Abstract
There is considerable debate as to whether human females bias the sex ratio of their offspring as a function of their own condition. We apply the Trivers–Willard prediction—that mothers in poor condition will overproduce daughters—to a novel measure of condition, namely wife rank within a polygynous marriage. Using a large-scale sample of over 95 000 Rwandan mothers, we show that lower-ranking polygynous wives do indeed have significantly more daughters than higher-ranking polygynous wives and monogamously married women. This effect remains when controlling for potential confounds such as maternal age. We discuss these results in reference to previous work on sex-ratio adjustment in humans.
Keywords: sex ratios, human, Rwanda, polygyny, Trivers–Willard hypothesis
1. Introduction
In their classic paper, Trivers & Willard (1973) argued that if maternal condition affects the reproductive success of sons and daughters differently, mothers should bias the sex ratio of their offspring accordingly. Where sons are more strongly affected, mothers in good condition should have more sons than daughters and should invest more in those sons. In contrast, mothers in relatively poor condition should concentrate their investment in daughters. The Trivers–Willard hypothesis has had rather mixed success when applied to vertebrates (e.g. Clutton-Brock et al. 1986; Brown & Silk 2002; Sheldon & West 2004).
Several recent studies in humans have documented patterns of sex-ratio variation consistent with the Trivers–Willard hypothesis (Bereczkei & Dunbar 1997; Gibson & Mace 2003; Hopcroft 2005; Almond & Edlund 2007; Cameron & Dalerum 2009), while others have found no effects (Zaldivar et al. 1991) or negligible effect sizes (Chacon-Puignau & Jaffe 1996). This is partly owing to differences in which aspects of female condition are considered. Measures used include the woman's education (Almond & Edlund 2007), the presence of an investing man (Almond & Edlund 2007), the man's economic resources (Hopcroft 2005; Cameron & Dalerum 2009) and the woman's upper-arm muscle mass (Gibson & Mace 2003).
Here, we apply the Trivers–Willard prediction to a novel measure of maternal situation, namely wife rank within polygynous marriage, and a novel population, that of Rwanda. Like most human societies (Marlowe 2000), Rwandan society allows polygynous marriage. While male reproductive success is always enhanced by adding an extra wife to the household, polygyny may be less beneficial for women. Each additional wife in the household dilutes available resources and male investment, and thus women should only be expected to join as lower-ranking wives when there are few or low-quality alternatives available (Hartung 1982; Pollet & Nettle 2009), or under coercion (e.g. Chisholm & Burbank 1991). In line with this argument, several studies have documented fitness costs involved in becoming a co-wife. Women in polygynous marriages have lower fertility (e.g. Dorjahn 1958) and suffer higher stress (e.g. Jankowiak et al. 2005) than women in monogamous marriages, while their children have higher mortality (e.g. Omariba & Boyle 2007) and poorer growth rates (see Bove & Valeggia 2009 for review). These costs appear to fall disproportionately on the lower-ranking wives and their children (Gibson & Mace 2007; Bove & Valeggia 2009). One previous study showed that women in polygynous marriages had relatively more daughters than those in monogamous marriages (Whiting 1993). However, this study did not take into account wife rank and used aggregated rather than individual-level data.
In view of this, and in line with the Trivers–Willard hypothesis, we predict that lower-ranking co-wives will bear significantly more daughters than wives of other ranks or monogamously married women.
2. Material and methods
The data are from a census of Rwanda conducted by the Ministère des Finances et de la Planification Economique, Commission Nationale du Recensement. The census was conducted by face-to-face interview during 14 days of fieldwork in August 2002 and aimed to represent all individuals present in the country. Data from a 10 per cent sample of households is freely available through IPUMS-I (Integrated Public Use Microdata Series International) and is designed to be representative of the complete census population (nindividuals = 843 392). From each of the households in the 10 per cent sample, we selected all married women who reported they had children and for whom data were complete (n = 96 880). We classified each of these women as either monogamously or polygynously married, and for the latter group as either the first, second or lower-order co-wife.
Rwanda had 12 provinces at the time of the survey (Minnesota Population Center 2007). As control variables we included mother's age at time of the census, province, urbanization (urban/rural), ownership of the dwelling (owned/rented/unknown) and mother's educational attainment (years of schooling) (table 1 of the electronic supplementary material). Our dependent variable was the son ratio: the number of sons ever born as a proportion of the number of children ever born, as reported by the mothers at the time of the survey. This variable ranges from 0 (all daughters) to 1 (all sons). Descriptive statistics for all the variables used are given in table 1 of the electronic supplementary material.
