Abstract
Reproductive altruism is an extreme form of altruism best typified by sterile castes in social insects and somatic cells in multicellular organisms. Although reproductive altruism is central to the evolution of multicellularity and eusociality, the mechanistic basis for the evolution of this behaviour is yet to be deciphered. Here, we report that the gene responsible for the permanent suppression of reproduction in the somatic cells of the multicellular green alga, Volvox carteri, evolved from a gene that in its unicellular relative, Chlamydomonas reinhardtii, is part of the general acclimation response to various environmental stress factors, which includes the temporary suppression of reproduction. Furthermore, we propose a model for the evolution of soma, in which by simulating the acclimation signal (i.e. a change in cellular redox status) in a developmental rather than environmental context, responses beneficial to a unicellular individual can be co-opted into an altruistic behaviour at the group level. The co-option of environmentally induced responses for reproductive altruism can contribute to the stability of this behaviour, as the loss of such responses would be costly for the individual. This hypothesis also predicts that temporally varying environments, which will select for more efficient acclimation responses, are likely to be more conducive to the evolution of reproductive altruism.
Keywords: reproductive altruism, evolution, acclimation, Volvox, Chlamydomonas, soma
1. Introduction
Reproductive altruism is an extreme form of altruism best exemplified by sterile castes in social insects and somatic cells in multicellular organisms (e.g. Buss 1987; Maynard Smith & Szathmáry 1997; Queller 2000). Although reproductive altruism is central to the emergence of multicellularity and eusociality, the mechanistic basis for the evolution of this behaviour is yet to be deciphered (e.g. Queller 2000; Toth & Robinson 2007). In eusocial insects, caste evolution is thought to have involved the remodelling of conserved regulatory circuits present in their solitary ancestors, including pathways associated with basic life-history traits such as nutrition, metabolism and reproduction (e.g. Toth & Robinson 2007). Similarly, we have suggested that the evolution of soma in multicellular lineages involved the co-option of life-history genes whose expression in their unicellular ancestors was conditioned on environmental cues (as an adaptive strategy to enhance survival at an immediate cost to reproduction), through shifting their expression from a temporal (environmentally induced) into a spatial (developmental) context (Nedelcu & Michod 2004, 2006).
To address this proposal, I am using a group of closely related green algae (Volvocales) comprising both unicellular species (e.g. Chlamydomonas reinhardtii) and multicellular forms with a complete division of labour between reproductive and somatic cells (e.g. Volvox carteri) (Kirk 1998). Volvox carteri consists of approximately 2000 permanently biflagellated sterile somatic cells and up to 16 non-flagellated reproductive cells. The terminal differentiation of somatic cells in V. carteri is dependent on the expression of regA—a master regulatory gene that encodes a transcription factor thought to repress several nuclear genes coding for chloroplast proteins (Kirk et al. 1999; Meissner et al. 1999). As a result, the growth (dependent on photosynthesis) and reproduction (dependent on growth) of somatic cells are suppressed. Repression of their reproductive potential is costly for somatic cells, but this behaviour is beneficial for the group as a whole, since it ensures its motility. In other words, somatic cells are altruistic, and regA is directly responsible for this behaviour (see discussion in Nedelcu & Michod 2006).
We identified regA's closest homologue in C. reinhardtii and showed that this gene (presently known as rls1; Duncan et al. 2007) is induced in the dark, when the expression of chloroplast proteins is downregulated (Nedelcu & Michod 2006). Based on those findings, we hypothesized that rls1 is generally induced under conditions when the temporary downregulation of photosynthesis is beneficial in terms of survival, though costly in terms of immediate reproduction (Nedelcu & Michod 2006). Such conditions include acclimation—a suite of physiological responses that restore the balance between produced and used energy, in response to various environmental changes (Grossman 2000). To test this hypothesis, the current study investigated the expression of rls1 under several environmental stress factors (phosphorus, sulphur and light deprivation) and compared the timing of rls1 induction with changes in C. reinhardtii's reproductive potential.
