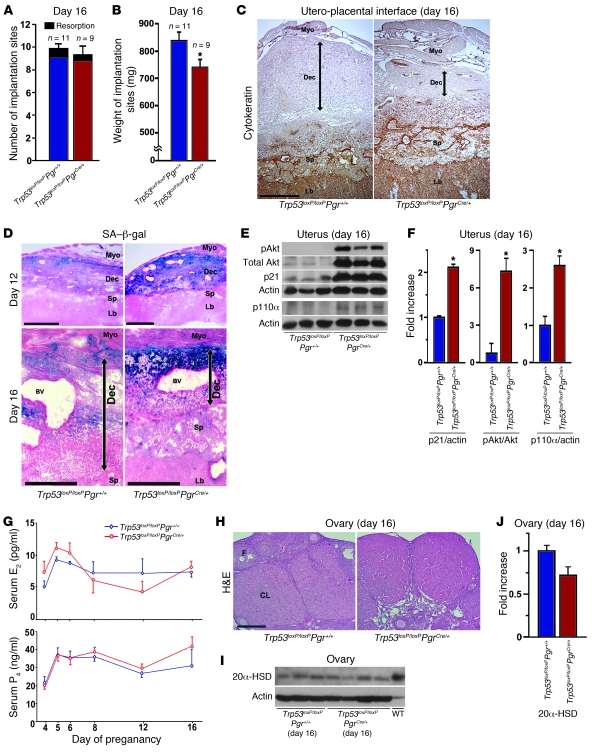
Despite this decidual growth restriction, Trp53loxP/loxPPgrCre/+ mice did not show increased incidence of resorption or pregnancy loss even on day 16 of pregnancy (Figure 5A). However, as expected, implantation sites were smaller in Trp53loxP/loxPPgrCre/+ dams (Figure 5B). Our observation of positive cytokeratin staining in the placenta, but its absence in the deciduum, clearly showed that decidual areas were smaller in Trp53loxP/loxPPgrCre/+ dams compared with Trp53loxP/loxPPgr+/+ dams (Figure 5C), suggestive of reduced decidual growth and thickness. The weights of both embryos and placentas were also low in Trp53loxP/loxPPgrCre/+ mice, despite comparable ratios of fetal to placental weight (Supplemental Figure 7). More interestingly, we found that increased SA–β-gal activity was sustained in the decidual layer on days 12 and 16 (Figure 5D), and that levels of p21, a senescence-associated marker (32, 35), were also upregulated in Trp53loxP/loxPPgrCre/+ uteri on day 16 (Figure 5, E and F). These findings suggest premature uterine senescence in Trp53loxP/loxPPgrCre/+ dams during pregnancy. Alterations in decidual growth and SA–β-gal activity (data not shown) or placental and fetal weights (Supplemental Figure 8A) in Trp53loxP/loxPPgrCre/+ dams were independent of embryonic genotypes. In addition, SA–β-gal activity was undetectable in fetuses or placentas (data not shown). In parallel to heightened p21 levels, pAkt levels were also higher in Trp53loxP/loxPPgrCre/+ uteri, probably resulting from increased PI3K activity caused by upregulated p110α in these mice (Figure 5, E and F). This was reflected in increased signals of pAkt immunostaining in Trp53loxP/loxPPgrCre/+ decidua (Supplemental Figure 9). These findings suggest that p21 and pAkt cooperatively promote premature decidual senescence and growth restriction that in turn affect fetoplacental growth in Trp53loxP/loxPPgrCre/+ dams. However, CD31-positve cells in the decidual vascular bed were comparable between Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ dams on day 16 (Supplemental Figure 9).
Figure 5. Decidual senescence persists in uteri deleted of Trp53.
(A and B) Number and weight of implantation sites in Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ mice on day 16 of pregnancy (mean ± SEM; *P < 0.05). (C) Uterine loss of p53 impeded decidual growth. Placental labyrinth and spongiotrophoblast layers were demarcated by cytokeratin-8 immunostaining. (D) Uterine Trp53 deletion enhanced SA–β-gal activity primarily in the decidual layer on days 12 and 16. (C and D) Decidual boundaries are demarcated by double-sided arrows. Myo, myometrium; Dec, decidua; Sp, spongiotrophoblast; Lb, labyrinth; BV, blood vessel. Scale bars: 500 μm. (E and F) Levels of p21, pAkt, and p110α were upregulated in day 16 uteri lacking p53. (E) Uterine samples from which placentas and fetuses had been removed were used for Western blotting. (F) Quantitative analysis of band intensities of pAkt were normalized against total Akt, and those of p21 and p110α were normalized against actin (mean ± SEM; *P < 0.05). In each group, 3 independent samples from different mice were examined. (G) Serum levels of E2 and P4 (mean ± SEM; P > 0.05). At least 4 independent samples were examined in each group. (H) Representative photomicrographs of H&E-stained histological sections of ovaries on day 16 of pregnancy. CL, corpus luteum; F, follicle. Scale bar: 200 μm. (I) Western blotting of ovarian 20α-HSD levels on day 16 of pregnancy. Day-20 WT mice were used as a positive control. (J) Quantitative analysis of 20α-HSD band intensities from I were normalized against actin (mean ± SEM; P > 0.05).
