Abstract
Single cell recording studies have resulted in a detailed understanding of motion-sensitive neurons in non-human primate visual cortex. However, it is not known to what extent response properties of motion-sensitive neurons in the non-human primate brain mirror response characteristics of motion-sensitive neurons in the human brain. Using a motion adaptation paradigm, the direction aftereffect, we show that changes in the activity of human motion-sensitive neurons to moving dot patterns that differ in dot density bear a strong resemblance to data from macaque monkey. We also show a division-like inhibition between neural populations tuned to opposite directions, which also mirrors neural-inhibitory behaviour in macaque. These findings strongly suggest that motion-sensitive neurons in human and non-human primates share common response and inhibitory characteristics.
Keywords: motion processing, motion adaptation, direction aftereffect, motion-sensitive neurons
1. Introduction
Decades of electrophysiological studies have resulted in a detailed characterization of response properties of neurons in monkey visual cortex, and of motion-sensitive neurons in particular (Dubner & Zeki 1974; Albright 1984; Newsome & Paré 1988; Lagae et al. 1989; Krekelberg & Albright 2005). This approach typically uses neuronal spiking as a measure of neural activity. However, it is not known to what extent motion-sensitive neurons in the non-human primate brain respond in a similar fashion to those in the human brain. While functional magnetic resonance imaging (fMRI) studies have established an effective means with which to study motion processing in the human brain (Watson et al. 1993; Tootell et al. 1995; Morrone et al. 2000; Bartels et al. 2008), it is not yet clear how neuronal spiking influences fMRI signal (Heeger & Ress 2002; Logothetis 2007, 2008; Viswanathan & Freeman 2007).
Previous studies have revealed a causal relationship between spike discharge of direction-sensitive neurons in monkey medial temporal (MT/V5) area and behavioural measurements of direction perception (Salzman et al. 1992; Ditterich et al. 2003). A number of factors determine the spike discharge of direction-sensitive neurons; including how well stimulus motion direction is matched to the neurons' preferred direction (Albright 1984; Snowden et al. 1992), and the ‘strength’ of the motion signal. Motion signal strength can be altered by manipulating motion coherence (Britten et al. 1993), stimulus contrast (Sclar et al. 1990) or dot density. In the case of dot density, random dot kinematograms (RDKs) have been used to demonstrate that motion-sensitive neurons rapidly increase their spiking as the number of moving dots within their receptive fields increases. This rapid increase in spike discharge plateaus at relatively low dot densities (Snowden et al. 1991, 1992). When additional dots moving in the neurons' anti-preferred direction are added, the resulting density-tuning functions indicate a division-like inhibitory interaction between neurons tuned to opposite directions (Snowden et al. 1991).
We made use of a motion-adaptation phenomenon, the direction aftereffect (DAE), to investigate whether motion-sensitive neurons in the human brain respond to varying dot density in a similar manner to macaque. The DAE describes the misperception of a motion direction following prolonged viewing of (adaptation to) a different direction of motion (Levinson & Sekuler 1976; Patterson & Becker 1996; Curran et al. 2006). Motion adapters that evoke a stronger response in targeted neurons usually result in greater changes in the neurons' direction tuning functions, which in turn, are thought to impact on DAE magnitude (Kohn 2007). If motion-sensitive neurons in the human brain respond in a similar manner to macaque, increasing adapter dot density will result in changes in neural spike discharge similar to those reported for macaque. Given the reported relationship between neural spiking and aftereffect magnitude, any changes in the levels of neural activity will be revealed through DAE measurements; with increasing neural activity leading to increasing DAE magnitude.
2. Material and methods
(a). Stimuli
Stimuli were displayed on a Mitsubishi 2070SB monitor, and comprised RDKs presented within a circular aperture (7 deg2), with each RDK containing equal numbers of black and white dots (dot diameter = 1.8 arcmin) against a mean luminance background (59 cd per m2). The monitor was driven by a Cambridge Research Systems Visage graphics board at a frame rate of 80 Hz.
