Abstract
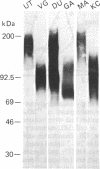
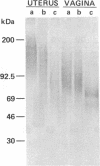
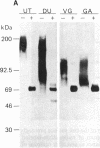
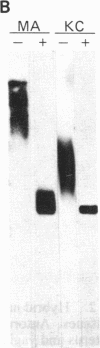
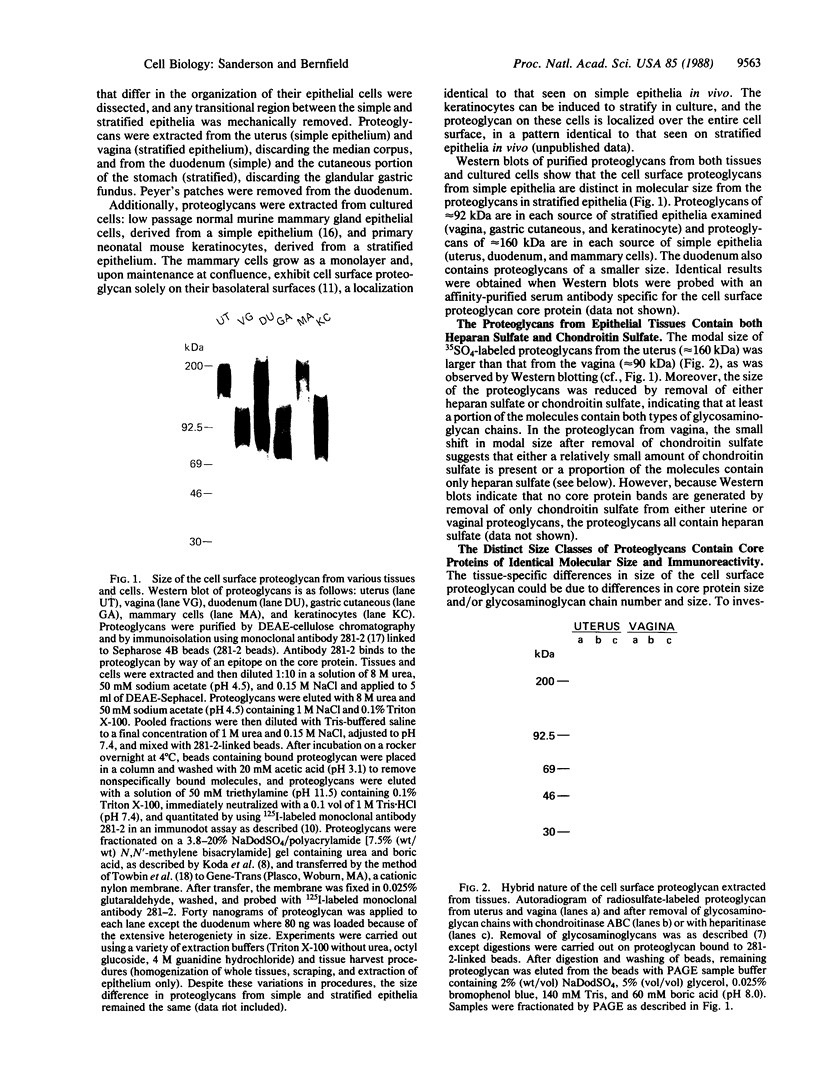
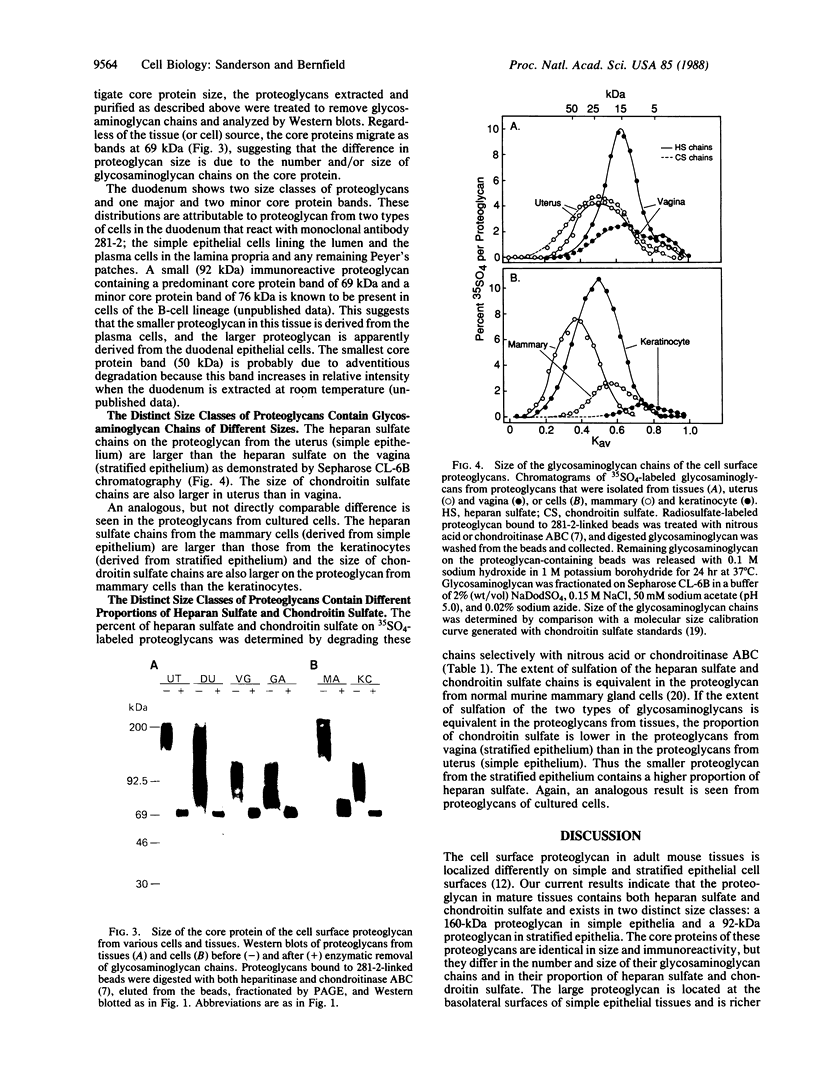
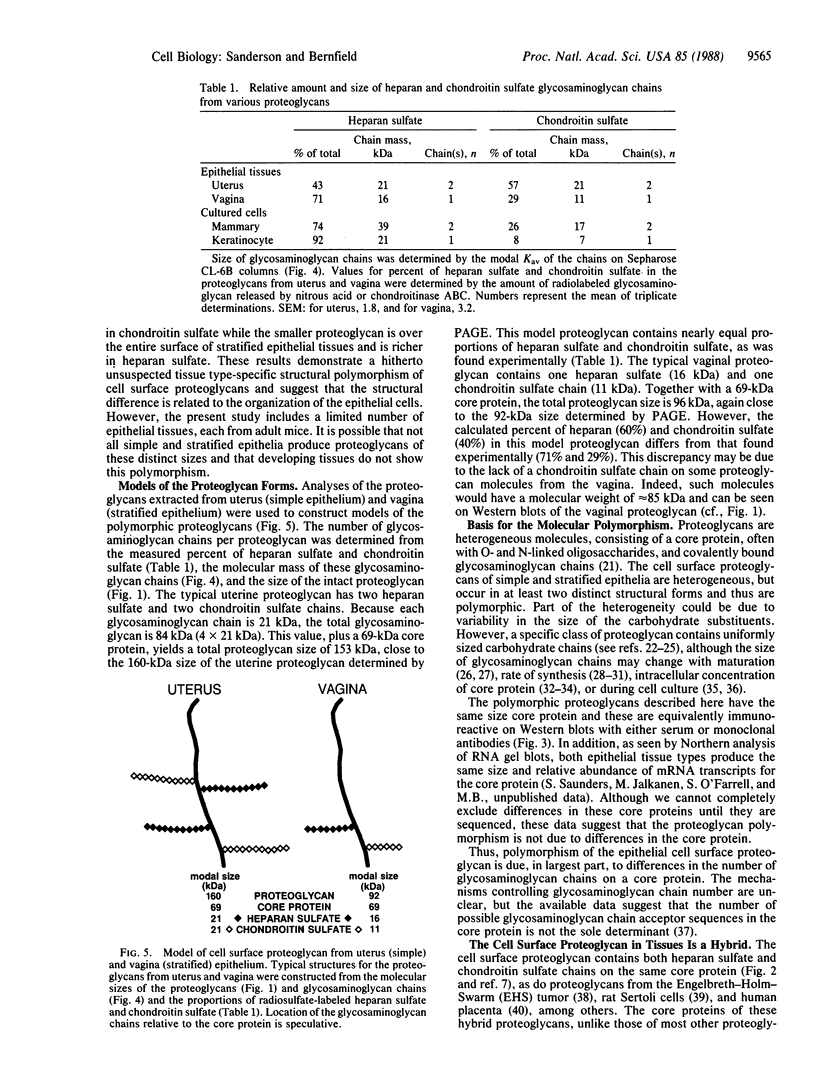
Epithelial cells are organized into either a single layer (simple epithelia) or multiple layers (stratified epithelia). Maintenance of these cellular organizations requires distinct adhesive mechanisms involving many cell surface molecules. One such molecule is a cell surface proteoglycan, named syndecan, that contains both heparan sulfate and chondroitin sulfate chains. This proteoglycan binds cells to fibrillar collagens and fibronectin and thus acts as a receptor for interstitial matrix. The proteoglycan is restricted to the basolateral surface of simple epithelial cells, but is located over the entire surface of stratified epithelial cells, even those surfaces not contacting matrix. We now show that the distinct localization in simple and stratified epithelia correlates with a distinct proteoglycan structure. The proteoglycan from simple epithelia (modal molecular size, 160 kDa) is larger than that from stratified epithelia (modal molecular size, 92 kDa), but their core proteins are identical in size and immunoreactivity. The proteoglycan from simple epithelia has more and larger heparan sulfate and chondroitin sulfate chains than the proteoglycan from stratified epithelia. Thus, the cell surface proteoglycan shows a tissue-specific structural polymorphism due to distinct posttranslational modifications. This polymorphism likely reflects distinct proteoglycan functions in simple and stratified epithelia, potentially meeting the different adhesive requirements of the cells in these different organizations.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourdon M. A., Oldberg A., Pierschbacher M., Ruoslahti E. Molecular cloning and sequence analysis of a chondroitin sulfate proteoglycan cDNA. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1321–1325. doi: 10.1073/pnas.82.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C. B., Kimura J. H., Hascall V. C. Effect of puromycin on cartilage proteoglycan structure and capacity to bind hyaluronic acid. Arch Biochem Biophys. 1984 May 1;230(2):594–604. doi: 10.1016/0003-9861(84)90440-5. [DOI] [PubMed] [Google Scholar]