Because ovarian hormones are known to influence pregnancy events, including decidual growth, parturition, and pregnancy outcome (36), we measured serum estradiol-17β (E2) and P4 levels in Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ dams at critical time points during pregnancy found them to be comparable between groups (Figure 5G). These hormone levels were consistent with functional corpora lutea with low and comparable levels of 20α–hydroxysteroid dehydrogenase (20α-HSD) in Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ dams on day 16 of pregnancy (Figure 5, H–J). This enzyme converts P4 to an inactive metabolite and is a known marker of luteolysis (37).
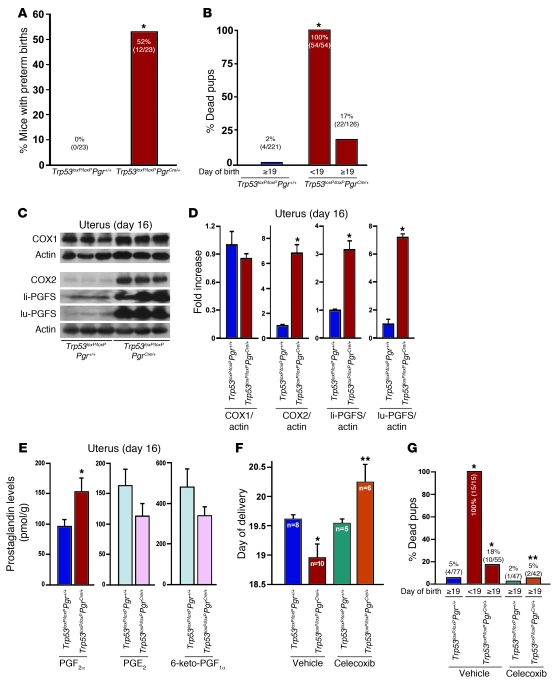
Although restricted decidual growth did not enhance the rate of resorption, a surprising event occurred: the incidence of premature birth significantly increased in Trp53loxP/loxPPgrCre/+ dams (Figure 6A). Specifically, whereas all Trp53loxP/loxPPgr+/+ dams experienced labor late on day 19 or early on day 20 with viable offspring, more than 50% of Trp53loxP/loxPPgrCre/+ dams delivered on days 17 or 18 (Table 1), with 100% of newborn pups dying during labor or just after birth (Figure 6B). Furthermore, 75% of Trp53loxP/loxPPgrCre/+ dams with preterm birth (9 of 12) showed signs of dystocia, with prolonged labor over a period of 12 hours (Table 1). The phenotype of preterm delivery was also observed in Trp53loxP/loxPPgrCre/+ dams on a CD1 background (Supplemental Figure 10), which suggests that this phenotype is not restricted to a specific genetic background. These findings led us to explore the underlying mechanism that triggers premature birth.
Figure 6. Uterine deletion of Trp53 promotes preterm birth through COX2/PGFS/PGF2α pathway.
(A) Preterm birth (deliveries earlier than day 19) occurred in 52% of Trp53loxP/loxPPgrCre/+ dams (*P < 0.05). Numbers in parentheses denote the ratio of dams with preterm birth/total dams. (B) All pups born before day 19 were dead around the time of delivery or died immediately after birth (*P < 0.05 versus Trp53loxP/loxPPgr+/+). Numbers in parentheses denote the ratio of dead pups/total pups. (C and D) Levels of COX2 and PGFS were upregulated in day 16 uteri lacking p53. (C) Uterine samples from which placentas and fetuses had been removed were used for Western blotting, and (D) quantitative analysis of band intensities were all normalized against actin (mean ± SEM of 3 independent samples from different mice; *P < 0.05). (E) Conditional loss of uterine p53 upregulated PGF2α levels in day 16 uteri. A total of 15 independent samples from 3 different mice were evaluated (mean ± SEM; *P < 0.05). (F and G) Preterm birth and neonatal deaths in Trp53loxP/loxPPgrCre/+ mice were reversed by oral gavage of celecoxib (10 mg/kg/dose, administered twice) on day 16. In G, numbers in parentheses denote the ratio of dead pups/total pups. *P < 0.05 versus vehicle-treated Trp53loxP/loxPPgr+/+; **P < 0.05 versus vehicle-treated Trp53loxP/loxPPgrCre/+.
Deletion of uterine Trp53 provokes preterm delivery via the pAkt/COX2 pathway.