(b). Procedure
During the initial motion adaptation phase (30 s duration), observers were presented with a random dot stimulus moving either 45° to the left or 45° to the right of vertical (upwards) at a constant speed of 2.5° s−1. The adapter direction was the same for all subsequent top-up phases. Both adapter and test stimuli had a central fixation spot to help maintain fixation. In the test phase following adaptation, observers judged whether the test stimulus (speed 2.5° s−1, duration 200 ms) was moving left or right of vertical up. To maintain adaptation, test phases alternated with adaptation top-up phases of 5 s duration. Test stimulus motion direction was chosen by an adaptive method of constant stimuli (adaptive probit estimation), a method that dynamically updates the set of stimulus motion directions being presented depending on the observer's previous responses (Treutwein 1995). The stimulus values are selected to optimize the estimation of the ‘point of subjective equality’, in our case the direction the test stimulus was moving when it was perceived as moving vertically upwards. DAE magnitude was taken as the difference between the two directions (perceived and actual). Half the psychometric functions were generated following adaptation to motion 45° clockwise to vertically upwards, and half were generated following adaptation to motion 45° anticlockwise to vertically upwards; thus controlling for any potential difference between subjective and objective measures of vertical. A range of adapter dot densities was used (4–40 dots per deg2). Observers generated four psychometric functions per adapter density condition, with each psychometric function being derived from 64 trials. Test stimulus dot density remained fixed at 40 dots per deg2. The interval between testing with different adapter densities was at least 15 min, thus ensuring recovery from adaptation.
3. Results and discussion
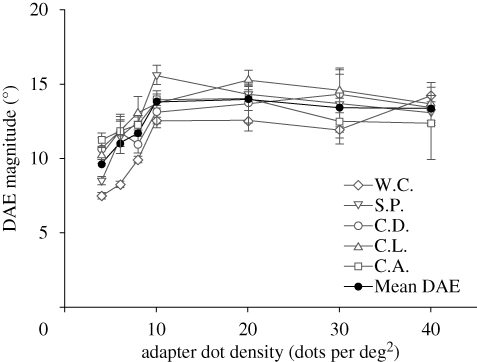
The results show the same pattern for all observers (figure 1). DAE magnitude rises sharply with increasing adapter dot density, and asymptotes at an adapter dot density of approximately 10 dots per deg2. This suggests that the underlying motion-sensitive neurons targeted by our adapter stimulus respond differentially to a range of low dot densities, and that their responses saturate at or around 10 dots per deg2. This is consistent with the macaque data (Snowden et al. 1991, 1992), in which an initial rapid increase in neuronal spiking asymptotes at dot densities up to 8 dots per deg2.
Figure 1.
Magnitude of the DAE as a function of adapter dot density. Light grey symbols plot individual DAE density functions; filled circles plot mean DAE density function. Following an initial rapid increase (mean slope = 0.66), DAE magnitude asymptotes at an adapter density of approximately 10 dots per deg2, and remains constant at higher densities (mean slope = −0.02). Error bars are ±1 s.e.
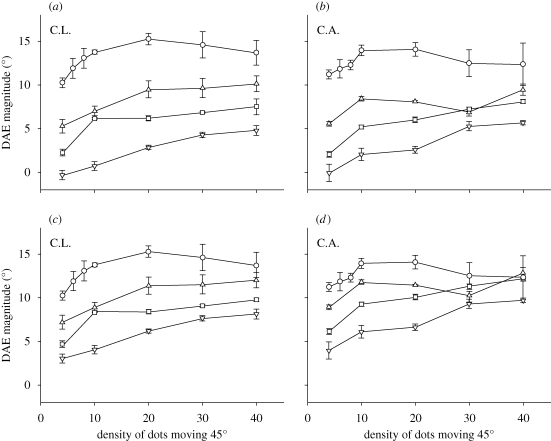
The data from our first experiment suggest that motion-sensitive neurons in human and macaque exhibit similar density-response functions. This raises the question do they also share similar inhibitory mechanisms? Macaque single cell recording data show that the addition of dots moving in the neuron's anti-preferred direction alters the shape of the neuron's density-response function (Snowden et al. 1991). The rise in neural spiking is less steep with increasing dot density; and continues to rise for a range of densities that cause no increase in response to the preferred direction alone. This is taken as evidence for division-like inhibition between neurons tuned to opposite directions. We repeated our experiment; but, this time the adapter stimuli contained additional dots moving in the opposite direction to the 45° direction dots. The density of these ‘opposite-direction’ dots was set to one of three values—1, 7 or 30 dots per deg2. As in the previous experiment, DAE magnitude was measured as a function of varying the number of dots in the adapter stimulus. The resulting data (figure 2a,b) bear a striking resemblance to macaque data. Adding dots moving in the opposite direction causes a clear reduction in DAE magnitude, and increasing their number further reduces DAE strength. In addition, the DAE density tuning functions continue to rise beyond the dot density at which the asymptotic DAE was reached in our first experiment. It is possible that reduced DAE magnitude is a consequence of the two superimposed motion directions inducing DAEs of opposite sign. However, the observed changes in the DAE density function cannot be explained solely in terms of a subtractive combination of opposing DAEs induced by the two directions. We had observers adapt to stimuli containing just the opposite-direction dots. The three dot densities (1, 7 and 30) induced small DAEs (C.L.: 1.9°, 2.2° and 3.3°; C.A.: 3.4°, 4.1° and 4.1°); but these small effects cannot account for the reduced DAEs found in experiment 2 (figure 2c,d).
