Abstract
In animal communication, complex displays usually have multiple functions and, male and female receivers often differ in their utilization and response to different aspects of these displays. The perceptual variability hypothesis suggests that different aspects of complex signals differ in their ability to be detected and processed by different receivers. Here, we tested whether receiver male and female Sceloporus graciosus lizards differ in visual motion detection by measuring the latency to the visual grasp response to a motion stimulus. We demonstrate that in lizards that largely exhibit complex motions as courtship signals, female lizards are faster than males at visually detecting motion. These results highlight that differential signal utilization by the sexes may be driven by variability in the capacity to detect different display properties.
Keywords: sex difference, visual performance, motion detection, complex signals, animal communication, Sceloporus graciosus
1. Introduction
In many animals, male and female receivers respond differently to the same sexual displays exhibited by conspecific males. When male and female receivers are receptive to different characteristics of the same display, display complexity is predicted to evolve as a result of dual functions of signals, such as mate attraction and rivalry (Berglund et al. 1996). Different aspects of complex displays are often not equally meaningful to different receivers and may be designed to send different messages to multiple receivers (content-based hypothesis; Hebets & Papaj 2005). For example, the multiple-receiver hypothesis suggests that different male signal elements evolve when multiple receivers (i.e. males and females) exert different forms of selection (inter- and intrasexual selection) on displays (Andersson et al. 2002). The red chest patch exhibited by male red-collared widowbirds (Euplectes ardens), for instance, is used during male–male territoriality, whereas the train of long tail feathers is favoured by females during the courtship season (Andersson et al. 2002). Alternatively, display complexity may be driven by variability in different receivers to detect, process, and respond to different display aspects (efficacy-based hypothesis; Hebets & Papaj 2005). Here, we consider receiver sex differences and test whether the difference in display utilization by males and females is accompanied by a sex difference in detection latency.
The perceptual variability hypothesis suggests that different aspects of complex signals differ in their capacity to be detected and processed by different receivers (Hebets & Papaj 2005). This is true in many animals that communicate acoustically; males and females often differ in recognition and/or discrimination of courtship calls (Searcy & Brenowitz 1988; Bernal et al. 2007). For example, in several primates, the sexes differ considerably in response to copulation calls (Hauser 2007; Balsby & Scarl 2008). However, receiver responsiveness and discrimination may differ between the sexes because males and females may ‘choose’ to act differently in reaction to the same sensory input (e.g. a conspecific signal) without differing in sensory ability and performance (Bernal et al. 2009). Receiver male and female túngara frogs (Physalaemus pustulosus), for instance, differ in the expression of egr-1, a putative marker of neural activity that is responsible for reproductive decision-making, resulting in sex differences in behavioural selectivity to male mating calls (Hoke et al. 2008). Alternatively, receiver differences may result from differences in sensory ability and performance between different male and female receivers. For example, reproductive female túngara frogs exhibit greater visual sensitivity to the coloration on the male vocal sac than males and non-reproductive females (Cummings et al. 2008). Thus, we predict that when separate receiver-specific signals evolve, different receivers (i.e. males and females) should respond differently to the same signals and also diverge in sensory response ability.
In visual communication systems that use complex signals, we might not only expect sex differences in responsiveness and visual ability, but sex differences should also be specific to the social context and physical properties of signals. For example, during aggressive contexts, male Sceloporus lizards exhibit displays that combine body colour patches with stilted postures; yet, during courtship contexts, male displays are primarily motion-based, consisting of a variety of complex, rapid head/body motions (Carpenter 1978). In Sceloporus graciosus, sexes differ in attentiveness to context-specific (aggressive versus courtship) male displays; males pay more attention to stilted body postures, whereas females are more attentive to the motion-based headbob displays (Martins et al. 2005). Sceloporus graciosus lizards are sexually dimorphic in coloration and signalling behaviour, exhibit complex displays and have shown to be a good model to study visual communication evolution (Martins 1993).