In many species, PGs such as PGE2 and PGF2α participate in labor as contractile mediators (38). The COX enzymes, COX1 and COX2, catalyze the rate-limiting step in PG biosynthesis from arachidonic acid (39). COX1 and COX2 convert arachidonic acid to PGH2, which is subsequently converted to PGF2α, PGE2, and PGI2 by PGF synthase (PGFS), microsomal PGE synthase–1 (mPGES1, encoded by Ptges1), and PGI synthase (PGIS, encoded by Ptgis), respectively (40). COX1-derived PGs are known to influence parturition in mice (41). However, to our surprise, levels of uterine COX1 remained unaltered in Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ dams on day 16 (Figure 6, C and D). Instead, levels of COX2 were elevated in Trp53loxP/loxPPgrCre/+ uteri compared with those in Trp53loxP/loxPPgr+/+ uteri on day 16 (Figure 6, C and D). It is highly probable that increased levels of COX2-derived PGs initiated the early onset of labor in Trp53loxP/loxPPgrCre/+ dams in the face of unaltered COX1 levels. Indeed, levels of PGF2α, but not other PGs, were significantly upregulated in Trp53-deleted uteri on day 16 of pregnancy (Figure 6E). Higher uterine PGF2α levels in Trp53loxP/loxPPgrCre/+ dams were independent of embryo genotypes (Supplemental Figure 8B). This upregulation is consistent with selective increases of liver- and lung-type PGFS in Trp53loxP/loxPPgrCre/+ compared with Trp53loxP/loxPPgr+/+ dams on day 16 (Figure 6, C and D). However, whereas levels of Ptges1 were lower in Trp53loxP/loxPPgrCre/+ uteri, levels of Ptgis and Hpgd (encoding 15-hydroxyprostaglandin dehydrogenase; 15-HPGD) were not appreciably different (Supplemental Figure 11). It is known that liver- and lung-type PGFSs are highly expressed in the mouse uterus just prior to parturition (42). PGF2α is known to play an important role in the initiation of parturition in mice (43). Studies in mice with deletion of PGF receptor FP (Ptgfr–/–) show that PGF2α acts on ovarian FP receptors to induce luteolysis, leading to the fall in P4 levels required for triggering labor (44). PGE2 and PGF2α are primarily metabolized by 15-HPGD (45, 46), and mice carrying 15-HPGD hypomorphic alleles show spontaneous preterm labor with increased levels of PGF2α in the absence of luteolysis and P4 withdrawal (47). We observed similar circulating levels of E2 and P4 in Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ dams without luteolysis on day 16 (Figure 5, G–J), which suggests that the onset of preterm labor in Trp53loxP/loxPPgrCre/+ mice is caused by direct contractile effects of higher COX2-derived PGF2α levels in the uterus, not by early luteolysis.
Oxytocin is uterotonic and acts via oxytocin receptor (OXTR; ref. 48), a contractile-associated protein that is upregulated during parturition (43). Oxtr is not induced in Ptgfr–/– uteri during parturition (44), which suggests that PGF2α induces OXTR at the time of labor. Interestingly, mice deficient in oxytocin or OXTR apparently show normal parturition (49, 50), raising questions regarding the critical roles of oxytocin/OXTR signaling in parturition in mice. We found a 45% increase in Oxtr expression in Trp53loxP/loxPPgrCre/+ uteri on day 16 (Supplemental Figure 12A), which suggests that perhaps increased PGF2α levels stimulated OXTR expression to complement contractile effects. Because activation of Akt induces COX2 in the mouse uterus (12), and because both COX2 and pAkt were clearly localized in Trp53loxP/loxPPgrCre/+ decidual cells (Supplemental Figure 9), it is highly likely that increased levels of uterine pAkt stimulate COX2-induced PGF2α levels to initiate preterm labor. This is supported by the finding of reversal of preterm delivery and neonatal death in Trp53loxP/loxPPgrCre/+ dams by oral gavage of the selective COX2 inhibitor celecoxib on day 16 (Figure 6, F and G). Taken together, these findings reinforce that deletion of uterine p53 induces premature delivery via activation of the Akt/COX2/PGFS/PGF2α signaling axis. Notably, cervical ripening was not evident in Trp53loxP/loxPPgrCre/+ dams on day 16 of pregnancy (Supplemental Figure 12B), in spite of heightened uterine PGF2α levels. This suggests that preterm uterine contraction without cervical ripening contributed to dystocia in Trp53loxP/loxPPgrCre/+ dams.