2. Material and methods
(a). Algal strain and growth conditions
A wall-less C. reinhardtii strain (CC2454) was obtained from the Chlamydomonas Center (http://www.chlamy.org/). Synchronized cultures were grown in Tris-acetate-phosphorus (TAP) medium (Gorman & Levine 1965), on a shaker (approx. 150 r.p.m.) at 25°C, on a 12 h L : 12 h D cycle. Phosphorus- and sulphur-deprived cultures were grown in TAP lacking phosphorus and sulphur, respectively (Chang et al. 2005); light-deprived cultures were grown in regular TAP, but flasks were covered in aluminium foil. To inhibit the photosynthetic electron flow, 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU; 50 mM stock in 95% ethanol) was added to a final concentration of 10 µM (Wykoff et al. 1998).
(b). Gene expression analyses
RNA was extracted from ca 1 × 107 cells, using the Qiagen RNeasy Plant Mini Kit, and the DNA was removed using an Invitrogen DNase I. cDNA synthesis and PCR were carried out with the Invitrogen Thermoscript RT–PCR system, the Invitrogen Platinum PCR SuperMix High Fidelity and the PCRx Enhancer System. Primers were designed across introns to distinguish between cDNA and genomic amplification products (Nedelcu & Michod 2006).
3. Results
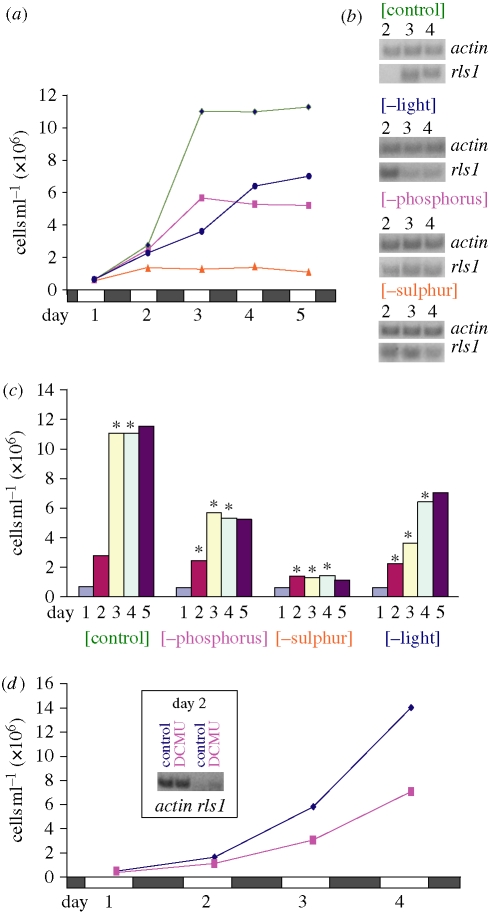
Chlamydomonas reinhardtii cultures were started at low density and grown in parallel under four distinct sets of conditions: (i) control (replete medium); (ii) without phosphorus; (iii) without sulphur; and (iv) dark. Under our conditions, in replete medium, cells grow four times in volume (during the light phase) and divide twice (during the dark phase) to produce four daughter cells; thus, the control culture quadrupled every 24 h during the logarithmic growth phase and entered the stationary phase by day 3 (figure 1a). On the other hand, as expected (e.g. Sager & Granick 1953; Wykoff et al. 1998), the reproductive potential of both nutrient- and light-deprived cultures was negatively affected (relative to the control culture), with the sulphur-deprived culture showing the most dramatic decline (figure 1a).
Figure 1.