Visual motion detection plays an integral role in Sceloporus communication. In a lizard's visual periphery, motion is the most effective stimulus for the visual grasp response (VGR) (Fleishman 1992). The VGR is a robust reflex shift of eye position that places an image on the fovea when something of importance is detected in the periphery (Ingle 1982). Because the VGR straightforwardly allows us to quantify how a lizard would naturally respond to a motion stimulus, we exploited the VGR to test whether male and female S. graciosus lizards differed in motion detection ability. Light intensity can affect VGR performance in diurnal lizards (Nava et al. 2009); thus, for comparison, we tested lizards under bright and dim light. Based on previous work (Martins et al. 2005), we tested the hypothesis that female S. graciosus lizards should detect motion faster than males.
2. Material and methods
We measured the latency of the VGR (methods similar to Nava et al. 2009) in 18 male and six female S. graciosus lizards (from pine scrub habitats in the San Gabriel Mountains of southern California, USA) to a motion stimulus under two light intensities (bright: 500 lx and dim: 10 lx). We randomly tested all lizards first under the bright lighting and then under the dim lighting. During each trial, we placed a lizard in the test arena (5.5 gal aquarium with three opaque white sides and one clear viewing side) under the test lighting. After 10 min, we presented a spinning stimulus flag (3.4° visual angle) in the lizard's visual periphery (approx. 45°) and recorded the amount of time before the lizard turned its eye towards the stimulus. The stimulus was a flat, circular flag (9 mm in diameter) diametrically painted black and white. We spun the stimulus flag at 1 Hz using a silent DC (1–6 V) geared motor. We selected 1 Hz because this is the lowest display frequency exhibited by S. graciosus (S. S. Nava 2006, unpublished data). A full-spectrum light (50 cm above the arena) regulated by a voltage divider illuminated the arena. Light intensities for each trial were measured using a digital illuminance meter (Minolta Camera, Japan). Ambient temperature was constant during all tests (26°C).
Because we measured each lizard in two light treatments, we analysed the response data using a repeated-measures model, testing the effects of sex and light on VGR latency. A residual analysis showed that the data do not conform to the normality and homoscedasticity assumptions of parametric tests; thus we used a non-parametric Wald test. Statistical analyses were conducted using SPSS 16.0.1.
3. Results
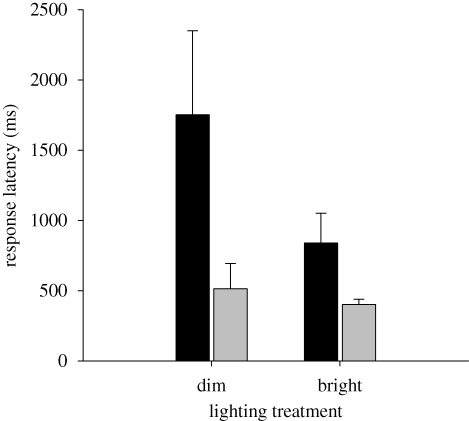
All lizards robustly exhibited the VGR towards the motion stimulus during all trials. For both lighting trials, males and females detected the motion stimulus relatively rapidly (less than 2.5 s); yet, reaction times for both sexes were lower under the bright lighting trials (figure 1). On average, females detected the motion stimulus approximately 52 per cent faster than did males under the bright lighting trials. Similarly, under the dim lighting trials, on average, females detected the motion stimulus approximately 70 per cent faster than did males (figure 1). The difference in detection latency between males and females was statistically significant (Wald χ2 = 6.3, d.f. = 1, p = 0.012). We did not find a significant main effect for lighting (Wald χ2 = 2.48, d.f. = 1, p = 0.11) or an interaction between sex and lighting (Wald χ2 = 1.50, d.f. = 1, p = 0.22).
Figure 1.
Mean VGR latency for male and female S. graciosus lizards under two light treatments. Error bars represent one standard error. Black bar, male; grey bar, female.
4. Discussion
Although multiple receivers may exert different selective pressures on the design of complex displays, variability between different receivers in the capacity to detect and process different signal aspects may also drive display complexity (Hebets & Papaj 2005). Previous research shows that female S. graciosus lizards are more attentive than males to the complex motion-based visual displays produced by males (Martins et al. 2005). Here, we demonstrate that female S. graciosus lizards are also faster than males in visually detecting motion.