Discussion
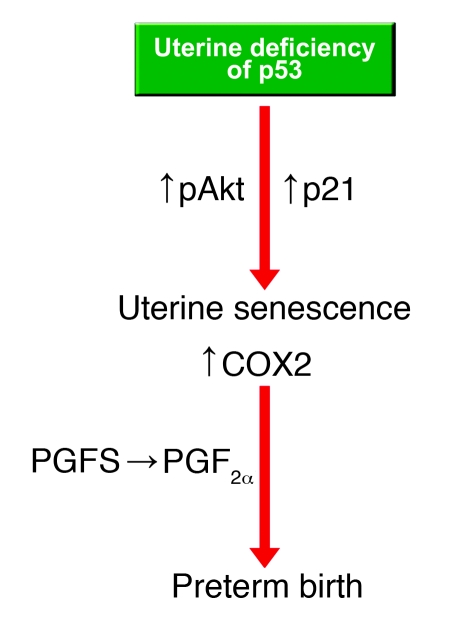
The highlight of the present study is that conditional deletion of uterine Trp53 in mice promoted terminal differentiation of decidual cells and premature uterine senescence, leading to preterm birth with dystocia and neonatal death. Because pAkt and p21 are known to be involved in terminal cell differentiation and senescence in other systems (32, 33), their increased levels in the deciduum missing p53 are likely causes of these observed effects. In pursuit of the underlying cause of premature birth, we found persistent signs of increased decidual senescence with increased levels of pAkt and COX2. Because pAkt is known to upregulate COX2 levels in various cell types, including uterine cells (12), and because COX-derived PGs are crucial for the initiation of labor (38), we strongly believe that an early rise in uterine pAkt and COX2 levels initiated premature labor in Trp53loxP/loxPPgrCre/+ dams. Our conclusion was supported by the observation of increased uterine levels of PGFS and PGF2α in the face of unaltered levels of COX1 and other PGs in these mice and the rescue of their preterm births by oral administration of celecoxib on day 16. Collectively, our results provide evidence that uterine deficiency of p53 evokes premature uterine senescence and elevates COX2-derived PGF2α levels that cooperatively trigger preterm birth (Figure 7).
Figure 7. Potential pathways that promote uterine senescence and preterm birth in Trp53loxP/loxPPgrCre/+ mice.
Our present observation is in contrast to the finding of only an implantation phenotype in Trp53–/– mice (6). The discrepancy between the prior study and our present one is likely due to different mouse models and/or experimental approaches used. However, the phenotype of difficult parturition in Trp53–/– females was previously reported in a review article as unpublished observations (51). Perhaps the parturition phenotype in Trp53–/– mice remained unnoticed for such a long time because most previous studies focused on tumorigenesis; however, this needs to be considered for future investigations using Trp53–/– females.
Lif, which is essential for implantation, is expressed in day 4 pregnant uteri at 2 different times (morning and night) in 2 different cell types (16). Our observation of reduced uterine Lif expression at similar levels on the morning of day 4 in both Trp53loxP/loxPPgrCre/+ and Lif+/– mice with normal implantation suggests that the mouse uterus is very sensitive to LIF. Thus, a lesser amount of LIF is sufficient for inducing implantation, similar to what was found for estrogen (52). After implantation, however, Lif expression is at low basal levels (53) and not required for pregnancy maintenance (54). In contrast, COX2 is expressed at several critical stages in a cell-specific manner during pregnancy. COX2 is expressed in the uterus not only at the time of blastocyst attachment (and is critical for this process; ref. 14), but also during decidualization and placentation, driving different functions. PGI2 and PGE2 are major PGs produced during implantation, decidualization, and placentation that participate in blastocyst attachment, trophoblast differentiation, and invasion via activation of the nuclear receptor PPARδ and/or the PGE2 receptors EP2 and EP4 (55, 56). Our present study shows that although uterine levels of COX2 were very low in Trp53loxP/loxPPgr+/+ dams on day 16 of pregnancy, levels of COX2 and PGF2α significantly rose in Trp53loxP/loxPPgrCre/+ dams on day 16, 1 day before the onset of preterm birth in these dams. The results suggest that the regulation of uterine LIF and COX2 are different during pregnancy.
p53 has been considered the “guardian of the genome” during pregnancy (57). One major role of the deciduum is to accommodate and support the developing embryo, and the roles of p53 in developing tissues are different from its roles in normal adult tissues or in cancerous tissues. Placental trophoblast cells in direct contact with the deciduum differentiate into large polyploid trophoblast giant cells. There is now evidence that downregulation of p53 leads to terminal differentiation of trophoblast cells (58). Decreased Trp53 expression with terminal differentiation is also observed in developing organs in mouse embryos (31). These and our present observations suggest differential roles of p53 between adult cells and cells during development.
Because PR is expressed in the myometrium (11), it is possible that myometrial deletion of Trp53 affects the parturition phenotype in Trp53loxP/loxPPgrCre/+ dams. However, increased COX2 expression was restricted to decidua in Trp53loxP/loxPPgrCre/+ dams, but was undetectable in the adjacent myometrium on day 16. Thus, COX2-derived PGF2α produced in the decidua is likely to influence myometrial contractions as a paracrine effector. This would then suggest that decidual changes can be transmitted to the myometrium for initiating labor.
One major advantage of using mice with conditional deletion of uterine Trp53 over that of systemically Trp53-deleted mice is circumvention of the effects on Trp53-deleted embryos. Hu et al. found the most implantation failures when they crossed Trp53–/– females with Trp53–/– males (6). This is an important issue in light of the observed anomalies in Trp53–/– embryos (8). Our present study found that decidual senescence and restricted growth was reflected in reduced growth of placentas and fetuses in Trp53loxP/loxPPgrCre/+ dams. This reduced fetoplacental growth appears secondary to restricted decidual growth, as we did not notice any sign of senescence in placentas or fetuses in Trp53loxP/loxPPgrCre/+ dams. This observation is consistent with the presence of Trp53 in placentas and embryos developing in Trp53loxP/loxPPgrCre/+ dams (Supplemental Figure 13). In addition, the ratio of Trp53loxP/loxPPgr+/+ to Trp53loxP/loxPPgrCre/+ genotype was comparable between live and dead pups born to Trp53loxP/loxPPgrCre/+ dams (data not shown), which suggests that fetal death in Trp53loxP/loxPPgrCre/+ dams is independent of embryo genotype.