We used linear mixed modelling to examine the independent effect of marriage type (only wife/first co-wife/second co-wife/third or lower-order co-wife) on son ratio. These models allow us to take into account possible correlated effects between variables (SPSS 2005). We tested models with random effects (slopes and intercepts) and estimated parameters using restricted maximum likelihood for the final model. From the subset of models including all parameters with significance p < 0.1, we selected the best-fitting model using Akaike's Information Criterion (AIC) and Schwarz's Bayesian Information Criterion (BIC) (see Kuha 2004). This model had absolute parameter, likelihood and Hessian convergence (SPSS 2005). For each parameter in the final model, we present estimates, standard errors and t-tests of significance. To confirm that lower-ranking wives were indeed in poorer condition than other wives, we tested whether they had lower fertility by constructing a similar linear mixed model but with number of children ever born, instead of son ratio, as the dependent variable.
3. Results
The linear mixed model for fertility showed that third or lower-ranking co-wives bore significantly fewer children than other mothers (β = −0.07 to −0.12; all p < 0.01; table 2 of the electronic supplementary material).
The best-fitting model for son ratio contained just mother's age, province and type of marriage, with no random intercept or slope, and gave a significantly better fit than a null model (χ2 = 143 884; d.f. = 16; p < 0.00 001). Mother's education, urbanization and ownership of the dwelling did not significantly predict son ratio and were excluded from the final model (p > 0.25). As women could have reproduced beyond the census point, we re-ran the analysis and limited the sample to women over age 45. This gave very similar estimates (table 3 of the electronic supplementary material), as did the second best-fitting model which also contained a random intercept (ΔAIC = 2; ΔBIC = 11.5).
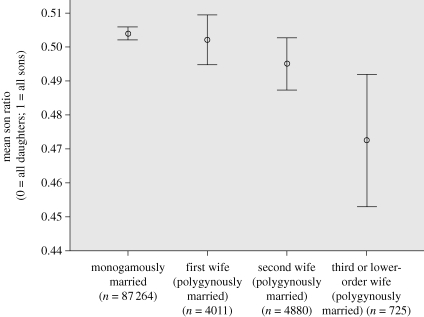
Third or lower-ranking wives had significantly more daughters than any other marriage type (table 1 and figure 1). These differences were significant and sizeable (β = 0.07–0.11), with the largest difference between third or lower-ranking wives (106 daughters for every 100 sons) and those in monogamous marriages (99 daughters for every 100 sons). By substituting reference categories, we compared other types of marriage. Son ratios did not differ between monogamously married and first or second co-wives (respectively, β = −0.005; p > 0.75 and β = 0.024; p = 0.1), nor between first and second co-wives (β = 0.029; p = 0.17). The effects were found while controlling for other variables in the model.
Table 1.
Parameter estimates and concomitant test characteristics from a linear mixed model for son ratio (number of sons ever born/number of children ever born).
| β | s.e. | t | p | ||
|---|---|---|---|---|---|
| intercept | −0.106 | 0.039 | −2.72 | 0.007 | |
| province | Kigali City | −0.019 | 0.019 | −0.978 | 0.328 |
| Kigali Ngali | −0.026 | 0.017 | −1.539 | 0.124 | |
| Gitarama | −0.014 | 0.017 | −0.797 | 0.425 | |
| Butare | −0.015 | 0.017 | −0.851 | 0.395 | |
| Gikongoro | −0.046 | 0.018 | −2.505 | 0.012 | |
| Cyangugu | −0.018 | 0.018 | −0.977 | 0.328 | |
| Kibuye | −0.048 | 0.019 | −2.580 | 0.010 | |
| Gisenyi | −0.034 | 0.017 | −2.028 | 0.043 | |
| Ruhengeri | −0.043 | 0.017 | −2.550 | 0.011 | |
| Byumba | −0.045 | 0.017 | −2.596 | 0.009 | |
| Kibungo | −0.029 | 0.017 | −1.645 | 0.100 | |
| Umutara | 0 | 0 | |||
| age | (1 s.d.) | −0.012 | 0.004 | −2.72 | 0.007 |
| marriage type | monogamously married | 0.113 | 0.038 | 3.03 | 0.002 |
| first wife (polygynous) | 0.112 | 0.041 | 2.928 | 0.003 | |
| second wife (polygynous) | 0.07 | 0.04 | 2.236 | 0.025 | |
| third or lower-order wife (polygynous) | 0 | 0 |
Figure 1.
Mean son ratio (number of sons ever born/number of children ever born) by marriage type. Bars represent 95 per cent confidence intervals.
The control variables showed that older women tended to have relatively more daughters than sons (β = −0.01; p = 0.007). Women who lived in Umutara, Gitarama, Kigali city and Butare had relatively more sons than women from other provinces (figure 1 of the electronic supplementary material), while women from Gikongoro, Kibuye and Byumba tended to have relatively more daughters than women from other provinces.