Many lizards rely largely on motion to detect prey and communicate by using motion patterns of the head and body (Carpenter 1978). In Sceloporus, male motion-based courtship displays directed to females are an important component of reproductive communication. In fact, females pay close attention to the complex structure and frequency of male motion displays to discriminate species and individual identity (Martins 1991; Martins et al. 2005). Female Sceloporus probably rely strongly on motion detection ability to notice motion-based signals directed towards them. Reproductive physiology and behaviour of S. graciosus females are also directly affected by the type of motion displays they observe; females exposed to courtship motion displays exhibit less rejection behaviour and produce more femoral pore secretions (Kelso & Martins 2008).
Our results support the idea that male and female receivers may not only differ in attentiveness and how they react to communicative motion signals (Martins et al. 2005), but also differ in how quickly they can detect motion. Motion detection sex differences may result from mechanistic sex differences along the visual pathway such as differences in eye size/placement, retinal morphology/physiology and neuronal responses. In house flies (Musca domestica), for example, only males use a set of photoreceptors known as the ‘love spot’ to search for and chase females (Hornstein et al. 2000). In vertebrates, however, visual response latency is more likely to be driven by hormonal amplification of sensory responses (Yilmaz et al. 2000). Furthermore, research on fishes (Sisneros et al. 2004) and frogs (Gordon & Gerhardt 2009) suggests that sensory performance, and potentially differences between sexes, are likely to be hormonally driven.
Together with previous studies on S. graciosus communication (Martins et al. 2005), our results support the efficacy-based, perceptual variability hypothesis (Hebets & Papaj 2005). Thus, we suggest that the different display characteristics exhibited by male lizards are well suited for the sex differences in visual performance. However, further work is needed to determine whether females develop faster motion detection to detect male signals or whether male courtship displays exploit female biases (sensu sensory exploitation). In other visual and non-visual communication systems, we might expect similar receiver sex differences in sensory performance, associated with complex signals. Thus, we suggest that to better understand the evolution of animal communication, researchers may consider not only receiver sex differences in behavioural tasks/responses (Bernal et al. 2009) but also in sensory ability and performance.
Acknowledgements
We thank Stephanie Dowdy-Nava, Greg Demas, Laura Hurley, Suresh Viswanathan and three anonymous reviewers for comments that improved earlier drafts of this manuscript. We thank Erin Kelso for lizard collection/care. M.C. was supported as a research intern in the NSF-funded REU program in Animal Behaviour at Indiana University's Center for the Integrative Study of Animal Behaviour. This research was supported through a predoctoral fellowship from the National Eye Institute to S.S.N.
References
- Andersson S., Pryke S. R., Ornborg J., Lawes M. J., Andersson M.2002Multiple receivers, multiple ornaments, and a trade-off between agonistic and epigamic signaling in a widowbird. Am. Nat. 160, 683–691 (doi:10.1086/342817) [DOI] [PubMed] [Google Scholar]
- Balsby T. J. S., Scarl J. C.2008Sex-specific responses to vocal convergence and divergence of contact calls in orange-fronted conures (Aratinga canicularis). Proc. R. Soc. B 275, 2147–2154 (doi:10.1098/rspb.2008.0517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund A., Bisazza A., Pilastro A.1996Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol. J. Linn. Soc. 58, 385–399 (doi:10.1111/j.1095-8312.1996.tb01442.x) [Google Scholar]