- David G., Bernfield M. R. Collagen reduces glycosaminoglycan degradation by cultured mammary epithelial cells: possible mechanism for basal lamina formation. Proc Natl Acad Sci U S A. 1979 Feb;76(2):786–790. doi: 10.1073/pnas.76.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G., Van Den Berghe H. Transformed mouse mammary epithelial cells synthesize undersulfated basement membrane proteoglycan. J Biol Chem. 1983 Jun 25;258(12):7338–7344. [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986;2:81–116. doi: 10.1146/annurev.cb.02.110186.000501. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Lyon M., Steward W. P. Structure and function of heparan sulphate proteoglycans. Biochem J. 1986 Jun 1;236(2):313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell J. R., Kimura J. H., Hascall V. C. Proteoglycan core protein families. Annu Rev Biochem. 1986;55:539–567. doi: 10.1146/annurev.bi.55.070186.002543. [DOI] [PubMed] [Google Scholar]
- Hassell J. R., Robey P. G., Barrach H. J., Wilczek J., Rennard S. I., Martin G. R. Isolation of a heparan sulfate-containing proteoglycan from basement membrane. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4494–4498. doi: 10.1073/pnas.77.8.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Hayashi M., Jalkanen M., Firestone J. H., Trelstad R. L., Bernfield M. Immunocytochemistry of cell surface heparan sulfate proteoglycan in mouse tissues. A light and electron microscopic study. J Histochem Cytochem. 1987 Oct;35(10):1079–1088. doi: 10.1177/35.10.2957423. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hök M., Kjellén L., Johansson S. Cell-surface glycosaminoglycans. Annu Rev Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- Isemura M., Sato N., Yamaguchi Y., Aikawa J., Munakata H., Hayashi N., Yosizawa Z., Nakamura T., Kubota A., Arakawa M. Isolation and characterization of fibronectin-binding proteoglycan carrying both heparan sulfate and dermatan sulfate chains from human placenta. J Biol Chem. 1987 Jun 25;262(18):8926–8933. [PubMed] [Google Scholar]