4. Discussion
Our findings show that low-ranking wives, of third order or lower, have lower fertility than other women, suggesting that they are in poorer condition. In line with the Trivers–Willard hypothesis, these low-ranking wives have relatively more daughters than higher-ranking and monogamously married wives. This fits with previous findings from non-Western cultures documenting female-biased sex ratios as a function of maternal condition (e.g. Bereczkei & Dunbar 1997). Mothers in poor condition, here lower-ranking co-wives in a polygynous marriage, may overproduce daughters because these give them greater fitness returns than sons.
The applicability of the Trivers–Willard hypothesis to our data from African polygynous societies rests on two key assumptions. The first is that lower-ranking wives receive a smaller share of their husband's resources than wives in other situations. Data from the Luo of Lake Victoria (Ssennyonga 1997), as well as studies of the health of women and their children (Gibson & Mace 2007; Bove & Valeggia 2009), suggest that this is the case. The second assumption is that the prospects of sons and daughters are affected differently by this unequal distribution of resources. This seems likely, given that male reproductive success is extremely variable in these societies and is strongly dependent on adult status and resources (Borgerhoff Mulder 1990; Cronk 1991; Nettle & Pollet 2008).
Our findings could also be explained by the maternal dominance hypothesis (Grant 1996), which states that dominant women are more likely to conceive sons because they have higher circulating levels of testosterone. Marriage rank could be related to testosterone levels in our study population, but we lack the data to investigate this. However, this hypothesis is a proximate explanation and is perfectly compatible with the ultimate explanation we have focused on here. Another possible proximate mechanism is the selective resorption of male foetuses by mothers in poor condition (Krackow 1995). Yet, the extent to which this drives adaptive sex-ratio variation in mammals is debated (Krackow 1995; Krüger et al. 2005). Alternatively, it is possible that the patterns we have documented are entirely non-adaptive and result from a greater mortality of male foetuses in poor-condition mothers (Kruuk et al. 1999). These demographic data do not allow us to distinguish male-biased mortality from selective resorption and other proximate mechanisms.
There are some limitations to our study. Some of the monogamous wives would subsequently have become polygynous, joined by one or more co-wives. Indeed, this may partly explain the close similarity in son ratios between monogamously married wives and first wives in polygynous marriages. If we had access to longitudinal data on marriage type and child production for this population, the distribution of women over the four marriage-type categories would be different. It is difficult to see, however, how this would alter the patterns of sex-ratio bias we describe.
References
- Almond D., Edlund L.2007Trivers–Willard at birth and one year: evidence from US natality data 1983–2001. Proc. R. Soc. B 274, 2491–2496 (doi:10.1098/rspb.2007.0524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereczkei T., Dunbar R. I. M.1997Female-biased reproductive strategies in a Hungarian Gypsy population. Proc. R. Soc. Lond. B 264, 17–22 (doi:10.1098/rspb.1997.0003) [Google Scholar]
- Borgerhoff Mulder M.1990Kipsigis women's preferences for wealthy men: evidence for female choice in mammals. Behav. Ecol. Sociobiol. 27, 255–264 [DOI] [PubMed] [Google Scholar]
- Bove R., Valeggia C. M.2009Polygyny and women's health in sub-Saharan Africa. Soc. Sci. Med. 68, 21–29 (doi:10.1016/j.socscimed.2008.09.045) [DOI] [PubMed] [Google Scholar]
- Brown G. R., Silk J. B.2002Reconsidering the null hypothesis: is maternal rank associated with sex ratios in primate groups? Proc. Natl Acad. Sci USA 99, 11 252–11 255 (doi:10.1073/pnas.162360599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron E. Z., Dalerum F.2009A Trivers–Willard effect in contemporary humans: male-biased sex ratios among billionaires. PLoS One 4, e4195 (doi:10.1371/journal.pone.0004195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon-Puignau G. C., Jaffe K.1996Sex ratio at birth deviations in modern Venezuela: the Trivers–Willard effect. Social Biol. 43, 257–270 [DOI] [PubMed] [Google Scholar]