Figure 2.
(a,b) Results of experiment 2, in which observers adapted to bidirectional random dot stimuli. DAE is plotted as a function of the density of the dots moving 45° from vertical. Additional dots moving in the opposite direction were also present in the adapter and had a density of 1 dot per deg2 (triangles), 7 dots per deg2 (squares), or 30 dots per deg2 (inverted triangles). The top DAE function (circles), in which there were no opposite-direction dots, is taken from experiment 1. The changes in the DAE density tuning function cannot be explained in terms of an opposing DAE induced by the opposite-direction dots. (c, d) When the small DAEs induced by the three opposite-direction densities are taken into account, there remains a clear difference in the tuning functions; thus demonstrating that changes in the DAE density tuning function are not solely attributable to a subtractive combination of the DAEs induced by the two directions.
The results from these two experiments provide compelling evidence that:
The responses of motion-sensitive neurons in the human brain to motion density mirror those reported for motion-sensitive neurons in macaque.
The division-like inhibition between macaque neurons tuned to opposite directions also applies to motion-sensitive neurons in the human brain.
It is only a matter of time before brain imaging technology reaches the point where spiking activity of human motion-sensitive neurons can be accurately recorded. Our data suggest that, when this point is reached, unequivocal evidence will be found for common response and inhibitory characteristics of motion-sensitive neurons in human and non-human primates.
Acknowledgements
We are grateful to A. Johnston, C. Benton and C. Clifford for their suggestions and comments.
References
- Albright T. D.1984Direction and orientation selectivity of neurons in visual area MT of the macaque. J. Neurophysiol. 52, 1106–1130 [DOI] [PubMed] [Google Scholar]
- Bartels A., Logothetis N. K., Moutoussis K.2008fMRI and its interpretations: an illustration on directional selectivity in area V5/MT. Trends Neurosci. 31, 444–453 (doi:10.1016/j.tins.2008.06.004) [DOI] [PubMed] [Google Scholar]
- Britten K. H., Shadlen M. N., Newsome W. T., Movshon J. A.1993Responses of neurons in macaque MT to stochastic motion signals. Visual Neurosci. 10, 1157–1169 (doi:10.1017/S0952523800010269) [DOI] [PubMed] [Google Scholar]
- Curran W., Clifford C. W., Benton C. P.2006The direction aftereffect is driven by adaptation of local motion detectors. Vision Res. 46, 4270–4278 (doi:10.1016/j.visres.2006.08.026) [DOI] [PubMed] [Google Scholar]
- Ditterich J., Mazurek M. E., Shalden M. N.2003Microstimulation of visual cortex affects the speed of perceptual decisions. Nat. Neurosci. 6, 891–898 (doi:10.1038/nn1094) [DOI] [PubMed] [Google Scholar]
- Dubner R., Zeki S. M.1974Response properties and receptive fields of cells in an anatomically defined region of the superior temporal sulcus in the monkey. Brain Res. 35, 528–532 (doi:10.1016/0006-8993(71)90494-X) [DOI] [PubMed] [Google Scholar]
- Heeger D. J., Ress D.2002What does fMRI tell us about neuronal activity? Nat. Rev. 3, 142–151 (doi:10.1038/nrn730) [DOI] [PubMed] [Google Scholar]