- Jalkanen M., Nguyen H., Rapraeger A., Kurn N., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells: localization on the cell surface with a monoclonal antibody. J Cell Biol. 1985 Sep;101(3):976–984. doi: 10.1083/jcb.101.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen M., Rapraeger A., Saunders S., Bernfield M. Cell surface proteoglycan of mouse mammary epithelial cells is shed by cleavage of its matrix-binding ectodomain from its membrane-associated domain. J Cell Biol. 1987 Dec;105(6 Pt 2):3087–3096. doi: 10.1083/jcb.105.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Koike Y., Ito Y., Suzuki S., Kimata K. Multiple forms of heparan sulfate proteoglycans in the Engelbreth-Holm-Swarm mouse tumor. The occurrence of high density forms bearing both heparan sulfate and chondroitin sulfate side chains. J Biol Chem. 1987 May 25;262(15):7180–7188. [PubMed] [Google Scholar]
- Kato Y., Kimata K., Ito K., Karasawa K., Suzuki S. Effect of beta-D-xyloside and cycloheximide on the synthesis of two types of proteochondroitin sulfate in chick embryo cartilage. J Biol Chem. 1978 Apr 25;253(8):2784–2789. [PubMed] [Google Scholar]
- Kimura J. H., Caputo C. B., Hascall V. C. The effect of cycloheximide on synthesis of proteoglycans by cultured chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1981 May 10;256(9):4368–4376. [PubMed] [Google Scholar]
- Koda J. E., Rapraeger A., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. Cell surface proteoglycan as a receptor for interstitial collagens. J Biol Chem. 1985 Jul 5;260(13):8157–8162. [PubMed] [Google Scholar]
- Ledbetter S. R., Tyree B., Hassell J. R., Horigan E. A. Identification of the precursor protein to basement membrane heparan sulfate proteoglycans. J Biol Chem. 1985 Jul 5;260(13):8106–8113. [PubMed] [Google Scholar]
- Lohmander S., Madsen K., Hinek A. Secretion of proteoglycans by chondrocytes. Influence of colchicine, cytochalasin B, and beta-D-xyloside. Arch Biochem Biophys. 1979 Jan;192(1):148–157. doi: 10.1016/0003-9861(79)90080-8. [DOI] [PubMed] [Google Scholar]