Chlamydomonas reinhardtii growth and rls1 expression during phosphorus, sulphur and light deprivation. (a) Growth curves of control and deprived C. reinhardtii cultures; open and solid boxes indicate the succession of light and dark phases. Green line, control; blue line, minus light; purple line, minus phosphorus; orange line, minus sulphur. (b) Expression of rls1 (RNA was extracted 4 h before the onset of dark; semi-quantitative RT–PCR; Nedelcu & Michod 2006) in the control and deprived cultures from (a); several independent experiments showed similar expression patterns (data not shown). (c) Correspondence between time of rls1 induction (indicated by asterisks) and decline in reproduction potential of control and deprived C. reinhardtii cultures from (a) (note that as cells divide during the dark phase, the induction of rls1 in a given day will affect the division in the next dark phase and will reflect in the cell counts of the following day). (d) Induction of rls1 in the presence of the photosynthetic electron transport inhibitor, DCMU (RNA was extracted 24 h after the addition of the inhibitor; i.e. 5.5 h into the light phase of day 2). Blue line, control; purple line, DCMU.
Interestingly, rls1 was expressed not only in the absence of light (Nedelcu & Michod 2006), but also under phosphorus and sulphur deprivation, as well as in the control culture as it entered the stationary phase (figure 1b). Furthermore, in all four treatments, the induction of rls1 corresponded with a decline in the reproduction potential of the population, independent of the culture's age and density. For instance, in the sulphur-deprived culture, rls1 was expressed as soon as day 2, at only 1.3×106 cells ml−1, whereas in the control culture, rls1 was induced starting with day 3, after the culture reached 1.1×107 cells ml−1; note that in both cases, cultures are in the stationary phase (figure 1c).
Acclimation involves both specific responses (e.g. scavenging for a specific nutrient) and general responses. The latter entail various photosynthetic and metabolic changes that ultimately result in the temporary cessation of cell growth and division (Grossman 2000). The fact that (i) rls1 is expressed under multiple stress conditions (figure 1b); (ii) its induction coincides with a decline in reproduction potential (figure 1c); and (iii) it codes for a putative transcriptional repressor (Nedelcu & Michod 2006), suggests that rls1 is part of the general acclimation response and might function as a regulator of acclimation in C. reinhardtii. In support of this suggestion is the finding that an inhibitor of the photosynthetic electron flow that triggers acclimation-like responses (i.e. DCMU; Wykoff et al. 1998) also induces the expression of rls1 (figure 1d).
4. Discussion
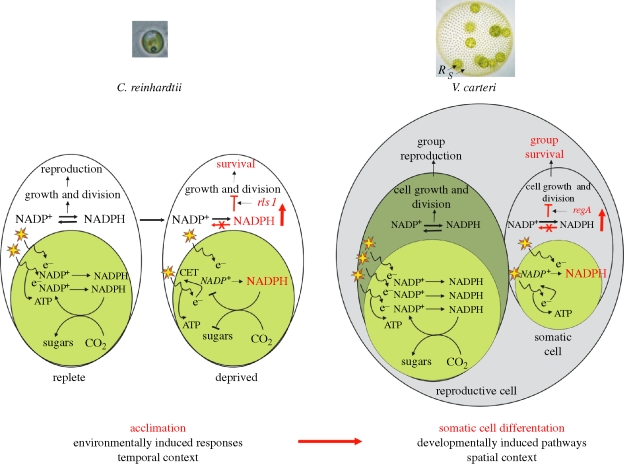
How can general acclimation responses be co-opted for reproductive altruism? In photosynthetic organisms, the flux of electrons through the electron-transport system (ETS) has to be balanced with the rate of adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH) consumption; imbalances between these processes can result in the generation of damaging reactive oxygen species (e.g. Wykoff et al. 1998). When a nutrient (such as sulphur or phosphorus) becomes limiting in the environment, ATP and NADPH consumption declines (figure 2). This results in an excess of excitation energy and a subsequent change in the redox state of the photosynthetic apparatus, which will trigger a suite of short- and long-term acclimation responses, including the downregulation of photosynthesis (to avoid the production of toxic oxygen radicals) and the subsequent cessation of cell growth and division (e.g. Wykoff et al. 1998; Pfannschmidt et al. 2009). Other environmental factors (e.g. cold, water stress) are also known to result in changes to cellular redox status and trigger acclimation responses (e.g. Eberhard et al. 2008). Thus, in principle, any factor that can elicit a similar redox change could prompt acclimation-like responses and ultimately induce cessation of cell division. In a group context, if the induction of such a redox change is restricted to a subset of cells, and if the suppression of reproduction in this subset of cells is beneficial to the group, reproductive altruism can evolve and be fixed.