Our observations of senescence-associated uterine growth restriction and preterm birth with conditional deletion of uterine Trp53 have uncovered a critical role of p53 in uterine biology and parturition involving the p21/Akt/COX2 pathway (Figure 7). This may then connect the observations that women at age 35 or older are at higher risk of premature birth (1) and that p53 activity diminishes in mice as they age (59).
Although inflammation and bacterial infection is considered a major cause of preterm labor in humans, many other causes remain unknown. Mice are often used to study the role of inflammation in preterm labor. However, mouse models for studying spontaneous preterm labor are very limited (43), since most mouse models, unlike humans, require P4 withdrawal for initiating labor. Therefore, we believe Trp53loxP/loxPPgrCre/+ mice serve as a novel model to study the underlying mechanism of spontaneous preterm labor without P4 withdrawal, which may be relevant to determining the causes of premature birth in humans.
Methods
Mice.
Trp53loxP/loxP mice (10), obtained from the Mouse Models of Human Cancers Consortium, PgrCre/+ mice (11), provided by J.B. Lydon and F.J. DeMayo (Baylor College of Medicine, Houston, Texas), and Lif+/– mice (16) were used in this study. To generate mice with uterine deletion of Trp53, Trp53loxP/loxP mice (FVB/129) were crossed with PgrCre/+ mice (C57BL6/129). The breeding scheme for generating mice with uterine deletion of p53 is shown in Supplemental Figure 14. For experiments, littermate Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ mice (F3 generation) and littermate Trp53loxP/+Pgr+/+ and Trp53loxP/+PgrCre/+ mice (F1 generation) on the mixed background were used. CD1 Trp53loxP/loxPPgrCre/+ females were generated by backcrossing Trp53loxP/+PgrCre/+ males on a mixed background with CD1 WT females for 10 generations. For the irradiation study, Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ dams were exposed to 9.5 Gy whole-body γ irradiation. RT-PCR, Northern hybridization, Western blotting, and immunostaining of Trp53 mRNA and p53 protein in uteri of Trp53loxP/loxPPgrCre/+ mice showed efficient deletion of Trp53. All mice used in this investigation were housed in the Cincinnati Children’s Hospital Medical Center Animal Care Facility according to National Institutes of Health and institutional guidelines for the use of laboratory animals. All protocols of the present study were reviewed and approved by Cincinnati Children’s Hospital Research Foundation Institutional Animal Care and Use Committee.
Analysis of ovulation, fertilization, implantation, and blastocyst transfer.
Mice were examined for ovulation, fertilization, and implantation as described previously (14, 36). Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ females were mated with Trp53loxP/loxPPgr+/+ fertile males to induce pregnancy. The day the vaginal plug was formed was considered day 1 of pregnancy. To examine ovulation and fertilization, mice were sacrificed on day 2 of pregnancy, oviducts were flushed with Whitten’s medium to recover ovulated eggs, and fertilization was assessed by the number of 2-cell embryos recovered. To examine preimplantation embryo development and oviductal embryo transfer, mice were sacrificed on day 4 of pregnancy and uteri were flushed with Whitten’s medium to recover blastocysts. To examine the attachment reaction and implantation, pregnant dams were sacrificed on day 4, 2200 h, and day 5, 0900 h, respectively. Implantation sites were visualized by intravenous injection of a Chicago blue dye solution, and the number of implantation sites — demarcated by distinct blue bands — was recorded. For blastocyst transfer, pseudopregnant recipients were generated by mating females with vasectomized males. Day 4 Trp53loxP/loxPPgr+/+ blastocysts were transferred into day 4 uteri of Trp53loxP/loxPPgr+/+ or Trp53loxP/loxPPgrCre/+ pseudopregnant recipients, and implantation sites were examined 96 h later (day 8).
Analysis of parturition.
Parturition events were monitored from day 17 through day 21 by observing mice daily, morning, noon, and evening. Preterm birth was defined as birth occurring earlier than day 19 of pregnancy. Dystocia was defined as difficult delivery lasting more than 12 hours. The selective COX2 inhibitor celecoxib was suspended in 0.5% (wt/vol) methylcellulose and 0.1% (vol/vol) polysorbate 80 dissolved in water by constant stirring and was given by oral gavage twice on day 16 of pregnancy (10 mg/kg/dose). This dose of celecoxib is known to selectively inhibit COX2 (60). The control group received vehicle alone.
Estrogen treatment.