- Mitchell D., Hardingham T. The effects of cycloheximide on the biosynthesis and secretion of proteoglycans by chondrocytes in culture. Biochem J. 1981 May 15;196(2):521–529. doi: 10.1042/bj1960521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrink B. Epithelial cell adhesion molecules. Exp Cell Res. 1986 Mar;163(1):1–21. doi: 10.1016/0014-4827(86)90554-9. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Hayman E. G., Ruoslahti E. Isolation of a chondroitin sulfate proteoglycan from a rat yolk sac tumor and immunochemical demonstration of its cell surface localization. J Biol Chem. 1981 Nov 10;256(21):10847–10852. [PubMed] [Google Scholar]
- Owens R. B., Smith H. S., Hackett A. J. Epithelial cell cultures from normal glandular tissue of mice. J Natl Cancer Inst. 1974 Jul;53(1):261–269. doi: 10.1093/jnci/53.1.261. [DOI] [PubMed] [Google Scholar]
- Poole A. R. Proteoglycans in health and disease: structures and functions. Biochem J. 1986 May 15;236(1):1–14. doi: 10.1042/bj2360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger A. C., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. A putative membrane proteoglycan associates quantitatively with lipid vesicles. J Biol Chem. 1983 Mar 25;258(6):3632–3636. [PubMed] [Google Scholar]
- Rapraeger A., Jalkanen M., Bernfield M. Cell surface proteoglycan associates with the cytoskeleton at the basolateral cell surface of mouse mammary epithelial cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2683–2696. doi: 10.1083/jcb.103.6.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger A., Jalkanen M., Endo E., Koda J., Bernfield M. The cell surface proteoglycan from mouse mammary epithelial cells bears chondroitin sulfate and heparan sulfate glycosaminoglycans. J Biol Chem. 1985 Sep 15;260(20):11046–11052. [PubMed] [Google Scholar]
- Robinson H. C., Horner A. A., Hök M., Ogren S., Lindahl U. A proteoglycan form of heparin and its degradation to single-chain molecules. J Biol Chem. 1978 Oct 10;253(19):6687–6693. [PubMed] [Google Scholar]