Figure 2.
A model for the co-option of acclimation responses into somatic cell differentiation in V. carteri (R and S denote reproductive and somatic cells); see text for discussion. Although many components are involved, for simplicity, changes in the redox status are symbolized by the over-reduction of the NADP pool (vertical red arrows) owing to either decreased NADPH consumption in nutrient-deprived Chlamydomonas or excess of excitation energy (owing to a higher surface/volume ratio) in Volvox somatic cells. The switch to cyclic electron transport (CET), which can maintain ATP synthesis (and thus vital processes) in acclimated Chlamydomonas cells (Eberhard et al. 2008) and possibly in Volvox somatic cells, is also indicated.
In V. carteri, the expression of regA is restricted (by an unknown mechanism) to cells whose size at the end of embryonic divisions falls below 8 µm (Kirk et al. 1993). As cell surface area and volume change at different rates, we propose that in these small cells, the ratio between membrane-bound proteins (including ETS and ETS-associated components) and soluble factors (including NADP+ and ADP) becomes skewed—relative to the ratio in larger cells, towards the former. Consequently, these small cells could experience an imbalance between the flux of electrons and the availability of final acceptors (i.e. NADPH+), which would result in a change in the intra-cellular redox status and the induction of acclimation-like responses, culminating with the suppression of their division (figure 2).
Hence, by simulating the general acclimation signal (i.e. a change in cellular redox status) in a developmental rather than environmental context, responses beneficial to a unicellular individual could be co-opted into an altruistic behaviour at the group level. As pleiotropy to other essential functions has been proposed to limit selfishness and allow stable cooperation to evolve (Foster et al. 2004), the co-option of environmentally induced responses for reproductive altruism can also contribute to the stability of this behaviour, as the loss of such responses would be costly for the individual. Furthermore, this hypothesis predicts that reproductive altruism is more likely to evolve in lineages with enhanced acclimation mechanisms, which can effectively and adaptively adjust their survival and reproduction potential in response to environmental changes. Because environments that vary in time (such as those in which volvocalean algae live; Kirk 1998) will select for enhanced and efficient acclimation responses (note that temporally varying environments have been shown to select for phenotypic plasticity—i.e. generalists, in C. reinhardtii; Reboud & Bell 1997), such environments are likely to be more conducive to the evolution of reproductive altruism. In this context, it is noteworthy that cast differentiation in social wasps is also thought to have evolved in variable environments, and specific adaptations to seasonal environments that control sequential shifts between life-cycle phases have been proposed as prerequisites to the evolution of sociality in this group (Hunt & Amdam 2005). As all organisms need to adjust their metabolism and reproduction potential to environmental changes, it is likely that analogous mechanisms apply to the evolution of reproductive altruism in other systems.
Acknowledgements
This work was supported by grants from the Natural Sciences and Engineering Council of Canada and the US National Science Foundation.