- Kohn A.2007Visual adaptation: physiology, mechanisms, and functional benefits. J. Neurophysiol. 97, 3155–3164 (doi:10.1152/jn.00086.2007) [DOI] [PubMed] [Google Scholar]
- Krekelberg B., Albright T. D.2005Motion mechanisms in macaque MT. J. Neurophysiol. 93, 2908–2921 (doi:10.1152/jn.00473.2004) [DOI] [PubMed] [Google Scholar]
- Lagae L., Gulyas B., Raiguel S., Orban G. A.1989Laminar analysis of motion information processing in macaque V5. Brain Res. 496, 361–367 (doi:10.1016/0006-8993(89)91089-5) [DOI] [PubMed] [Google Scholar]
- Levinson E., Sekuler R.1976Adaptation alters perceived direction of motion. Vision Res. 16, 779–781 (doi:10.1016/0042-6989(76)90189-9) [DOI] [PubMed] [Google Scholar]
- Logothetis N. K.2007The ins and outs of fMRI signals. Nat. Neurosci. 10, 1230–1232 (doi:10.1038/nn1007-1230) [DOI] [PubMed] [Google Scholar]
- Logothetis N. K.2008What we can do and what we cannot do with fMRI. Nature 453, 869–878 (doi:10.1038/nature06976) [DOI] [PubMed] [Google Scholar]
- Morrone M. C., Tosetti M., Montanaro D., Fiorentini A., Cioni G., Burr D. C.2000A cortical area that responds specifically to optic flow, revealed by fMRI. Nat. Neurosci. 3, 1322–1328 (doi:10.1038/81860) [DOI] [PubMed] [Google Scholar]
- Newsome W. T., Paré E. B.1988A selective impairment of motion perception following lesions of the middle temporal visual area (MT). J. Neurosci. 8, 2201–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R., Becker S.1996Direction-selective adaptation and simultaneous contrast induced by stereoscopic (cyclopean) motion. Vision Res. 36, 1773–1781 (doi:10.1016/0042-6989(95)00239-1) [DOI] [PubMed] [Google Scholar]
- Salzman C. D., Murasugi C. M., Britten K. H., Newsome M. T.1992Microstimulation in visual area MT: effects on direction discrimination performance. J. Neurosci. 12, 2331–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclar G., Maunsell J. H., Lennie P.1990Coding of image contrast in central visual pathways of the macaque monkey. Vision Res. 30, 1–10 (doi:10.1016/0042-6989(90)90123-3) [DOI] [PubMed] [Google Scholar]
- Snowden R. J., Treue S., Erickson R. G., Andersen R. A.1991The response of area MT and V1 neurons to transparent motion. J. Neurosci. 11, 2768–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden R. J., Treue S., Andersen R. A.1992The response of neurons in areas V1 and MT of the alert rhesus monkey to moving random dot patterns. Exp Brain Res. 88, 389–400 (doi:10.1007/BF02259114) [DOI] [PubMed] [Google Scholar]
- Tootell R. B., Reppas J. B., Dale A. M., Look R. B., Sereno M. I., Malach R., Brady T. J., Rosen B. R.1995Visual motion aftereffect in human cortical area MT revealed by functional magnetic resonance imaging. Nature 375, 139–141 (doi:10.1038/375139a0) [DOI] [PubMed] [Google Scholar]
- Treutwein B.1995Adaptive psychophysical procedures. Vision Res. 35, 2503–2522 [PubMed] [Google Scholar]
- Viswanathan A., Freeman R. D.2007Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat. Neurosci. 10, 1308–1312 (doi:10.1038/nn1977) [DOI] [PubMed] [Google Scholar]
- Watson J. D. G., Myers R., Frackowiak R. S. J., Hajnal J. V., Woods R. P., Mazziotta J. C., Shipp S., Zeki S.1993Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cerebral Cortex 3, 79–94 (doi:10.1093/cercor/3.2.79) [DOI] [PubMed] [Google Scholar]