- Chisholm J., Burbank V.1991Monogamy and polygyny in South East Arnhem Land: male coercion and female choice. Ethol. Sociobiol. 12, 291–313 (doi:10.1016/0162-3095(91)90022-I) [Google Scholar]
- Clutton-Brock T. H., Albon S. D., Guinness F. E.1986Great expectations: dominance, breeding success and offspring sex ratios in red deer. Anim. Behav. 34, 460–471 [Google Scholar]
- Cronk L.1991Wealth, status and reproductive success among the Mukogodo of Kenya. Am. Anthropol. 93, 345–360 (doi:10.1525/aa.1991.93.2.02a00040) [Google Scholar]
- Dorjahn V. R.1958Fertility, polygyny, and their interrelations in Temne society. Am. Anthropol. 60, 838–860 (doi:10.1525/aa.1958.60.5.02a00050) [Google Scholar]
- Gibson M. A., Mace R.2003Strong mothers bear more sons in rural Ethiopia. Proc. R. Soc. Lond. B 270, S108–S109 (doi:10.1098/rsbl.2003.0031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M. A., Mace R.2007Polygyny, reproductive success and child health in rural Ethiopia: why marry a married man? J. Biosoc. Sci. 39, 287–300 (doi:10.1017/S0021932006001441) [DOI] [PubMed] [Google Scholar]
- Grant V. J.1996Sex determination and the maternal dominance hypothesis. Hum. Reprod. 11, 2371–2375 [DOI] [PubMed] [Google Scholar]
- Hartung J.1982Polygyny and inheritance of wealth. Curr. Anthropol. 23, 1–12 (doi:10.1086/202775) [Google Scholar]
- Hopcroft R. L.2005Parental status and differential investment in sons and daughters: Trivers–Willard revisited. Soc. Forces 83, 1111–1136 (doi:10.1353/sof.2005.0035) [Google Scholar]
- Jankowiak W., Sudakov M., Wilreker B. C.2005Co-wife conflict and co-operation. Ethnology 44, 81–98 [Google Scholar]
- Krackow S.1995Potential mechanisms for sex ratio adjustment in birds and mammals. Biol. Rev. 70, 225–241 (doi:10.1111/j.1469-185X.1995.tb01066.x) [DOI] [PubMed] [Google Scholar]
- Krüger O., Radford A. N., Anderson C., Liversidge R.2005Successful sons or superior daughters: sex-ratio variation in springbok. Proc. R. Soc. B 272, 375–381 (doi:10.1098/rspb.2004.2943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruuk L. E. B., Clutton-Brock T. H., Albon S. D., Pemberton J. M., Guinness F. E.1999Population density affects sex ratio variation in red deer. Nature 399, 459–461 (doi:10.1038/20917) [DOI] [PubMed] [Google Scholar]
- Kuha J.2004AIC and BIC: comparisons of assumptions and performance. Sociol. Methods Res. 33, 188–229 (doi:10.1177/0049124103262065) [Google Scholar]
- Marlowe F.2000Paternal investment and the human mating system. Behav. Process. 51, 45–61 (doi:10.1016/S0376-6357(00)00118-2) [DOI] [PubMed] [Google Scholar]
- Minnesota Population Center 2007Integrated public use microdata series—International: version 3.0 Minneapolis, MN: University of Minnesota [Google Scholar]
- Nettle D., Pollet T. V.2008Natural selection for male wealth. Am. Nat. 172, 658–666 (doi:10.1086/591690) [DOI] [PubMed] [Google Scholar]
- Omariba D. W. R., Boyle M. H.2007Family structure and child mortality in sub-Saharan Africa: cross-national effects of polygyny. J. Marriage Fam. 69, 528–543 [Google Scholar]
- Pollet T. V., Nettle D.2009Market forces affect patterns of polygyny in Uganda. Proc. Natl Acad. Sci. USA 106, 2114–2117 (doi:10.1073/pnas.0810016106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon B. C., West S. A.2004Maternal dominance, maternal condition, and offspring sex ratio variation in ungulate mammals. Am. Nat. 163, 40–54 (doi:10.1086/381003) [DOI] [PubMed] [Google Scholar]
- SPSS 2005Linear mixed-effects modeling in SPSS: an introduction to the MIXED procedure Chicago, IL: SPSS Inc [Google Scholar]
- Ssennyonga J. W.1997Polygyny and resource allocation in the Lake Victoria basin. In African families and the crisis of social change (eds Weisner T. S., Bradley C., Kilbride P. L.), pp. 268–282 Westport, CT: Bergin and Garvey [Google Scholar]
- Trivers R. L., Willard D. E.1973Natural selection of parental ability to vary the sex ratio of offspring. Science 191, 249–263 (doi:10.1126/science.1108197) [DOI] [PubMed] [Google Scholar]
- Whiting J. W. M.1993The effect of polygyny on sex ratio at birth. Am. Anthropol. 95, 435–442 (doi:10.1525/aa.1993.95.2.02a00090) [Google Scholar]
- Zaldivar M. E., Lizarralde R., Beckerman S.1991Unbiased sex ratios among the Bari: an evolutionary interpretation. Hum. Ecol. 19, 469–498 (doi:10.1007/BF00889791) [DOI] [PubMed] [Google Scholar]