- Saunders S., Bernfield M. Cell surface proteoglycan binds mouse mammary epithelial cells to fibronectin and behaves as a receptor for interstitial matrix. J Cell Biol. 1988 Feb;106(2):423–430. doi: 10.1083/jcb.106.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick B. P., Walsh C. J., Jenkins-West T. Sulfated proteoglycans and sulfated proteins in guinea pig megakaryocytes and platelets in vivo. Relevance to megakaryocyte maturation and platelet activation. J Biol Chem. 1988 Jan 15;263(2):1052–1062. [PubMed] [Google Scholar]
- Schwartz N. B. Regulation of chondroitin sulfate synthesis. Effect of beta-xylosides on synthesis of chondroitin sulfate proteoglycan, chondroitin sulfate chains, and core protein. J Biol Chem. 1977 Sep 25;252(18):6316–6321. [PubMed] [Google Scholar]
- Schwartz N. B. Synthesis and secretion of an altered chondroitin sulfate proteoglycan. J Biol Chem. 1979 Apr 10;254(7):2271–2277. [PubMed] [Google Scholar]
- Simons K., Fuller S. D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Fritz I. B. Structural characterization of proteoglycans produced by testicular peritubular cells and Sertoli cells. J Biol Chem. 1985 Sep 25;260(21):11874–11883. [PubMed] [Google Scholar]
- Stevens R. L., Lee T. D., Seldin D. C., Austen K. F., Befus A. D., Bienenstock J. Intestinal mucosal mast cells from rats infected with Nippostrongylus brasiliensis contain protease-resistant chondroitin sulfate di-B proteoglycans. J Immunol. 1986 Jul 1;137(1):291–295. [PubMed] [Google Scholar]
- Tantravahi R. V., Stevens R. L., Austen K. F., Weis J. H. A single gene in mast cells encodes the core peptides of heparin and chondroitin sulfate proteoglycans. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9207–9210. doi: 10.1073/pnas.83.23.9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonar E. J., Buckwalter J. A., Kuettner K. E. Maturation-related differences in the structure and composition of proteoglycans synthesized by chondrocytes from bovine articular cartilage. J Biol Chem. 1986 Feb 15;261(5):2467–2474. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A. Properties of fractionated chondroitin sulphate from ox nasal septa. Biochem J. 1971 May;122(4):477–485. doi: 10.1042/bj1220477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurt R. W., Leid R. W., Jr, Austen K. F. Native heparin from rat peritoneal mast cells. J Biol Chem. 1977 Jan 25;252(2):518–521. [PubMed] [Google Scholar]