To determine the effects of uterine p53 deficiency on estrogen-induced uterine Lif expression, Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ females were ovariectomized and rested for 2 weeks. They were then given a single subcutaneous injection of E2 (100 ng in 0.1 ml oil per mouse; ref. 14). The control group of mice received only the vehicle (0.1 ml oil). Mice were killed after 24 hours, and uteri were collected for Northern hybridization.
Preparation of decidual cells.
Decidual tissues were isolated from day 8 pregnant mice after dissecting out the embryos. Isolated decidual tissues were passed through various gauges of needles to prepare single-cell suspensions for flow cytometry analysis.
In situ hybridization.
Paraformaldehyde-fixed frozen sections were hybridized with 35S-labeled cRNA probes as described previously (13). To compare mRNA localization between Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ tissues, sections of tissues from both genotypes were placed onto the same slide and processed for hybridization. Mouse-specific cRNA probes for Ptgs2, Lif, Hoxa10, and Bmp2 were used for hybridization.
Northern hybridization.
Total RNA (6 μg) was denatured, separated by formaldehyde-agarose gel electrophoresis, and transferred onto nylon membranes. Cross-linked blots were prehybridized, hybridized, and washed as described previously (13). 32P-labeled cRNA probes for mouse Trp53, Lif, Hoxa10, Bmp2, Ptges1, Ptgis, Hpgd, and Rpl7 were used for hybridization. The hybrids were detected by autoradiography. Rpl7 served as a housekeeping gene. The same blots were used for quantitative analyses of each mRNA.
Western blotting.
Protein extraction and Western blotting were performed as described previously (12). Antibodies to p53 (Santa Cruz Biotechnology), actin (Santa Cruz Biotechnology), pAkt (Ser473; Cell Signaling), total Akt (Cell Signaling), p110α (Cell Signaling), p21 (Santa Cruz Biotechnology), 20α-HSD (gift from G. Gibori, University of Illinois at Chicago), COX1 (gift from D. DeWitt, Michigan State University, East Lansing), COX2 (Cayman), and liver- and lung-type PGFS (gifts from K. Watanabe, University of East Asia, Shimonoseki, Japan) were used. The same blots were used for quantitative analyses of each protein. Bands were visualized by using an ECL kit (GE Healthcare). Actin served as a loading control.
Immunohistochemistry.
Immunostaining of p53, PR, COX2, Ki67, pAkt, cytokeratin, and CD31 was performed in formalin-fixed, paraffin-embedded sections using specific antibodies to p53 (Lab Vision Cooperation), PR (Invitrogen), COX2 (generated in our laboratory), Ki67 (Lab Vision Cooperation), pAkt (Ser473; Cell Signaling), cytokeratin-8 (Developmental Studies Hybridoma Bank), and CD31 (Santa Cruz Biotechnology), as described previously (12). To compare intensity of immunostaining between Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ tissues, tissue sections from both genotypes on each day of pregnancy were processed onto the same slide.
Flow cytometry.
Staining of cells for FACS analysis was performed as previously described (29). Cycle TEST PLUS DNA Reagent Kit (BD) was used for DNA ploidy analysis.
SA–β-gal staining.
Staining of SA–β-gal activity was performed as described previously (35). To compare the intensity of SA–β-gal staining between Trp53loxP/loxPPgr+/+ and Trp53loxP/loxPPgrCre/+ tissues, sections of tissues from both genotypes were processed on the same slide. In brief, frozen sections were fixed in 0.5% glutaraldehyde in PBS and stained for 6 hours in PBS (pH 5.5) containing 1 mM MgCl2, 1 mg/ml X-gal, and 5 mM each of potassium ferricyanide and potassium ferrocyanide. Sections were counterstained with eosin.
RT-PCR.
RT-PCR was performed as previously described (12). The housekeeping gene Actb (which encodes β-actin) was used as an internal control.
Measurement of E2 and P4 levels.
Mouse blood samples were collected on days 4, 5, 6, 8, 12, and 16 of pregnancy. Serum levels of E2 and P4 were measured by EIA kits (Cayman).
Measurement of PG profiles.
Implantation sites from which fetuses and placentas had been removed were collected on day 16 of pregnancy. Methanolic extracts of tissues were partially purified by passing through solid phase cartridges, and PGs were quantified by liquid chromatography–tandem mass spectrometry as previously described (61).
Masson trichrome staining.
Paraffin-embedded sections were stained with Masson trichrome. Collagen fibrils show blue staining.
Statistics.
Statistical analyses were performed using 2-tailed Student’s t test and Fisher exact probability test as appropriate. P values less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Erin L. Adams for editing the manuscript. We are grateful to John B. Lydon and Francesco J. DeMayo for providing us with the original breeding pair of PR-Cre mice and to Geula Gibori, David DeWitt, and Kikuko Watanabe for providing us with specific antibodies. Flow cytometry analyses were performed in the Flow Cytometry Core at Cincinnati Children’s Hospital Medical Center supported by an NIH Center grant (AR47363). This study was supported in part by NIH grants (HD12304, DA006668 and DA01822). Y. Hirota is supported by the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2010;120(3):803–815. doi:10.1172/JCI40051.