References
- Buss L. W.1987The evolution of individuality Princeton, NJ: Princeton University [Google Scholar]
- Chang C. W., Moseley J. L., Wykoff D., Grossman A. R.2005The LPB1 gene is important for acclimation of Chlamydomonas reinhardtii to phosphorus and sulfur deprivation. Plant Physiol. 138, 319–329 (doi:10.1104/pp.105.059550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan L., Nishii I., Harryman A., Buckley S., Howard A., Friedman N. R., Miller S. M.2007The VARL gene family and the evolutionary origins of the master cell-type regulatory gene, regA, in Volvox carteri. J. Mol. Evol. 65, 1–11 (doi:10.1007/s00239-006-0225-5) [DOI] [PubMed] [Google Scholar]
- Eberhard S., Finazzi G., Wollman F. A.2008The dynamics of photosynthesis. Ann. Rev. Genet. 42, 463–515 (doi:10.1146/annurev.genet.42.110807.091452) [DOI] [PubMed] [Google Scholar]
- Foster K. R., Shaulsky G., Strassmann J. E., Queller D. C., Thompson C. R. L.2004Pleiotropy as a mechanism to stabilize cooperation. Nature 431, 693–696 (doi:10.1038/nature02894) [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P.1965Cytochrome and plastocyanin: their sequences in photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc. Natl Acad. Sci. USA 54, 1665–1669 (doi:10.1073/pnas.54.6.1665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A.2000Acclimation of Chlamydomonas reinhardtii to its nutrient environment. Protist 151, 201–224 (doi:10.1078/1434-4610-00020) [DOI] [PubMed] [Google Scholar]
- Hunt J. H., Amdam G. V.2005Bivoltinism as an antecedent to eusociality in the paper wasp genus Polistes. Science 308, 264–267 (doi:10.1126/science.1109724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk D. L.1998Volvox. Molecular genetic origins of multicellularity and cellular differentiation New York, NY: Cambridge University Press [Google Scholar]
- Kirk M., Ransick A., McRae S. E., Kirk D. L.1993The relationship between cell size and cell fate in Volvox carteri. J. Cell Biol. 123, 191–208 (doi:10.1083/jcb.123.1.191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk M., Stark K., Miller S., Muller W., Taillon B., Gruber H., Schmitt R., Kirk D. L.1999regA, a Volvox gene that plays a central role in germ soma differentiation, encodes a novel regulatory protein. Development 126, 639–647 [DOI] [PubMed] [Google Scholar]
- Maynard Smith J., Szathmáry E.1997The major transitions in evolution Oxford, UK: Oxford University Press [Google Scholar]
- Meissner M., Stark K., Cresnar B., Kirk D. L., Schmitt R.1999Volvox germline-specific genes that are putative targets of regA repression encode chloroplast proteins. Curr. Genet. 36, 363–370 (doi:10.1007/s002940050511) [DOI] [PubMed] [Google Scholar]
- Nedelcu A. M., Michod R. E.2004Evolvability, modularity, and individuality during the transition to multicellularity in volvocalean green algae. In Modularity in development and evolution (eds Schlosser G., Wagner G.), pp. 466–489 Chicago, IL: University of Chicago Press [Google Scholar]
- Nedelcu A. M., Michod R. E.2006The evolutionary origin of an altruistic gene. Mol. Biol. Evol. 23, 1460–1464 (doi:10.1093/molbev/msl016) [DOI] [PubMed] [Google Scholar]
- Pfannschmidt T., Brautigam K., Wagner R., Dietzel L., Schroter Y., Steiner S., Nykytenko A.2009Potential regulation of gene expression in photosynthetic cells by redox and energy state: approaches towards better understanding. Ann. Bot. 103, 599–607 (doi:10.1093/aob/mcn081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queller D. C.2000Relatedness and the fraternal major transitions. Phil. Trans. R. Soc. Lond. B 355, 1647–1655 (doi:10.1098/rstb.2000.0727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboud X., Bell G.1997Experimental evolution in Chlamydomonas. III. Evolution of specialist and generalist types in environments that vary in space and time. Heredity 78, 507–514 (doi:10.1038/hdy.1997.79) [Google Scholar]
- Sager R., Granick S.1953Nutritional studies with Chlamydomonas reinhardi. Ann. NY Acad. Sci. 56, 831–838 (doi:10.1111/j.1749-6632.1953.tb30261.x) [DOI] [PubMed] [Google Scholar]
- Toth A. L., Robinson G. E.2007Evo-devo and the evolution of social behavior. Trends Genet. 23, 334–341 (doi:10.1016/j.tig.2007.05.001) [DOI] [PubMed] [Google Scholar]
- Wykoff D. D., Davies J. P., Melis A., Grossman A. R.1998The regulation of photosynthetic electron transport during nutrient deprivation in Chlamydomonas reinhardtii. Plant Physiol. 117, 129–139 (doi:10.1104/pp.117.1.129) [DOI] [PMC free article] [PubMed] [Google Scholar]