- Bernal X. E., Rand A. S., Ryan M. J.2007Sex differences in response to nonconspecific advertisement calls: receiver permissiveness in male and female túngara frogs. Anim. Behav. 73, 955–964 (doi:10.1016/j.anbehav.2006.10.018) [Google Scholar]
- Bernal X. E., Rand A. S., Ryan M. J.2009Task differences confound sex differences in receiver permissiveness in túngara frogs. Proc. R. Soc. B 276, 1323–1329 (doi:10.1098/rspb.2008.0935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. C.1978Comparative display behavior in the genus Sceloporus (Iguanidae). Contrib. Biol. Geol. Milwaukee Public Mus. 18, 1–71 [Google Scholar]
- Cummings M. E., Bernal X. E., Reynaga R., Rand A. S., Ryan M. J.2008Visual sensitivity to a conspicuous male cue varies by reproductive state in Physalaemus pustulosus females. J. Exp. Biol. 211, 1203–1210 (doi:10.1242/jeb.012963) [DOI] [PubMed] [Google Scholar]
- Fleishman L. J.1992The influence of the sensory system and the environment on motion patterns in the visual-displays of anoline lizards and other vertebrates. Am. Nat. 139, S36–S61 (doi:10.1086/285304) [Google Scholar]
- Gordon N. M., Gerhardt H. C.2009Hormonal modulation of phonotaxis and advertisement-call preferences in the gray treefrog (Hyla versicolor). Horm. Behav. 55, 121–127 (doi:10.1016/j.yhbeh.2008.09.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser M. D.2007When males call, females listen: sex differences in responsiveness to rhesus monkey, Macaca mulatta, copulation calls. Anim. Behav. 73, 1059–1065 (doi:10.1016/j.anbehav.2006.11.006) [Google Scholar]
- Hebets E. A., Papaj D. R.2005Complex signal function: developing a framework of testable hypotheses. Behav. Ecol. Sociobiol. 57, 197–214 (doi:10.1007/s00265-004-0865-7) [Google Scholar]
- Hoke K. L., Ryan M. J., Wilczynski W.2008Candidate neural locus for sex differences in reproductive decisions. Biol. Lett. 4, 518–521 (doi:10.1098/rsbl.2008.0192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstein E. P., O'Carroll D. C., Anderson J. C., Laughlin S. B.2000Sexual dimorphism matches photoreceptor performance to behavioural requirements. Proc. R. Soc. Lond. B 267, 2111–2117 (doi:10.1098/rspb.2000.1257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle D.1982Organization of visuomotor behaviors in vertebrates. In Analysis of visual behavior (eds Goodale M. A., Ingle D. J., Mansfield R. J. W.), pp. 67–109 Cambridge, MA: Massachusetts Institution of Technology Press [Google Scholar]
- Kelso E. C., Martins E. P.2008Effects of two courtship display components on female reproductive behaviour and physiology in the sagebrush lizard. Anim. Behav. 75, 639–646 (doi:10.1016/j.anbehav.2007.07.017) [Google Scholar]
- Martins E. P.1991Individual and sex-differences in the use of the push-up display by the sagebrush lizard Sceloporus graciosus. Anim. Behav. 41, 403–416 (doi:10.1016/S0003-3472(05)80841-3) [Google Scholar]
- Martins E. P.1993A comparative study of the evolution of Sceloporus push-up displays. Am. Nat. 142, 994–1018 (doi:10.1086/285585) [DOI] [PubMed] [Google Scholar]
- Martins E. P., Ord T. J., Davenport S. W.2005Combining motions into complex displays: playbacks with a robotic lizard. Behav. Ecol. Sociobiol. 58, 351–360 (doi:10.1007/s00265-005-0954-2) [Google Scholar]
- Nava S. S., Conway M. A., Martins E. P.2009Divergence of visual motion detection in diurnal geckos that inhabit bright and dark habitats. Funct. Ecol. 23, 794–799 (doi:10.1111/j.1365-2435.2009.01565.x) [Google Scholar]
- Searcy W. A., Brenowitz E. A.1988Sexual differences in species recognition of avian song. Nature 332, 152–154 (doi:10.1038/332152a0) [Google Scholar]
- Sisneros J. A., Forlano P. M., Deitcher D. L., Bass A. H.2004Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science 305, 404–407 (doi:10.1126/science.1097218) [DOI] [PubMed] [Google Scholar]
- Yilmaz H., Erkin E., Mavioglu H., Lacin S.2000Effects of oestrogen replacement therapy on pattern reversal visual evoked potentials. Eur. J. Neurol. 7, 217–221 (doi:10.1046/j.1468-1331.2000.00053.x) [DOI] [PubMed] [Google Scholar]