References
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 3. Behrman RE, Butler AS, eds.Preterm Birth. Washington, DC: The National Academies Press; 2007. [PubMed] [Google Scholar]
- 4.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7(3):185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 5.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 6.Hu W, Feng Z, Teresky AK, Levine AJ. p53 regulates maternal reproduction through LIF. Nature. 2007;450(7170):721–724. doi: 10.1038/nature05993. [DOI] [PubMed] [Google Scholar]
- 7.Donehower LA, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 8.Sah VP, Attardi LD, Mulligan GJ, Williams BO, Bronson RT, Jacks T. A subset of p53-deficient embryos exhibit exencephaly. Nat Genet. 1995;10(2):175–180. doi: 10.1038/ng0695-175. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz D, Goldfinger N, Kam Z, Rotter V. p53 controls low DNA damage-dependent premeiotic checkpoint and facilitates DNA repair during spermatogenesis. Cell Growth Differ. 1999;10(10):665–675. [PubMed] [Google Scholar]
- 10.Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29(4):418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 11.Soyal SM, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41(2):58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- 12.Daikoku T, et al. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 2008;68(14):5619–5627. doi: 10.1158/0008-5472.CAN-08-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das SK, et al. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120(5):1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 14.Lim H, et al. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91(2):197–208. doi: 10.1016/S0092-8674(00)80402-X. [DOI] [PubMed] [Google Scholar]
- 15.Stewart CL, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359(6390):76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 16.Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol Endocrinol. 2000;14(8):1147–1161. doi: 10.1210/me.14.8.1147. [DOI] [PubMed] [Google Scholar]
- 17.Song H, Lim H. Evidence for heterodimeric association of leukemia inhibitory factor (LIF) receptor and gp130 in the mouse uterus for LIF signaling during blastocyst implantation. Reproduction. 2006;131(2):341–349. doi: 10.1530/rep.1.00956. [DOI] [PubMed] [Google Scholar]
- 18.Yue L, et al. Cyclin G1 and cyclin G2 are expressed in the periimplantation mouse uterus in a cell-specific and progesterone-dependent manner: evidence for aberrant regulation with Hoxa-10 deficiency. Endocrinology. 2005;146(5):2424–2433. doi: 10.1210/en.2004-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooke PS, Fujii DK, Cunha GR. Vaginal and uterine stroma maintain their inductive properties following primary culture. In Vitro Cell Dev Biol. 1987;23(3):159–166. doi: 10.1007/BF02623575. [DOI] [PubMed] [Google Scholar]
- 20.Lim H, Ma L, Ma WG, Maas RL, Dey SK. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol. 1999;13(6):1005–1017. doi: 10.1210/me.13.6.1005. [DOI] [PubMed] [Google Scholar]
- 21.Paria BC, et al. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci U S A. 2001;98(3):1047–1052. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KY, et al. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27(15):5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pohnke Y, et al. Wild-type p53 protein is up-regulated upon cyclic adenosine monophosphate-induced differentiation of human endometrial stromal cells. J Clin Endocrinol Metab. 2004;89(10):5233–5244. doi: 10.1210/jc.2004-0012. [DOI] [PubMed] [Google Scholar]
- 24.Yoshino O, et al. Akt as a possible intracellular mediator for decidualization in human endometrial stromal cells. Mol Hum Reprod. 2003;9(5):265–269. doi: 10.1093/molehr/gag035. [DOI] [PubMed] [Google Scholar]
- 25.Astanehe A, et al. Mechanisms underlying p53 regulation of PIK3CA transcription in ovarian surface epithelium and in ovarian cancer. J Cell Sci. 2008;121(Pt 5):664–674. doi: 10.1242/jcs.013029. [DOI] [PubMed] [Google Scholar]
- 26.Parker SB, et al. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995;267(5200):1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- 27.Macleod KF, et al. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995;9(8):935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 28.Tan J, Raja S, Davis MK, Tawfik O, Dey SK, Das SK. Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mech Dev. 2002;111(1–2):99–113. doi: 10.1016/S0925-4773(01)00614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cross SM, et al. A p53-dependent mouse spindle checkpoint. Science. 1995;267(5202):1353–1356. doi: 10.1126/science.7871434. [DOI] [PubMed] [Google Scholar]
- 30.Baccini V, et al. Role of p21(Cip1/Waf1) in cell-cycle exit of endomitotic megakaryocytes. Blood. 2001;98(12):3274–3282. doi: 10.1182/blood.V98.12.3274. [DOI] [PubMed] [Google Scholar]
- 31.Schmid P, Lorenz A, Hameister H, Montenarh M. Expression of p53 during mouse embryogenesis. Development. 1991;113(3):857–865. doi: 10.1242/dev.113.3.857. [DOI] [PubMed] [Google Scholar]
- 32.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436(7051):725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Dowbenko D, Lasky LA. AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J Biol Chem. 2002;277(13):11352–11361. doi: 10.1074/jbc.M109062200. [DOI] [PubMed] [Google Scholar]
- 35.Krizhanovsky V, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134(4):657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tranguch S, Wang H, Daikoku T, Xie H, Smith DF, Dey SK. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest. 2007;117(7):1824–1834. doi: 10.1172/JCI31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28(1):117–149. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- 38.Challis JR, Lye SJ, Gibb W. Prostaglandins and parturition. Ann N Y Acad Sci. 1997;828:254–267. doi: 10.1111/j.1749-6632.1997.tb48546.x. [DOI] [PubMed] [Google Scholar]
- 39.Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol. 1996;62:167–215. doi: 10.1016/s0065-2776(08)60430-7. [DOI] [PubMed] [Google Scholar]
- 40.Helliwell RJ, Adams LF, Mitchell MD. Prostaglandin synthases: recent developments and a novel hypothesis. Prostaglandins Leukot Essent Fatty Acids. 2004;70(2):101–113. doi: 10.1016/j.plefa.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Gross GA, et al. Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc Natl Acad Sci U S A. 1998;95(20):11875–11879. doi: 10.1073/pnas.95.20.11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winchester SK, et al. Coordinate regulation of prostaglandin metabolism for induction of parturition in mice. Endocrinology. 2002;143(7):2593–2598. doi: 10.1210/en.143.7.2593. [DOI] [PubMed] [Google Scholar]
- 43.Ratajczak CK, Muglia LJ. Insights into parturition biology from genetically altered mice. Pediatr Res. 2008;64(6):581–589. doi: 10.1203/PDR.0b013e31818718d2. [DOI] [PubMed] [Google Scholar]
- 44.Sugimoto Y, et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997;277(5326):681–683. doi: 10.1126/science.277.5326.681. [DOI] [PubMed] [Google Scholar]
- 45.Okita RT, Okita JR. Prostaglandin-metabolizing enzymes during pregnancy: characterization of NAD(+)-dependent prostaglandin dehydrogenase, carbonyl reductase, and cytochrome P450-dependent prostaglandin omega-hydroxylase. Crit Rev Biochem Mol Biol. 1996;31(2):101–126. doi: 10.3109/10409239609106581. [DOI] [PubMed] [Google Scholar]
- 46.Tai HH, Ensor CM, Tong M, Zhou H, Yan F.Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat. August 200268–69483–493. [DOI] [PubMed] [Google Scholar]
- 47.Roizen JD, Asada M, Tong M, Tai HH, Muglia LJ. Preterm birth without progesterone withdrawal in 15-hydroxyprostaglandin dehydrogenase hypomorphic mice. Mol Endocrinol. 2008;22(1):105–112. doi: 10.1210/me.2007-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 49.Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci U S A. 1996;93(21):11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takayanagi Y, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102(44):16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donehower LA. The p53-deficient mouse: a model for basic and applied cancer studies. Semin Cancer Biol. 1996;7(5):269–278. doi: 10.1006/scbi.1996.0035. [DOI] [PubMed] [Google Scholar]
- 52.Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100(5):2963–2968. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhatt H, Brunet LJ, Stewart CL. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc Natl Acad Sci U S A. 1991;88(24):11408–11412. doi: 10.1073/pnas.88.24.11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen JR, Cheng JG, Shatzer T, Sewell L, Hernandez L, Stewart CL. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology. 2000;141(12):4365–4372. doi: 10.1210/en.141.12.4365. [DOI] [PubMed] [Google Scholar]
- 55.Lim H, et al. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev. 1999;13(12):1561–1574. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H, et al. Stage-specific integration of maternal and embryonic peroxisome proliferator-activated receptor delta signaling is critical to pregnancy success. J Biol Chem. 2007;282(52):37770–37782. doi: 10.1074/jbc.M706577200. [DOI] [PubMed] [Google Scholar]
- 57.Brosens JJ, Gellersen B. Death or survival--progesterone-dependent cell fate decisions in the human endometrial stroma. J Mol Endocrinol. 2006;36(3):389–398. doi: 10.1677/jme.1.02060. [DOI] [PubMed] [Google Scholar]
- 58.Soloveva V, Linzer DI. Differentiation of placental trophoblast giant cells requires downregulation of p53 and Rb. Placenta. 2004;25(1):29–36. doi: 10.1016/S0143-4004(03)00215-7. [DOI] [PubMed] [Google Scholar]
- 59.Feng Z, Hu W, Teresky AK, Hernando E, Cordon-Cardo C, Levine AJ. Declining p53 function in the aging process: a possible mechanism for the increased tumor incidence in older populations. Proc Natl Acad Sci U S A. 2007;104(42):16633–16638. doi: 10.1073/pnas.0708043104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reese J, Paria BC, Brown N, Zhao X, Morrow JD, Dey SK. Coordinated regulation of fetal and maternal prostaglandins directs successful birth and postnatal adaptation in the mouse. Proc Natl Acad Sci U S A. 2000;97(17):9759–9764. doi: 10.1073/pnas.97.17.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang SM, et al. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci. 2008;28(51):13727–13737. doi: 10.1523/JNEUROSCI.5741-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.